The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL)
Abstract
1. Introduction
2. Systemic ALK-Positive Anaplastic Large Cell Lymphoma (ALK+ ALCL)
2.1. Definition and Clinical Features
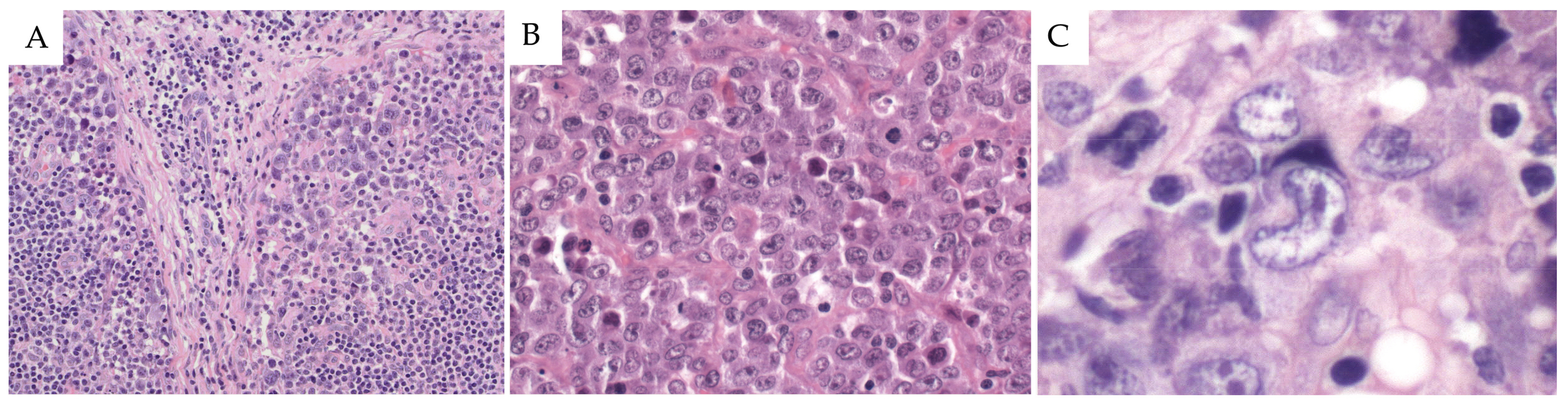
2.2. Morphological Features
2.2.1. Common Pattern
2.2.2. Small Cell Pattern
2.2.3. Lymphohistiocytic Pattern
2.2.4. Hodgkin’s-Like Pattern
2.2.5. Composite Pattern
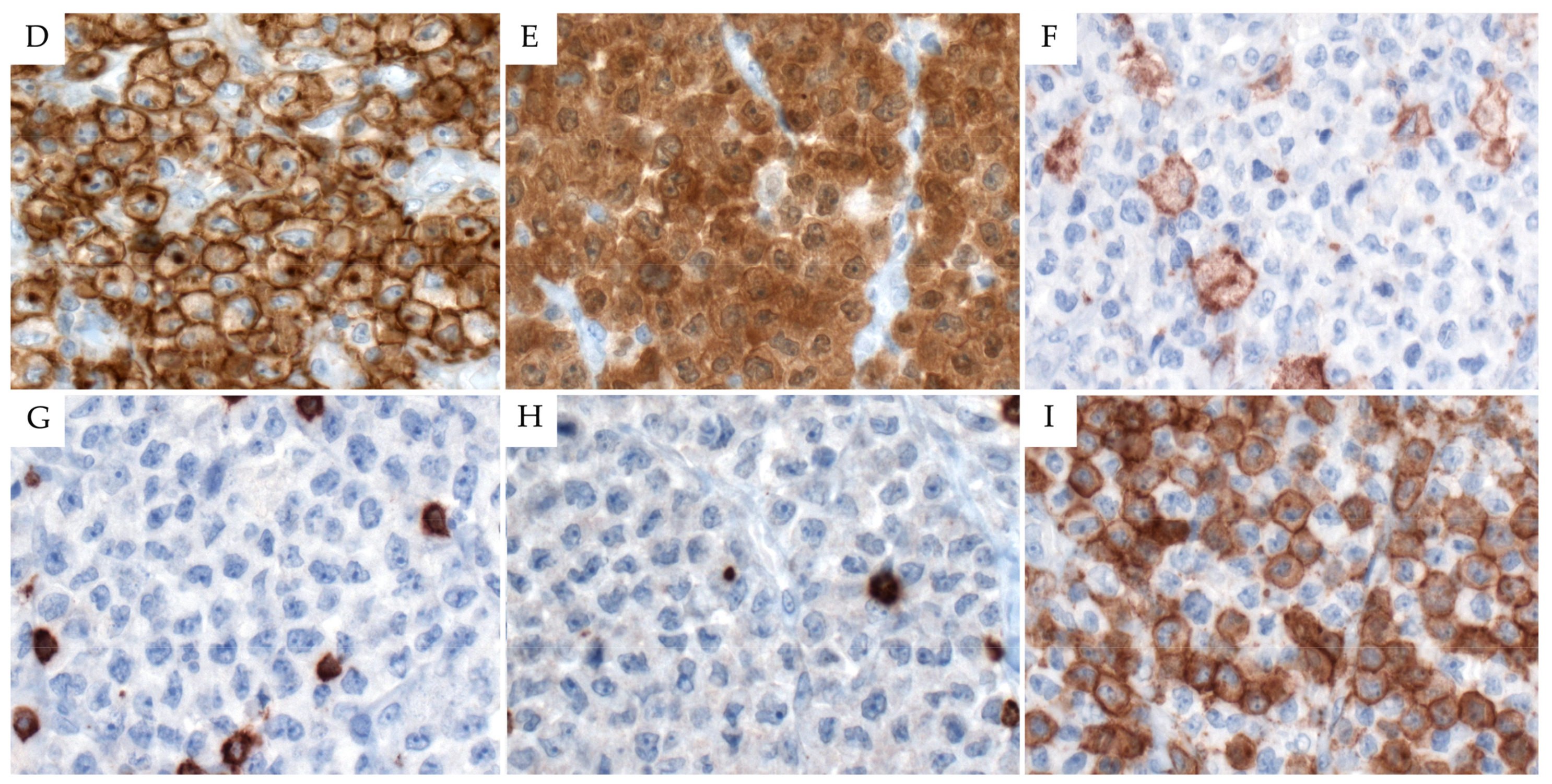
2.3. Immunophenotype
2.4. Genetic and Molecular Findings
2.5. Differential Diagnosis
3. Systemic ALK-Negative Anaplastic Large Cell Lymphoma (ALK− ALCL)
3.1. Definition and Clinical Features
3.2. Morphological Features
3.3. Immunophenotype
3.4. Genetic and Molecular Findings
3.5. Differential Diagnosis
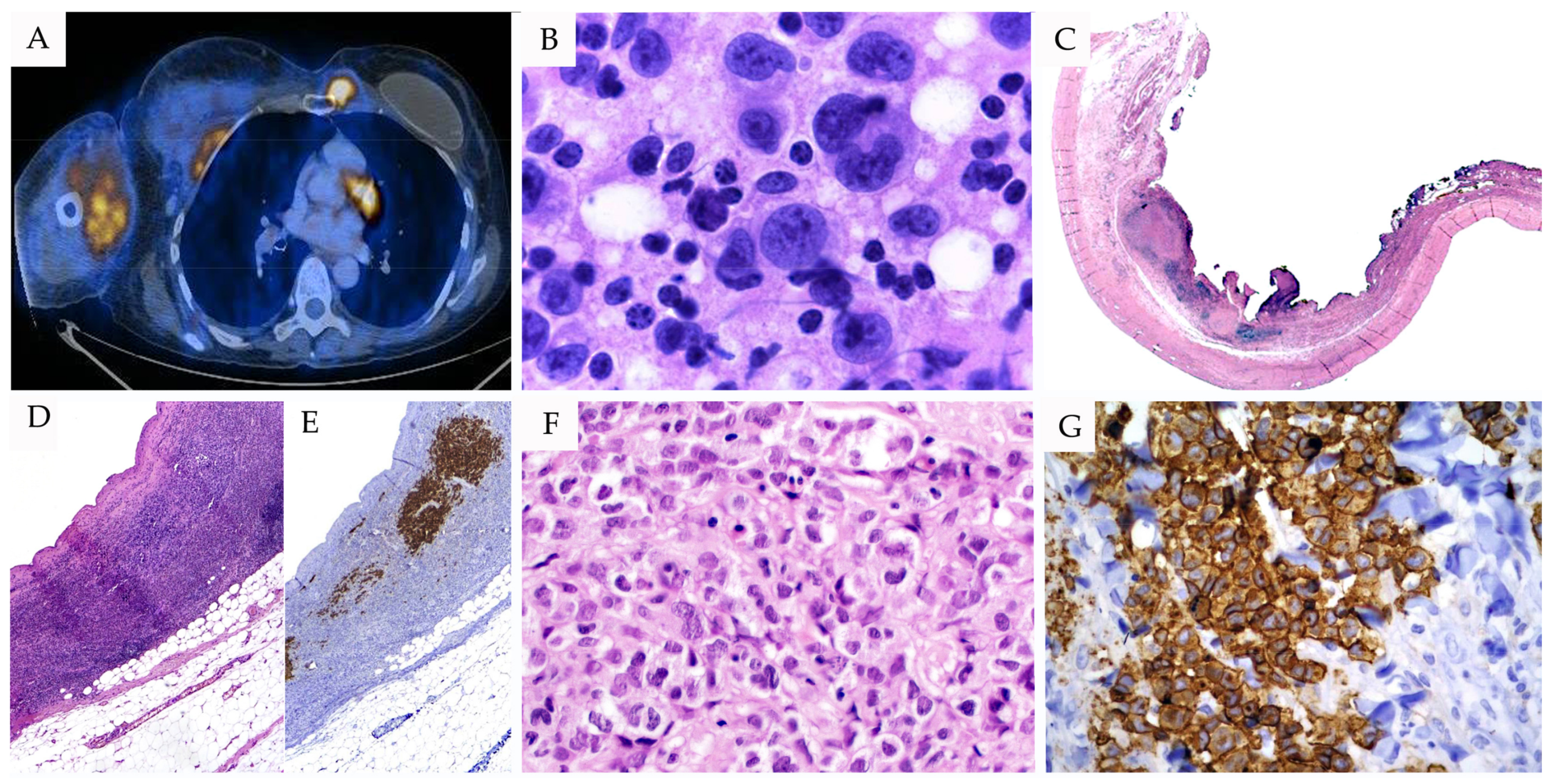
4. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BI-ALCL)
4.1. Definition and Clinical Features
4.2. Morphological Features
4.3. Immunophenotype
4.4. Genetic and Molecular Findings
4.5. Differential Diagnosis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swerdlow, S.H.C.E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Arber, D.A.; Hasserjian, R.P.; Le Beau, M.M.; Orazi, A.; et al. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; ISBN 978-92-832-4494-3. [Google Scholar]
- Stein, H.; Mason, D.Y.; Gerdes, J.; O’Connor, N.; Wainscoat, J.; Pallesen, G.; Gatter, K.; Falini, B.; Delsol, G.; Lemke, H.; et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66, 848–858. [Google Scholar] [PubMed]
- Stansfeld, A.G.; Diebold, J.; Noel, H.; Kapanci, Y.; Rilke, F.; Kelenyi, G.; Sundstrom, C.; Lennert, K.; van Unnik, J.A.; Mioduszewska, O.; et al. Updated Kiel classification for lymphomas. Lancet 1988, 1, 292–293. [Google Scholar] [CrossRef]
- Harris, N.L.; Jaffe, E.S.; Stein, H.; Banks, P.M.; Chan, J.K.; Cleary, M.L.; Delsol, G.; De Wolf-Peeters, C.; Falini, B.; Gatter, K.C.; et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84, 1361–1392. [Google Scholar] [CrossRef]
- O’Connor, N.T.; Stein, H.; Gatter, K.C.; Wainscoat, J.S.; Crick, J.; Al Saati, T.; Falini, B.; Delsol, G.; Mason, D.Y. Genotypic analysis of large cell lymphomas which express the Ki-1 antigen. Histopathology 1987, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Herbst, H.; Tippelmann, G.; Anagnostopoulos, I.; Gerdes, J.; Schwarting, R.; Boehm, T.; Pileri, S.; Jones, D.B.; Stein, H. Immunoglobulin and T-cell receptor gene rearrangements in Hodgkin’s disease and Ki-1-positive anaplastic large cell lymphoma: Dissociation between phenotype and genotype. Leuk. Res. 1989, 13, 103–116. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Researh on Cancer (IARC): Lyon, France, 2008; Volume 2, ISBN 978-92-832-2431-0. [Google Scholar]
- Savage, K.J.; Harris, N.L.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Connors, J.M.; Rimsza, L.; Pileri, S.A.; Chhanabhai, M.; Gascoyne, R.D.; et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008, 111, 5496–5504. [Google Scholar] [CrossRef] [PubMed]
- Ten Berge, R.L.; de Bruin, P.C.; Oudejans, J.J.; Ossenkoppele, G.J.; van der Valk, P.; Meijer, C.J. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology 2003, 43, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Delas, A.; Gaulard, P.; Haioun, C.; Moreau, A.; Xerri, L.; Traverse-Glehen, A.; Rousset, T.; Quintin-Roue, I.; Petrella, T.; et al. Breast implant-associated anaplastic large cell lymphoma: Two distinct clinicopathological variants with different outcomes. Ann. Oncol. 2016, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A.; Group, B.F.M. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Pileri, S.; Zinzani, P.L.; Carbone, A.; Zagonel, V.; Wolf-Peeters, C.; Verhoef, G.; Menestrina, F.; Todeschini, G.; Paulli, M.; et al. ALK+ lymphoma: Clinico-pathological findings and outcome. Blood 1999, 93, 2697–2706. [Google Scholar] [PubMed]
- Stein, H.; Foss, H.D.; Durkop, H.; Marafioti, T.; Delsol, G.; Pulford, K.; Pileri, S.; Falini, B. CD30(+) anaplastic large cell lymphoma: A review of its histopathologic, genetic, and clinical features. Blood 2000, 96, 3681–3695. [Google Scholar] [PubMed]
- Filippa, D.A.; Ladanyi, M.; Wollner, N.; Straus, D.J.; O’Brien, J.P.; Portlock, C.; Gangi, M.; Sun, M. CD30 (Ki-1)-positive malignant lymphomas: Clinical, immunophenotypic, histologic, and genetic characteristics and differences with Hodgkin’s disease. Blood 1996, 87, 2905–2917. [Google Scholar] [PubMed]
- Kadin, M.E.; Carpenter, C. Systemic and primary cutaneous anaplastic large cell lymphomas. Semin. Hematol. 2003, 40, 244–256. [Google Scholar] [CrossRef]
