Venous Thromboembolism in Asian Patients with Pancreatic Cancer Following Palliative Chemotherapy: Low Incidence but a Negative Prognosticator for Those with Early Onset
Abstract
1. Introduction
2. Result
2.1. Patients’ Characteristics
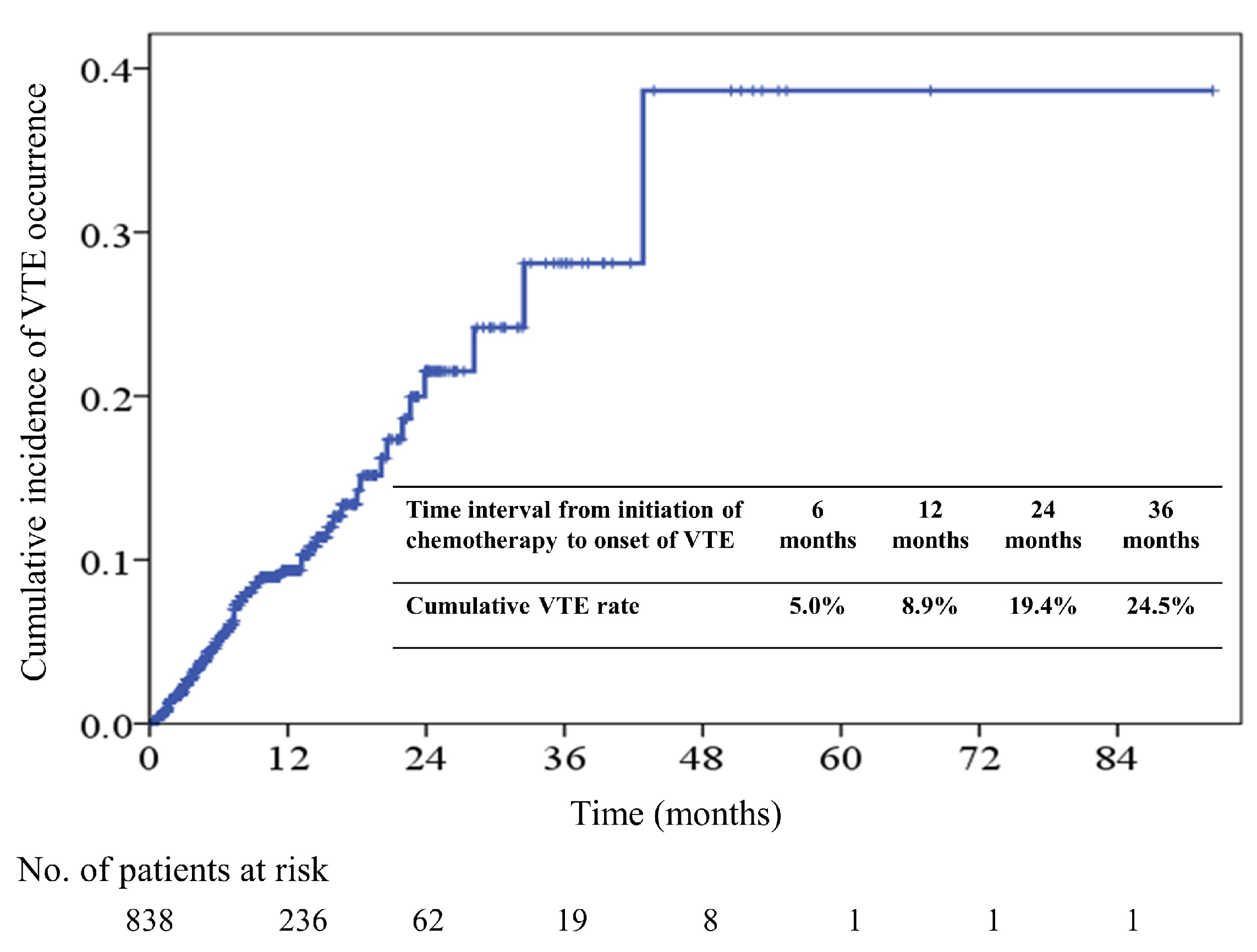
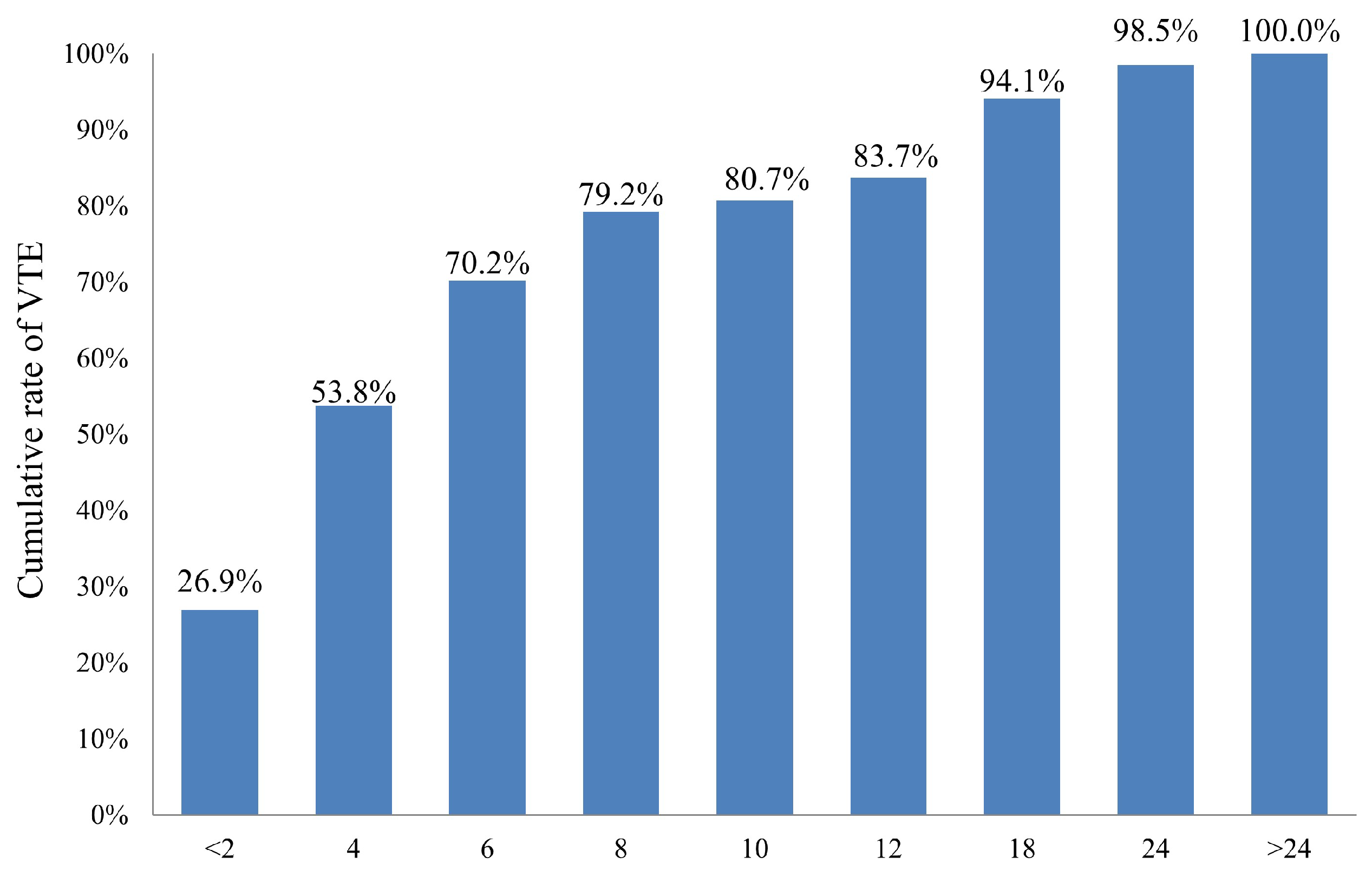
2.2. Incidence and Predictors of Venous Thromboembolism (VTE)
2.3. Association of Khorana Risk Score with VTE Incidence
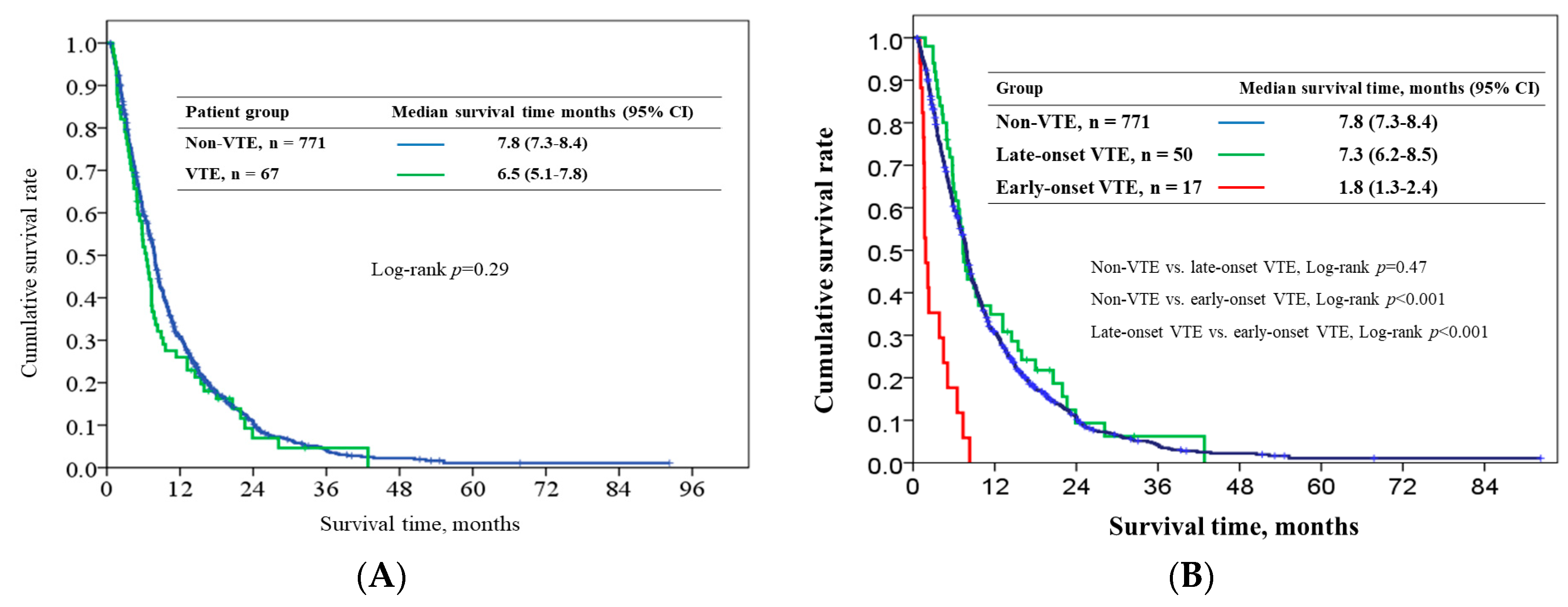
2.4. Survival Outcome
3. Discussion
4. Patients and Methods
4.1. Patient Selection
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greco, P.S.; Bazzi, A.A.; McLean, K.; Reynolds, R.K.; Spencer, R.J.; Johnston, C.M.; Liu, J.R.; Uppal, S. Incidence and timing of thromboembolic events in patients with ovarian cancer undergoing neoadjuvant chemotherapy. Obstet. Gynecol. 2017, 129, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.L. Cancer-associated thrombosis: Focus on extended therapy with dalteparin. J. Support. Oncol. 2006, 4, 115–120. [Google Scholar] [PubMed]
- Lopez, J.A.; Kearon, C.; Lee, A.Y. Deep venous thrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2004, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Piccioli, A.; Girolami, A. Cancer and venous thromboembolism: An overview. Haematologica 1999, 84, 437–445. [Google Scholar] [PubMed]
- Hedderich, G.S.; O’Connor, R.J.; Reid, E.C.; Mulder, D.S. Caval tumor thrombus complicating renal cell carcinoma: A surgical challenge. Surgery 1987, 102, 614–621. [Google Scholar] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Sondergaard, F.; Pedersen, L.A.; Fryzek, J.P.; Cetin, K.; Acquavella, J.; Baron, J.A.; Sørensen, H.T. Hospitalisation for venous thromboembolism in cancer patients and the general population: A population-based cohort study in Denmark, 1997–2006. Br. J. Cancer 2010, 103, 947–953. [Google Scholar] [CrossRef]
- Byrne, M.; Reynolds, J.V.; O’Donnell, J.S.; Keogan, M.; White, B.; Byrne, M.; Murphy, S.; Maher, S.G.; Pidgeon, G.P. Long-term activation of the pro-coagulant response after neoadjuvant chemoradiation and major cancer surgery. Br. J. Cancer 2010, 102, 73–79. [Google Scholar] [CrossRef]
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef]
- Chew, T.W.; Gau, C.S.; Wen, Y.W.; Shen, L.J.; Mullins, C.D.; Hsiao, F.Y. Epidemiology, clinical profile and treatment patterns of venous thromboembolism in cancer patients in Taiwan: A population-based study. BMC Cancer 2015, 15, 298. [Google Scholar] [CrossRef]
