Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial
Abstract
:1. Introduction
2. Results
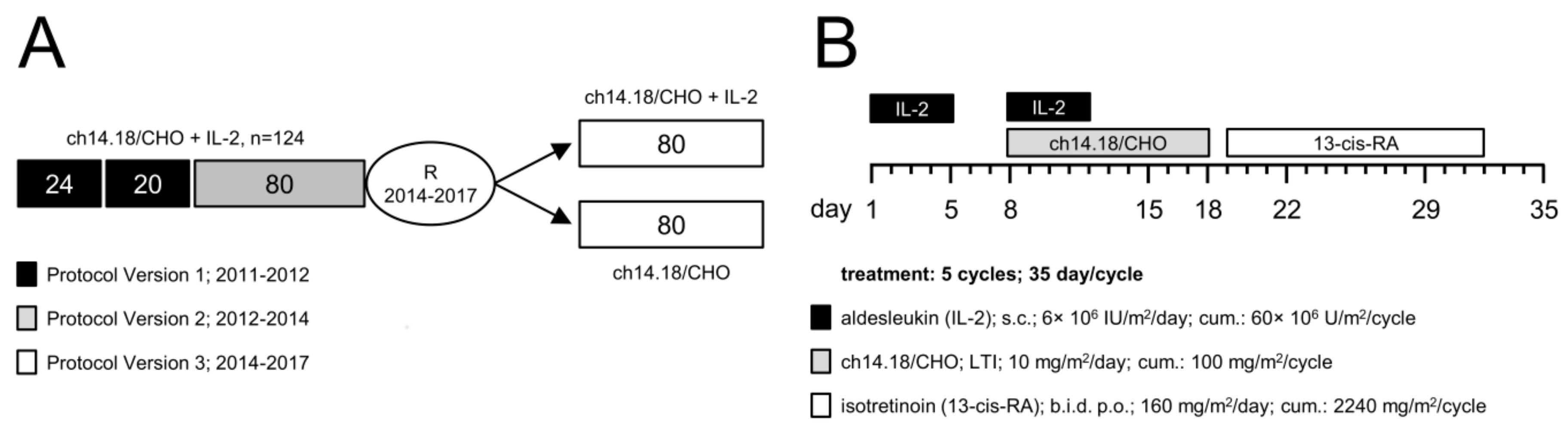
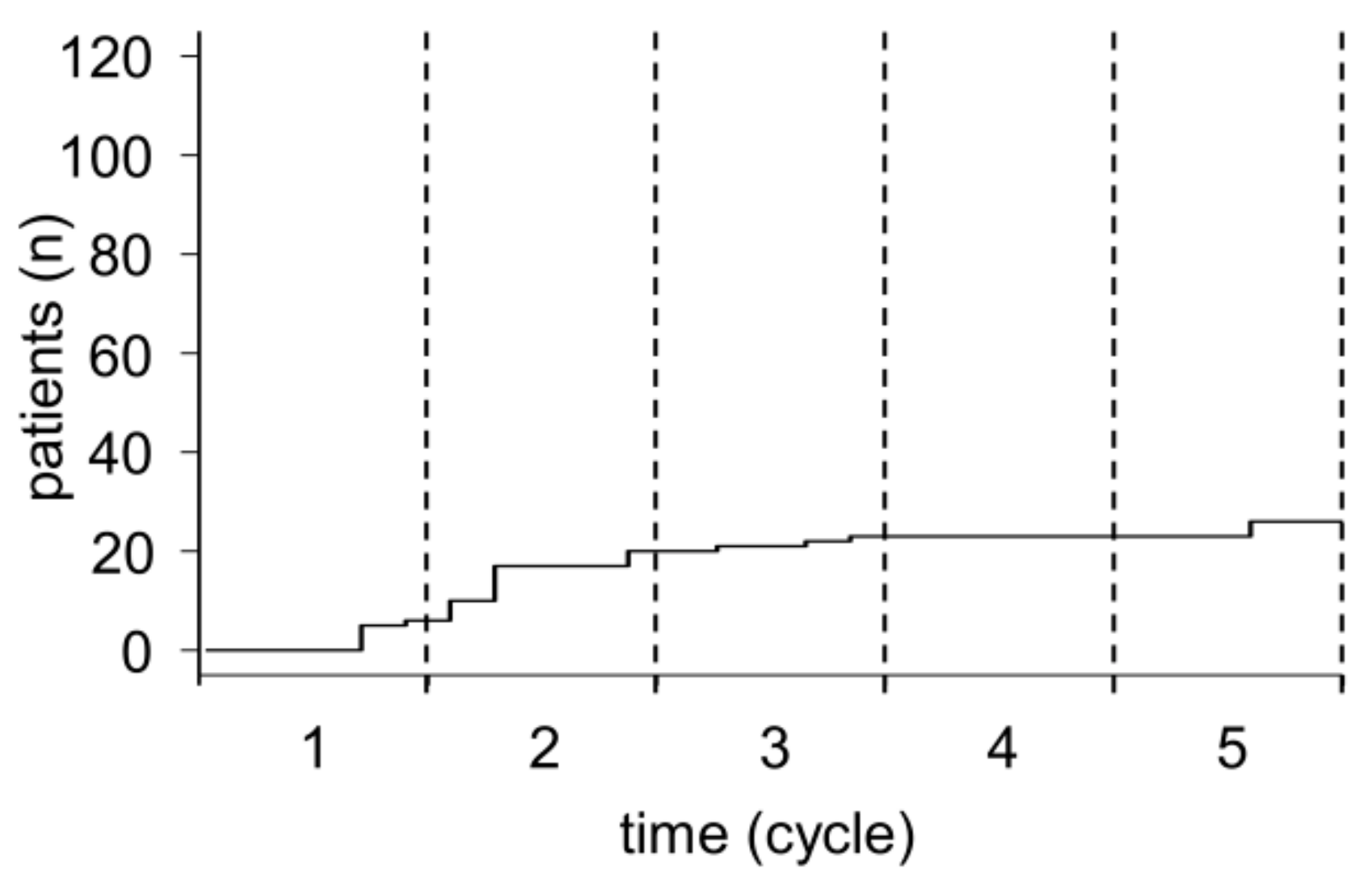
2.1. Patient Selection and Treatment
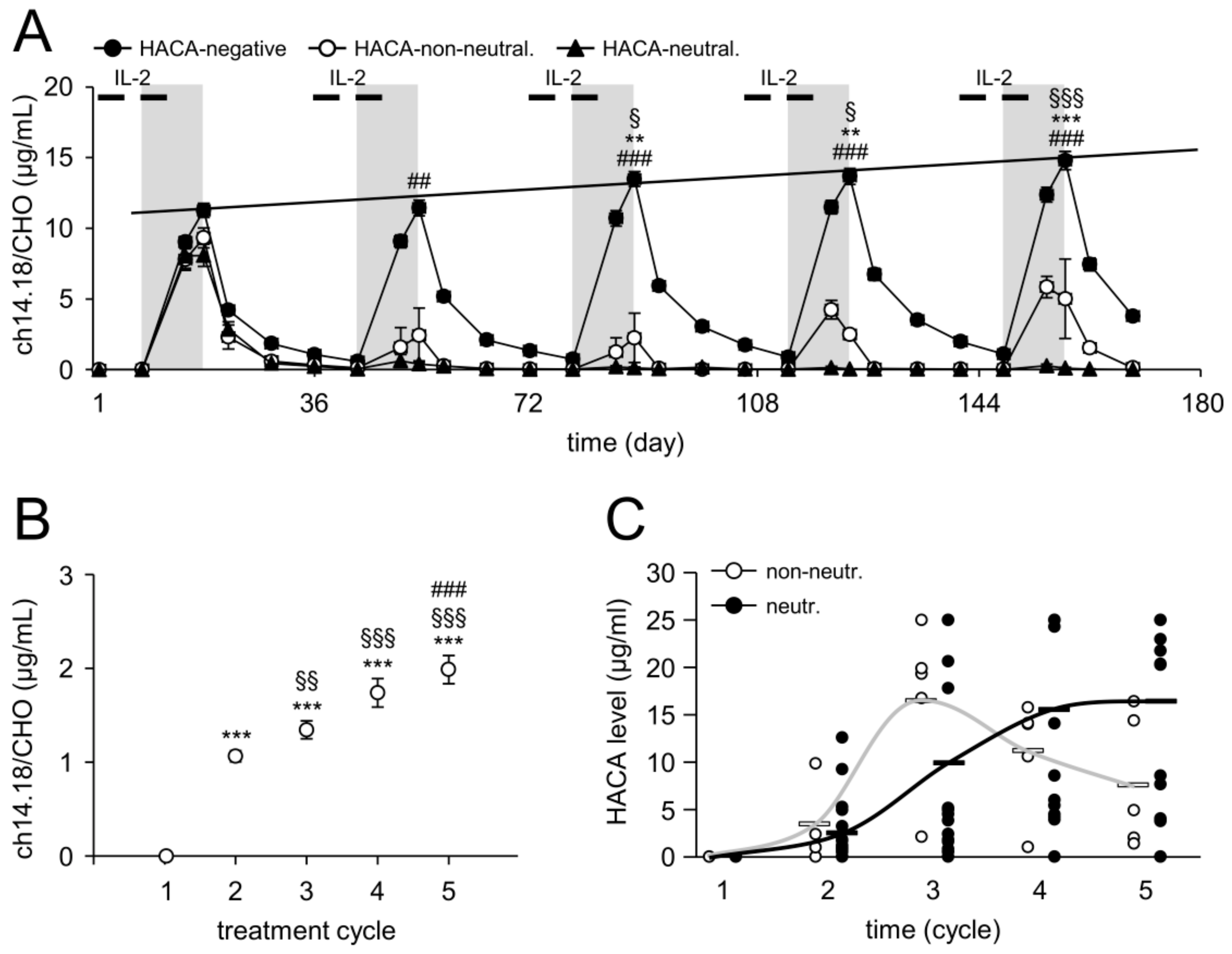
2.2. Time Line of HACA Development in Treated Patients
2.3. Serum Concentration of ch14.18/CHO
2.4. Impact of HACA on ch14.18/CHO Serum Concentration-Time Curves
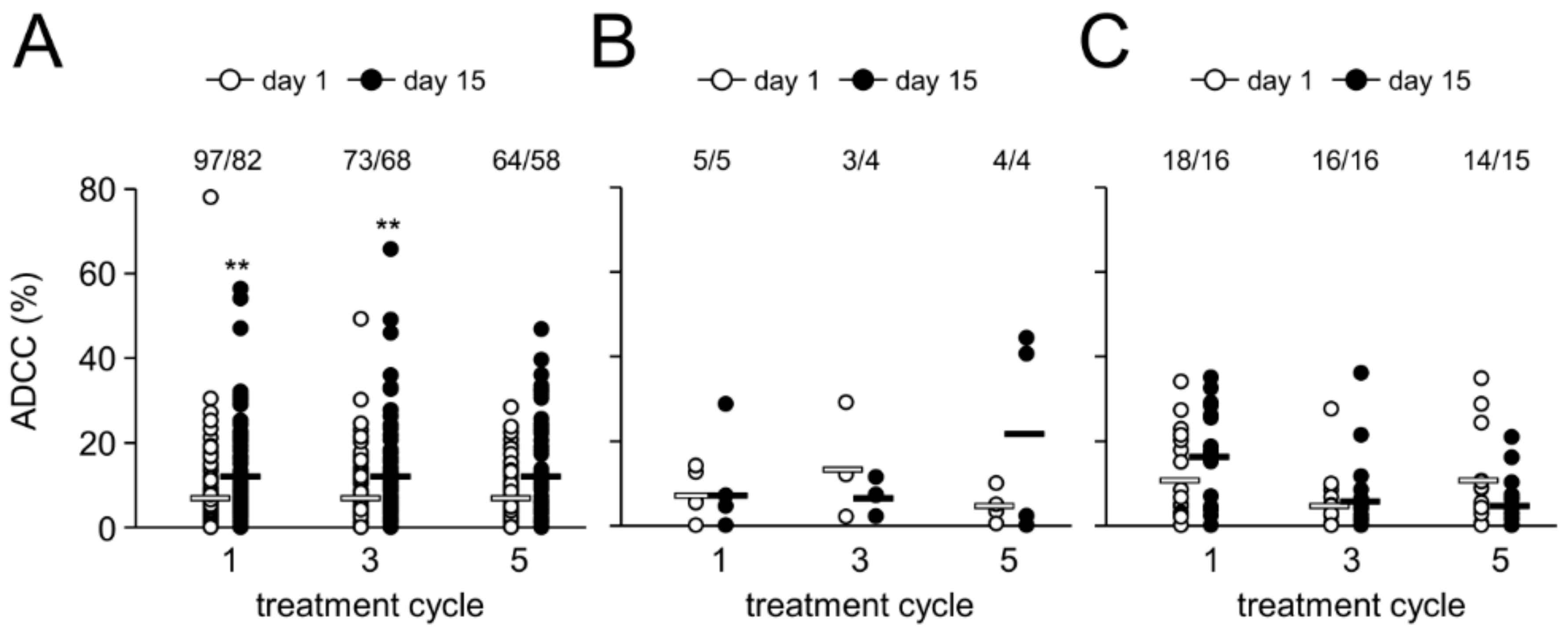
2.5. Antibody-Dependent Cell-Mediated Cytotoxicity
2.6. Effect of HACA on Antibody-Dependent Cell-Mediated Cytotoxicity
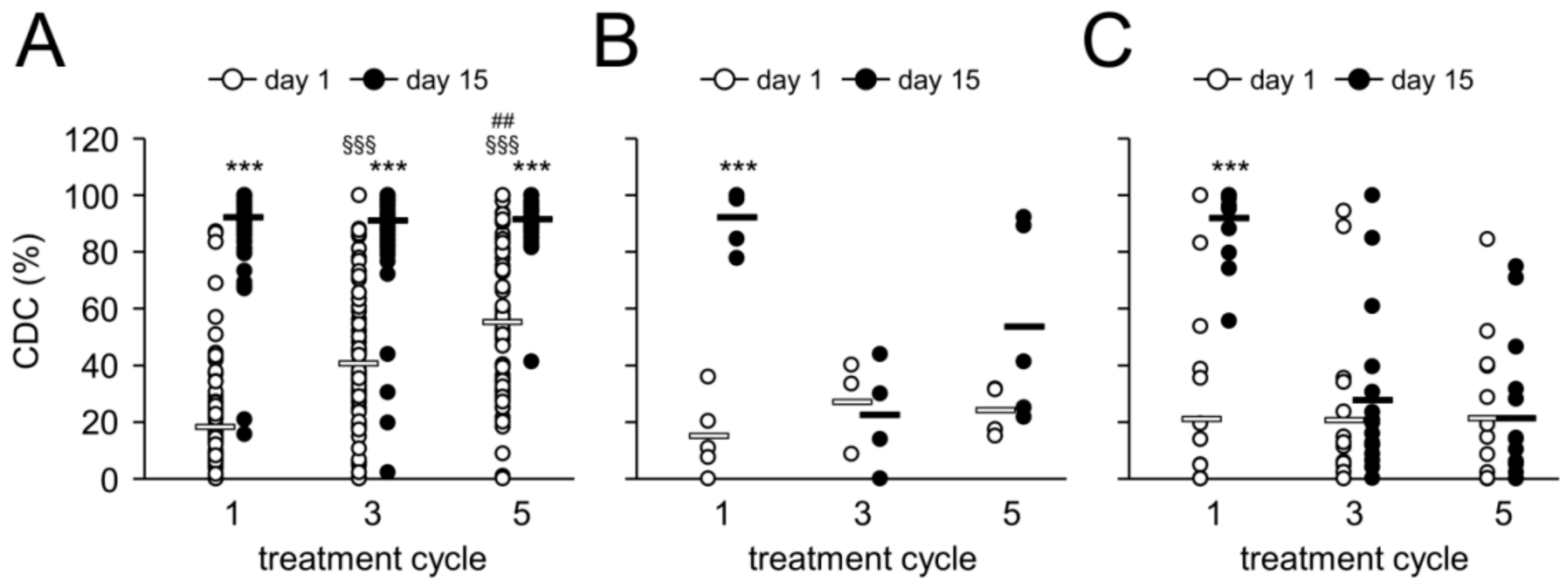
2.7. Complement-Dependent Cytotoxicity
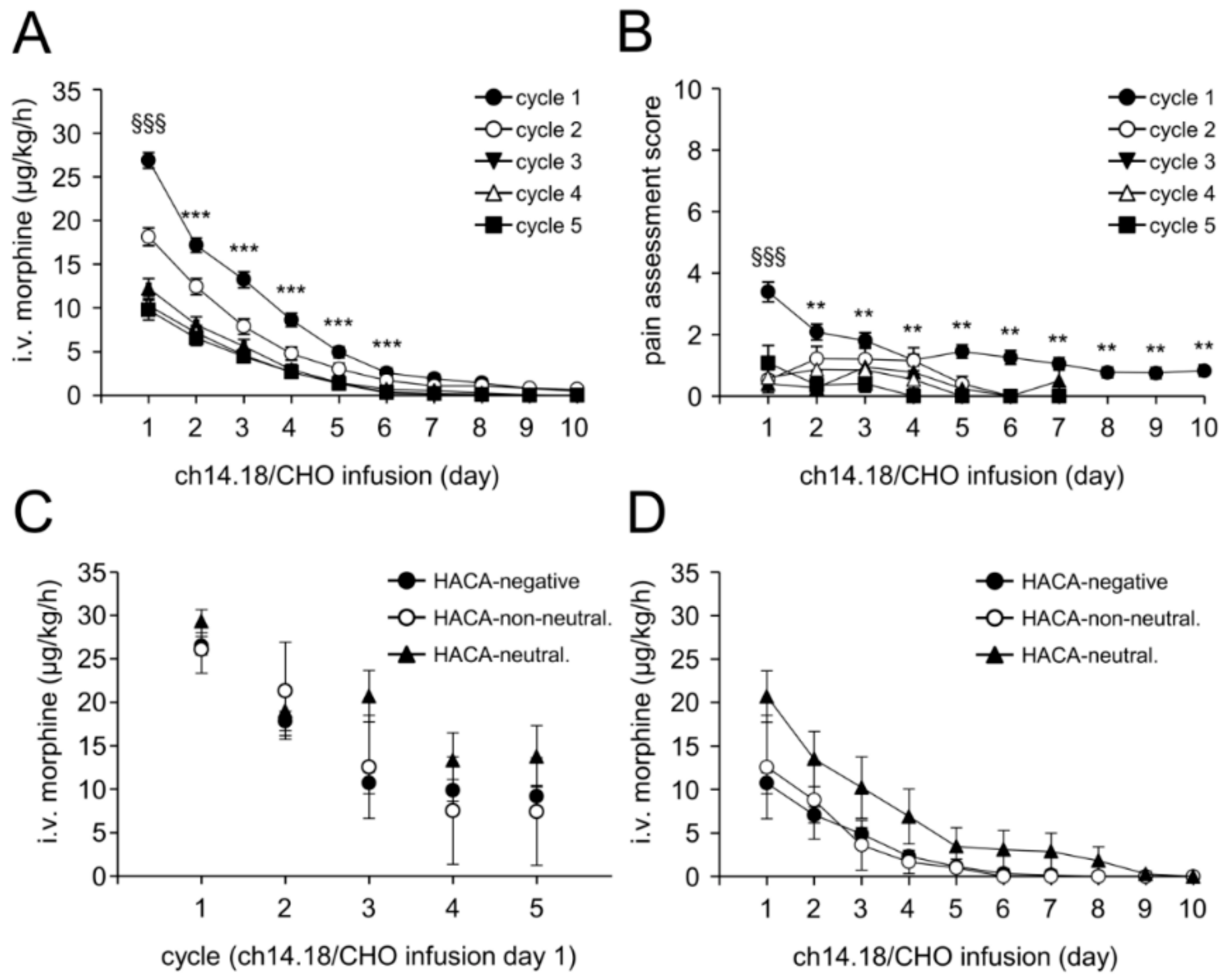
2.8. Treatment Toxicity