- Ellin, F.; Landstrom, J.; Jerkeman, M.; Relander, T. Central nervous system relapse in peripheral T-cell lymphomas: A Swedish Lymphoma Registry study. Blood 2015, 126, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Narita, Y.; Miyakita, Y.; Ohno, M.; Fukushima, S.; Maruyama, T.; Muragaki, Y.; Shibui, S. Clinical presentation of anaplastic large-cell lymphoma in the central nervous system. Mol. Clin. Oncol. 2013, 1, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Mori, T.; Reiter, A.; Woessman, W.; Rosolen, A.; Wrobel, G.; Zsiros, J.; Uyttebroeck, A.; Marky, I.; Le Deley, M.C.; et al. Central nervous system involvement in anaplastic large cell lymphoma in childhood: Results from a multicentre European and Japanese study. Pediatr. Blood Cancer 2013, 60, E118-121. [Google Scholar] [CrossRef] [PubMed]
- Onciu, M.; Behm, F.G.; Raimondi, S.C.; Moore, S.; Harwood, E.L.; Pui, C.H.; Sandlund, J.T. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am. J. Clin. Pathol. 2003, 120, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Paillard, C.; Ducassou, S.; Perel, Y.; Plantaz, D.; Strullu, M.; Eischen, A.; Lutz, P.; Lamant, L.; Le Deley, M.C.; et al. Paediatric anaplastic large cell lymphoma with leukaemic presentation in children: A report of nine French cases. Br. J. Haematol. 2014, 165, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Falini, B.; Banham, A.H.; Codrington, D.; Roberton, H.; Hatton, C.; Mason, D.Y. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood 2000, 96, 1605–1607. [Google Scholar] [PubMed]
- Ait-Tahar, K.; Cerundolo, V.; Banham, A.H.; Hatton, C.; Blanchard, T.; Kusec, R.; Becker, M.; Smith, G.L.; Pulford, K. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int. J. Cancer 2006, 118, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ait-Tahar, K.; Damm-Welk, C.; Burkhardt, B.; Zimmermann, M.; Klapper, W.; Reiter, A.; Pulford, K.; Woessmann, W. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 2010, 115, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Brousset, P.; Schlaifer, D.; Payen, C.; Robert, A.; Rubie, H.; Huguet-Rigal, F.; Delsol, G. Bone marrow involvement in anaplastic large cell lymphoma. Immunohistochemical detection of minimal disease and its prognostic significance. Am. J. Clin. Pathol. 1995, 103, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Benharroch, D.; Meguerian-Bedoyan, Z.; Lamant, L.; Amin, C.; Brugieres, L.; Terrier-Lacombe, M.J.; Haralambieva, E.; Pulford, K.; Pileri, S.; Morris, S.W.; et al. ALK-positive lymphoma: A single disease with a broad spectrum of morphology. Blood 1998, 91, 2076–2084. [Google Scholar] [PubMed]
- Delsol, G.; Lamant, L.; Mariame, B.; Pulford, K.; Dastugue, N.; Brousset, P.; Rigal-Huguet, F.; al Saati, T.; Cerretti, D.P.; Morris, S.W.; et al. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood 1997, 89, 1483–1490. [Google Scholar] [PubMed]
- Falini, B.; Bigerna, B.; Fizzotti, M.; Pulford, K.; Pileri, S.A.; Delsol, G.; Carbone, A.; Paulli, M.; Magrini, U.; Menestrina, F.; et al. ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am. J. Pathol. 1998, 153, 875–886. [Google Scholar] [CrossRef]
- Jaffe, E.S. Anaplastic large cell lymphoma: The shifting sands of diagnostic hematopathology. Mod. Pathol. 2001, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Pillon, M.; d’Amore, E.S.; Santoro, N.; Lombardi, A.; Fagioli, F.; Zanesco, L.; Rosolen, A. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia 2005, 19, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Sueki, Y.; Nozaki, Y.; Kawashima, I.; Yamamoto, T.; Nakajima, K.; Mitumori, T.; Kirito, K. Anaplastic large cell lymphoma with paraneoplastic neutrophilia: An association between IL-17 elevation and aggressive disease progression. Int. J. Hematol. 2014, 99, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Lamant, L.; McCarthy, K.; d’Amore, E.; Klapper, W.; Nakagawa, A.; Fraga, M.; Maldyk, J.; Simonitsch-Klupp, I.; Oschlies, I.; Delsol, G.; et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: Results of the ALCL99 study. J. Clin. Oncol. 2011, 29, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Bayle, C.; Charpentier, A.; Duchayne, E.; Manel, A.M.; Pages, M.P.; Robert, A.; Lamant, L.; Dastugue, N.; Bertrand, Y.; Dijoud, F.; et al. Leukaemic presentation of small cell variant anaplastic large cell lymphoma: Report of four cases. Br. J. Haematol. 1999, 104, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kinney, M.C.; Collins, R.D.; Greer, J.P.; Whitlock, J.A.; Sioutos, N.; Kadin, M.E. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am. J. Surg. Pathol. 1993, 17, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.; Falini, B.; Delsol, G.; Stein, H.; Baglioni, P.; Poggi, S.; Martelli, M.F.; Rivano, M.T.; Mason, D.Y.; Stansfeld, A.G. Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology 1990, 16, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, L.; Del Vecchio, M.T.; Kraft, R.; Megha, T.; Barbini, P.; Cevenini, G.; Poggi, S.; Pileri, S.; Tosi, P.; Cottier, H. Hodgkin’s disease and CD30-positive anaplastic large cell lymphomas—A continuous spectrum of malignant disorders. A quantitative morphometric and immunohistologic study. Am. J. Pathol. 1990, 137, 1047–1057. [Google Scholar] [PubMed]