- Gade, I.L.; Braekkan, S.K.; Naess, I.A.; Hansen, J.B.; Cannegieter, S.C.; Overvad, K.; Jensvoll, H.; Hammerstrøm, J.; Blix, K.; Tjønneland, A.; et al. The impact of initial cancer stage on the incidence of venous thromboembolism: The Scandinavian Thrombosis and Cancer (STAC) Cohort. J. Thromb. Haemost. 2017, 15, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Bounameaux, H.; Brenner, B.; Cajfinger, F.; Debourdeau, P.; Khorana, A.A.; Pabinger, I.; Solymoss, S.; Douketis, J.; Kakkar, A. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016, 17, e452–e466. [Google Scholar] [CrossRef]
- Khorana, A.A.; Carrier, M.; Garcia, D.A.; Lee, A.Y. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 81–91. [Google Scholar] [CrossRef]
- Maraveyas, A.; Waters, J.; Roy, R.; Fyfe, D.; Propper, D.; Lofts, F.; Sgouros, J.; Gardiner, E.; Wedgwood, K.; Ettelaie, C.; et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur. J. Cancer 2012, 48, 1283–1292. [Google Scholar] [CrossRef]
- Pelzer, U.; Opitz, B.; Deutschinoff, G.; Stauch, M.; Reitzig, P.C.; Hahnfeld, S.; Müller, L.; Grunewald, M.; Stieler, J.M.; Sinn, M.; et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: Outcomes from the CONKO-004 trial. J. Clin. Oncol. 2015, 33, 2028–2034. [Google Scholar] [CrossRef]
- Yu, Y.B.; Gau, J.P.; Liu, C.Y.; Yang, M.H.; Chiang, S.C.; Hsu, H.C.; Hong, Y.C.; Hsiao, L.T.; Liu, J.H.; Chiou, T.J.; et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb. Haemost. 2012, 108, 225–235. [Google Scholar] [PubMed]
- Wang, K.L.; Yap, E.S.; Goto, S.; Zhang, S.; Siu, C.W.; Chiang, C.E. The diagnosis and treatment of venous thromboembolism in asian patients. Thromb. J. 2018, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, J.H.; Lee, K.W.; Bang, S.M.; Hwang, J.H.; Oh, D.; Lee, J.S. Venous thromboembolism in patients with pancreatic adenocarcinoma: Lower incidence in Asian ethnicity. Thromb. Res. 2008, 122, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Timp, J.F.; Braekhan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Sasaki, M.; Hosoi, H.; Sakamoto, Y.; Morizane, C.; Ueno, H.; Okusaka, T. Incidence and risk factors for venous thromboembolism in patients with pretreated advanced pancreatic carcinoma. Oncotarget 2018, 9, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Lee, M.Y.; Yoon, J.; Kim, H.J.; Kim, K.H.; Kim, S.H.; Lee, S.C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; et al. The incidence of venous thromboembolism is not lowin Korean patients with advanced pancreatic cancer. Blood Res. 2018, 53, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Ro, Y.S.; Cho, J.; Park, Y.; Lee, J.H.; Hwang, J.H.; Choi, H.J.; Lee, S. Characteristics of Venous Thromboembolism in Pancreatic Adenocarcinoma in East Asian Ethnics: A Large Population-Based Observational Study. Medicine (Baltimore) 2016, 95, e3472. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119, 648–655. [Google Scholar] [CrossRef]
- Epstein, A.S.; Soff, G.A.; Capanu, M.; Crosbie, C.; Shah, M.A.; Kelsen, D.P.; Denton, B.; Gardos, S.; O’Reilly, E.M. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer 2012, 118, 3053–3061. [Google Scholar] [CrossRef]
- Ishigaki, K.; Nakai, Y.; Isayama, H.; Saito, K.; Hamada, T.; Takahara, N.; Mizuno, S.; Mohri, D.; Kogure, H.; Matsubara, S.; et al. Thromboembolisms in advanced pancreatic cancer: A retrospective analysis of 475 patients. Pancreas 2017, 46, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Rybicki, L.; McCrae, K.R.; Khorana, A.A. Predicting risk of venous thromboembolism in hospitalized cancer patients: Utility of a risk assessment tool. Am. J. Hematol. 2017, 92, 501–507. [Google Scholar] [CrossRef]
- Ramos, J.D.; Casey, M.F.; Crabb, S.J.; Bamias, A.; Harshman, L.C.; Wong, Y.N.; Bellmunt, J.; De Giorgi, U.; Ladoire, S.; Powles, T.; et al. Venous thromboembolism in metastatic urothelial carcinoma or variant histologies: Incidence, associative factors, and effect on survival. Cancer Med. 2017, 6, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.T.; Walker, A.J.; Baig, S.; Card, T.R.; Kirwan, C.C.; Grainge, M.J. Venous thromboembolism and mortality in breast cancer: Cohort study with systematic review and meta-analysis. BMC Cancer 2017, 17, 747. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Hironaka, S.; Minashi, K.; Denda, T.; Shimokawa, M.; Yamaguchi, T. Cumulative incidence, risk factors and prognostic impact of venous thromboembolism in Japanese patients with advanced gastric cancer. Jpn. J. Clin. Oncol. 2017, 47, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Wun, T.; White, R.H. Venous thromboembolism (VTE) in patients with cancer: Epidemiology and risk factors. Cancer Invest. 2009, 27 (Suppl 1), 63–74. