Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery
Abstract
:1. Introduction
2. Patients and Methods
2.1. Database
2.2. Selection of Patients and Controls
2.3. Statistical Analysis
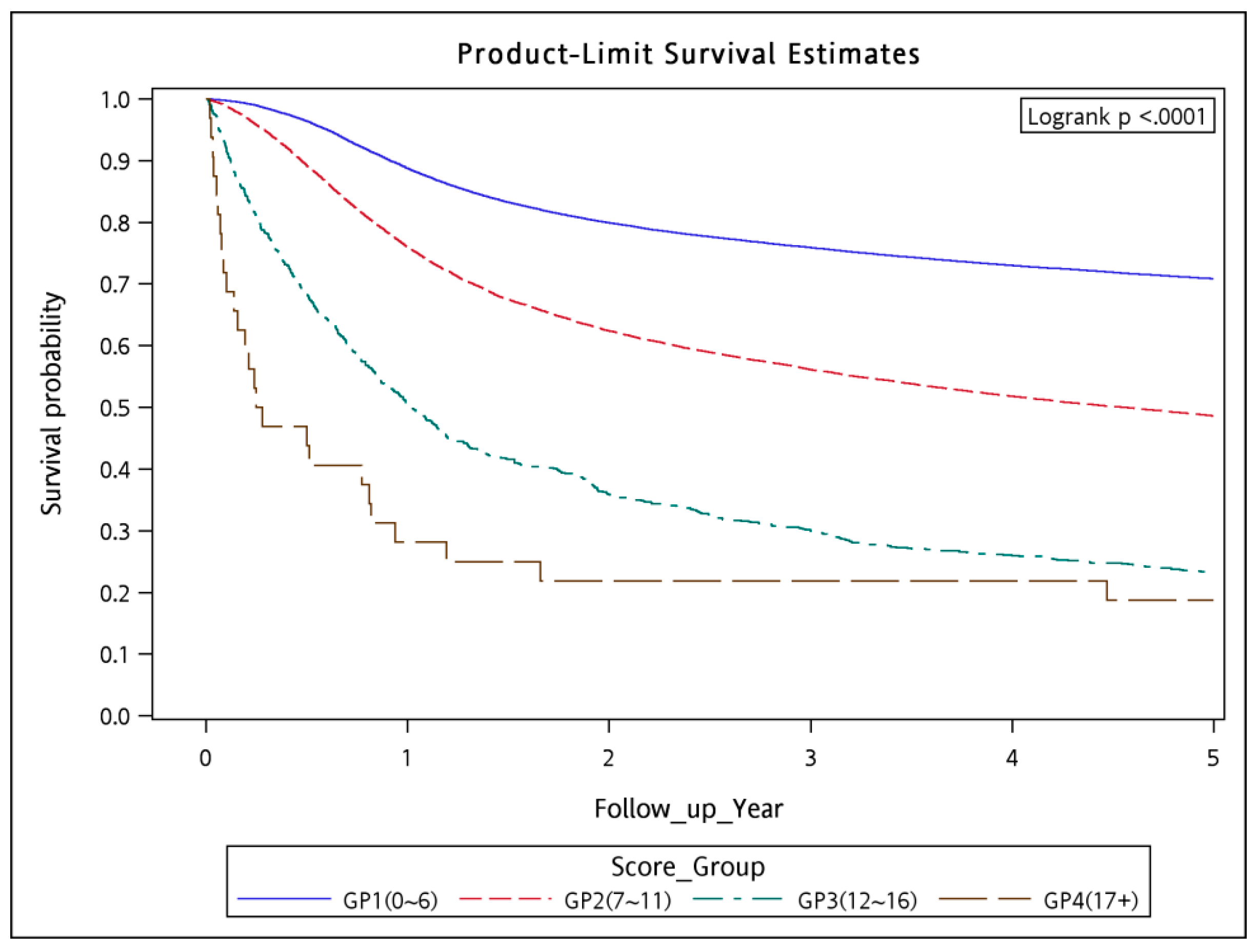
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| ARDS | Adult Respiratory Distress Syndrome |
| ASA | American Society of Anesthesiologists |
| CAD | Coronal Arterial Disease |
| CCI | Charlson Comorbidity Index |
| CCRT | Concurrent Chemoradiotherapy |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CVA | Cerebral Vascular Accident |
| DIC | Disseminated Intravascular Coagulation |
| ESRD | End-Stage Renal Disease |
| HBV | Hepatitis B |
| HCV | Hepatitis C |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HR | Hazard Ratio |
| HTN | Diabetes Mellitus DM Hypertension |
| ICD-9-CM | International Classification of Diseases Ninth Revision Clinical Modification |
| LA-HNSCC | Locoregionally Advanced Head and Neck Squamous Cell Carcinoma |
| MI | Myocardial Infarction |
| PUD | Peptic Ulcer Disease |
| PVD | Peripheral Vascular Disease |
| RT | Radiotherapy |
| TCRD | Taiwan Cancer Registry Database |
| TIA | Transient Ischemic Attack |
References
- Chen, J.H.; Yen, Y.C.; Chen, T.M.; Yuan, K.S.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; Chang, C.L.; Wu, S.Y. Survival prognostic factors for metachronous second primary head and neck squamous cell carcinoma. Cancer Med. 2017, 6, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Yuan, K.S.; Wu, S.Y. High-dose or low-dose cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer. Head Neck 2017, 39, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yen, Y.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; Chen, T.M.; et al. Outcomes of Induction Chemotherapy for Head and Neck Cancer Patients: A Combined Study of Two National Cohorts in Taiwan. Medicine (Baltimore) 2016, 95, e2845. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yen, Y.C.; Yang, H.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; et al. Curative-intent aggressive treatment improves survival in elderly patients with locally advanced head and neck squamous cell carcinoma and high comorbidity index. Medicine (Baltimore) 2016, 95, e3268. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Wu, C.C.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Locoregionally recurrent head and neck squamous cell carcinoma: Incidence, survival, prognostic factors, and treatment outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration, M.o.H.a.W. Taiwan Cancer Registry Report, 2017 Edition. Available online: http://www.hpa.gov.tw/BHPNet/Web/Stat/StatisticsShow.aspx?No=201404160001 (accessed on 28 December 2017).