- Vassallo, J.; Lamant, L.; Brugieres, L.; Gaillard, F.; Campo, E.; Brousset, P.; Delsol, G. ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin's lymphoma: Report of 10 cases. Am. J. Surg. Pathol. 2006, 30, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Buchanan, R.; Fletcher, C.D. Sarcomatoid variant of anaplastic large-cell Ki-1 lymphoma. Am. J. Surg. Pathol. 1990, 14, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Gruss, H.J.; Dower, S.K. Tumor necrosis factor ligand superfamily: Involvement in the pathology of malignant lymphomas. Blood 1995, 85, 3378–3404. [Google Scholar] [PubMed]
- Andreesen, R.; Osterholz, J.; Lohr, G.W.; Bross, K.J. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood 1984, 63, 1299–1302. [Google Scholar] [PubMed]
- Chiarle, R.; Podda, A.; Prolla, G.; Gong, J.; Thorbecke, G.J.; Inghirami, G. CD30 in normal and neoplastic cells. Clin. Immunol. 1999, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Latza, U.; Foss, H.D.; Durkop, H.; Eitelbach, F.; Dieckmann, K.P.; Loy, V.; Unger, M.; Pizzolo, G.; Stein, H. CD30 antigen in embryonal carcinoma and embryogenesis and release of the soluble molecule. Am. J. Pathol. 1995, 146, 463–471. [Google Scholar] [PubMed]
- Hittmair, A.; Rogatsch, H.; Hobisch, A.; Mikuz, G.; Feichtinger, H. CD30 expression in seminoma. Hum. Pathol. 1996, 27, 1166–1171. [Google Scholar] [CrossRef]
- Durkop, H.; Foss, H.D.; Eitelbach, F.; Anagnostopoulos, I.; Latza, U.; Pileri, S.; Stein, H. Expression of the CD30 antigen in non-lymphoid tissues and cells. J. Pathol. 2000, 190, 613–618. [Google Scholar] [CrossRef]
- Went, P.; Agostinelli, C.; Gallamini, A.; Piccaluga, P.P.; Ascani, S.; Sabattini, E.; Bacci, F.; Falini, B.; Motta, T.; Paulli, M.; et al. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J. Clin. Oncol. 2006, 24, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Lamant, L.; Morris, S.W.; Butler, L.H.; Wood, K.M.; Stroud, D.; Delsol, G.; Mason, D.Y. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89, 1394–1404. [Google Scholar] [PubMed]
- Lamant, L.; Gascoyne, R.D.; Duplantier, M.M.; Armstrong, F.; Raghab, A.; Chhanabhai, M.; Rajcan-Separovic, E.; Raghab, J.; Delsol, G.; Espinos, E. Non-muscle myosin heavy chain (MYH9): A new partner fused to ALK in anaplastic large cell lymphoma. Genes Chromosom. Cancer 2003, 37, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Touriol, C.; Greenland, C.; Lamant, L.; Pulford, K.; Bernard, F.; Rousset, T.; Mason, D.Y.; Delsol, G. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 2000, 95, 3204–3207. [Google Scholar] [PubMed]
- Tort, F.; Pinyol, M.; Pulford, K.; Roncador, G.; Hernandez, L.; Nayach, I.; Kluin-Nelemans, H.C.; Kluin, P.; Touriol, C.; Delsol, G.; et al. Molecular characterization of a new ALK translocation involving moesin (MSN-ALK) in anaplastic large cell lymphoma. Lab. Investig. 2001, 81, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bonzheim, I.; Geissinger, E.; Roth, S.; Zettl, A.; Marx, A.; Rosenwald, A.; Muller-Hermelink, H.K.; Rudiger, T. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood 2004, 104, 3358–3360. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, C.; Martinengo, C.; Voena, C.; Tondat, F.; Riera, L.; di Celle, P.F.; Inghirami, G.; Chiarle, R. NPM-ALK oncogenic tyrosine kinase controls T-cell identity by transcriptional regulation and epigenetic silencing in lymphoma cells. Cancer Res. 2009, 69, 8611–8619. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Law, M.E.; Inwards, D.J.; Dogan, A.; McClure, R.F.; Macon, W.R. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod. Pathol. 2010, 23, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kinney, M.C.; Higgins, R.A.; Medina, E.A. Anaplastic large cell lymphoma: Twenty-five years of discovery. Arch. Pathol. Lab. Med. 2011, 135, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Kotylo, P.; Neiman, R.S.; Braziel, R.M. Comparison of fascin expression in anaplastic large cell lymphoma and Hodgkin disease. Am. J. Clin. Pathol. 2003, 119, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.F.; Pinkus, J.L.; Pinkus, G.S. Clusterin, a marker for anaplastic large cell lymphoma immunohistochemical profile in hematopoietic and nonhematopoietic malignant neoplasms. Am. J. Clin. Pathol. 2004, 121, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, C.H.; DeMello, D.E.; Gale, G.B. Pediatric CD56+ anaplastic large cell lymphoma: A review of the literature. Arch. Pathol. Lab. Med. 2006, 130, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Honorat, J.F.; Ragab, A.; Lamant, L.; Delsol, G.; Ragab-Thomas, J. SHP1 tyrosine phosphatase negatively regulates NPM-ALK tyrosine kinase signaling. Blood 2006, 107, 4130–4138. [Google Scholar] [CrossRef] [PubMed]