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, A.; Wun, T.; Khatri, V.; Chew, H.K.; Harvey, D.; Zhou, H.; White, R.H. Venous thromboembolism in patients with colorectal cancer: Incidence and effect on survival. J. Clin. Oncol. 2006, 24, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Mellemkjaer, L.; Steffensen, F.H.; Olsen, J.H.; Nielsen, G.L. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N. Engl. J. Med. 1998, 338, 1169–1173. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Lyman, G.H. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005, 104, 2822–2829. [Google Scholar] [CrossRef]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N. The thrombophilic state induced by therapeutic agents in the cancer patient. Semin. Thromb. Hemost. 1999, 25, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Otten, H.M.; Mathijssen, J.; ten Cate, H.; Soesan, M.; Inghels, M.; Richel, D.J.; Prins, M.H. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: An underestimated phenomenon. Arch. Intern. Med. 2004, 164, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J. Clin. Oncol. 2003, 21, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Silver, S.M.; Djulbegovic, B.; Samaras, A.T.; Blau, C.A.; Gleason, K.J.; Barnato, S.E.; Elverman, K.M.; Courtney, D.M.; McKoy, J.M.; et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008, 299, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Bohlius, J.; Wilson, J.; Seidenfeld, J.; Piper, M.; Schwarzer, G.; Sandercock, J.; Trelle, S.; Weingart, O.; Bayliss, S.; Djulbegovic, B.; et al. Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J. Natl. Cancer Inst. 2006, 98, 708–714. [Google Scholar] [CrossRef]
- Chou, W.C.; Wang, F.; Cheng, Y.F.; Chen, M.F.; Lu, C.H.; Wang, C.H.; Lin, Y.C.; Yeh, T.S. A simple risk stratification model that predicts 1-year postoperative mortality rate in patients with solid-organ cancer. Cancer Med. 2015, 4, 1687–1696. [Google Scholar] [CrossRef]
- Chou, W.C.; Liu, K.H.; Lu, C.H.; Hung, Y.S.; Chen, M.F.; Cheng, Y.F.; Wang, C.H.; Lin, Y.C.; Yeh, T.S. To operate or not: Prediction of 3-month postoperative mortality in geriatric cancer patients. J. Cancer 2016, 7, 14–21. [Google Scholar] [CrossRef]

| Variable | Category | All Patients, n = 838 (%) | Patients Without VTE, n = 771 (%) | VTE Patients, n = 67 (%) | p |
|---|---|---|---|---|---|
| Age, year | median (range) | 62 (23–89) | 62 (23–89) | 63(43–83) | 0.86 |
