Treatment of Pain in Cancer: Towards Personalised Medicine
Abstract
:1. Introduction
2. Pain Prevalence: Reasons for Lack of Improvement in Cancer Pain
2.1. Patients do Not Report Pain Spontaneously
2.2. Measurement of Pain
2.3. Assessment of Undertreatment
2.4. Undertreatment
2.5. Pain and/or Patients More Complex?
2.5.1. Pain Characteristics
2.5.2. Patient Characteristics
2.6. Interventions to do Better?
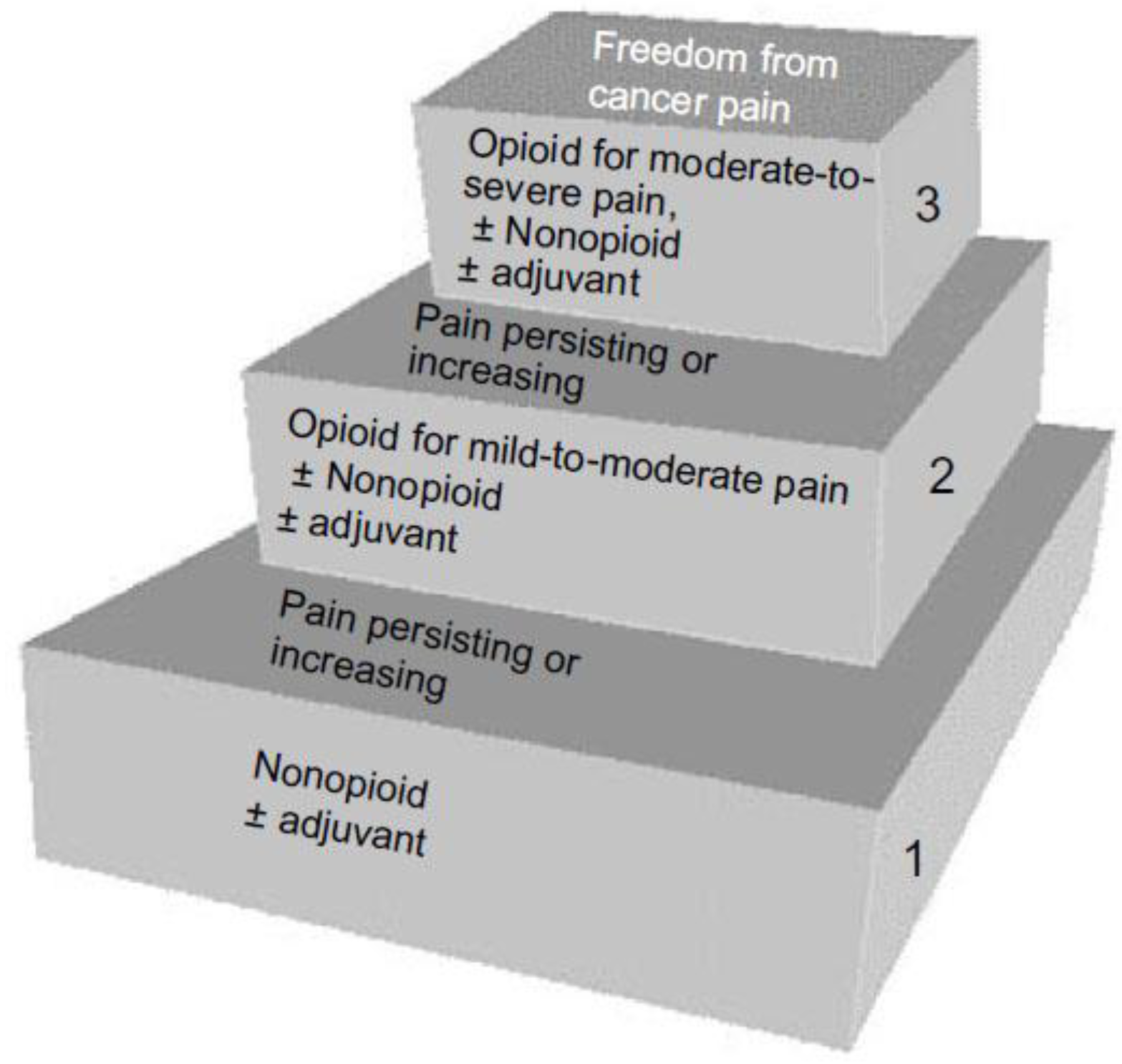
2.6.1. Pharmacological Interventions
2.6.2. Nonpharmacological Interventions
3. General Considerations Concerning “Cancer Pain”
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van den Beuken-van Everdingen, M.H.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann. Oncol. 2007, 18, 1437–1449. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef]
- Serlin, R.C.; Mendoza, T.R.; Nakamura, Y.; Edwards, K.R.; Cleeland, C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995, 61, 277–284. [Google Scholar] [CrossRef]
- Woodruff, R.K. Cancer Pain; Asperula Pty Ltd.: Heidelberg, Australia, 1997. [Google Scholar]
- Graeff de, A.; Verhagen, E.H.; Besse, T.C.; Crul, B.J.P.; Krol, R.J.A. Palliatieve Zorg: Richtlijnen voor de Praktijk; Vereniging van Integrale Kankercentra: Utrecht, The Netherlands, 2010; Volume 1, pp. 565–623. [Google Scholar]
- Larue, F.; Colleau, S.M.; Brasseur, L.; Cleeland, C.S. Multicentre study of cancer pain and its treatment in France. BMJ 1995, 310, 1034–1037. [Google Scholar] [CrossRef] [Green Version]
- Torrance, N.; Smith, B.H.; Bennett, M.I.; Lee, A.J. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain 2006, 7, 281–289. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain 2007, 132, 312–320. [Google Scholar] [CrossRef]
- Mantyh, P.W. Bone cancer pain: From mechanism to therapy. Curr. Opin. Support Palliat. Care 2014, 8, 83–90. [Google Scholar] [CrossRef]
- Penalba, V.; Deshields, T.L.; Klinkenberg, D. Gaps in communication between cancer patients and healthcare providers: Symptom distress and patients’ intentions to disclose. Support Care Cancer 2018. [Google Scholar] [CrossRef]
- Kwon, J.H. Overcoming Barriers in Cancer Pain Management. J. Clin. Oncol. 2014, 32, 1727–1733. [Google Scholar] [CrossRef]
