Abstract
Aptamer-related technologies represent a revolutionary advancement in the capacity to rapidly develop new classes of targeting ligands. Structurally distinct RNA and DNA oligonucleotides, aptamers mimic small, protein-binding molecules and exhibit high binding affinity and selectivity. Although their molecular weight is relatively small—approximately one-tenth that of monoclonal antibodies—their complex tertiary folded structures create sufficient recognition surface area for tight interaction with target molecules. Additionally, unlike antibodies, aptamers can be readily chemically synthesized and modified. In addition, aptamers’ long storage period and low immunogenicity are favorable properties for clinical utility. Due to their flexibility of chemical modification, aptamers are conjugated to other chemical entities including chemotherapeutic agents, siRNA, nanoparticles, and solid phase surfaces for therapeutic and diagnostic applications. However, as relatively small sized oligonucleotides, aptamers present several challenges for successful clinical translation. Their short plasma half-lives due to nuclease degradation and rapid renal excretion necessitate further structural modification of aptamers for clinical application. Since the US Food and Drug Administration (FDA) approval of the first aptamer drug, Macugen® (pegaptanib), which treats wet-age-related macular degeneration, several aptamer therapeutics for oncology have followed and shown promise in pre-clinical models as well as clinical trials. This review discusses the advantages and challenges of aptamers and introduces therapeutic aptamers under investigation and in clinical trials for cancer treatments.
1. Advantages of Aptamers
Molecularly targeted therapy is broadly adopted for treatment of many cancer types as an opportunity to inhibit oncogene function. Currently, chimeric monoclonal antibodies as well as small molecule inhibitors are the clinical mainstays in this class of agents. Aptamers, or so called “chemical antibodies,” represent a new class of molecular targeting agents as a result of their unique properties, such as ease of synthesis and modification as well as high affinity binding and excellent safety profiles.
The structural base of aptamers is composed of short DNA or RNA oligonucleotides ranging around 15–100 nt that form complex tertiary or quadruplex structures through hybridization of complementary sequences [1]. Large surface areas, despite their small molecular weight (5–30 kDa), permit high-affinity binding to their molecular targets [1]. The dissociation constant (Kd) of an aptamer’s target is generally in the range of several micro- to pico-molars [2,3], which is comparable to antibody therapeutics. In contrast, small molecule inhibitors (Tyrosine kinase inhibitors: TKI) function as ATP mimetics, thus their sizes are small enough to occupy the ATP binding pocket of the intracellular domain of a receptor tyrosine kinase and are responsible for their relatively large Kd [4]. For example, gefitinib (Iressa®, AstraZeneca, Chesire, UK), the TKI for EGFR, binds with wild-type EGFR kinase at a Kd of 53.5 nM [5], while the Kd of cetuximab (Erbitux®, ImClone, Branchburg, NJ, USA), an inhibitory anti-EGFR antibody, is far smaller, 2.3 nM [6]. A nuclease resistant 2′-fluoropyrimidines-containing RNA aptamer, named CL4 and E07, a 2′-fluoropyrimidine modified anti-EGFR aptamer display Kd comparable to antibody, at 10 nM [7] and 2.4 nM, respectively [8].
Aptamer backbones are synthesized automatically through cell-free assembly that enables cost effective and rapid bulk production with minimal batch-to-batch variation. Additionally, aptamers’ structural stability affords them an extensive storage period as well as the ability to withstand a broad range of temperatures. They are stable at ambient temperature and heat resistant, thus their functional tertiary structure is readily regenerated following heat denaturation. Another significant advantage of aptamers is their capacity for site-specific chemical modifications. Oligonucleotide sugar, base, and phosphate backbone modifications as well as a variety of unnatural oligonucleotides make up the wide repertoire of chemical alterations available to aptamers. Methods for base substitutions including 2′-fluoro- [9,10,11], 2′-amino-, 2′-azido-, 2′-hydroxymethyl-, and 2′-methoxypyrimidines and 2′-methoxypurines have been established [12,13,14,15]. Phosphorothioate and phosphorodithioate substitutions are another option for the backbone modification [16]. Such chemical modifications of the DNA backbone provide resistance against nucleases, as was first shown by Eckstein’s group [17], and often increase binding affinity [16]. The introduction of functional groups in the aptamer backbone permits conjugation to other drugs, siRNA [18,19], and nanoparticles [20,21,22], further broadening their application as multivalent therapeutics [23,24,25,26,27,28,29,30].
2. Challenges and Possible Solutions in Aptamer Therapeutics
2.1. Aptamer Stability
For treatment of malignancies, it is ideal that drugs remain in circulation for extended periods to increase chance of cancer cell exposure to drugs. Thus, the pharmacokinetic profile and bioavailability of injectable drugs are critical determinants of therapeutic efficacy. In this respect, humanized antibodies are superior to other drug entities, displaying circulation half-lives from days to weeks [31]. Unmodified nucleotides, however, may have a serum half-life as short as few minutes [32]. This unfavorable pharmaceutical property represents one the critical challenges facing realistic clinical application of aptamers. Two contributing factors for this are their susceptibility to nuclease degradation and renal excretion. Nucleases are abundantly present in biological fluids, and both exo- and endo-nucleases cleave phosphodiester bonds of single and double stranded oligonucleotides [1]. The average time of oligonucleotide decay in the blood depends on their structure, and ranges from several minutes to several tens of minutes [1]. Since such a short half-life is undesirable for therapeutic applications, oligonucleotides are typically chemically modified to improve the serum stability [3]. For instance, simple alteration of the 2′-OH group on ribose to 2′-amino (NH2) or 2′-fluoro-modified sugars on pyrimidines can successfully confer resistance to breakdown in the serum for over 2 days [33] and remains present in the eyes after 28 days [34]. Thus, current clinically used aptamers [35] employ chemical modifications such as replacing 2’-OH with fluoro [8,36], NH2 [13,37], or O-methyl [38,39] groups at the 2′ position or capping the 3′ end with inverted thymidine to overcome nuclease degradation. Anti-human TNFα RNA aptamer which exhibits increased stability with the 2′-NH2 and 2′-fluoro modifications is one example using these methods. While degradation half-life of the unmodified TNFα RNA aptamer in the serum was several minutes, 2′-NH2 and 2′-fluoro modifications extended to 8 h [13]. Another study indicated that a RNA aptamer with 2-O-methyl-modification of all nucleotides in the backbone was stable in RNase rich mycoplasma-contaminated media for more than 4 h, whereas the stability of the modification on pyrimidines only was less than 30 min [39]. Thiophosphate-substituted aptamers, called thioaptamers, enhance stability by replacing one (monothioaptamer) or both (dithioaptamer) of the non-bridging phosphoryl oxygens in the oligonucleotide phosphate backbone with sulfur [40]. In addition to stabilization, thioation enhanced binding affinity as well, owing to decreased negative charge on DNA backbone [40]. Dithioaptamer developed by Gorenstein’s group [16] showed an increased binding affinity up to 28–600 fold compared to monothioaptamer [41]. “Mirror aptamers” (Spiegelmers) with oligonucleotide backbones composed of l-ribose (RNA Spiegelmers) or l-deoxy-ribose (DNA Spiegelmers) also lead to nuclease resistance. Spiegelmers, l-form aptamers that are natural d-form chiral inversions are not recognized by nucleases, and therefore display increased stability compared to their natural d-nucleic acid counterparts [42]. Pfander et al. developed a novel initiator nucleotide that resulted in an aldehyde modification of the 5′-end of RNA, which can be further modified with amino- or hydrazine-functionalized groups [43]. A38, an unmodified DNA aptamer for Vaccinia virus, also exhibits high stability [44] that may result from three-dimensional structures protecting the 3′- and 5′-termini of the aptamer from exonucleases. Examples of unmodified aptamer structures that confer high nuclease degradation resistance include closed ring structures at both terminal ligations or ligation of several different aptamers, forming closed structures with multiple specificities [45,46]. The degradation half-life of unligated “open” TS-1 aptamer (DNA aptamer bearing L-selectin-hairpin head) was 4.8 and 16 h in pooled human serum and plasma respectively, while the ligated “closed” TS-1 aptamer significantly increased the stability against nucleases to 9.5 and 50 h, respectively [45].
2.2. Renal Excretion
The primary excretion routes of injectable drugs are through the kidneys and liver. With aptamer sizes ranging from 5 to 30 kDa, the average diameter of aptamers is less than 5 nm [47], so most aptamers are smaller than the renal filtration threshold of glomeruli (i.e., 50 kDa) and are excreted from the kidney, regardless of their nuclease resistance. One common solution to overcome rapid renal excretion is augmentation of the aptamer’s overall size through conjugation with high molecular weight moieties such as polyethylene glycol (PEG) [48], cholesterol [49,50], protein [51,52], multimerization [53,54,55], or nanomaterials [56,57,58]. PEG is a US Food and Drug Administration (FDA) approved [59] hydrophilic biocompatible material that has been adopted in 12 biopharmaceuticals currently marketed for human use [60]. PEG is commercially available in a variety of sizes (0.3 to 10,000 kDa), with different terminal functional groups for chemical conjugation. For aptamer application, PEG is widely used for enlargement of the size [61] as well as addition of stealth effect to increase their retention in circulation. Macugen® (OSI Pharmaceuticals, Melville, USA) is composed of a RNA aptamer backbone conjugated with 40 kDa PEG, and exhibits prolonged plasma half-life of 9.3 h via intravenous injection, 12 h via subcutaneous injection [33], and 94 h via vitreous humor [34]. Similarly, conjugation of 40 kDa PEG to a 2′-fluoro-/2′-O-methyl modified aptamer resulted in an increase of the circulation half-life to 23 h in a mouse model [48]. However, high molecular weight PEG conjugation may cause a functional hindrance of the aptamer binding to its target [62]. Thus the size of PEG as well as type and length of linkage of PEG to aptamer should be determined in a case-by-case fashion. Cholesterol and sugar-based polymers are alternative options for this purpose. Cholesterol conjugation to factor IXa specific aptamer prolonged its half-life significantly in pigs, increasing from only 5 to 10 min for the unmodified aptamer up to 1 to 1.5 h for the conjugated ones [49].