| Sex | 0.52 | ||||
| male | 497(59.3) | 460 (59.6) | 37 (56.1) | ||
| female | 341 (40.7) | 311 (40.3) | 30 (44.8) | ||
| BMI, kg/m2 | median (range) | 22.5 (13–36.2) | 22.4 (13.0–36.0) | 22.8 (15.9–36.2) | 0.25 |
| BMI, kg/m2 | >35 | 3 (0.4) | 2 (0.3) | 1 (1.5) | 0.22 |
| ECOG PS | 0.48 | ||||
| 0–1 | 597 (71.2) | 545 (70.7) | 52 (77.6) | ||
| 2 | 206 (24.6) | 193 (25.0) | 13 (19.4) | ||
| 3 | 35 (4.2) | 33 (4.3) | 2 (3.0) | ||
| CCI | 0.79 | ||||
| 0 | 227 (27.1) | 211 (27.4) | 16 (23.9) | ||
| 1 | 292 (34.8) | 265 (34.4) | 27 (40.3) | ||
| 2 | 193 (23.0) | 179 (23.2) | 14 (20.9) | ||
| >3 | 126(15.0) | 116 (15.0) | 10 (14.9) | ||
| Diabetes mellitus | presence | 313 (37.4) | 292 (37.9) | 21 (31.3) | 0.37 |
| Hypertension | presence | 332 (39.6) | 302 (39.2) | 30 (44.8) | 0.36 |
| Cerebrovascular disease | presence | 30 (3.6) | 28 (3.6) | 2 (3.0) | 0.99 |
| Coronary artery disease | presence | 52 (6.2) | 46 (6.0) | 6 (9.0) | 0.29 |
| Arrhythmia | presence | 13 (1.6) | 12 (1.6) | 1 (1.5) | 0.99 |
| Tumor site | 0.12 | ||||
| head | 343 (40.9) | 320 (41.5) | 23 (34.3) | ||
| body | 148 (17.7) | 140 (18.2) | 8 (11.9) | ||
| tail | 171 (20.4) | 156 (20.2) | 15 (22.4) | ||
| overlapping | 176 (21.0) | 155 (20.1) | 21 (31.3) | ||
| Tumor stage, seventh AJCC | 0.35 | ||||
| III | 183 (21.8) | 172 (22.3) | 11 (16.4) | ||
| IV | 655 (78.2) | 599 (77.7) | 56 (83.6) | ||
| Tumor grade | 0.53 | ||||
| well to moderate differentiation | 93 (11.1) | 83 (10.8) | 10 (14.9) | ||
| poorly differentiation | 92 (11.0) | 86 (11.2) | 6 (9) | ||
| unclassified or unknown | 653 (77.9) | 602 (78.1) | 51 (76.1) | ||
| Jaundice under drainage | presence | 272 (32.5) | 248 (32.2) | 24 (32.2) | 0.59 |
| Metastatic organ | liver | 438 (52.3) | 394 (51.1) | 44 (65.7) | 0.03 |
| peritoneum | 239 (28.5) | 222 (28.8) | 17 (25.4) | 0.67 | |
| lymph nodes | 150 (17.9) | 138 (17.9) | 12 (17.9) | 0.99 | |
| lung | 98 (11.7) | 88 (11.4) | 10 (14.9) | 0.43 | |
| CEA, ng/mL | >5 | 427 (51.0) | 387 (50.2) | 40 (59.7) | 0.16 |
| CA19-9, u/mL | >37 | 666 (79.5) | 610 (79.1) | 56 (83.6) | 0.43 |
| Platelet count, 109/L | >350 | 86 (10.3) | 81 (10.5) | 5 (7.5) | 0.53 |
| Hemoglobin, g/dL | >10 | 734 (87.6) | 674 (87.4) | 60 (89.6) | 0.70 |
| Leukocyte count, 109/L | >11 | 167 (19.9) | 146 (18.9) | 21 (31.3) | 0.024 |
| Chemotherapy regimen had been exposure | Gemcitabine | 792 (94.5) | 726 (94.2) | 66 (98.5) | 0.17 |