- McGuirt, W.F.; Loevy, S.; McCabe, B.F.; Krause, C.J. The risks of major head and neck surgery in the aged population. Laryngoscope 1977, 87, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Gueret, G.; Bourgain, J.L.; Luboinski, B. Sudden death after major head and neck surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.G.; Moreno-Torres, A.; Campos, R. Radical neck dissection. Evaluation of 230 consecutive cases. Arch. Otolaryngol. 1971, 94, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yarington, C.T., Jr.; Yonkers, A.J.; Beddoe, G.M. Radical neck dissection. Mortality and morbidity. Arch. Otolaryngol. 1973, 97, 306–308. [Google Scholar] [CrossRef] [PubMed]
- MacComb, W.S. Mortality from radical neck dissection. Am. J. Surg. 1968, 115, 352–354. [Google Scholar] [CrossRef]
- Shaha, A.R. Neck dissection: An operation in evolution. World J. Surg. Oncol. 2005, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, E.N.; Gastman, B.R. Neck dissection: An operation in evolution: Hayes Martin lecture. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm—General principles. Nat. Clin. Pract. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase IB KEYNOTE-012 expansion cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum-and Cetuximab-Refractory Head and Neck Cancer: Results from a Single-Arm, Phase II Study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Iqbal, F.; Pollock, B.E.; Link, M.J.; Stien, K.; Garces, Y.I.; Brown, P.D.; Foote, R.L. Long-term follow-up of stereotactic radiosurgery for head and neck malignancies. Head Neck 2015, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Mageras, G.S.; Mechalakos, J. Planning in the IGRT context: Closing the loop. Semin. Radiat. Oncol. 2007, 17, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Puri, D.R.; Blanco, A.I.; Chao, K.S. Intensity-modulated radiation therapy in head and neck cancers: An update. Head Neck 2007, 29, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.C.; Chang, J.H.; Lin, W.C.; Chiou, J.F.; Chang, Y.C.; Chang, C.L.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.; et al. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Tsai, H.C.; Lin, W.C.; Chang, J.H.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Austin, P.C.; Beattie, W.S.; Hux, J.E.; Laupacis, A. A population-based study of anesthesia consultation before major noncardiac surgery. Arch. Intern. Med. 2009, 169, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Ding, Y.F.; Hsu, H.L.; Chang, J.H.; Yuan, K.S.; Wu, A.T.H.; Chow, J.M.; Chang, C.L.; Chen, S.U.; Wu, S.Y. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. Cancer 2017, 123, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M.; Duncan, P.G.; Tate, R.B. Does anesthesia contribute to operative mortality? JAMA 1988, 260, 2859–2863. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Dagash, H.; Pierro, A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br. J. Surg. 2007, 94, 145–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, R.L.; Perruccio, A.V.; Gandhi, R.; Mahomed, N.N. The role of surgeon volume on patient outcome in total knee arthroplasty: A systematic review of the literature. BMC Musculoskelet. Disord. 2012, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.C.; Tang, C.H.; Lin, H.C.; Tsai, C.S.; Chen, C.S.; Li, C.Y. Association between surgeon and hospital volume in coronary artery bypass graft surgery outcomes: A population-based study. Ann. Thorac. Surg. 2006, 81, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhorst-de van der, S.; van Leeuwen, P.A.; Kuik, D.J.; Klop, W.M.; Sauerwein, H.P.; Snow, G.B.; Quak, J.J. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 1999, 86, 519–527. [Google Scholar] [CrossRef]
- Sylvester, M.J.; Marchiano, E.; Park, R.C.; Baredes, S.; Eloy, J.A. Impact of chronic obstructive pulmonary disease on patients undergoing laryngectomy for laryngeal cancer. Laryngoscope 2017, 127, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.T.; Bohl, D.D.; Basques, B.A.; Arzeno, A.H.; Grauer, J.N. Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery? Am. J. Orthop. (Belle Mead NJ) 2017, 46, E177–E185. [Google Scholar] [PubMed]
- Volpi, A.; De Vita, C.; Franzosi, M.G.; Geraci, E.; Maggioni, A.P.; Mauri, F.; Negri, E.; Santoro, E.; Tavazzi, L.; Tognoni, G. Determinants of 6-month mortality in survivors of myocardial infarction after thrombolysis. Results of the GISSI-2 data base. The Ad hoc Working Group of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-2 Data Base. Circulation 1993, 88, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, T.G.; Libman, R.B.; Frankel, M.; Tilley, B.C.; Morgenstern, L.B.; Lu, M.; Broderick, J.P.; Lewandowski, C.A.; Marler, J.R.; Levine, S.R.; et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N. Engl. J. Med. 1999, 340, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Liu, L.; Wang, D.; Wang, C.; Li, H.; Meng, X.; Cui, L.; Jia, J.; Dong, Q.; et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. The viable myocardium: Epidemiology, detection, and clinical implications. Lancet 1998, 351, 815–819. [Google Scholar] [CrossRef]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Sack, G.H., Jr.; Levin, J.; Bell, W.R. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: Clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977, 56, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Myers, J.L.; Mazur, M.T. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am. J. Surg. Pathol. 