- Street, R.L., Jr.; Tancredi, D.J.; Slee, C.; Kalauokalani, D.K.; Dean, D.E.; Franks, P.; Kravitz, R.L. A pathway linking patient participation in cancer consultations to pain control. Psychooncology 2014, 23, 1111–1117. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; Geerling, J.I.; Mostovaya, I.; Vissers, K.C.P.; de Graeff, A.; Reyners, A.K.L.; van der Linden, Y.M. A systematic review of the effectiveness of patient-based educational interventions to improve cancer-related pain. Cancer Treat. Rev. 2018, 63, 96–103. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, H.; Park, E.C.; Park, K. Attitude and knowledge of physicians about cancer pain management: Young doctors of South Korea in their early career. Jpn. J. Clin. Oncol. 2011, 41, 783–791. [Google Scholar] [CrossRef]
- Te Boveldt, N.D.; Vernooij-Dassen, M.J.; Jansen, A.; Vissers, K.C.; Engels, Y. Pain is not systematically registered in Dutch medical oncology outpatients. Pain Pract. 2015, 15, 364–370. [Google Scholar] [CrossRef]
- Besse, K.; Vernooij-Dassen, M.; Vissers, K.; Engels, Y. The Impact of a National Guideline on the Management of Cancer Pain on the Practice of Pain Assessment and Registration. Pain Pract. 2016, 16, 148–153. [Google Scholar] [CrossRef]
- Kasasbeh, M.A.M.; McCabe, C.; Payne, S. Cancer-related pain management: A review of knowledge and attitudes of healthcare professionals. Eur. J. Cancer Care (Engl.) 2017, 26. [Google Scholar] [CrossRef]
- Shvartzman, P.; Friger, M.; Shani, A.; Barak, F.; Yoram, C.; Singer, Y. Pain control in ambulatory cancer patients–can we do better? J. Pain Symptom Manag. 2003, 26, 716–722. [Google Scholar] [CrossRef]
- Ward, S.E.; Goldberg, N.; Miller-McCauley, V.; Mueller, C.; Nolan, A.; Pawlik-Plank, D.; Robbins, A.; Stormoen, D.; Weissman, D.E. Patient-related barriers to management of cancer pain. Pain 1993, 52, 319–324. [Google Scholar] [CrossRef]
- Sakakibara, N.; Higashi, T.; Yamashita, I.; Yoshimoto, T.; Matoba, M. Negative pain management index scores do not necessarily indicate inadequate pain management: A cross-sectional study. BMC Palliat. Care 2018, 17, 102. [Google Scholar] [CrossRef]
- Gibbins, J.; Bhatia, R.; Forbes, K.; Reid, C.M. What do patients with advanced incurable cancer want from the management of their pain? A qualitative study. Palliat. Med. 2014, 28, 71–78. [Google Scholar] [CrossRef]
- Deandrea, S.; Montanari, M.; Moja, L.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [Green Version]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol. 2014, 32, 4149–4154. [Google Scholar] [CrossRef]