| 5-FU or S-1 | 592 (70.6) | 540 (70.0) | 52 (77.6) | 0.45 | |
| Platinum | 433 (51.7) | 401 (51.9) | 32 (48.5) | 0.61 | |
| Khorana risk score | 0.33 | ||||
| 2 | 60 (7.2) | 56 (7.3) | 4 (6.0) | ||
| 3 | 591 (70.5) | 549 (71.2) | 42 (62.7) | ||
| 4 | 162 (19.3) | 144 (18.7) | 18 (26.9) | ||
| 5 | 25 (3.0) | 22 (2.9) | 3 (4.5) |
| Location of thrombosis | n | % |
|---|---|---|
| DVT, lower limb | 34 | 50.7% |
| DVT, upper limb | 7 | 10.4% |
| PE | 14 | 20.9% |
| Concomitant DVT and PE | 8 | 11.9% |
| Concomitant DVT and IVC thrombosis | 2 | 3.0% |
| Concomitant upper and lower limb DVT | 1 | 1.5% |
| Concomitant lower limb DVT and PVT | 1 | 1.5% |
| Clinical Factors | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | SE | 95% CI | p | HR | 95% CI | p | |
| Clinical characteristics | |||||||
| Male (vs. female) | 1.20 | 0.25 | 0.73–1.98 | 0.48 | |||
| Age > 65 years (vs. ≤ 65 years) | 0.83 | 0.26 | 0.50–1.39 | 0.49 | |||
| Presence of hypertension (vs. no hypertension) | 1.26 | 0.26 | 0.76–2.08 | 0.37 | |||
| Presence of diabetes mellitus (vs. no diabetes mellitus) | 0.75 | 0.27 | 0.44–1.28 | 0.29 | |||
| Presence of coronary artery disease (vs. no coronary artery disease) | 1.55 | 0.45 | 0.64–3.78 | 0.33 | |||
| Presence of cerebrovascular disease (vs. no cerebrovascular disease) | 0.82 | 0.74 | 0.19–3.50 | 0.79 | |||
| ECOG PS 0–1 (vs. > 1) | 0.70 | 0.30 | 0.38–1.26 | 0.23 | |||
| CCI 0 (vs. > 0) | 1.20 | 0.30 | 0.67–2.15 | 0.54 | |||
| BMI > 23 kg/m2 (≤23 kg/m2) | 1.06 | 0.26 | 0.64–1.75 | 0.82 | |||
| Tumor characteristics | |||||||
| Tumor site at pancreatic head (vs. other sites) | 0.74 | 0.27 | 0.44–1.24 | 0.25 | |||
| Presence of jaundice under drainage (vs. no jaundice) | 1.18 | 0.27 | 0.70–1.98 | 0.54 | |||
| Stage IV disease (vs. stage III) | 1.46 | 0.34 | 0.75–2.85 | 0.27 | |||
| Presence of liver metastases (vs. no liver metastases) | 1.83 | 0.27 | 1.08–3.09 | 0.024 | 1.65 | 1.03–3.99 | 0.046 |
| Presence of peritoneal metastases (vs. no peritoneal metastases) | 0.84 | 0.29 | 0.48–1.49 | 0.55 | |||
| Presence of lung metastases (vs. no lung metastases) | 1.36 | 0.36 | 0.67–2.76 | 0.39 | |||
| Presence of distant lymph nodes metastases (vs. no distant lymph node metastases) | 1.01 | 0.33 | 0.52–1.92 | 0.99 | |||
| Biological data | |||||||
| CEA > 5 ng/mL (vs. < 5 ng/mL) | 1.47 | 0.26 | 0.88–2.44 | 0.14 | 1.27 | 0.75–2.14 | 0.37 |