2.3. Safety of Aptamers
Several factors including aptamer sequence, dose (single and cumulative), route, and chemical modifications may contribute to adverse effects [63]. Intravenously administered aptamers are generally well tolerated; however, a few adverse effects related to dose and sequence have been reported [64,65]. Studies have shown that intravenously administered aptamers diffuse rapidly into the tissues of various organs, accumulating primarily in the liver, kidney, and spleen [66]. Accumulation of oligonucleotides in various tissues has been qualitatively measured by immunohistochemistry, in situ hybridization, or histopathology [67,68]. A study by Geary et al. found the accumulation of human TNFα antisense phosphorothioate aptamer in the kidneys, livers, lymph nodes, and spleens of mice and primates [66]. Administration of aptamers via peritoneum (100 mg/kg) and vein (50 mg/kg) caused reversible kidney and liver abnormalities in mice with a recovery period of 4 to 13 weeks [69,70,71]. Infiltration of mononuclear cells and hypertrophy of Kupffer cells in the liver as well as basophilic granules in the cytoplasm of tubular epithelial cells in the kidneys were reported [71,72,73].
Liver metabolism of oligonucleotides may result in hepatotoxicity. Kang et al. reported no definitive evidence of sinusoidal dilation, Kupffer cell hypertrophy, or perivascular inflammatory infiltrate in the liver after intravenous bolus injection of E-selectin antagonistic thioaptamer (ESTA) at a dose up to 500 μg twice weekly for 4 weeks, compared to saline injected control mice [74]. A slight elevation of transaminases aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was noted, although the levels were still within normal range. Similarly, Phase I and II clinical trials of mipomersen, a phosphorothioate antisense oligonucleotide for apolipoprotein B synthesis inhibitor, showed transient dose-dependent elevation of AST and ALT over 5-week treatment (30–400 mg/week) [75,76]. In a randomized, placebo-controlled, dose-escalation Phase II study of mipomersen for 5–13-week treatment, an increase of ALT was noted among 17% of patients (10 of 59 cases), to a level more than three times the upper limit of the normal range [76]. In phase III trials, elevation of ALT with grade 2–4 (3-fold greater than upper limit of normal) was observed in 17.9% of patients (28 out of 156) who received mipomersen for 26 weeks treatment compared to the placebo arm [77,78,79,80].
Unintended anticoagulation effects have also been reported in ISIS 2302, a 20-mer antisense phosphorothioate oligonucleotide targeting human intercellular adhesion molecule-1 (ICAM-1) mRNA [81]. Prolongation of prothrombin and thrombin coagulation times was observed in in vitro assays in human plasma at concentrations of ISIS 2302 >100 μg/mL. Additionally, in a placebo-controlled trial of ISIS 2302 for the treatment of Crohn’s disease, intravenous infusion of ISIS 2302 (2 mg/kg) over 2 h caused a transient (2–4 h after dosing) increase in the activated partial thromboplastin time by approximately 10 seconds, compared to saline placebo infusion [82]. However, no increase in bleeding time was noted.
Complement activation through the interaction of oligonucleotides has been reported. Repeated bolus intravenous injections of PEGylated E-selectin thioaptamer at a dose of 128 μg resulted in a 2-fold increase of plasma C3a level in mice compared with saline injected mice, though the level of increase was far less than the level of symptomatic hypersensitive reaction [62]. Henry et al. reported that a single dose of intravenous 10-min infusion of 20 mg/kg ISIS 2302 caused an increase of complement split products Bb and C5a (100- and 7-fold, respectively), accompanied by changes in blood pressure, neutrophil count, and serum cytokine levels (i.e., increases in IL-6, MCP-1, and IL-12) in primates. These changes were observed at or near the end of infusion, which led to death in one of the three primates approximately 4 h after infusion. This study demonstrated that complement pathway activation in primates was related to rapid infusion of phosphorothioate oligonucleotides [83]. Co-administration of CAB-2m, a complement activation inhibitor, alleviated complement pathway activation along with other clinical symptoms [84].
Since aptamers are synthetic nucleic acids, it is possible for them to be recognized by the innate immune system via germline-encoded-pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) and retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) [85]. Activation of Toll-like Receptor 3 (TLR 3), TLR 7, TLR 8 or TLR 9 induces immunity upon recognizing pathogen-derived nucleic acids [86]. Accordingly, TLR9 responds to unmethylated CpG motifs in DNA, TLR3 to double-stranded RNA, and TLR7 and TLR8 to single-stranded RNA [87]. Studies showed that the endocytosis of unmethylated CpG motif of DNA into dendritic cells or B cells expressing TLR9 triggers both innate and adaptive immune responses [88,89] and induces inflamatory cascades [88,89,90]. The CpG motif consists of an unmethylated CpG dinucleotide flanked by particular base contexts, such as two 5′-purines and two 3′-pyrimidines [91]. For example, the CpG motif with the “TCAACGTT” sequence was shown to induce B cell activation, whereas a similar sequence, “TCATCGAT”, had no effect on B cell activation. Interestingly, immunogenicity was either absent or limited even if pegaptanib doses 1000-fold higher than clinically required were intravenously administered to primates [92,93]. While development of antibodies against synthetic oligonucleotides is uncommon [93], it is noteworthy that antibodies to PEG may cause an adverse effect due to frequent exposure to PEGylated products [94]. In a phase II trial of pegloticase with 10 kDa PEG for refractory gout at a dose of 8 mg intravenous infusion every 3 weeks, 13 of 30 (43%) patients with pre-existing antibody against PEG experienced more frequent infusion reactions (eight of 13 patients, 62%) than patients without (five of 17 patients, 29%) [95].
3. Aptamers as Therapeutics under Investigation in Oncology
The main strategy of aptamer-based therapeutics in oncology application is a blockade of protein-protein or receptor-ligand interactions as an antagonist. Accordingly, aptamers have been utilized as antagonists against oncoproteins or their ligands, targeting moieties for drug delivery in multivalent therapeutics. In this section, we specifically focus on therapeutic aptamers with antagonistic functions under pre-clinical investigation for anti-cancer therapy (Figure 1 and Table 1). In addition, antidote aptamers for controlled therapy are also discussed.
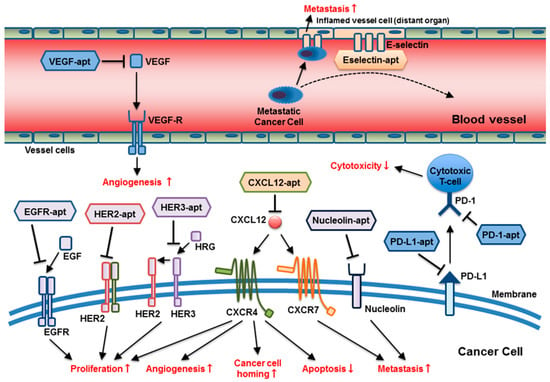
Figure 1.
The broad range of molecular targets and targeting mechanisms of anti-cancer aptamers: Aptamers target multiple molecular pathways involving tumor progression and metastasis, including cancer cell proliferation, cell homing, apoptosis suppression, metastasis, impairment of T-cell cytotoxicity, and angiogenesis at different locations. (apt = aptamer).

Table 1.
Molecularly targeted anti-cancer aptamers with confirmed in vivo anti-cancer efficacy.
3.1. Aptamers as Tools for Cancer Therapy
3.1.1. RNA Aptamer Targeting VEGF
Although the VEGF targeted aptamer pegaptanib is currently approved for use in ophthalmology (see the Clinical Trials section), it was originally designed for use in cancer treatment [148,149]. The cancer treatment strategy of anti-VEGF aptamer is inhibition of VEGF-associated tumor vessel formation by binding to VEGF thus blocking the VEGF/VEGF-R interaction [29]. Daily treatment of mice bearing human A673 rhabdomyosarcoma cell xenograft tumor with anti-VEGF PEGylated RNA aptamer (EYE001) at a dose of 10 mg/kg inhibited tumor growth by 74% relative to the control [149]. Recently, an aptamer-antibody hybrid complex has been prepared to improve the pharmacokinetics, using cotinine-specific antibody and cotinine-binding pegaptanib aptamer [52]. This hybrid complex showed prolongation of half-life in serum to 8.2 h. In an A549 (human lung adenocarcinoma cells) xenograft mouse model, systemic administration of the pegaptanib-antibody complex inhibited tumor angiogenesis and reduced tumor growth (<50%) compared to the human-IgG control mice group. This effect was comparable to that observed for bevacizumab, an anti-VEGF monoclonal antibody.
3.1.2. Aptamers Targeting the Epidermal Growth Factor Receptor (EGFR)
EGFR (ErbB1) is a receptor tyrosine kinase (RTK) that is often mutated and overexpressed in many types of solid tumors, resulting in tumor growth [150]. Thus, EGFR serves as an excellent therapeutic target, and anti-EGFR antibodies as well as TKI have been widely adopted for clinical use to block the downstream signaling cascade [150]. Similar to anti-EGFR antibody, the binding of anti-EGFR aptamer to the extracellular domain of EGFR blocks subsequent phosphorylation and downstream signaling, i.e., PI3K/AKT and MAPK signaling [29]. Several anti-EGFR aptamers have been developed and demonstrated antitumor effect in various cancers (vulvar carcinoma, lung carcinoma, breast cancer, glioblastoma, and epidermoid carcinoma) [7,8,108,109,110]. Esposito et al. reported that CL4, an anti-EGFR 2′-fluoro pyrimidine RNA aptamer, inhibited EGFR-mediated signal pathways, induced apoptosis, and inhibited tumor growth in a mouse xenograft model of human non-small-cell lung cancer (NSCLC) [7]. Li et al. identified an anti-EGFR 2′-fluoro pyrimidine modified aptamer (E07) that binds both wild-type EGFR as well as EGFR variant III (EGFRvIII), the most common deletion mutant of extracellular domain mutations that precludes ligand binding. The binding of E07 to EGFR expressing A431 epidermoid carcinoma cells led to inhibition of EGFR autophosphorylation and cell proliferation in three-dimensional cultures [8].