1986, 10, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, W.T. The effects of pre-existing dementia on surgical outcomes in emergent and nonemergent general surgical procedures: Assessing differences in surgical risk with dementia. BMC Geriatr. 2018, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Patel, U.A.; Samant, S.; Yang, A.; Smith, S.S. Liver disease in patients undergoing head and neck surgery: Incidence and risk for postoperative complications. Laryngoscope 2017, 127, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, J.F.; Lacy, P.D.; Basu, A.; Spitznagel, E.L. Development of a new head and neck cancer-specific comorbidity index. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Dees, E.C.; O’Reilly, S.; Goodman, S.N.; Sartorius, S.; Levine, M.A.; Jones, R.J.; Grochow, L.B.; Donehower, R.C.; Fetting, J.H. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Investig. 2000, 18, 521–529. [Google Scholar] [CrossRef]
- Gomez, H.; Mas, L.; Casanova, L.; Pen, D.L.; Santillana, S.; Valdivia, S.; Otero, J.; Rodriguez, W.; Carracedo, C.; Vallejos, C. Elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage colony-stimulating factor: Identification of two age subgroups with differing hematologic toxicity. J. Clin. Oncol. 1998, 16, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.E.; Stella, P.J.; Geyer, S.M.; Bonner, J.A.; McGinnis, W.L.; Mailliard, J.A.; Brindle, J.; Jatoi, A.; Jett, J.R. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J. Clin. Oncol. 2003, 21, 3201–3206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Ioannidis, J.P. Nationwide Population Science: Lessons from the Taiwan National Health Insurance Research Database. JAMA Intern. Med. 2015, 175, 1527–1529. [Google Scholar] [CrossRef] [PubMed]

| Factor | Death No. | Death Rate | Survival No. | Survival Rate | p Value |
|---|---|---|---|---|---|
| Number of patients | 1287 | 53,793 | |||
| Age (years) | <0.0001 | ||||
| 18–29 | 2 | 0.16% | 696 | 1.29% | |
| 30–39 | 40 | 3.11% | 4978 | 9.25% | |
| 40–49 | 231 | 17.95% | 14,379 | 26.73% | |
| 50–59 | 352 | 27.35% | 16,611 | 30.88% | |
| 60–69 | 263 | 20.44% | 10,046 | 18.68% | |
| ≥70 | 399 | 31.00% | 7083 | 13.17% | |
| Sex | <0.0001 | ||||
| Female | 195 | 7.38% | 5697 | 10.59% | |
| Male | 1192 | 92.62% | 48,096 | 89.41% | |
| Comorbidity | |||||
| DM | 80 | 6.22% | 1804 | 3.35% | <0.0001 |
| HTN | 477 | 37.06% | 16,260 | 30.23% | <0.0001 |
| Pneumonia | 250 | 19.43% | 2665 | 4.95% | <0.0001 |
| COPD | 240 | 18.65% | 4517 | 8.40% | <0.0001 |
| Hepatitis B | 8 | 0.62% | 425 | 0.79% | 0.4989 |
| Hepatitis C | 32 | 2.49% | 1003 | 1.86% | 0.1045 |
| Implanted pacemaker | 2 | 0.16% | 22 | 0.04% | 0.0518 |
| MI, CVA, TIA, angina, or CAD | 280 | 21.76% | 6957 | 12.93% | <0.0001 |
| Heart valve dysfunction | 35 | 2.72% | 659 | 1.23% | <0.0001 |