- Lamant, L.; de Reynies, A.; Duplantier, M.M.; Rickman, D.S.; Sabourdy, F.; Giuriato, S.; Brugieres, L.; Gaulard, P.; Espinos, E.; Delsol, G. Gene-expression profiling of systemic anaplastic large-cell lymphoma reveals differences based on ALK status and two distinct morphologic ALK+ subtypes. Blood 2007, 109, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Martinez, L.; Pittaluga, S.; Miething, C.; Klier, M.; Rudelius, M.; Davies-Hill, T.; Anastasov, N.; Martinez, A.; Vivero, A.; Duyster, J.; et al. NPM-ALK-dependent expression of the transcription factor CCAAT/enhancer binding protein beta in ALK-positive anaplastic large cell lymphoma. Blood 2006, 108, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Bonzheim, I.; Steinhilber, J.; Montes-Mojarro, I.A.; Ortiz-Hidalgo, C.; Klapper, W.; Fend, F.; Quintanilla-Martinez, L. EMMPRIN (CD147) is induced by C/EBPβ and is differentially expressed in ALK+ and ALK− anaplastic large-cell lymphoma. Lab. Investig. 2017, 97, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Liang, X.M.; Hou, J.H.; Huan, Y.L.; Wu, Q.L.; Xiao, Y.B. Study of CD44v6 protein expression in intraductal papilloma and its malignant transformation of breast. Ai Zheng 2002, 21, 615–618. [Google Scholar] [PubMed]
- Liang, X.; Golitz, L.E.; Smoller, B.R.; Meech, S.J.; Odom, L.F.; Williams, S.A.; Ryder, J.W. Association of expression of CD44v6 with systemic anaplastic large cell lymphoma: Comparison with primary cutaneous anaplastic large cell lymphoma. Am. J. Clin. Pathol. 2002, 117, 276–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlette, E.J.; Medeiros, L.J.; Goy, A.; Lai, R.; Rassidakis, G.Z. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J. Clin. Oncol. 2004, 22, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Hsi, E.D.; Said, J.; Macon, W.R.; Rodig, S.J.; Ondrejka, S.L.; Gascoyne, R.D.; Morgan, E.A.; Dorfman, D.M.; Maurer, M.J.; Dogan, A. Diagnostic accuracy of a defined immunophenotypic and molecular genetic approach for peripheral T/NK-cell lymphomas. A North American PTCL study group project. Am. J. Surg. Pathol. 2014, 38, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Fizzotti, M.; Pucciarini, A.; Bigerna, B.; Marafioti, T.; Gambacorta, M.; Pacini, R.; Alunni, C.; Natali-Tanci, L.; Ugolini, B.; et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000, 95, 2084–2092. [Google Scholar] [PubMed]
- Saglam, A.; Uner, A.H. Immunohistochemical expression of Mum-1, Oct-2 and Bcl-6 in systemic anaplastic large cell lymphomas. Tumori 2011, 97, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Pulford, K.; Pucciarini, A.; Carbone, A.; De Wolf-Peeters, C.; Cordell, J.; Fizzotti, M.; Santucci, A.; Pelicci, P.G.; Pileri, S.; et al. Lymphomas expressing ALK fusion protein(s) other than NPM-ALK. Blood 1999, 94, 3509–3515. [Google Scholar] [PubMed]
- Cools, J.; Wlodarska, I.; Somers, R.; Mentens, N.; Pedeutour, F.; Maes, B.; De Wolf-Peeters, C.; Pauwels, P.; Hagemeijer, A.; Marynen, P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 2002, 34, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Vasmatzis, G.; Asmann, Y.W.; Davila, J.; Middha, S.; Eckloff, B.W.; Johnson, S.H.; Porcher, J.C.; Ansell, S.M.; Caride, A. Novel TRAF1-ALK fusion identified by deep RNA sequencing of anaplastic large cell lymphoma. Genes Chromosomes Cancer 2013, 52, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Pinyol, M.; Hernandez, S.; Bea, S.; Pulford, K.; Rosenwald, A.; Lamant, L.; Falini, B.; Ott, G.; Mason, D.Y.; et al. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 1999, 94, 3265–3268. [Google Scholar] [PubMed]
- Lamant, L.; Dastugue, N.; Pulford, K.; Delsol, G.; Mariame, B. A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood 1999, 93, 3088–3095. [Google Scholar] [PubMed]
- Mason, D.Y.; Pulford, K.A.; Bischof, D.; Kuefer, M.U.; Butler, L.H.; Lamant, L.; Delsol, G.; Morris, S.W. Nucleolar localization of the nucleophosmin-anaplastic lymphoma kinase is not required for malignant transformation. Cancer Res. 1998, 58, 1057–1062. [Google Scholar] [PubMed]
- Rosenwald, A.; Ott, G.; Pulford, K.; Katzenberger, T.; Kuhl, J.; Kalla, J.; Ott, M.M.; Mason, D.Y.; Muller-Hermelink, H.K. t(1;2)(q21;p23) and t(2;3)(p23;q21): Two novel variant translocations of the t(2;5)(p23;q35) in anaplastic large cell lymphoma. Blood 1999, 94, 362–364. [Google Scholar] [PubMed]
- Trinei, M.; Lanfrancone, L.; Campo, E.; Pulford, K.; Mason, D.Y.; Pelicci, P.G.; Falini, B. A new variant anaplastic lymphoma kinase (ALK)-fusion protein (ATIC-ALK) in a case of ALK-positive anaplastic large cell lymphoma. Cancer Res. 2000, 60, 793–798. [Google Scholar] [PubMed]
- Wlodarska, I.; De Wolf-Peeters, C.; Falini, B.; Verhoef, G.; Morris, S.W.; Hagemeijer, A.; Van den Berghe, H. The cryptic inv(2)(p23q35) defines a new molecular genetic subtype of ALK-positive anaplastic large-cell lymphoma. Blood 1998, 92, 2688–2695. [Google Scholar] [PubMed]