- Breuer, B.; Fleishman, S.B.; Cruciani, R.A.; Portenoy, R.K. Medical oncologists’ attitudes and practice in cancer pain management: A national survey. J. Clin. Oncol. 2011, 29, 4769–4775. [Google Scholar] [CrossRef]
- Martens, M.J.M.; Janssen, D.J.A.; Schols, J.; van den Beuken-van Everdingen, M.H.J. Opioid Prescribing Behavior in Long-Term Geriatric Care in the Netherlands. J. Am. Med. Dir. Assoc. 2018, 19, 974–980. [Google Scholar] [CrossRef]
- Hoang, H.T.; Sabia, M.; Torjman, M.; Goldberg, M.E. The importance of medical education in the changing field of pain medicine. Pain Manag. 2014, 4, 437–443. [Google Scholar] [CrossRef]
- Fainsinger, R.L.; Nekolaichuk, C.; Lawlor, P.; Hagen, N.; Bercovitch, M.; Fisch, M.; Galloway, L.; Kaye, G.; Landman, W.; Spruyt, O.; et al. An international multicentre validation study of a pain classification system for cancer patients. Eur. J. Cancer 2010, 46, 2896–2904. [Google Scholar] [CrossRef]
- Oosterling, A.; te Boveldt, N.; Verhagen, C.; van der Graaf, W.T.; Van Ham, M.; Van der Drift, M.; Vissers, K.; Engels, Y. Neuropathic Pain Components in Patients with Cancer: Prevalence, Treatment, and Interference with Daily Activities. Pain Pract. 2016, 16, 413–421. [Google Scholar] [CrossRef]
- Rayment, C.; Hjermstad, M.J.; Aass, N.; Kaasa, S.; Caraceni, A.; Strasser, F.; Heitzer, E.; Fainsinger, R.; Bennett, M.I. Neuropathic cancer pain: Prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat. Med. 2013, 27, 714–721. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Kaasa, S.; Caraceni, A.; Loge, J.H.; Pedersen, T.; Haugen, D.F.; Aass, N. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat. Care 2016, 6, 344–352. [Google Scholar] [CrossRef]
- Caraceni, A.; Portenoy, R.K. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain 1999, 82, 263–274. [Google Scholar] [CrossRef]
- Zech, D.F.; Grond, S.; Lynch, J.; Hertel, D.; Lehmann, K.A. Validation of World Health Organization Guidelines for cancer pain relief: A 10-year prospective study. Pain 1995, 63, 65–76. [Google Scholar] [CrossRef]
- Bennett, M.I.; Rayment, C.; Hjermstad, M.; Aass, N.; Caraceni, A.; Kaasa, S. Prevalence and aetiology of neuropathic pain in cancer patients: A systematic review. Pain 2012, 153, 359–365. [Google Scholar] [CrossRef]