3.1.3. Aptamers Targeting HER2 and HER3
HERs including HER2 (ErbB-2), HER3 (ErbB-3), and HER4 (ErbB-4) are a part of the EGFR family and transduce growth-promoting signals in response to dimerization of extracellular domains upon ligand binding [151]. Overexpression of HER2 is observed in approximately 10–15% of breast cancers and currently monoclonal antibody or TKI is used to block its function. Weekly intraperitoneal injection of trimeric DNA anti-HER2 aptamer at a dose of 40 μg exhibited a 2-fold higher tumor inhibitory effect on N87 human gastric cancer cells implanted in CD-1 mice compared to the HER2 monoclonal antibody (Ab431, 160 μg) [112]. In a recent study, HER3 has been shown to play a role in mediating resistance to HER2 and phosphoinositide 3-kinase (PI3K) pathway-directed therapies due to its feedback regulation via AKT signaling [152]. As a result, HER3 is being examined as a direct therapeutic target [152]. The first RNA aptamer against HER3 (A30) has been shown to block interaction between HER3 and its ligand (HRG; heregulin). Binding of HRG disrupts HER3 oligomerization and leads to the formation of signaling-competent heterodimers, preferentially with HER2. Binding of A30 to HER3 inhibited HRG-dependent tyrosine phosphorylation of HER2 and the HRG-induced growth response of MCF7 breast cancer cells in vitro [153].
3.1.4. DNA Aptamer Targeting Nucleolin
The abundant non-ribosomal protein, nucleolin, which shows abnormally increased cell membrane localization in several types of cancer, interacts with key oncogenes and plays an integral role in cellular proliferation, invasion, and apoptosis [100,154,155]. Nucleolin expressed on the cell surface interacts with ligands implicated in cell differentiation, survival, inflammation, angiogenesis, and tumor development [156]. AS1411 is a guanine quadruplex aptamer that binds to nucleolin, inducing growth inhibition in vitro and in vivo human xenograft models (renal cancer, lung cancer, MX1 breast cancer, and pancreatic cancer) [100]. Notably, AS1411 was discovered by screening for anti-proliferative activity among antisense oligonucleotides rather than the SELEX method typical in aptamer development [100]. The underlying mechanism of nucleolin-associated anti-proliferative effect involves binding to cell surface nucleolin, internalization of the aptamer-nucleolin complex, subsequent cell cycle arrest, and apoptosis [100,155]. AS1411 is currently in clinical trials for treatment of leukemia and renal cell carcinoma (see the Clinical Trials section) and is in pre-clinical investigation for other hematologic and solid malignancies. Recent in vitro studies have found that AS1411 displays activity against lymphoma through blockade of secondary targets (nuclear factor-κB and B-cell lymphoma 2) [157,158] and that in combination therapy with doxorubicin it reduced diffuse large B-cell lymphoma (DLBCL) cell survival [159]. Xenograft tumor models derived from A498 renal cancer, SKMES lung cancer, and MX1 breast cancer cells showed that intravenous administration of AS1411 delays tumor growth [100]. Moreover, combination therapy with AS1411 and gemcitabine, a chemotherapeutic agent used to treat pancreatic and non–small cell lung cancer in clinical practice, resulted in an increased antitumor effect compared to gemcitabine alone in xenograft model of human pancreatic cancer [100].
3.1.5. DNA Aptamer Targeting PD-1/PD-L1
Programmed cell death 1 (PD-1; also known as CD279) is expressed in several cell types including T-lymphocytes, specifically CD8 tumor-infiltrating lymphocytes, which are in charge of directly eradicating tumor cells [160]. The interaction between PD-1 expressed on the surface of T-lymphocytes and PD-L1 expressed on cancer cells leads to an impairment of CD8 cytotoxicity. Prodeus et al. developed a 40 kDa PEGylated DNA aptamer, PEG-MP7, that specifically binds to PD-1, decreases tumor growth, and increases survival in mouse tumor models [139]. Their study found that PD-L1-mediated suppression of IL-2 secretion was functionally inhibited in primary T-cells treated with the base aptamer MP7 or a known anti-PD-1 antibody, but not control aptamer. Furthermore, an in vivo study using a murine colon cancer model (MC38 cells stably expressing human CEA; MC38.CEA) showed that PEG-MP7 effectively inhibited PD-1: PD-L1 interaction, showing efficacy equal to anti-PD-1 antibody in suppressing PD-L1+ carcinoma cell growth. Additional study by Lai et al. reported the PD-L1 antagonizing DNA aptamer (aptPD-L1) for the blockade of the binding between human PD-1 and PD-L1 [140]. Repeated intraperitoneal administrations of aptPD-L1 (1.2 mg/kg, 4 times a week) led to significant tumor growth inhibition compared to random-sequence aptamer administration in both the CT26 colon cancer cell line and LL/2 Lewis lung cancer cell line murine syngeneic tumor model mice.
3.1.6. RNA Aptamer Targeting SDF-1 (CXCL12)
CXCL12 (C-X-C motif chemokine ligand 12), also known as SDF-1 (stromal cell-derived factor-1), is a critical chemokine involved in tumor metastasis, angiogenesis, cancer cell homing, and proliferation [161,162]. NOX-A12 is a PEGylated mirror-image RNA aptamer that displays high binding affinity to CXCL12 at a Kd of 0.2 nM [104]. Through binding to CXCL12, NOX-A12 inhibits signaling on both its receptors, CXCR4 and CXCR7, thus preventing angiogenesis as well as tumor cell proliferation, invasion, and metastasis [104]. Based on the efficacy of NOX-A12 as a treatment in a pre-clinical study of hematologic malignancies, it is currently in clinical trials for treatment of leukemia (CLL) and multiple myeloma (see Clinical Trials section). In addition, CXCL12 is expressed in the tumor microenvironment mainly by cancer-associated fibroblasts [163] and has been indicated for resistance to checkpoint inhibitors such as anti-PD-L1 through T-cell exclusion [164]. Zboralski et al. showed that NOX-A12 broke the immune-privileged status of the tumor microenvironment by paving the way for immune effector cells to enter into the tumor [107]. In this study, combination of NOX-A12 and anti-PD-1 (NOX-A12: 20 mg/kg s.c., every other day, anti-PD-1: 10 mg/kg i.p. twice weekly) resulted in significant tumor size reduction, superior to either vehicle, anti-PD-1 monotherapy, or NOX-A12 monotherapy in a murine syngeneic CT-26 colon cancer model.
3.1.7. Thioaptamer Targeting E-Selectin
E-selectin is an adhesion molecule expressed on the luminal surface of inflamed blood vessels which mediates hematogenous metastasis by assisting shear-resistant adhesion of circulating tumor cells to the inflamed vessel surface under dynamic blood flow [128]. An E-selectin antagonistic thioaptamer (ESTA) identified from cell-SELEX demonstrated the ability to inhibit adhesion of circulating cells to E-selectin expressing endothelial cells with binding affinity and IC50 of 47 nM and 63–83 nM, respectively. This accounts for >10,000 times higher affinity and 1000 times lower IC50 to its natural ligands (sLex, Kd = 100–2000 uM; IC50 = 100–750 uM) [128]. Accordingly, a single intravenous injection of ESTA effectively prevented hematogenous metastasis of CD44high breast cancer cells to a level equal to baseline by abrogating their adhesion to E-selectin-expressing pre-metastatic vascular niches in both syngeneic and xenogeneic forced breast cancer metastasis models [129]. Additionally, truncated ESTA conjugated with 10 kDa PEG extended its serum half-life and led to improvement of its anti-metastasis activity compared to parental ESTA [62]. Additionally, twice weekly intravenous administration of PEGylated ESTA at a 128 μg dose was well tolerated; no symptomatic changes in the ALT or AST levels, C3a, C5a complement levels, inflammation, or tissue damage (kidney, lung, or heart) were noted. Based on its E-selectin specific binding ability, ESTA was also used as a targeting moiety for nano- and microparticles [165]. ESTA conjugation to liposome enhanced tumor targeting in mouse models of breast cancer and conjugation of ESTA to porous silicon microparticles allowed bone marrow targeting in mice [166,167]. This exemplifies the versatile applications of aptamers as therapeutic agents and targeting moieties.
3.1.8. RNA Aptamer Targeting CD40
CD40Apt, a 2-fluoro-RNA aptamer against CD40, has shown antitumor effect on CD40-expressing A20 lymphoma cells in vitro and in vivo. Soldevilla et al. reported that CD40Apt-SMG1-shRNA chimera improved survival of BALB/c mice that were intravenously inoculated with A20 lymphoma cells compared to CD40Apt-control-shRNA or untreated mice [145].
3.1.9. DNA Aptamer Targeting CTLA-4
Monoclonal antibodies targeting cytotoxic T lymphocyte antigen-4 (CTLA-4) and PD-1/PD-L1 axis are now a part of routine clinical practice [168]. Huang et al. reported a novel high-affinity CTLA-4-antagonizing DNA aptamer that promoted lymphocyte proliferation and inhibited tumor growth in a murine syngeneic tumor model with mouse TC-1 lung cancer cells [146].
3.1.10. RNA/DNA Aptamer Targeting C5a
A synergistic antitumor effect through inhibition of C5a/C5a receptor-1 and PD-1 signaling has been reported. Ajona et al. showed that blockade of PD-1 via RMP1-14 antibody and signaling inhibition of complement C5a/CD5a receptor via AON-D21 L-aptamer, reduced tumor growth and metastasis in syngeneic models of lung cancer. This study further showed a complete reversal of CD8 T-cell exhaustion contributed prolonged survival in mice receiving dual therapy [147].