| ESRD | 0 | 0.00% | 0 | 0.00% | |
| Sepsis | 172 | 13.36% | 882 | 1.64% | <0.0001 |
| CKD | 165 | 12.82% | 1754 | 3.26% | <0.0001 |
| Heart failure | 92 | 7.15% | 1000 | 1.86% | <0.0001 |
| DIC | 5 | 0.39% | 5 | 0.01% | <0.0001 |
| ARDS | 4 | 0.31% | 18 | 0.03% | <0.0001 |
| Aortic aneurysm | 4 | 0.31% | 58 | 0.11% | 0.0319 |
| PVD | 33 | 2.56% | 707 | 1.31% | 0.0001 |
| PUD | 268 | 20.82% | 7394 | 13.75% | <0.0001 |
| Dementia | 71 | 5.52% | 1100 | 2.04% | <0.0001 |
| Chronic pulmonary disease | 222 | 17.25% | 4810 | 8.94% | <0.0001 |
| Connective tissue disease | 23 | 1.79% | 614 | 1.14% | 0.0323 |
| Mild liver disease | 255 | 19.81% | 7733 | 14.38% | <0.0001 |
| Hemiplegia | 107 | 8.31% | 1970 | 3.66% | <0.0001 |
| Moderate or severe renal disease | 166 | 12.90% | 1757 | 3.27% | <0.0001 |
| Any non-HNSCC Solid Cancer | 659 | 51.20% | 14,591 | 27.12% | <0.0001 |
| Leukemia | 2 | 0.16% | 33 | 0.06% | 0.1858 |
| Lymphoma | 22 | 1.71% | 647 | 1.20% | 0.101 |
| Moderate or severe liver disease | 89 | 6.92% | 2487 | 4.62% | 0.0001 |
| Metastatic non-HNSCC solid cancer | 548 | 42.58% | 13,390 | 24.89% | <0.0001 |
| Smoking | 1158 | 89.98% | 48,413 | 90.00% | 0.8989 |
| Previous thoracic surgery | 6 | 0.47% | 268 | 0.50% | 0.6767 |
| Obesity | 11 | 1.24% | 699 | 1.30% | 0.7756 |
| Asthma | 10 | 0.78% | 429 | 0.80% | 0.9573 |
| Bowel obstruction | 3 | 0.23% | 123 | 0.23% | 0.8457 |
| Factor | HR | 95% CI | p Value |
|---|---|---|---|
| Age (years) | |||
| 18–29 | 1 | (Reference) | |
| ≥30 | 1.277 | 2.077, 32.989 | 0.0027 |
| ≥40 | 1.463 | 2.546, 4.709 | <0.0001 |
| ≥50 | 2.19 | 1.916, 2.503 | <0.0001 |
| ≥60 | 2.245 | 2.012, 2.504 | <0.0001 |
| ≥70 | 2.915 | 2.59, 3.28 | <0.0001 |
| Sex | |||
| Female | 1 | (Reference) | |
| Male | 1.48 | 1.201, 1.824 | 0.0002 |
| Comorbidities | |||
| DM | 1.894 | 1.51, 2.374 | <0.0001 |
| HTN | 1.356 | 1.211, 1.518 | <0.0001 |
| Pneumonia | 4.476 | 3.898, 5.138 | <0.0001 |
| COPD | 2.466 | 2.143, 2.838 | <0.0001 |
| Hepatitis B | 0.787 | 0.393, 1.576 | 0.4986 |
| Hepatitis C | 1.342 | 0.945, 1.905 | 0.1004 |
| Implanted pacemaker | 3.716 | 0.928, 14.874 | 0.0636 |
| MI, CVA, TIA, angina, or CAD | 1.857 | 1.627, 2.12 | <0.0001 |
| Heart valve dysfunction | 2.224 | 1.589, 3.111 | <0.0001 |
| Sepsis | 8.647 | 7.364, 10.153 | <0.0001 |
| CKD | 4.245 | 3.605, 4.999 | <0.0001 |
| Heart failure | 3.965 | 3.207, 4.902 | <0.0001 |
| DIC | 32.834 | 13.643, 79.019 | <0.0001 |
| ARDS | 9.058 | 3.395, 24.166 | <0.0001 |
| Aortic aneurysm | 2.818 | 1.056, 7.519 | 0.0385 |
| PAD | 1.962 | 1.389, 2.772 | 0.0001 |
| PUD | 1.64 | 1.433, 1.876 | <0.0001 |
| Dementia | 2.735 | 2.153, 3.475 | <0.0001 |
| Chronic pulmonary disease | 2.1 | 1.817, 2.427 | <0.0001 |
| Connective tissue disease | 1.567 | 1.037, 2.367 | 0.0328 |