- Deandrea, S.; Corli, O.; Consonni, D.; Villani, W.; Greco, M.T.; Apolone, G. Prevalence of breakthrough cancer pain: A systematic review and a pooled analysis of published literature. J. Pain Symptom Manag. 2014, 47, 57–76. [Google Scholar] [CrossRef]
- Roberto, A.; Deandrea, S.; Greco, M.T.; Corli, O.; Negri, E.; Pizzuto, M.; Ruggeri, F. Prevalence of Neuropathic Pain in Cancer Patients: Pooled Estimates from a Systematic Review of Published Literature and Results from a Survey Conducted in 50 Italian Palliative Care Centers. J. Pain Symptom Manag. 2016, 51, 1091–1102. [Google Scholar] [CrossRef]
- Bouhassira, D.; Luporsi, E.; Krakowski, I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 2017, 158, 1118–1125. [Google Scholar] [CrossRef]
- Davies, A.N.; Dickman, A.; Reid, C.; Stevens, A.-M.; Zeppetella, G. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur. J. Pain 2009, 13, 331–338. [Google Scholar] [CrossRef]
- Smith, H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs 2012, 26, 509–535. [Google Scholar] [CrossRef]
- Davies, A.N.; Elsner, F.; Filbet, M.J.; Porta-Sales, J.; Ripamonti, C.; Santini, D.; Webber, K. Breakthrough cancer pain (BTcP) management: A review of international and national guidelines. BMJ Support Palliat. Care 2018, 8, 241–249. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Jensen, T.S.; Kamerman, P.R.; McNicol, E.; Moore, A.; et al. Neuropathic pain clinical trials: Factors associated with decreases in estimated drug efficacy. Pain 2018. [Google Scholar] [CrossRef]
- Tranvag, E.J.; Norheim, O.F.; Ottersen, T. Clinical decision making in cancer care: A review of current and future roles of patient age. BMC Cancer 2018, 18, 546. [Google Scholar] [CrossRef]
- Nygaard, H.A.; Naik, M.; Ruths, S.; Kruger, K. Clinically important renal impairment in various groups of old persons. Scand. J. Prim. Health Care 2004, 22, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Sverrisdottir, E.; Lund, T.M.; Olesen, A.E.; Drewes, A.M.; Christrup, L.L.; Kreilgaard, M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur. J. Pharm. Sci. 2015, 74, 45–62. [Google Scholar] [CrossRef]
- Ashby, M.; Fleming, B.; Wood, M.; Somogyi, A. Plasma morphine and glucuronide (M3G and M6G) concentrations in hospice inpatients. J. Pain Symptom Manag. 1997, 14, 157–167. [Google Scholar] [CrossRef]