3.1.11. Thioaptamer Targeting Annexin A2
Given the central role angiogenesis plays in cancer progression, therapies have sought to target immature tumor blood vessels, yet have only achieved limited efficacy due to treatment induced-hypoxia. Mangala et al. developed a novel approach for the identification of target thioaptanmers using patient-derived endothelial cells [169]. Thioaptamers specific to tumor associated endothelial cells isolated from ovarian cancer patients were isolated following repeated cycles of negative and positive selection. This unbiased selection method allows for a selection of aptamers that specifically bind to tumor vessels but not to normal vessels. Mass spectrometry data identified a molecular target, the tumor endothelial cell specific membrane protein annexin A2. Treatment with the aptamer/microRNA-inhibitor complex restored tight junction function and improved chemotherapy delivery in orthotopic ovarian cancer mouse models, reducing tumor growth.
3.1.12. Bispecific Aptamers
Bispecific aptamers have been generated to specifically and simultaneously interact with two independent targets. RNA-based bispecific CD44-EpCAM aptamer is capable of blocking CD44 and EpCAM simultaneously by fusing single CD44 and EpCAM aptamers with a double stranded RNA adaptor. Bispecific CD44-EpCAM aptamer suppressed intraperitoneal tumor outgrowth more significantly than individual CD44 and EpCAM aptamers did alone or in combination through enhanced targeting of cancer cells. Bispecific aptamers are well adapted for anti-cancer immunotherapy with minimal toxicity. Co-stimulatory ligand binding of tumor antigen-specific lymphocytes is one immunotherapy approach to boost the antitumor immune response [170]. However, the use of agonistic antibodies such as anti-4-1BB or anti-CD28 have proven difficult due to activation of the lymphocytes expressing the co-stimulatory receptor. In fact, multi-organ toxicity following the administration of agonistic CD28 antibodies such as TGN1412 led to early termination of clinical trial [171]. Liver toxicity is a concern with 4-1BB-mediated treatment of cancer [172]. To alleviate possible adverse effects, bispecific aptamers were developed to bridge between cancer cells and co-stimulatory receptors. For example, bispecific aptamer PSMA-41BB is composed of PSMA and the agonistic 4-1BB aptamers. PSMA binding to cancer cells and 4-1BB binding to the co-stimulatory receptor trigger its activation. Similarly, bispecific aptamers such as VEGF-4-1BB, CD28-MRP1, and CD16a-C-Met have been generated [136,137,138]. These bispecific aptamers have shown higher anti-tumor efficacy relative to non-targeted, co-stimulation reagents or their corresponding monoclonal antibody, achieving the same effect with lower toxicity [136,173,174].
3.2. Antidote Aptamer for Controlled Therapy
Eradication of malignant cells is currently the main goal of cancer therapies. Therapeutic efficacy and toxicity are often two sides of the same coin and the balance is fundamental to determining therapeutic efficacy. Humanized monoclonal antibodies have shown circulation half-lives as long as several weeks, enabling less frequent drug administration. Such patient-friendly regimens not only improve quality of life, but are also expected to have enhanced anti-tumor efficacy due to continuous drug exposure. However, unanticipated toxicity from such long-circulating drugs may potentially be prolonged unless otherwise inactivated. Antidote aptamers are a strategy to control the action time of therapeutic aptamers using the corresponding complementary sequence. Hybridization with the antidote leads to a conformational change and loss of target binding. Attempts to control drug action time using this method have been confirmed in animal experiments [175,176]. Rusconi et al. showed that coagulation factor IXa antagonistic aptamer Peg-9.3t’s potent anticoagulant activity was efficiently reversed by administration of the complementary oligonucleotide (antidote oligonucleotide 5–2) within 10 min in human plasma. An antidote oligonucleotide for R9D-14T, a RNA aptamer that binds prothrombin and thrombin pro/exosite I, has been shown to swiftly (<2 min) reverse F9D-14T’s anti-coagulation activity in an assay containing human plasma [176].
4. Clinical Trials of Aptamer Application in Oncology
As previously discussed, renal filtration, nuclease degradation, and safety profile present some of the major hurdles for aptamer therapeutics. However, the degree of such challenges may vary with route of administration as well as the targeted site. The best example of an aptamer therapeutic that has overcome these difficulties is pegaptanib, the first only aptamer to receive FDA marketing approval. In 2004, following almost a decade of development and testing, the efficacy of the RNA aptamer commercially known as Macugen® (Eyetech Pharmaceuticals, New York, NY, USA) in treating neovascular age-related macular (AMD) degeneration was confirmed by clinical trial [177]. Through intravitreous administration every 6 weeks over a 48-week course, pegaptanib acts as an antagonist to vascular endothelial growth factor (VEGF), which plays a role in pathologic angiogenesis. In addition to abnormal neovascularization, VEGF contributes to increased vascular permeability and other effects important not only in the development of macular degeneration, but also cancer [9,178,179]. Given the significant role VEGF plays in tumors it was initially thought that pegaptanib may display anti-cancer properties as well. Unfortunately, preclinical models failed to substantiate this hypothesis and the drug has not been tested in a clinical trial for oncology application [29]. The bioavailability of intravitrious injections may contribute to the difference in pegaptanib’s efficacy in AMD and solid tumors, since the former route does not necessitate extended circulation time to reach the target as it does in tumors. Further preclinical studies, however, are underway to address novel ways of improving the biostability of aptamers like pegaptanib [52].
To date, two therapeutic aptamers have made successful transition to clinical trials for oncology, although several ongoing trials have incorporated the development of targeted aptamers for either detection or monitoring into their study design. The first aptamer in clinical trials for cancer treatment was AS1411, a nucleolin-targeting DNA aptamer [100]. Its unique G-rich quadruplex structure as well as pegylation aid the pharmacokinetic profile of AS1411, providing nuclease evasion and an extended half-life [100]. Two clinical trials of AS1411 have assessed safety and efficacy in advanced solid tumors and renal cell carcinoma, respectively (NCT00881244 [180] and NCT00740441 [181]), while a third examined AS1411 in treatment of acute myeloid leukemia (AML) (NCT00512083 [182]). In its initial dose-escalation Phase I clinical study, AS1411 was administered as a continuous intravenous infusion at doses ranging from 1–40 mg/kg/day for 4 to 7 days in up to two cycles of treatment for patients with advanced solid tumors and was found to be well tolerated without serious side effects. Given the promising results of initial clinical assessment, a Phase II trial was conducted in patients with metastatic refractory renal cell carcinoma (RCC), but found the drug to have limited activity in unselected patients, indicating the need for biomarkers for AS1411 responsive tumors. Pre-clinical studies demonstrating that AS1411 targets cell-membrane nucleolin in leukemia cells [183] and results in cell death for acute myeloid leukemia (AML) cell lines [184] indicated its efficacy in hematologic malignancies as well. Another Phase II trial examined the effect of combination therapy with AS1411 and high-dose cytarabine in relapsed/refractory AML patients and found improved response rates among patients receiving combination therapy with continuous infusion of either 10 or 40 mg/kg/day dosing of AS1411 when compared to the control cytarabine only group [185]. Several pre-clinical models are also investigating its role as a potential transport mechanism for other anti-cancer drugs (See the under investigation section), possibly expanding its impact from hematologic malignancies to solid as well.
The second therapeutic aptamer to launch clinical trials for cancer is NOX-A12 (Olaptesed Pegol, NOXXON), an antagonist of the chemokine CXCL12 (or SDF1). Thus far, NOX-A12 has only completed clinical trials for hematologic malignancies, although a new two-part trial (NCT03168139 [186]) is currently recruiting patients with colorectal or pancreatic cancer. NOX-A12 is an L-form RNA aptamer (or Spiegelmer) whose inverted stereochemistry provides nuclear resistance, while PEGylation at the 3′-terminus provides further enhancement of its pharmacokinetic parameters. Pre-clinical models showed that NOX-A12 mobilized white blood cells, hematopoietic stem cells, and progenitor cells in the peripheral blood of mice and healthy human volunteers [104] and Phase I trials (NCT00976378 [187] and NCT01194934 [188]) established its safety profile in healthy volunteers. Pre-clinical models demonstrated that co-treatment of multiple myeloma (MM) cells with NOX-A12 inhibited chemotaxis of MM to bone marrow, inhibiting cell adhesion-mediated drug resistance and sensitizing them to chemotherapy [189]. NOX-A12 also decreased BM niche microenvironment receptivity to MM cells, halting an essential step in disease progression in mouse models [190]. Thus, a Phase II trial (NCT01521533 [191]) comparing single dose intravenous injections of NOX-A12 alone and in combination with bortezomib and dexamethasone (VD) in multiple myeloma (MM) patients who had received previous treatment was performed. Results from this trial indicated that NOX-A12 enhanced the efficacy of VD treatment without increasing treatment toxicity [192]. Pre-clinical models in chronic lymphocytic leukemia (CLL) also showed that NOX-A12 effectively prevented chemotaxis of cancer cells towards CXCL12 and sensitized the cells to chemotherapies in bone marrow stem cell (BMSC) co-cultures [106]. Phase II study (NCT01486797 [193]) testing the efficacy of NOX-A12 in combination with bendamustine and rituximab chemotherapy in patients with relapsed CLL also found improved overall response rates as well as increasing rates of remission in patients treated with NOX-A12 [194]. The aptamer is currently in the recruitment stage of a two-part, phase I/II clinical trial of testing the drug in combination with pembrolizumab in metastatic colorectal and pancreatic cancer (NCT03168139 [186]).
5. Conclusions and Future Perspectives
In the era of personalized medicine, targeted therapy has become an integral part of cancer treatment in conjunction with conventional chemo- and radiotherapy, and many molecularly targeted drugs are under investigation and in the drug development pipeline [195]. Aptamers have evolved rapidly in the past 10 years as evidenced by the steep increase in related peer-review publications and US patents, and have been highlighted as the next generation of anti-cancer drug owing to their unique characteristics (high affinity binding, low immunogenicity, ease of synthesis, and chemical modification). A range of molecules involved in tumor progression and metastasis, present at different sites (i.e., circulation, cancer cell, tumor stroma, tumor associated vessel, and pre-metastic vascular niche), have been targeted by aptamers. Pre-clinical investigations of aptamers have shown promising efficacies as well as safety profiles. Creative chemistry has effectively addressed the primary challenges of aptamer therapeutics. Mounting evidence supports the competencies of aptamers for clinical utility in treating cancer, and currently, two anti-cancer aptamers have launched clinical trials. As safe, high-affinity therapeutics, aptamers present an exceptional opportunity to make personalized medicine a reality for more patients with cancer.