| Mild liver disease | 1.465 | 1.277, 1.68 | <0.0001 |
| Hemiplegia | 2.35 | 1.928, 2.865 | <0.0001 |
| Moderate or severe renal disease | 4.272 | 3.629, 5.028 | <0.0001 |
| Any non-HNSCC Solid Cancer | 2.753 | 1.917, 3.953 | <0.0001 |
| Leukemia | 2.506 | 0.626, 10.032 | 0.1941 |
| Lymphoma | 1.423 | 0.934, 2.168 | 0.1008 |
| Moderate or severe liver disease | 1.525 | 1.229, 1.891 | 0.0001 |
| Metastatic non-HNSCC solid cancer | 2.21 | 1.979, 2.468 | <0.0001 |
| Smoking | 0.964 | 0.405, 1.459 | 0.3571 |
| Previous thoracic surgery | 1.168 | 0.778, 2.589 | 0.6734 |
| Obesity | 1.473 | 0.746, 1.896 | 0.8197 |
| Asthma | 1.384 | 0.804, 8.09 | 0.7592 |
| Bowel obstruction | 1.132 | 0.494, 2.873 | 0.8112 |
| Factor | HR | 95% CI | p Value |
|---|---|---|---|
| Age (years) | |||
| 18–29 | 1 | (Reference) | |
| ≥30 | 1.012 | 0.486, 3.336 | 0.3348 |
| ≥40 | 1.125 | 1.304, 2.555 | 0.0004 |
| ≥50 | 1.309 | 1.107, 1.547 | 0.0016 |
| ≥60 | 1.218 | 1.037, 1.432 | 0.0166 |
| ≥70 | 1.902 | 1.618, 2.236 | <0.0001 |
| Sex | |||
| Female | 1 | (Reference) | |
| Male | 1.439 | 1.163, 1.78 | 0.0008 |
| Comorbidities | |||
| DM | 1.188 | 0.942, 1.5 | 0.1463 |
| HTN | 0.811 | 0.713, 0.922 | 0.0014 |
| Pneumonia | 2.093 | 1.79, 2.447 | <0.0001 |
| COPD | 1.262 | 1.007, 1.581 | 0.0431 |
| Hepatitis B | 0.864 | 0.429, 1.741 | 0.6823 |
| Hepatitis C | 0.746 | 0.488, 1.142 | 0.1772 |
| Implanted pacemaker | 1.453 | 0.36, 5.873 | 0.6001 |
| MI, CVA, TIA, angina, or CAD | 0.891 | 0.745, 1.066 | 0.2072 |
| Heart valve dysfunction | 1.215 | 0.858, 1.721 | 0.2716 |
| Sepsis | 4.079 | 3.418, 4.869 | <0.0001 |
| CKD | 1.117 | 0.658, 1.897 | 0.6818 |
| Heart failure | 2.037 | 1.617, 2.567 | <0.0001 |
| DIC | 7.585 | 3.105, 18.53 | <0.0001 |
| ARDS | 4.04 | 1.494, 10.923 | 0.0059 |
| Aortic aneurysm | 1.059 | 0.394, 2.845 | 0.9093 |
| PAD | 1.107 | 0.777, 1.578 | 0.5733 |
| PUD | 1.063 | 0.923, 1.225 | 0.3953 |
| Dementia | 1.583 | 1.234, 2.029 | 0.0003 |
| Chronic pulmonary disease | 0.97 | 0.774, 1.216 | 0.7916 |
| Connective tissue disease | 1.173 | 0.772, 1.781 | 0.4551 |
| Mild liver disease | 1.211 | 1.043, 1.407 | 0.0121 |
| Hemiplegia | 1.426 | 1.117, 1.821 | 0.0044 |
| Moderate or severe renal disease | 2.092 | 1.235, 3.544 | 0.0061 |
| Any non-HNSCC solid cancer | 2.306 | 1.599, 3.325 | <0.0001 |
| Leukemia | 1.989 | 0.496, 7.981 | 0.332 |
| Lymphoma | 1.455 | 0.952, 2.225 | 0.0832 |
| Moderate or severe liver disease | 1.212 | 1.104, 1.519 | 0.0025 |
| Metastatic non-HNSCC solid Cancer | 2.144 | 1.916, 2.399 | <0.0001 |
| Smoking | 0.846 | 0.618, 1.126 | 0.5753 |
| Previous thoracic surgery | 0.957 | 0.901, 1.976 | 0.8251 |
| Obesity | 1.015 | 0.879, 1.705 | 0.9088 |
| Asthma | 1.203 | 0.798, 1.511 | 0.8603 |