- Smith, G.D.; Smith, M.T. The excitatory behavioral and antianalgesic pharmacology of normorphine-3-glucuronide after intracerebroventricular administration to rats. J. Pharmacol. Exp. Ther. 1998, 285, 1157–1162. [Google Scholar]
- Sande, T.A.; Laird, B.J.; Fallon, M.T. The use of opioids in cancer patients with renal impairment-a systematic review. Support Care Cancer 2017, 25, 661–675. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Coaccioli, S.; Skaper, S.D.; Varrassi, G. Chronic Pain in the Elderly: The Case for New Therapeutic Strategies. Pain Phys. 2015, 18, E863–E876. [Google Scholar]
- Carlson, C.L. Effectiveness of the World Health Organization cancer pain relief guidelines: An integrative review. J. Pain Res. 2016, 9, 515–534. [Google Scholar] [CrossRef]
- Walker, V.A.; Hoskin, P.J.; Hanks, G.W.; White, I.D. Evaluation of the WHO analgesic guidelines for cancer pain in a hospital-based palliative care unit. J. Pain Symptom Manag. 1988, 3, 145–149. [Google Scholar] [CrossRef]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; de Conno, F.; Fallon, M.; Hanna, M.; et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Stockler, M.; Vardy, J.; Pillai, A.; Warr, D. Acetaminophen (paracetamol) improves pain and well-being in people with advanced cancer already receiving a strong opioid regimen: A randomized, double-blind, placebo-controlled cross-over trial. J. Clin. Oncol. 2004, 22, 3389–3394. [Google Scholar] [CrossRef]
- Tasmacioglu, B.; Aydinli, I.; Keskinbora, K.; Pekel, A.F.; Salihoglu, T.; Sonsuz, A. Effect of intravenous administration of paracetamol on morphine consumption in cancer pain control. Support Care Cancer 2009, 17, 1475–1481. [Google Scholar] [CrossRef]
- Israel, F.J.; Parker, G.; Charles, M.; Reymond, L. Lack of benefit from paracetamol (acetaminophen) for palliative cancer patients requiring high-dose strong opioids: A randomized, double-blind, placebo-controlled, crossover trial. J. Pain Symptom Manag. 2010, 39, 548–554. [Google Scholar] [CrossRef]
- Cubero, D.I.; del Giglio, A. Early switching from morphine to methadone is not improved by acetaminophen in the analgesia of oncologic patients: A prospective, randomized, double-blind, placebo-controlled study. Support Care Cancer 2010, 18, 235–242. [Google Scholar] [CrossRef]
- Axelsson, B.; Borup, S. Is there an additive analgesic effect of paracetamol at step 3? A double-blind randomized controlled study. Palliat. Med. 2003, 17, 724–725. [Google Scholar] [CrossRef]