Acknowledgments
This work was supported by the National Institutes of Health (1R01CA160271-01A1 to T.T).
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [PubMed]
- Gewirtz, A.M. Oligonucleotide therapeutics: Clothing the emperor. Curr. Opin. Mol. Ther. 1999, 1, 297–306. [Google Scholar] [PubMed]
- Brody, E.N.; Gold, L. Aptamers as therapeutic and diagnostic agents. J. Biotechnol. 2000, 74, 5–13. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Liu, Y.-C.; Chen, N.-Y.; Tsai, C.-F.; Wang, Y.-T.; Chen, Y.-J.; Hsu, T.-L.; Yang, P.-C.; Wong, C.-H. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Boggon, T.J.; Li, Y.; Woo, M.S.; Greulich, H.; Meyerson, M.; Eck, M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Passaro, D.; Longobardo, I.; Condorelli, G.; Marotta, P.; Affuso, A.; de Franciscis, V.; Cerchia, L. A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death. PLoS ONE 2011, 6, e24071. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Nguyen, H.H.; Byrom, M.; Ellington, A.D. Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS ONE 2011, 6, e20299. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2′-fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Khati, M.; Schuman, M.; Ibrahim, J.; Sattentau, Q.; Gordon, S.; James, W. Neutralization of infectivity of diverse R5 clinical isolates of human immunodeficiency virus type 1 by gp120-binding 2′F-RNA aptamers. J. Virol. 2003, 77, 12692–12698. [Google Scholar] [CrossRef] [PubMed]
- Layzer, J.M.; McCaffrey, A.P.; Tanner, A.K.; Huang, Z.; Kay, M.A.; Sullenger, B.A. In vivo activity of nuclease-resistant siRNAs. RNA 2004, 10, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, D.; Green, L.S.; Bell, C.; Lynott, C.K.; Gill, N.; Vargeese, C.; Kirschenheuter, G.; McGee, D.P.; Abesinghe, P.; Pieken, W.A.; et al. Potent 2′-amino-2′-deoxypyrimidine RNA inhibitors of basic fibroblast growth factor. Biochemistry 1995, 34, 11363–11372. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gao, X.; Zhang, Z. Isolation and characterization of 2′-amino-modified RNA aptamers for human TNFα. Genom. Proteom. Bioinform. 2004, 2, 32–42. [Google Scholar] [CrossRef]
- Padilla, R.; Sousa, R. A Y639F/H784A T7 RNA polymerase double mutant displays superior properties for synthesizing RNAs with non-canonical NTPs. Nucleic Acids Res. 2002, 30, e138. [Google Scholar] [CrossRef] [PubMed]
- Dellafiore, M.A.; Montserrat, J.M.; Iribarren, A.M. Modified nucleoside triphosphates for in vitro selection techniques. Front. Chem. 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.E.; Lokesh, G.L.R. Development of phosphorothioate DNA and DNA thioaptamers. Biomedicines 2017, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Clercq, E.D.; Eckstein, F.; Merigan, T.C. Interferon induction increased through chemical modification of a synthetic polyribonucleotide. Science 1969, 165, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Giangrande, P.H. Aptamer-siRNA chimeras: Discovery, progress, and future prospects. Biomedicines 2017, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G. A review of therapeutic aptamer conjugates with emphasis on new approaches. Pharmaceuticals 2013, 6, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, V.C.; Kavruk, M.; Dilek, O.; Bayrac, A.T. Aptamers: Molecular tools for medical diagnosis. Curr. Top. Med. Chem. 2015, 15, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.B.; Gu, M.B. Aptamer-based sandwich-type biosensors. J. Biol. Eng. 2017, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Ma, H.; Liu, J.; Ali, M.M.; Mahmood, M.A.; Labanieh, L.; Lu, M.; Iqbal, S.M.; Zhang, Q.; Zhao, W.; Wan, Y. Nucleic acid aptamers in cancer research, diagnosis and therapy. Chem. Soc. Rev. 2015, 44, 1240–1256. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, E.K.; Palomaki, T.; Pasanen, M. Oligonucleotide-based pharmaceuticals: Non-clinical and clinical safety signals and non-clinical testing strategies. Regul. Toxicol. Pharmacol. 2017, 90, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Yu, Z.; Zhao, X.; Lu, S.; Wang, Z. Aptamers in hematological malignancies and their potential therapeutic implications. Crit. Rev. Oncol. Hematol. 2016, 106, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A. Aptamers in therapeutics. J. Clin. Diagn. Res. 2016, 10, BE01-06. [Google Scholar] [CrossRef] [PubMed]
- Poolsup, S.; Kim, C.Y. Therapeutic applications of synthetic nucleic acid aptamers. Curr. Opin. Biotechnol. 2017, 48, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yazdian-Robati, R.; Arab, A.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Application of aptamers in treatment and diagnosis of leukemia. Int. J. Pharm. 2017, 529, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wilson, G.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget 2016, 7, 13446–13463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Zhang, X.B.; Ye, M.; Tan, W. Nucleic acid aptamer-mediated drug delivery for targeted cancer therapy. ChemMedChem 2015, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Robbie, G.J.; Criste, R.; Dall’Acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A novel investigational fc-modified humanized monoclonal antibody, motavizumab-yte, has an extended half-life in healthy adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.C.; Tidmarsh, G.F.; Bock, L.C.; Toole, J.J.; Leung, L.L. In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood 1993, 81, 3271–3276. [Google Scholar] [PubMed]
- Tucker, C.E.; Chen, L.S.; Judkins, M.B.; Farmer, J.A.; Gill, S.C.; Drolet, D.W. Detection and plasma pharmacokinetics of an anti-vascular endothelial growth factor oligonucleotide-aptamer (nx1838) in rhesus monkeys. J. Chromatogr. B Biomed. Sci. Appl. 1999, 732, 203–212. [Google Scholar] [CrossRef]
- Drolet, D.W.; Nelson, J.; Tucker, C.E.; Zack, P.M.; Nixon, K.; Bolin, R.; Judkins, M.B.; Farmer, J.A.; Wolf, J.L.; Gill, S.C.; et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (nx1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res. 2000, 17, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Keating, G.M. Pegaptanib: In exudative age-related macular degeneration. Drugs 2005, 65, 1571–1577, discussion 1578–1579. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, N.; White, S.J.; Bunka, D.H.; Song, L.; Stead, S.; Tarbin, J.; Sharman, M.; Zhou, D.; Stockley, P.G. Toggled RNA aptamers against aminoglycosides allowing facile detection of antibiotics using gold nanoparticle assays. Anal. Chem. 2012, 84, 6595–6602. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Sugimoto, N. Molecular evolution of functional nucleic acids with chemical modifications. Molecules 2010, 15, 5423–5444. [Google Scholar] [CrossRef] [PubMed]
- Lebars, I.; Richard, T.; Di Primo, C.; Toulme, J.J. LNA derivatives of a kissing aptamer targeted to the trans-activating responsive RNA element of hiv-1. Blood Cells Mol. Dis. 2007, 38, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.J.; Stockdale, K.R.; Huang, L.; Horswill, A.R.; Behlke, M.A.; McNamara, J.O., 2nd. Degradation of nuclease-stabilized RNA oligonucleotides in mycoplasma-contaminated cell culture media. Nucleic Acid Ther. 2012, 22, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Thiviyanathan, V.; Somasunderam, A.D.; Gorenstein, D.G. Combinatorial selection and delivery of thioaptamers. Biochem. Soc. Trans. 2007, 35, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.E.; Yang, X.; Fennewald, S.M.; King, D.J.; Bassett, S.E.; Venkitachalam, S.; Herzog, N.; Luxon, B.A.; Gorenstein, D.G. Solution structure and design of dithiophosphate backbone aptamers targeting transcription factor nf-kappab. Bioorg. Chem. 2002, 30, 396–419. [Google Scholar] [CrossRef]
- Maasch, C.; Buchner, K.; Eulberg, D.; Vonhoff, S.; Klussmann, S. Physicochemical stability of NOX-E36, a 40mer L-RNA (spiegelmer) for therapeutic applications. Nucleic Acids Symp. Ser. 2008, 52, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Pfander, S.; Fiammengo, R.; Kirin, S.I.; Metzler-Nolte, N.; Jaschke, A. Reversible site-specific tagging of enzymatically synthesized RNAs using aldehyde-hydrazine chemistry and protease-cleavable linkers. Nucleic Acids Res. 2007, 35, e25. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, A.; Kurth, A.; Dunkhorst, A.; Panke, O.; Sielaff, H.; Junge, W.; Muth, D.; Scheller, F.; Stocklein, W.; Dahmen, C.; et al. One-step selection of vaccinia virus-binding DNA aptamers by monolex. BMC Biotechnol. 2007, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Di Giusto, D.A.; Knox, S.M.; Lai, Y.; Tyrelle, G.D.; Aung, M.T.; King, G.C. Multitasking by multivalent circular DNA aptamers. Chembiochem 2006, 7, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nakamura, Y.; Ohuchi, S. Inhibitory RNA aptamer against sp6 RNA polymerase. Biochem. Biophys. Res. Commun. 2012, 420, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Guo, P. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 2010, 5, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, P.E.; Lewis, S.D.; Silva, R.F.; Preiss, J.R.; Horwitz, L.R.; Pendergrast, P.S.; McCauley, T.G.; Kurz, J.C.; Epstein, D.M.; Wilson, C.; et al. Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem. Biol. 2005, 12, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, C.P.; Roberts, J.D.; Pitoc, G.A.; Nimjee, S.M.; White, R.R.; Quick, G., Jr.; Scardino, E.; Fay, W.P.; Sullenger, B.A. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat. Biotechnol. 2004, 22, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.H.; Kim, J.H.; Noh, Y.H.; Noh, G.J.; Lee, S.W. Pharmacokinetics of a cholesterol-conjugated aptamer against the hepatitis C virus (HCV) NS5B protein. Mol. Ther. Nucleic Acids 2015, 4, e254. [Google Scholar] [CrossRef] [PubMed]