- Abate, F.; Todaro, M.; van der Krogt, J.A.; Boi, M.; Landra, I.; Machiorlatti, R.; Tabbo, F.; Messana, K.; Abele, C.; Barreca, A.; et al. A novel patient-derived tumorgraft model with TRAF1-ALK anaplastic large-cell lymphoma translocation. Leukemia 2015, 29, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Lamant, L.; Espinos, E.; Jiang, Q.; Xue, L.; Turturro, F.; Delsol, G.; Morris, S.W. The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cell. Mol. Life Sci. 2004, 61, 2939–2953. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Morris, S.W.; Turturro, F. Anaplastic lymphoma kinase proteins in growth control and cancer. J. Cell. Physiol. 2004, 199, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Shiota, M.; Iwahara, T.; Seki, N.; Satoh, H.; Mori, S.; Yamamoto, T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc. Natl. Acad. Sci. USA 1996, 93, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Bischof, D.; Pulford, K.; Mason, D.Y.; Morris, S.W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin’s lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol. Cell. Biol. 1997, 17, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Zamo, A.; Chiarle, R.; Piva, R.; Howes, J.; Fan, Y.; Chilosi, M.; Levy, D.E.; Inghirami, G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Anastasov, N.; Bonzheim, I.; Rudelius, M.; Klier, M.; Dau, T.; Angermeier, D.; Duyster, J.; Pittaluga, S.; Fend, F.; Raffeld, M.; et al. C/EBPβ expression in ALK-positive anaplastic large cell lymphomas is required for cell proliferation and is induced by the STAT3 signaling pathway. Haematologica 2010, 95, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Slupianek, A.; Nieborowska-Skorska, M.; Hoser, G.; Morrione, A.; Majewski, M.; Xue, L.; Morris, S.W.; Wasik, M.A.; Skorski, T. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 2001, 61, 2194–2199. [Google Scholar] [PubMed]
- Bai, R.Y.; Dieter, P.; Peschel, C.; Morris, S.W.; Duyster, J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-γ to mediate its mitogenicity. Mol. Cell. Biol. 1998, 18, 6951–6961. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.T.; Zhao, C.; Zhang, Q.; Wasik, M.A. Nucleophosmin-anaplastic lymphoma kinase: The ultimate oncogene and therapeutic target. Blood 2017, 129, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Inghirami, G.; Chiarle, R.; Simmons, W.J.; Piva, R.; Schlessinger, K.; Levy, D.E. New and old functions of STAT3: A pivotal target for individualized treatment of cancer. Cell Cycle 2005, 4, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Halasa, K.; Liu, X.; Wang, H.Y.; Cheng, M.; Baldwin, D.; Tobias, J.W.; Schuster, S.J.; Woetmann, A.; Zhang, Q.; et al. Malignant transformation of CD4+ T lymphocytes mediated by oncogenic kinase NPM/ALK recapitulates IL-2-induced cell signaling and gene expression reprogramming. J. Immunol. 2013, 191, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Chiarle, R.; Simmons, W.J.; Cai, H.; Dhall, G.; Zamo, A.; Raz, R.; Karras, J.G.; Levy, D.E.; Inghirami, G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 2005, 11, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Weilemann, A.; Grau, M.; Erdmann, T.; Merkel, O.; Sobhiafshar, U.; Anagnostopoulos, I.; Hummel, M.; Siegert, A.; Hayford, C.; Madle, H.; et al. Essential role of IRF4 and MYC signaling for survival of anaplastic large cell lymphoma. Blood 2015, 125, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bandini, C.; Pupuleku, A.; Spaccarotella, E.; Pellegrino, E.; Wang, R.; Vitale, N.; Duval, C.; Cantarella, D.; Rinaldi, A.; Provero, P.; et al. IRF4 Mediates the Oncogenic Effects of STAT3 in Anaplastic Large Cell Lymphomas. Cancers (Basel) 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Emre, N.C.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lollies, A.; Hartmann, S.; Schneider, M.; Bracht, T.; Weiss, A.L.; Arnolds, J.; Klein-Hitpass, L.; Sitek, B.; Hansmann, M.L.; Kuppers, R.; et al. An oncogenic axis of STAT-mediated BATF3 upregulation causing MYC activity in classical Hodgkin lymphoma and anaplastic large cell lymphoma. Leukemia 2018, 32, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Ingham, R.J. The pathobiology of the oncogenic tyrosine kinase NPM-ALK: A brief update. Ther. Adv. Hematol. 2013, 4, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, P.; Young, L.C.; Lai, R.; Li, L. Proteome-wide identification of novel binding partners to the oncogenic fusion gene protein, NPM-ALK, using tandem affinity purification and mass spectrometry. Am. J. Pathol. 2009, 174, 361–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marzec, M.; Liu, X.; Wong, W.; Yang, Y.; Pasha, T.; Kantekure, K.; Zhang, P.; Woetmann, A.; Cheng, M.; Odum, N.; et al. Oncogenic kinase NPM/ALK induces expression of HIF1alpha mRNA. Oncogene 2011, 30, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, C.; Poggio, T.; Menotti, M.; Scalzo, M.S.; Mastini, C.; Ambrogio, C.; Pellegrino, E.; Riera, L.; Piva, R.; Ribatti, D.; et al. ALK-dependent control of hypoxia-inducible factors mediates tumor growth and metastasis. Cancer Res. 2014, 74, 6094–6106. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.D.; Wu, F.; Ye, X.; Sharon, D.; Hitt, M.; McMullen, T.P.; Hegazy, S.A.; Gelebart, P.; Yang, J.; et al. The expression and oncogenic effects of the embryonic stem cell marker SALL4 in ALK-positive anaplastic large cell lymphoma. Cell Signal. 2012, 24, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, P.; Hegazy, S.A.; Wang, P.; Bone, K.M.; Anand, M.; Sharon, D.; Hitt, M.; Pearson, J.D.; Ingham, R.J.; Ma, Y.; et al. Aberrant expression and biological significance of Sox2, an embryonic stem cell transcriptional factor, in ALK-positive anaplastic large cell lymphoma. Blood Cancer J. 2012, 2, e82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Wu, F.; Li, M.; Sharon, D.; Ingham, R.J.; Hitt, M.; McMullen, T.P.; Lai, R. Aberrant expression of the transcriptional factor Twist1 promotes invasiveness in ALK-positive anaplastic large cell lymphoma. Cell Signal. 2012, 24, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Abate, F.; Lasorsa, E.; Tabbo, F.; Gaudiano, M.; Chiesa, N.; Di Giacomo, F.; Spaccarotella, E.; Barbarossa, L.; Ercole, E.; et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 2015, 27, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, M.; Zhang, Q.; Witkiewicz, A.; Marzec, M.; Potoczek, M.; Liu, X.; Wang, H.Y.; Milone, M.; Basu, S.; Mauger, J.; et al. γc-Signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J. Immunol. 2008, 181, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Desjobert, C.; Renalier, M.H.; Bergalet, J.; Dejean, E.; Joseph, N.; Kruczynski, A.; Soulier, J.; Espinos, E.; Meggetto, F.; Cavaille, J.; et al. MiR-29a down-regulation in ALK-positive anaplastic large cell lymphomas contributes to apoptosis blockade through MCL-1 overexpression. Blood 2011, 117, 6627–6637. [Google Scholar] [CrossRef] [PubMed]
- Piva, R.; Pellegrino, E.; Mattioli, M.; Agnelli, L.; Lombardi, L.; Boccalatte, F.; Costa, G.; Ruggeri, B.A.; Cheng, M.; Chiarle, R.; et al. Functional validation of the anaplastic lymphoma kinase signature identifies CEBPB and BCL2A1 as critical target genes. J. Clin. Investig. 2006, 116, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Bonzheim, I.; Irmler, M.; Klier-Richter, M.; Steinhilber, J.; Anastasov, N.; Schafer, S.; Adam, P.; Beckers, J.; Raffeld, M.; Fend, F.; et al. Identification of C/EBPβ target genes in ALK+ anaplastic large cell lymphoma (ALCL) by gene expression profiling and chromatin immunoprecipitation. PLoS ONE 2013, 8, e64544. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Koivunen, J.; Ogino, A.; Yanagita, M.; Nikiforow, S.; Zheng, W.; Lathan, C.; Marcoux, J.P.; Du, J.; Okuda, K.; et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011, 71, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Salaverria, I.; Bea, S.; Lopez-Guillermo, A.; Lespinet, V.; Pinyol, M.; Burkhardt, B.; Lamant, L.; Zettl, A.; Horsman, D.; Gascoyne, R.; et al. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br. J. Haematol. 2008, 140, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, R.D.; Lamant, L.; Martin-Subero, J.I.; Lestou, V.S.; Harris, N.L.; Muller-Hermelink, H.K.; Seymour, J.F.; Campbell, L.J.; Horsman, D.E.; Auvigne, I.; et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: Report of 6 cases. Blood 2003, 102, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Weisenburger, D.D.; Greiner, T.C.; Vose, J.M.; McKeithan, T.; Kucuk, C.; Geng, H.; Deffenbacher, K.; Smith, L.; Dybkaer, K.; et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Scarfo, I.; Pellegrino, E.; Mereu, E.; Kwee, I.; Agnelli, L.; Bergaggio, E.; Garaffo, G.; Vitale, N.; Caputo, M.; Machiorlatti, R.; et al. Identification of a new subclass of ALK-negative ALCL expressing aberrant levels of ERBB4 transcripts. Blood 2016, 127, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, L.; Mereu, E.; Pellegrino, E.; Limongi, T.; Kwee, I.; Bergaggio, E.; Ponzoni, M.; Zamo, A.; Iqbal, J.; Piccaluga, P.P.; et al. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood 2012, 120, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Boi, M.; Rinaldi, A.; Kwee, I.; Bonetti, P.; Todaro, M.; Tabbo, F.; Piva, R.; Rancoita, P.M.; Matolcsy, A.; Timar, B.; et al. PRDM1/BLIMP1 is commonly inactivated in anaplastic large T-cell lymphoma. Blood 2013, 122, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Thompson, E.R.; Jones, K.; Arnau, G.M.; Lade, S.; Markham, J.F.; Li, J.; Deva, A.; Johnstone, R.W.; Khot, A.; et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica 2016, 101, e387–e390. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Pileri, S.; Stein, H.; Dieneman, D.; Dallenbach, F.; Delsol, G.; Minelli, O.; Poggi, S.; Martelli, M.F.; Pallesen, G.; et al. Variable expression of leucocyte-common (CD45) antigen in CD30 (Ki1)-positive anaplastic large-cell lymphoma: Implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Hum. Pathol. 1990, 21, 624–629. [Google Scholar] [CrossRef]