- Nikles, J.; Mitchell, G.K.; Hardy, J.; Senior, H.; Carmont, S.-A.; Schluter, P.J.; Vora, R.; Currow, D.; Yelland, M. Single-patient multiple crossover studies to determine the effectiveness of paracetamol in relieving pain suffered by patients with advanced cancer taking regular opioids: A pilot study. Palliat. Med. 2016, 30, 800–802. [Google Scholar] [CrossRef]
- Axelsson, B.; Stellborn, P.; Strom, G. Analgesic effect of paracetamol on cancer related pain in concurrent strong opioid therapy. A prospective clinical study. Acta Oncol. 2008, 47, 891–895. [Google Scholar] [CrossRef] [Green Version]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst. Rev. 2017, 7, CD012637. [Google Scholar]
- Nicholson, A.B. Methadone for cancer pain. Cochrane Database Syst. Rev. 2007, CD003971. [Google Scholar] [CrossRef]
- Hadley, G.; Derry, S.; Moore, R.A.; Wiffen, P.J. Transdermal fentanyl for cancer pain. Cochrane Database Syst. Rev. 2013, CD010270. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Bromham, N.; Taubert, M.; Arnold, S.; Hilgart, J.S. Buprenorphine for treating cancer pain. Cochrane Database Syst. Rev. 2015, CD009596. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Bennett, M.I.; Arnold, S.; Bromham, N.; Hilgart, J.S. Oxycodone for cancer-related pain. Cochrane Database Syst. Rev. 2015, CD003870. [Google Scholar] [CrossRef]
- Matic, M.; de Wildt, S.N.; Tibboel, D.; van Schaik, R.H.N. Analgesia and Opioids: A Pharmacogenetics Shortlist for Implementation in Clinical Practice. Clin. Chem. 2017, 63, 1204–1213. [Google Scholar] [CrossRef]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef]
- Haumann, J.; Geurts, J.W.; van Kuijk, S.M.; Kremer, B.; Joosten, E.A.; van den Beuken-van Everdingen, M.H. Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer. Eur. J. Cancer 2016, 65, 121–129. [Google Scholar] [CrossRef]
- Swartjes, M.; Morariu, A.; Niesters, M.; Aarts, L.; Dahan, A. Nonselective and NR2B-selective N-methyl-d-aspartic acid receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology 2011, 115, 165–174. [Google Scholar] [CrossRef]
- Haumann, J.; van Kuijk, S.M.J.; Joosten, E.A.; van den Beuken-van Everdingen, M.H.J. The Association between Patient Characteristics and Opioid Treatment Response in Neuropathic and Nociceptive Pain Due to Cancer. J. Palliat. Med. 2018. [Google Scholar] [CrossRef]