- Dougan, H.; Lyster, D.M.; Vo, C.V.; Stafford, A.; Weitz, J.I.; Hobbs, J.B. Extending the lifetime of anticoagulant oligodeoxynucleotide aptamers in blood. Nucl. Med. Biol. 2000, 27, 289–297. [Google Scholar] [CrossRef]
- Heo, K.; Min, S.W.; Sung, H.J.; Kim, H.G.; Kim, H.J.; Kim, Y.H.; Choi, B.K.; Han, S.; Chung, S.; Lee, E.S.; et al. An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. J. Control. Release 2016, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Dennis, D.M.; Morey, T.; Yang, L.; Tan, W. Engineering dendritic aptamer assemblies as superior inhibitors of protein function. Chem. Asian J. 2010, 5, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Montesarchio, D. Polyvalent nucleic acid aptamers and modulation of their activity: A focus on the thrombin binding aptamer. Pharmacol. Ther. 2012, 136, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Soule, E.E.; Bompiani, K.M.; Woodruff, R.S.; Sullenger, B.A. Targeting two coagulation cascade proteases with a bivalent aptamer yields a potent and antidote-controllable anticoagulant. Nucleic Acid Ther. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.C.; Collins, B.D.; Zhang, T.; Green, L.S.; Sebesta, D.P.; Bell, C.; Kellogg, E.; Gill, S.C.; Magallanez, A.; Knauer, S.; et al. Liposome-anchored vascular endothelial growth factor aptamers. Bioconj. Chem. 1998, 9, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Soontornworajit, B.; Martin, J.; Sullenger, B.A.; Gilboa, E.; Wang, Y. A hybrid DNA aptamer-dendrimer nanomaterial for targeted cell labeling. Macromol. Biosci. 2009, 9, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rashid, F.; Shah, A.; Awan, H.M.; Wu, M.; Liu, A.; Wang, J.; Zhu, T.; Luo, Z.; Shan, G. The isolation of an RNA aptamer targeting to p53 protein with single amino acid mutation. Proc. Natl. Acad. Sci. USA 2015, 112, 10002–10007. [Google Scholar] [CrossRef] [PubMed]
- Milla, P.; Dosio, F.; Cattel, L. Pegylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. Pegylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Veronese, F.M. State of the art in pegylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kamal, M.; Kang, S.A.; Zhang, R.; Lokesh, G.L.; Thiviyanathan, V.; Hasan, N.; Woo, S.; Zhao, D.; Leslie, M.; et al. E-selectin targeting pegylated-thioaptamer prevents breast cancer metastases. Mol. Ther. Nucleic Acids 2016, 5, e399. [Google Scholar] [CrossRef] [PubMed]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Stessl, M.; Noe, C.R.; Winkler, J. Off-Target Effects and Safety Aspects of Phosphorothioate Oligonucleotides; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Geary, R.S.; Yu, R.Z.; Watanabe, T.; Henry, S.P.; Hardee, G.E.; Chappell, A.; Matson, J.; Sasmor, H.; Cummins, L.; Levin, A.A. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: Comparison across species. Drug Metab. Dispos. 2003, 31, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Farman, C.A.; Kornbrust, D.J. Oligodeoxynucleotide studies in primates: Antisense and immune stimulatory indications. Toxicol. Pathol. 2003, 31 (Suppl. 1), 119–122. [Google Scholar] [PubMed]
- Goebl, N.; Berridge, B.; Wroblewski, V.J.; Brown-Augsburger, P.L. Development of a sensitive and specific in situ hybridization technique for the cellular localization of antisense oligodeoxynucleotide drugs in tissue sections. Toxicol. Pathol. 2007, 35, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, U.M.; Perez, J.R.; Becker, J.M.; Narayanan, R. In vivo toxicological effects of rel a antisense phosphorothioates in CD-1 mice. Antisense Res. Dev. 1994, 4, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.P.; Zuckerman, J.E.; Rojko, J.; Hall, W.C.; Harman, R.J.; Kitchen, D.; Crooke, S.T. Toxicological properties of several novel oligonucleotide analogs in mice. Anticancer Drug Des. 1997, 12, 1–14. [Google Scholar] [PubMed]
- Bouchard, P.R.; Hutabarat, R.M.; Thompson, K.M. Discovery and development of therapeutic aptamers. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.P.; Taylor, J.; Midgley, L.; Levin, A.A.; Kornbrust, D.J. Evaluation of the toxicity of ISIS 2302, a phosphorothioate oligonucleotide, in a 4-week study in CD-1 mice. Antisense Nucleic Acid Drug Dev. 1997, 7, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.P.; Templin, M.V.; Gillett, N.; Rojko, J.; Levin, A.A. Correlation of toxicity and pharmacokinetic properties of a phosphorothioate oligonucleotide designed to inhibit ICAM-1. Toxicol. Pathol. 1999, 27, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Tsolmon, B.; Mann, A.P.; Zheng, W.; Zhao, L.; Zhao, Y.D.; Volk, D.E.; Lokesh, G.L.; Morris, L.; Gupta, V.; et al. Safety evaluation of intravenously administered mono-thioated aptamer against e-selectin in mice. Toxicol. Appl. Pharmacol. 2015, 287, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Desai, K.; Macapinlac, M.; Wadler, S.; Goldberg, G.; Fields, A.; Einstein, M.; Volterra, F.; Wong, B.; Martin, R.; et al. A phase I safety and dose escalation trial of docetaxel combined with GEM231, a second generation antisense oligonucleotide targeting protein kinase a R1α in patients with advanced solid cancers. Investig. New Drugs 2006, 24, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Akdim, F.; Stroes, E.S.; Sijbrands, E.J.; Tribble, D.L.; Trip, M.D.; Jukema, J.W.; Flaim, J.D.; Su, J.; Yu, R.; Baker, B.F.; et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J. Am. Coll. Cardiol. 2010, 55, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Goldberg, T. Mipomersen (kynamro): A novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. Pharm. Ther. 2014, 39, 119–122. [Google Scholar]
- Raal, F.J.; Santos, R.D.; Blom, D.J.; Marais, A.D.; Charng, M.J.; Cromwell, W.C.; Lachmann, R.H.; Gaudet, D.; Tan, J.L.; Chasan-Taber, S.; et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of ldl cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 998–1006. [Google Scholar] [CrossRef]
- McGowan, M.P.; Tardif, J.C.; Ceska, R.; Burgess, L.J.; Soran, H.; Gouni-Berthold, I.; Wagener, G.; Chasan-Taber, S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS ONE 2012, 7, e49006. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Dufour, R.; Gagne, C.; Gaudet, D.; East, C.; Donovan, J.M.; Chin, W.; Tribble, D.L.; McGowan, M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: Results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012, 126, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Lan, H.C. Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex. Blood 1998, 92, 1617–1625. [Google Scholar] [PubMed]
- Yacyshyn, B.R.; Bowen-Yacyshyn, M.B.; Jewell, L.; Tami, J.A.; Bennett, C.F.; Kisner, D.L.; Shanahan, W.R., Jr. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn’s disease. Gastroenterology 1998, 114, 1133–1142. [Google Scholar] [CrossRef]
- Henry, S.P.; Beattie, G.; Yeh, G.; Chappel, A.; Giclas, P.; Mortari, A.; Jagels, M.A.; Kornbrust, D.J.; Levin, A.A. Complement activation is responsible for acute toxicities in rhesus monkeys treated with a phosphorothioate oligodeoxynucleotide. Int. Immunopharmacol. 2002, 2, 1657–1666. [Google Scholar] [CrossRef]
- Henry, S.P.; Jagels, M.A.; Hugli, T.E.; Manalili, S.; Geary, R.S.; Giclas, P.C.; Levin, A.A. Mechanism of alternative complement pathway dysregulation by a phosphorothioate oligonucleotide in monkey and human serum. Nucleic Acid Ther. 2014, 24, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Steinle, H.; Michel, T.; Schlensak, C.; Wendel, H.P. Potential capacity of aptamers to trigger immune activation in human blood. PLoS ONE 2013, 8, e68810. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Muller, C.; Podszuweit, A.; Montino, C.; Vollmer, J.; Forsbach, A. Toll-like receptor (TLR) 3 immune modulation by unformulated small interfering RNA or DNA and the role of CD14 (in TLR-mediated effects). Immunology 2012, 136, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Mechanisms and applications of immune stimulatory CpG oligodeoxynucleotides. Biochim. Biophys. Acta 1999, 1489, 107–116. [Google Scholar] [CrossRef]
- Krieg, A.M. Therapeutic potential of toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Yi, A.K.; Beaucage, S.L.; Conover, J.; Krieg, A.M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 1996, 93, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.W.; Rittenhouse, K.; Modi, M.; Patel, M. Local tolerance and systemic safety of pegaptanib sodium in the dog and rabbit. J. Ocul. Pharmacol. Ther. 2007, 23, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; Esposito, C.L.; Camorani, S.; Catuogno, S.; Franciscis, V. Coupling aptamers to short interfering RNAs as therapeutics. Pharmaceuticals 2011, 4, 1434–1449. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly(ethylene glycol) adversely affects peg-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Kachi, S.; Silva, R.L.; Umeda, N.; Hackett, S.F.; McCauley, D.; McCauley, T.; Zoltoski, A.; Epstein, D.M.; Campochiaro, P.A. Intraocular injection of an aptamer that binds PDGF-B: A potential treatment for proliferative retinopathies. J. Cell. Physiol. 2006, 207, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Sennino, B.; Falcon, B.L.; McCauley, D.; Le, T.; McCauley, T.; Kurz, J.C.; Haskell, A.; Epstein, D.M.; McDonald, D.M. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007, 67, 7358–7367. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Li, J.J.; Bay, B.H.; Yung, L.Y. Investigating the antiproliferative activity of high affinity DNA aptamer on cancer cells. PLoS ONE 2013, 8, e50964. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, A.R.; Sarikaya, H.; Riederer, F.; Goadsby, P.J. Postoperative hemicrania continua-like headache—A case series. J. Headache Pain 2015, 16, 526. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Kelland, L.R. Discovery and development of anticancer aptamers. Mol. Cancer Ther. 2006, 5, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Mongelard, F.; Bouvet, P. As-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 2010, 12, 107–114. [Google Scholar] [PubMed]