- Griffin, C.A.; Hawkins, A.L.; Dvorak, C.; Henkle, C.; Ellingham, T.; Perlman, E.J. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999, 59, 2776–2780. [Google Scholar] [PubMed]
- Marino-Enriquez, A.; Dal Cin, P. ALK as a paradigm of oncogenic promiscuity: Different mechanisms of activation and different fusion partners drive tumors of different lineages. Cancer Genet. 2013, 206, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Lamant, L.; Algar, E.; Delsol, G.; Tsang, W.Y.; Lee, K.C.; Tiedemann, K.; Chow, C.W. ALK+ histiocytosis: A novel type of systemic histiocytic proliferative disorder of early infancy. Blood 2008, 112, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Sibon, D.; Fournier, M.; Briere, J.; Lamant, L.; Haioun, C.; Coiffier, B.; Bologna, S.; Morel, P.; Gabarre, J.; Hermine, O.; et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J. Clin. Oncol. 2012, 30, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Desouki, M.M.; Post, G.R.; Cherry, D.; Lazarchick, J. PAX-5: A valuable immunohistochemical marker in the differential diagnosis of lymphoid neoplasms. Clin. Med. Res. 2010, 8, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Arun, I.; Roy, P.; Arora, N.; Bhave, S.J.; Nair, R.; Chandy, M. PAX-5 Positivity in Anaplastic Lymphoma Kinase-Negative Anaplastic Large Cell Lymphoma: A Case Report and Review of Literature. Int. J. Surg. Pathol. 2017, 25, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, T.; Kiel, M.J.; Sahasrabuddhe, A.A.; Rolland, D.; Dixon, C.A.; Bailey, N.G.; Betz, B.L.; Brown, N.A.; Hristov, A.C.; Wilcox, R.A.; et al. A novel recurrent NPM1-TYK2 gene fusion in cutaneous CD30-positive lymphoproliferative disorders. Blood 2014, 124, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
- Abate, F.; Zairis, S.; Ficarra, E.; Acquaviva, A.; Wiggins, C.H.; Frattini, V.; Lasorella, A.; Iavarone, A.; Inghirami, G.; Rabadan, R. Pegasus: A comprehensive annotation and prediction tool for detection of driver gene fusions in cancer. BMC Syst. Biol. 2014, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Medeiros, L.J.; Rassidakis, G.Z.; Yared, M.A.; Tsioli, P.; Leventaki, V.; Schmitt-Graeff, A.; Herling, M.; Amin, H.M.; Lai, R. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK− anaplastic large cell lymphoma. Clin. Cancer Res. 2003, 9, 3692–3699. [Google Scholar] [PubMed]
- Feldman, A.L.; Dogan, A.; Smith, D.I.; Law, M.E.; Ansell, S.M.; Johnson, S.H.; Porcher, J.C.; Ozsan, N.; Wieben, E.D.; Eckloff, B.W.; et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood 2011, 117, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Vasmatzis, G.; Johnson, S.H.; Knudson, R.A.; Ketterling, R.P.; Braggio, E.; Fonseca, R.; Viswanatha, D.S.; Law, M.E.; Kip, N.S.; Ozsan, N.; et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood 2012, 120, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Feldman, A.L. Genetics of anaplastic large cell lymphoma. Leuk. Lymphoma 2016, 57, 21–27. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Dao, L.N.; McPhail, E.D.; Jaffe, E.S.; Said, J.; Swerdlow, S.H.; Sattler, C.A.; Ketterling, R.P.; Sidhu, J.S.; Hsi, E.D.; et al. Morphologic Features of ALK-negative Anaplastic Large Cell Lymphomas With DUSP22 Rearrangements. Am. J. Surg. Pathol. 2016, 40, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Law, M.; Remstein, E.D.; Macon, W.R.; Erickson, L.A.; Grogg, K.L.; Kurtin, P.J.; Dogan, A. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia 2009, 23, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu-Monette, Z.Y.; Balasubramanyam, A.; Manyam, G.C.; Visco, C.; Tzankov, A.; Liu, W.M.; Miranda, R.N.; Zhang, L.; Montes-Moreno, S.; et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2013, 121, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Imai, Y.; Ota, S.; Yamamoto, G.; Takahashi, T.; Fukayama, M.; Kurokawa, M. CD30-positive anaplastic variant diffuse large B cell lymphoma: A rare case presented with cutaneous involvement. Int. J. Hematol. 2010, 92, 550–552. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Gaulard, P. CD30+ lymphoproliferative disorders. Haematologica 2010, 95, 1627–1630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Brody, G.S.; Deapen, D.; Taylor, C.R.; Pinter-Brown, L.; House-Lightner, S.R.; Andersen, J.S.; Carlson, G.; Lechner, M.G.; Epstein, A.L. Anaplastic large cell lymphoma occurring in women with breast implants: Analysis of 173 cases. Plast. Reconstr. Surg. 2015, 135, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Aladily, T.N.; Medeiros, L.J.; Amin, M.B.; Haideri, N.; Ye, D.; Azevedo, S.J.; Jorgensen, J.L.; de Peralta-Venturina, M.; Mustafa, E.B.; Young, K.H.; et al. Anaplastic large cell lymphoma associated with breast implants: A report of 13 cases. Am. J. Surg. Pathol. 