- Corli, O.; Roberto, A.; Bennett, M.I.; Galli, F.; Corsi, N.; Rulli, E.; Antonione, R. Nonresponsiveness and Susceptibility of Opioid Side Effects Related to Cancer Patients’ Clinical Characteristics: A Post-Hoc Analysis. Pain Pract. 2018, 18, 748–757. [Google Scholar] [CrossRef]
- Hochstenbach, L.M.; Zwakhalen, S.M.; Courtens, A.M.; van Kleef, M.; de Witte, L.P. Feasibility of a mobile and web-based intervention to support self-management in outpatients with cancer pain. Eur. J. Oncol. Nurs. 2016, 23, 97–105. [Google Scholar] [CrossRef]
- Adam, R.; Bond, C.; Murchie, P. Educational interventions for cancer pain. A systematic review of systematic reviews with nested narrative review of randomized controlled trials. Patient Educ. Couns. 2015, 98, 269–282. [Google Scholar] [CrossRef]
- Coelho, A.; Parola, V.; Cardoso, D.; Bravo, M.E.; Apostolo, J. Use of non-pharmacological interventions for comforting patients in palliative care: A scoping review. JBI Database Syst. Rev Implement Rep. 2017, 15, 1867–1904. [Google Scholar] [CrossRef]
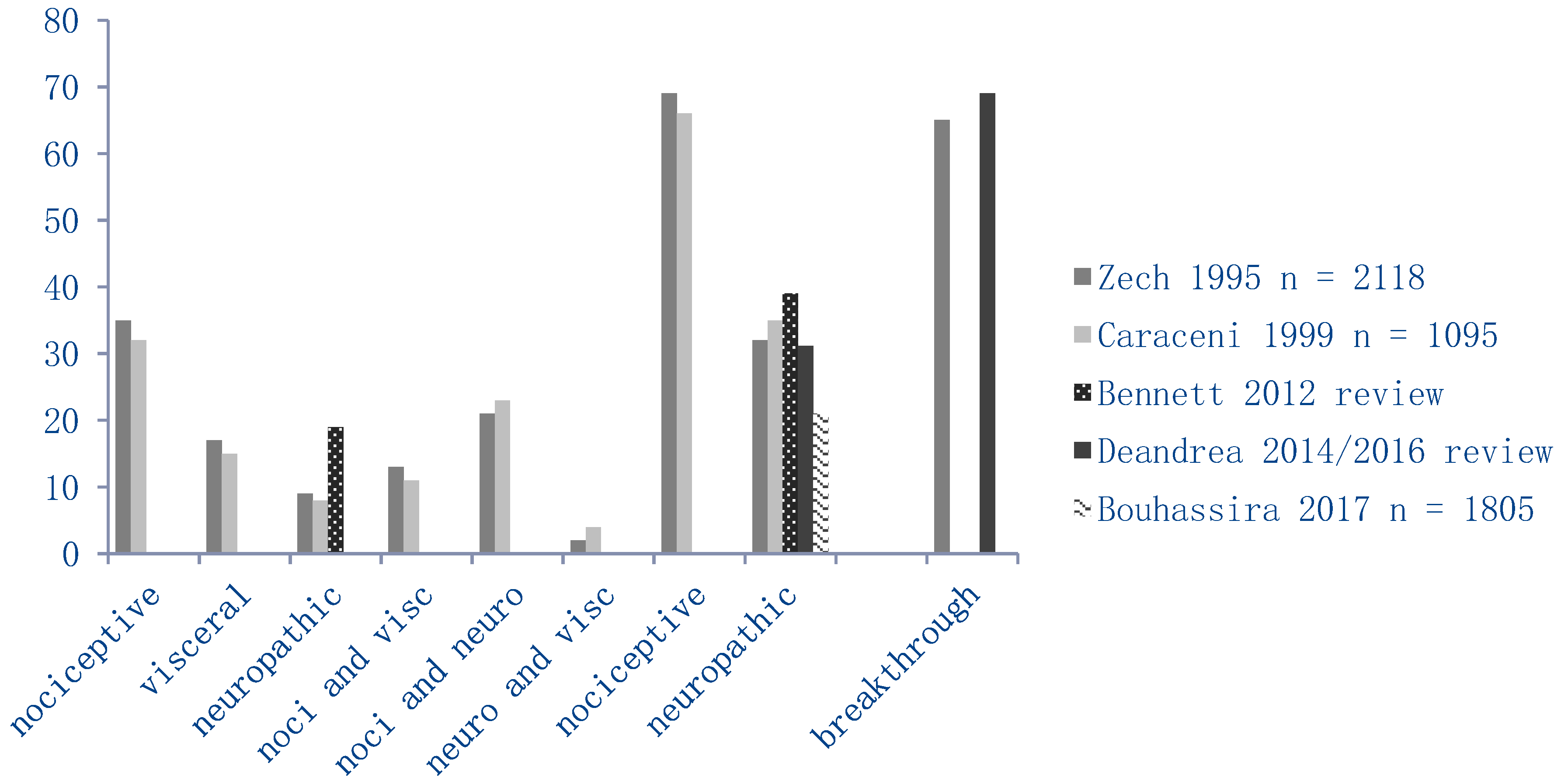
| PAIN (%) | NRS >4 (%) | |||
|---|---|---|---|---|
| 1966–2005 | 2005–2014 | 1966–2005 | 2005–2014 | |
| after curative treatment | 33 | 39 | not reported | 27 |
| on anticancer treatment | 59 | 55 | 36 | 32 |
| extensive/metastatic disease | 64 | 66 | 45 | 52 |
| all stages | 53 | 51 | 31 | 33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Beuken-van Everdingen, M.H.J.; Van Kuijk, S.M.J.; Janssen, D.J.A.; Joosten, E.A.J. Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers 2018, 10, 502. https://doi.org/10.3390/cancers10120502
Van den Beuken-van Everdingen MHJ, Van Kuijk SMJ, Janssen DJA, Joosten EAJ. Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers. 2018; 10(12):502. https://doi.org/10.3390/cancers10120502
Chicago/Turabian StyleVan den Beuken-van Everdingen, Marieke H. J., Sander M. J. Van Kuijk, Daisy J. A. Janssen, and Elbert A. J. Joosten. 2018. "Treatment of Pain in Cancer: Towards Personalised Medicine" Cancers 10, no. 12: 502. https://doi.org/10.3390/cancers10120502
APA StyleVan den Beuken-van Everdingen, M. H. J., Van Kuijk, S. M. J., Janssen, D. J. A., & Joosten, E. A. J. (2018). Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers, 10(12), 502. https://doi.org/10.3390/cancers10120502