- Fan, X.; Sun, L.; Li, K.; Yang, X.; Cai, B.; Zhang, Y.; Zhu, Y.; Ma, Y.; Guan, Z.; Wu, Y.; et al. The bioactivity of d-/l-isonucleoside- and 2′-deoxyinosine-incorporated aptamer AS1411s including DNA replication/microrna expression. Mol. Ther. Nucleic Acids 2017, 9, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Vater, A.; Sahlmann, J.; Kroger, N.; Zollner, S.; Lioznov, M.; Maasch, C.; Buchner, K.; Vossmeyer, D.; Schwoebel, F.; Purschke, W.G.; et al. Hematopoietic stem and progenitor cell mobilization in mice and humans by a first-in-class mirror-image oligonucleotide inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Alomran, R.; Chernikova, S.B.; Lartey, F.; Stafford, J.; Jang, T.; Merchant, M.; Zboralski, D.; Zollner, S.; Kruschinski, A.; et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Zboralski, D.; Hoehlig, K.; Eulberg, D.; Fromming, A.; Vater, A. Increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol. Res. 2017, 5, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Tamuly, D.; Allen, P.B.; Kim, Y.T.; Bachoo, R.; Ellington, A.D.; Iqbal, S.M. Proliferation and migration of tumor cells in tapered channels. Biomed. Microdevices 2013, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C.; Nagel-Wolfrum, K.; Kunz, C.; Wittig, I.; Butz, K.; Hoppe-Seyler, F.; Groner, B. Sequence-specific peptide aptamers, interacting with the intracellular domain of the epidermal growth factor receptor, interfere with STAT3 activation and inhibit the growth of tumor cells. J. Biol. Chem. 2003, 278, 37610–37621. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Song, Y.L.; Zhu, Z.; Li, X.L.; Zou, Y.; Yang, H.T.; Wang, J.J.; Yao, P.S.; Pan, R.J.; Yang, C.J.; et al. Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem. Biophys. Res. Commun. 2014, 453, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jeong, S. In vitro selection of RNA aptamer and specific targeting of ErbB2 in breast cancer cells. Nucleic Acid Ther. 2011, 21, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, G.; Maron, R.; Mancini, M.; Schechter, B.; Sela, M.; Yarden, Y. Aptamer to ErbB-2/HER2 enhances degradation of the target and inhibits tumorigenic growth. Proc. Natl. Acad. Sci. USA 2013, 110, 8170–8175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, I.S.; Park, S.A.; Kim, Y.; Lee, J.E.; Noh, D.Y.; Kim, K.T.; Ryu, S.H.; Suh, P.G. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol. Ther. 2013, 21, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Stephens, A.W.; Gould, T.; Chang, Y.F.; Lynott, C.K.; Heil, J.; Borkowski, S.; Hilger, C.S.; Cook, G.; Warren, S.; et al. Tumor targeting by an aptamer. J. Nucl. Med. 2006, 47, 668–678. [Google Scholar] [PubMed]
- Daniels, D.A.; Chen, H.; Hicke, B.J.; Swiderek, K.M.; Gold, L. A tenascin-c aptamer identified by tumor cell selex: Systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA 2003, 100, 15416–15421. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Marion, C.; Chang, Y.F.; Gould, T.; Lynott, C.K.; Parma, D.; Schmidt, P.G.; Warren, S. Tenascin-C aptamers are generated using tumor cells and purified protein. J. Biol. Chem. 2001, 276, 48644–48654. [Google Scholar] [CrossRef] [PubMed]
- Zamay, T.N.; Kolovskaya, O.S.; Glazyrin, Y.E.; Zamay, G.S.; Kuznetsova, S.A.; Spivak, E.A.; Wehbe, M.; Savitskaya, A.G.; Zubkova, O.A.; Kadkina, A.; et al. DNA-aptamer targeting vimentin for tumor therapy in vivo. Nucleic Acid Ther. 2014, 24, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, S.R.; Kim, N.Y.; Lee, S.H.; Jeong, J.S.; Lee, S.W. An RNA aptamer that binds carcinoembryonic antigen inhibits hepatic metastasis of colon cancer cells in mice. Gastroenterology 2012, 143, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Maeda, S.; Takeuchi, M.; Inoue, H.; Higashimoto, Y.; Yamagishi, S. DNA aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab. Investig. 2014, 94, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Zhang, X.; Rabbani, Z.N.; Liu, Y.; Reddy, S.K.; Su, Z.; Salahuddin, F.K.; Viles, K.; Giangrande, P.H.; Dewhirst, M.W.; et al. RNA aptamer-targeted inhibition of NF-κB suppresses non-small cell lung cancer resistance to doxorubicin. Mol. Ther. 2008, 16, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; Esposito, C.L.; Camorani, S.; Rienzo, A.; Stasio, L.; Insabato, L.; Affuso, A.; de Franciscis, V. Targeting AXL with an high-affinity inhibitory aptamer. Mol. Ther. 2012, 20, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Guo, H.; Russell, M.B.; Liu, Y.; Sullenger, B.A.; Kuo, P.C. RNA aptamer blockade of osteopontin inhibits growth and metastasis of mda-mb231 breast cancer cells. Mol. Ther. 2009, 17, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Talbot, L.J.; Mi, Z.; Bhattacharya, S.D.; Kim, V.; Guo, H.; Kuo, P.C. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells. Surgery 2011, 150, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Ma, Y.; Pei, X.; Liu, Q.; Lu, B.; Jin, L.; Wang, J.; Liu, J. A cell-based single-stranded DNA aptamer specifically targets gastric cancer. Int. J. Biochem. Cell Biol. 2014, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.Y.; Yuan, A.H.; Chen, W.; Shi, X.S.; Miao, Y. A DNA aptamer with high affinity and specificity for molecular recognition and targeting therapy of gastric cancer. BMC Cancer 2014, 14, 699. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Ding, H.; Xu, H.; Zhao, Q.; Li, J.; Li, H.; Xia, W.; Su, X.; Chen, Y.; et al. Aptamer BC15 against heterogeneous nuclear ribonucleoprotein A1 has potential value in diagnosis and therapy of hepatocarcinoma. Nucleic Acid Ther. 2012, 22, 391–398. [Google Scholar] [PubMed]
- Dassie, J.P.; Hernandez, L.I.; Thomas, G.S.; Long, M.E.; Rockey, W.M.; Howell, C.A.; Chen, Y.; Hernandez, F.J.; Liu, X.Y.; Wilson, M.E.; et al. Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen. Mol. Ther. 2014, 22, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.P.; Somasunderam, A.; Nieves-Alicea, R.; Li, X.; Hu, A.; Sood, A.K.; Ferrari, M.; Gorenstein, D.G.; Tanaka, T. Identification of thioaptamer ligand against E-selectin: Potential application for inflamed vasculature targeting. PLoS ONE 2010, 5, e13050. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Hasan, N.; Mann, A.P.; Zheng, W.; Zhao, L.; Morris, L.; Zhu, W.; Zhao, Y.D.; Suh, K.S.; Dooley, W.C.; et al. Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Mol. Ther. 2015, 23, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar]
- McNamara, J.O.; Kolonias, D.; Pastor, F.; Mittler, R.S.; Chen, L.; Giangrande, P.H.; Sullenger, B.; Gilboa, E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008, 118, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Dollins, C.M.; Nair, S.; Boczkowski, D.; Lee, J.; Layzer, J.M.; Gilboa, E.; Sullenger, B.A. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 2008, 15, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Pratico, E.D.; Sullenger, B.A.; Nair, S.K. Identification and characterization of an agonistic aptamer against the T cell costimulatory receptor, OX40. Nucleic Acid Ther. 2013, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Soldevilla, M.M.; Villanueva, H.; Kolonias, D.; Inoges, S.; de Cerio, A.L.; Kandzia, R.; Klimyuk, V.; Gleba, Y.; Gilboa, E.; et al. CD28 aptamers as powerful immune response modulators. Mol. Ther. Nucleic Acids 2013, 2, e98. [Google Scholar] [CrossRef] [PubMed]
- Santulli-Marotto, S.; Nair, S.K.; Rusconi, C.; Sullenger, B.; Gilboa, E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 2003, 63, 7483–7489. [Google Scholar] [PubMed]
- Pastor, F.; Kolonias, D.; McNamara, J.O., 2nd; Gilboa, E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol. Ther. 2011, 19, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.P.; Vande Woude, G.F.; Boerner, S.A.; LoRusso, P.M. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 2009, 15, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Schrand, B.; Berezhnoy, A.; Brenneman, R.; Williams, A.; Levay, A.; Kong, L.Y.; Rao, G.; Zhou, S.; Heimberger, A.B.; Gilboa, E. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol. Res. 2014, 2, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Abdul-Wahid, A.; Fischer, N.W.; Huang, E.H.; Cydzik, M.; Gariepy, J. Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol. Ther. Nucleic Acids 2015, 4, e237. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Huang, B.T.; Wang, J.W.; Lin, P.Y.; Yang, P.C. A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol. Ther. Nucleic Acids 2016, 5, e397. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, A.; Stewart, C.A.; McNamara, J.O., 2nd; Thiel, W.; Giangrande, P.; Trinchieri, G.; Gilboa, E. Isolation and optimization of murine IL-10 receptor blocking oligonucleotide aptamers using high-throughput sequencing. Mol. Ther. 2012, 20, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; De La Fuente, A.C.; Vella, J.L.; Zoso, A.; Inverardi, L.; Serafini, P. Aptamer-mediated blockade of IL4α triggers apoptosis of mdscs and limits tumor progression. Cancer Res. 2012, 72, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, S.; Yu, X.; Huang, S.; Liu, H.Y. Simultaneous targeting of CD44 and epcam with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics 2017, 7, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Stubbs, S.; Soldevilla, M.M.; Villanueva, H.; Mancheno, U.; Bendandi, M.; Pastor, F. Identification of TIM3 2′-fluoro oligonucleotide aptamer by HT-SELEX for cancer immunotherapy. Oncotarget 2016, 7, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Villanueva, H.; Bendandi, M.; Inoges, S.; Lopez-Diaz de Cerio, A.; Pastor, F. 2-fluoro-RNA oligonucleotide CD40 targeted aptamers for the control of B lymphoma and bone-marrow aplasia. Biomaterials 2015, 67, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Lai, W.Y.; Chang, Y.C.; Wang, J.W.; Yeh, S.D.; Lin, E.P.; Yang, P.C. A CTLA-4 antagonizing DNA aptamer with antitumor effect. Mol. Ther. Nucleic Acids 2017, 8, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Ortiz-Espinosa, S.; Moreno, H.; Lozano, T.; Pajares, M.J.; Agorreta, J.; Bertolo, C.; Lasarte, J.J.; Vicent, S.; Hoehlig, K.; et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 2017, 7, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the eye: Aptamers in ocular disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Eyetech Study, G. Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina 2002, 22, 143–152. [Google Scholar] [CrossRef]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Gala, K.; Chandarlapaty, S. Molecular pathways: HER3 targeted therapy. Clin. Cancer Res. 2014, 20, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chernis, G.A.; Hoang, V.Q.; Landgraf, R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc. Natl. Acad. Sci. USA 2003, 100, 9226–9231. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Koutsioumpa, M.; Papadimitriou, E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat. Anticancer Drug Discov. 2014, 9, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Girvan, A.C.; Teng, Y.; Casson, L.K.; Thomas, S.D.; Jüliger, S.; Ball, M.W.; Klein, J.B.; Pierce, W.M.; Barve, S.S.; Bates, P.J. AGRO100 inhibits activation of nuclear factor-κB (NF-κB) by forming a complex with NF-κB essential modulator (NEMO) and nucleolin. Mol. Cancer Ther. 2006, 5, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Zhu, H.; Khashab, T.; Ye, Q.; George, B.; Mathur, R.; Singh, R.K.; Berkova, Z.; Wise, J.F.; Braun, F.K.; et al. Targeting nucleolin for better survival in diffuse large B-cell lymphoma. Leukemia 2017, 32, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Robbins, P.F.; Yao, X.; Li, Y.F.; Turcotte, S.; Tran, E.; Wunderlich, J.R.; Mixon, A.; Farid, S.; Dudley, M.E.; et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J. Clin. Investig. 2014, 124, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, S.G.; Hägele, H.; Kulkarni, O.P.; Endlich, K.; Segerer, S.; Eulberg, D.; Klussmann, S.; Anders, H.-J. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 2009, 52, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Marasca, R.; Maffei, R. NOX-A12: Mobilizing CLL away from home. Blood 2014, 123, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, Y.; Liu, J.; Mok, S.C.; Xue, F.; Zhang, W. CXCL12/CXCR4: A symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 2016, 35, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.P.; Bhavane, R.C.; Somasunderam, A.; Liz Montalvo-Ortiz, B.; Ghaghada, K.B.; Volk, D.; Nieves-Alicea, R.; Suh, K.S.; Ferrari, M.; Annapragada, A.; et al. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget 2011, 2, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.P.; Tanaka, T.; Somasunderam, A.; Liu, X.; Gorenstein, D.G.; Ferrari, M. E-selectin-targeted porous silicon particle for nanoparticle delivery to the bone marrow. Adv. Mater. 2011, 23, H278–H282. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Huang, Y.; Mu, C.; Zhang, G.; Xu, R.; Guo, X.; Xia, X.; Volk, D.E.; Lokesh, G.L.; Thiviyanathan, V.; et al. Bone marrow endothelium-targeted therapeutics for metastatic breast cancer. J. Control. Release 2014, 187, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Mangala, L.S.; Wang, H.; Jiang, D.; Wu, S.Y.; Somasunderam, A.; Volk, D.E.; Lokesh, G.L.; Li, X.; Pradeep, S.; Yang, X.; et al. Improving vascular maturation using noncoding RNAs increases antitumor effect of chemotherapy. JCI Insight 2016, 1, e87754. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Hervas-Stubbs, S.; Glennie, M.; Pardoll, D.M.; Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 2007, 7, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Attarwala, H. TGN1412: From discovery to disaster. J. Young Pharm. 2010, 2, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Curran, M.A. 4-1BB agonists: Multi-potent potentiators of tumor immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Schrand, B.; Berezhnoy, A.; Brenneman, R.; Williams, A.; Levay, A.; Gilboa, E. Reducing toxicity of 4-1BB costimulation: Targeting 4-1BB ligands to the tumor stroma with BI-specific aptamer conjugates. Oncoimmunology 2015, 4, e970918. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Villanueva, H.; Casares, N.; Lasarte, J.J.; Bendandi, M.; Inoges, S.; Lopez-Diaz de Cerio, A.; Pastor, F. MRP1-CD28 bi-specific oligonucleotide aptamers: Target costimulation to drug-resistant melanoma cancer stem cells. Oncotarget 2016, 7, 23182–23196. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, C.P.; Scardino, E.; Layzer, J.; Pitoc, G.A.; Ortel, T.L.; Monroe, D.; Sullenger, B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 2002, 419, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Bompiani, K.M.; Monroe, D.M.; Church, F.C.; Sullenger, B.A. A high affinity, antidote-controllable prothrombin and thrombin-binding RNA aptamer inhibits thrombin generation and thrombin activity. J. Thromb. Haemost. 2012, 10, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.M.; Shima, D.T.; Calias, P.; Cunningham, E.T., Jr.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Dis. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, D.; Green, L.S.; Bell, C.; Janjic, N. Inhibition of receptor binding by high-affinity RNA ligands to vascular endothelial growth factor. Biochemistry 1994, 33, 10450–10456. [Google Scholar] [CrossRef] [PubMed]
- Green, L.S.; Jellinek, D.; Bell, C.; Beebe, L.A.; Feistner, B.D.; Gill, S.C.; Jucker, F.M.; Janjić, N. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem. Biol. 1995, 2, 683–695. [Google Scholar] [CrossRef]
- Study of AS1411 in Advanced Solid Tumours. Available online: https://ClinicalTrials.gov/show/NCT00881244 (accessed on 12 December 2017).
- A phase II Study of AS1411 in Renal Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT00740441 (accessed on 12 December 2017).
- Phase II Study of AS1411 Combined with Cytarabine to Treat Acute Myeloid Leukemia. Available online: https: //ClinicalTrials.gov/show/NCT00512083 (accessed on 12 December 2017).
- Soundararajan, S.; Wang, L.; Sridharan, V.; Chen, W.; Courtenay-Luck, N.; Jones, D.; Spicer, E.K.; Fernandes, D.J. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol. 2009, 76, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sridharan, V.; Soundararajan, S.; Otake, Y.; Stuart, R.; Jones, D.; Fernandes, D. Activity and mechanism of action of AS1411 in acute myeloid leukemia cells. Blood 2007, 110, 1604. [Google Scholar]
- Stuart, R.K.; Stockerl-Goldstein, K.; Cooper, M.; Devetten, M.; Herzig, R.; Medeiros, B.; Schiller, G.; Wei, A.; Acton, G.; Rizzieri, D. Randomized phase ii trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML). J. Clin. Oncol. 2009, 27, 7019. [Google Scholar]
- Olaptesed (NOX-A12) Alone and in Combination with Pembrolizumab in Colorectal and Pancreatic Cancer. Available online: https://ClinicalTrials.gov/show/NCT03168139 (accessed on 12 December 2017).
- NOX-A12 First-in-Human (FIH) Study. Available online: https://ClinicalTrials.gov/show/NCT00976378 (accessed on 12 December 2017).
- NOX-A12 Multiple Ascending Dose Study in Healthy Volunteers. Available online: https://ClinicalTrials.gov/show/NCT01194934 (accessed on 12 December 2017).
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Muller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. Cxcl12 and Cxcr7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017, 179, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Purschke, W.G.; Moschetta, M.; Buchner, K.; Maasch, C.; Zboralski, D.; Zollner, S.; Vonhoff, S.; Mishima, Y.; et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014, 9, 118–128. [Google Scholar] [CrossRef] [PubMed]
- NOX-A12 in Combination with Bortezomib and Dexamethasone in Relapsed Multiple Myeloma. Available online: https://ClinicalTrials.gov/show/NCT01521533 (accessed on 12 December 2017).
- Ludwig, H.; Weisel, K.; Petrucci, M.T.; Leleu, X.; Cafro, A.M.; Garderet, L.; Leitgeb, C.; Foa, R.; Greil, R.; Yakoub-Agha, I.; et al. Olaptesed pegol, an anti-CXCL12/SDF-1 spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: A phase IIa study. Leukemia 2017, 31, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- NOX-A12 in Combination with Bendamustine and Rituximab in Relapsed Chronic Lymphocytic Leukemia (CLL). Available online: https://ClinicalTrials.gov/show/NCT01486797 (accessed on 12 December 2017).
- Steurer, M.; Montillo, M.; Scarfò, L.; Mauro, F.R.; Andel, J.; Wildner, S.; Trentin, L.; Janssens, A.; Burgstaller, S.; Kruschinski, A.; et al. Results from a phase IIa study of the anti-CXCL12 spiegelmer olaptesed pegol (NOX-A12) in combination with bendamustine/rituximab in patients with chronic lymphocytic leukemia. Blood 2014, 124, 1996. [Google Scholar]
- Buffery, D. The 2015 oncology drug pipeline: Innovation drives the race to cure cancer. Am. Health Drug Benefits 2015, 8, 216–222. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).