| CA19-9 > 37 u/mL (≤37 u/mL) | 1.34 | 0.34 | 0.69–2.62 | 0.39 | |||
| Platelet count > 350 × 109/L (vs. ≤ 350 × 109/L) | 0.69 | 0.48 | 0.27–1.76 | 0.43 | |||
| Hemoglobin > 10 g/dL (vs. ≤ 10 g/dL) | 1.23 | 0.41 | 0.55–2.78 | 0.61 | |||
| Leukocyte count > 11,000/μL (vs. ≤ 11,000/μL) | 1.95 | 0.28 | 1.13–3.38 | 0.016 | 1.75 | 1.07–3.03 | 0.032 |
| Use of anticancer drugs | |||||||
| Chemotherapy with platinum (vs. without platinum) | 0.90 | 0.26 | 0.55–1.48 | 0.68 | |||
| Chemotherapy with gemcitabine (vs. without gemcitabine) | 4.09 | 1.02 | 0.56–30.2 | 0.17 | 4.27 | 0.58–21.0 | 0.16 |
| Chemotherapy with 5-FU or S-1 (vs. without 5-FU or S-1) | 1.21 | 0.27 | 0.72–2.03 | 0.48 | |||
| Khorana risk score 4–5 (vs. score 2–3) | 1.66 | 0.28 | 0.97–2.87 | 0.067 | 1.12 | 0.36–2.64 | 0.66 |
| Khorana Risk Score | Overall, n = 838 | 2, n = 60 | 3, n = 591 | 4, n = 162 | 5, n = 25 |
|---|---|---|---|---|---|
| Venous thromboembolism, n | 67 | 4 | 42 | 18 | 3 |
| Person-years | 676.9 | 39.2 | 510.7 | 113.8 | 13.2 |
| Incidence rate, per 1000 person-years | 99.0 | 102.0 | 82.2 | 158.2 | 227.3 |
| Crude HR (95% CI) | 1 | 0.90 (0.32–2.50) | 1.73 (0.59–5.12) | 2.24 (0.50–10.0) | |
| Adjusted HR* (95% CI) | 1 | 0.97 (0.35–2.70) | 1.20 (0.31–4.67) | 1.23(0.21–7.19) |
| Clinical Factor | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | SE | 95% CI | p | HR | 95% CI | p | |
| Clinical characteristics | |||||||
| Male (vs. female) | 1.31 | 0.08 | 1.13–1.52 | <0.001 | 1.27 | 1.09–1.47 | 0.002 |
| Age > 65 years (vs. age ≤ 65) | 1.21 | 0.07 | 1.05–1.40 | 0.011 | 1.07 | 0.91–1.25 | 0.41 |
| ECOG PS 0–1 (vs. ECOG PS >1) | 0.37 | 0.08 | 0.31–0.43 | <0.001 | 1.78 | 1.48–2.14 | <0.001 |
| CCI 0 (vs. CCI > 0) | 0.66 | 0.08 | 0.56–0.78 | <0.001 | 1.37 | 1.15–1.63 | <0.001 |
| BMI > 23 kg/m2 (vs. ≤ 23 kg/m2) | 0.93 | 0.07 | 0.81–1.08 | 0.33 | |||
| Tumor characteristics | |||||||
| Tumor site at pancreatic head (vs. other sites) | 1.07 | 0.07 | 0.93–1.24 | 0.35 | |||
| Presence of jaundice under drainage (vs. no jaundice) | 1.11 | 0.08 | 0.95–1.29 | 0.21 | |||
| Stage IV disease (vs. stage III) | 1.99 | 0.09 | 1.66–2.40 | <0.001 | 2.04 | 1.69–2.46 | <0.001 |
| Biological data | |||||||
| CEA > 5 ng/mL (vs. ≤ 5 ng/mL) | 1.28 | 0.07 | 1.11–1.48 | 0.001 | 1.10 | 0.95–1.28 | 0.21 |
| CA19-9 > 37 u/mL (vs. ≤ 37 u/mL) | 1.06 | 0.09 | 0.88–1.26 | 0.56 | |||
| Platelet count > 350 × 109/L (vs. ≤ 350 × 109/L) | 1.09 | 0.09 | 0.87–1.40 | 0.44 | |||