| Bowel obstruction | 1.047 | 0.505, 1.984 | 0.9221 |
| Factor | HR | 95% CI | p Value | Points |
|---|---|---|---|---|
| Age (years) | ||||
| ≥40 | 1.913 | 1.376, 2.659 | 0.0001 | 1 |
| ≥50 | 1.309 | 1.107, 1.547 | 0.0016 | 1 |
| ≥60 | 1.221 | 1.039, 1.435 | 0.0154 | 1 |
| ≥70 | 1.894 | 1.613, 2.225 | <0.0001 | 1 |
| Sex | ||||
| Female | 1 | (Reference) | ||
| Male | 1.425 | 1.153, 1.76 | 0.001 | 1 |
| Comorbidities | ||||
| HTN | 0.803 | 0.708, 1.11 | 0.1006 | 0 |
| Pneumonia | 2.092 | 1.79, 2.446 | <0.0001 | 2 |
| COPD | 1.227 | 1.052, 1.431 | 0.0093 | 1 |
| Sepsis | 4.161 | 3.492, 4.958 | <0.0001 | 4 |
| Heart failure | 2.056 | 1.646, 2.566 | <0.0001 | 2 |
| DIC | 7.683 | 3.152, 18.728 | <0.0001 | 7 |
| ARDS | 3.897 | 1.444, 10.52 | 0.0073 | 3 |
| Dementia | 1.598 | 1.247, 2.048 | 0.0002 | 1 |
| Mild liver disease | 1.251 | 1.087, 1.439 | 0.0018 | 1 |
| Hemiplegia | 1.342 | 1.089, 1.654 | 0.0059 | 1 |
| Moderate or severe renal disease | 2.361 | 1.983, 2.81 | <0.0001 | 2 |
| Any non-HNSCC solid cancer | 2.289 | 1.588, 3.3 | <0.0001 | 2 |
| Moderate or severe liver disease | 1.284 | 1.034, 1.473 | 0.0083 | 1 |
| Metastatic non-HNSCC solid cancer | 2.142 | 1.915, 2.397 | <0.0001 | 2 |
| Score | No of Patient | No of Death | Death Rate |
|---|---|---|---|
| 0 | 107 | 0 | 0.00% |
| 1 | 277 | 0 | 0.00% |
| 2 | 1104 | 5 | 0.45% |
| 3 | 4289 | 16 | 0.37% |
| 4 | 8948 | 67 | 0.75% |
| 5 | 11,317 | 124 | 1.10% |
| 6 | 10,621 | 167 | 1.57% |
| 7 | 8423 | 228 | 2.71% |
| 8 | 4621 | 168 | 3.64% |
| 9 | 2514 | 154 | 6.13% |
| 10 | 1263 | 109 | 8.63% |
| 11 | 681 | 68 | 9.99% |
| 12 | 393 | 68 | 17.30% |
| 13 | 236 | 44 | 18.64% |
| 14 | 149 | 32 | 21.48% |
| 15 | 70 | 15 | 21.43% |
| 16 | 35 | 7 | 20.00% |
| 17 | 26 | 12 | 46.15% |
| 18+ | 6 | 3 | 50.00% |
| Total | 55,080 | 1287 | 2.34% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Chen, T.-M.; Kao, Y.-W.; Lin, K.-C.; Yuan, K.S.-P.; Wu, A.T.H.; Shia, B.-C.; Wu, S.-Y. Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery. Cancers 2018, 10, 392. https://doi.org/10.3390/cancers10100392
Qin L, Chen T-M, Kao Y-W, Lin K-C, Yuan KS-P, Wu ATH, Shia B-C, Wu S-Y. Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery. Cancers. 2018; 10(10):392. https://doi.org/10.3390/cancers10100392
Chicago/Turabian StyleQin, Lei, Tsung-Ming Chen, Yi-Wei Kao, Kuan-Chou Lin, Kevin Sheng-Po Yuan, Alexander T. H. Wu, Ben-Chang Shia, and Szu-Yuan Wu. 2018. "Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery" Cancers 10, no. 10: 392. https://doi.org/10.3390/cancers10100392
APA StyleQin, L., Chen, T.-M., Kao, Y.-W., Lin, K.-C., Yuan, K. S.-P., Wu, A. T. H., Shia, B.-C., & Wu, S.-Y. (2018). Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery. Cancers, 10(10), 392. https://doi.org/10.3390/cancers10100392