2012, 36, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Aladily, T.N.; Medeiros, L.J.; Alayed, K.; Miranda, R.N. Breast implant-associated anaplastic large cell lymphoma: A newly recognized entity that needs further refinement of its definition. Leuk. Lymphoma 2012, 53, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shokrollahi, K.; Rozen, W.M.; Conyers, R.; Wright, P.; Kenner, L.; Turner, S.D.; Whitaker, I.S. Anaplastic large cell lymphoma (ALCL) and breast implants: Breaking down the evidence. Mutat. Res. Rev. Mutat. Res. 2014, 762, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.N.; Lin, L.; Talwalkar, S.S.; Manning, J.T.; Medeiros, L.J. Anaplastic large cell lymphoma involving the breast: A clinicopathologic study of 6 cases and review of the literature. Arch. Pathol. Lab. Med. 2009, 133, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Popplewell, L.; Thomas, S.H.; Huang, Q.; Chang, K.L.; Forman, S.J. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk. Lymphoma 2011, 52, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Story, S.K.; Schowalter, M.K.; Geskin, L.J. Breast implant-associated ALCL: A unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist 2013, 18, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wei, S. Breast implant-associated anaplastic large cell lymphoma: Review of a distinct clinicopathologic entity. Arch. Pathol. Lab. Med. 2014, 138, 842–846. [Google Scholar] [CrossRef] [PubMed]

| 1. Systemic ALK-positive ALCL (ALK+ ALCL) |
| Morphological variants: |
| 1.1. Common pattern 1.2. Small cell pattern 1.3. Lymphohistiocytic pattern 1.4. Hodgkin’s-like pattern 1.5. Composite pattern |
| 2. Systemic ALK-negative ALCL (ALK− ALCL) |
| Genetic variants: |
| 2.1. DUSP-22-rearranged ALCL 2.2. TP63-rearranged ALCL 2.3. Triple-negative ALCL 2.4. Others: ERBB4-aberrant expression |
| 3. Breast implant-associated ALCL (BI-ALCL) |
| Clinical and morphological variants |
| 3.1. Seroma (in situ pattern) 3.2. Palpable mass (infiltrative pattern), related to systemic involvement |
| 4. Primary cutaneous ALCL (pC-ALCL) |
| Marker | ALK+ ALCL | ALK− ALCL | BI-Associated ALCL |
|---|---|---|---|
| CD30 | + | + | + |
| ALK | + | − | − |
| EMA | + | + | + |
| CD3 | −/+ | +/− | −/+ |
| CD4 | +/− | +/− | +/− |
| CD8 | − | −/+ | −/+ |
| CD5 | −/+ | −/+ | −/+ |
| CD2 | +/− | −/+ | −/+ |
| TCR BF1 | − | − | N/A |
| TIA1 | +/− | +/− | −/+ |
| BCL6 1 | −/+ | −/+ | N/A |
| IRF4/MUM1 | + | + | +/− |
| Disease | Chromosomal Rearrangements | Gene Expression | Chromosomal Imbalances |
|---|---|---|---|
| ALK+ ALCL | Most common rearrangement (80%): NPM1-ALK, t(2;5)(p23;q35) [7] Other variant translocations: TPM3-ALK, t(1;2)(p23;q25) [72] ATIC-ALK, inv(2)(p23;q35) [75] TFG-ALK, t(2;3)(p23;q12) [71] CTLC-ALK, t(2;17)(p23;q23) [49,111] MSN-ALK, t(2;X)(p23;q11–12) [50] TPM4-ALK, t(2;19)(p23;p13) [69] MYH9-ALK, t(2;22)(p23;q11) [48] RNF213-ALK, t(2;17)(p23;q25) [69] TRAF-1-ALK, t(2;9)(p23;q33) [70] | BCL-6, PTNP12, SERPINA1, CEBPB [59] STAT3 regulators: IL-6, IL3IRA [112] | Present in 58% of the cases: Gains of 6q, 7p, 17p, and 17q24 Losses of 4q, 13-q21, and 11q14 [110] |
| ALK− ALCL | IRF4-DUSP-22, 6p25.3 (30%) TP63-TBL1RXR1, 3q28 (8%) JAK-1 and STAT3 mutations variants resulting in STAT3 or JAK-1 constitutive activation: NKB2, ROS1 NCOR2-ROS1 NFKB2-TYK2 PABPC4-TYK2 | ERBB4 overexpression (24% of the cases) [113] TNFSRF8, BATF, TMOD1, CCR7, CNTFR, IL22, and IL21 [59,114] | Present in 65% of the cases: Gains of 1q, 6p21, and 7p Losses of 6q21 (PRDM1), 13q, and 17p13 (TP53) [110,115] |
| BI-ALCL | No chromosomal translocations have been described to date | Not known | Gains of 19p Losses of 10p and 1p [116] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Mojarro, I.A.; Steinhilber, J.; Bonzheim, I.; Quintanilla-Martinez, L.; Fend, F. The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL). Cancers 2018, 10, 107. https://doi.org/10.3390/cancers10040107
Montes-Mojarro IA, Steinhilber J, Bonzheim I, Quintanilla-Martinez L, Fend F. The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL). Cancers. 2018; 10(4):107. https://doi.org/10.3390/cancers10040107
Chicago/Turabian StyleMontes-Mojarro, Ivonne A., Julia Steinhilber, Irina Bonzheim, Leticia Quintanilla-Martinez, and Falko Fend. 2018. "The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL)" Cancers 10, no. 4: 107. https://doi.org/10.3390/cancers10040107
APA StyleMontes-Mojarro, I. A., Steinhilber, J., Bonzheim, I., Quintanilla-Martinez, L., & Fend, F. (2018). The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL). Cancers, 10(4), 107. https://doi.org/10.3390/cancers10040107