| Hemoglobin > 10 g/dL (vs. ≤ 10 g/dL) | 0.64 | 0.11 | 0.51–0.79 | <0.001 | 1.15 | 0.92–1.45 | 0.22 |
| Leukocyte count > 11,000/μL (vs. ≤ 11,000/μL) | 1.69 | 0.09 | 1.41–2.01 | <0.001 | 1.24 | 1.03–1.49 | 0.02 |
| Use of anticancer drugs | |||||||
| Chemotherapy with platinum (vs. without platinum) | 0.62 | 0.07 | 0.53–0.71 | <0.001 | 1.42 | 1.21–1.66 | <0.001 |
| Chemotherapy with gemcitabine (vs. without gemcitabine) | 0.50 | 0.16 | 0.37–0.68 | <0.001 | 2.22 | 1.59–3.10 | <0.001 |
| Chemotherapy with 5-FU or S-1 (vs. without 5-FU or S-1) | 0.44 | 0.08 | 0.38–0.51 | <0.001 | 1.93 | 1.64–2.27 | <0.001 |
| Occurrence of VTE | |||||||
| Occurrence of any VTE (vs. no VTE) | 1.15 | 0.13 | 0.89–1.50 | 0.29 | |||
| Early onset of venous thromboembolism (vs. no VTE) | 4.86 | 0.24 | 3.02–7.80 | <0.001 | 3.80 | 2.34–6.18 | <0.001 |
| Late onset of venous thrombosis (vs. no VTE) | 0.88 | 0.16 | 0.64–1.91 | 0.39 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-S.; Hung, C.-Y.; Chang, H.; Liu, C.-T.; Chen, Y.-Y.; Lu, C.-H.; Chang, P.-H.; Hung, Y.-S.; Chou, W.-C. Venous Thromboembolism in Asian Patients with Pancreatic Cancer Following Palliative Chemotherapy: Low Incidence but a Negative Prognosticator for Those with Early Onset. Cancers 2018, 10, 501. https://doi.org/10.3390/cancers10120501
Chen J-S, Hung C-Y, Chang H, Liu C-T, Chen Y-Y, Lu C-H, Chang P-H, Hung Y-S, Chou W-C. Venous Thromboembolism in Asian Patients with Pancreatic Cancer Following Palliative Chemotherapy: Low Incidence but a Negative Prognosticator for Those with Early Onset. Cancers. 2018; 10(12):501. https://doi.org/10.3390/cancers10120501
Chicago/Turabian StyleChen, Jen-Shi, Chia-Yen Hung, Hung Chang, Chien-Ting Liu, Yen-Yang Chen, Chang-Hsien Lu, Pei-Hung Chang, Yu-Shin Hung, and Wen-Chi Chou. 2018. "Venous Thromboembolism in Asian Patients with Pancreatic Cancer Following Palliative Chemotherapy: Low Incidence but a Negative Prognosticator for Those with Early Onset" Cancers 10, no. 12: 501. https://doi.org/10.3390/cancers10120501
APA StyleChen, J.-S., Hung, C.-Y., Chang, H., Liu, C.-T., Chen, Y.-Y., Lu, C.-H., Chang, P.-H., Hung, Y.-S., & Chou, W.-C. (2018). Venous Thromboembolism in Asian Patients with Pancreatic Cancer Following Palliative Chemotherapy: Low Incidence but a Negative Prognosticator for Those with Early Onset. Cancers, 10(12), 501. https://doi.org/10.3390/cancers10120501