2.9. Impact of HACA on Treatment Toxicity
2.10. Evaluation of an Anti-Anti-Id Response
3. Discussion
4. Materials and Methods
4.1. Ethic Statement
4.2. Patients
4.3. Sampling
4.4. Analysis of Treatment Toxicity
4.5. Analysis of ch14.18/CHO Levels in Patient Serum
4.6. Evaluation of Human Anti-Chimeric Ab Response
4.7. Analysis of ADCC and CDC
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perez Horta, Z.; Goldberg, J.L.; Sondel, P.M. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 2016, 8, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Fest, S.; Kunert, R.; Katinger, H.; Pistoia, V.; Michon, J.; Lewis, G.; Ladenstein, R.; Lode, H.N. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol. Immunol. 2005, 42, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Weixler, S.; Baykan, B.; Bleeke, M.; Kunert, R.; Katinger, D.; Pribill, I.; Glander, P.; Bauer, S.; Pistoia, V.; et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: A SIOPEN Phase 1 study. Mabs 2013, 5, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Lazarus, H.; Miraldi, F.D.; Abramowsky, C.R.; Kallick, S.; Saarinen, U.M.; Spitzer, T.; Strandjord, S.E.; Coccia, P.F.; Berger, N.A. Ganglioside GD2 specific monoclonal antibody 3F8: A phase I study in patients with neuroblastoma and malignant melanoma. J. Clin. Oncol. 1987, 5, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Anderson, K.; Lang, P.; Dopfer, R.; Klingebiel, T.; Schrappe, M.; Reuland, P.; Gillies, S.D.; Reisfeld, R.A.; Neithammer, D. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur. J. Cancer 1995, 31A, 261–267. [Google Scholar] [CrossRef]
- Yu, A.L.; Uttenreuther-Fischer, M.M.; Huang, C.S.; Tsui, C.C.; Gillies, S.D.; Reisfeld, R.A.; Kung, F.H. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J. Clin. Oncol. 1998, 16, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Yu, A.L.; Sorkin, L.S. Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain 1997, 69, 145–151. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Otto, M.; Baldwin, W.M., 3rd; Vail, E.; Gillies, S.D.; Handgretinger, R.; Barfield, R.C.; Ming Yu, H.; Yu, A.L. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010, 149, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, D.L.; Goldberg, J.L.; Faughnan, L.G.; Wu, J.; Mao, S.; Furman, W.L.; Santana, V.M.; Navid, F. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr. Blood Cancer 2015, 62, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Ehlert, K.; Endres, S.; Pill, L.; Siebert, N.; Kietz, S.; Brock, P.; Garaventa, A.; Valteau-Couanet, D.; Janzek, E.; et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD2 antibody ch14.18/CHO. Mabs 2018, 10, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Eger, C.; Seidel, D.; Juttner, M.; Zumpe, M.; Wegner, D.; Kietz, S.; Ehlert, K.; Veal, G.J.; Siegmund, W.; et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. Mabs 2016, 8, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Seidel, D.; Eger, C.; Brackrock, D.; Reker, D.; Schmidt, M.; Lode, H.N. Validated detection of anti-GD2 antibody ch14.18/CHO in serum of neuroblastoma patients using anti-idiotype antibody ganglidiomab. J. Immunol. Methods 2013, 398–399, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Seidel, D.; Eger, C.; Juttner, M.; Lode, H.N. Functional bioassays for immune monitoring of high-risk neuroblastoma patients treated with ch14.18/CHO anti-GD2 antibody. PLoS ONE 2014, 9, e107692. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Eger, C.; Seidel, D.; Juttner, M.; Lode, H.N. Validated detection of human anti-chimeric immune responses in serum of neuroblastoma patients treated with ch14.18/CHO. J. Immunol. Methods 2014, 407, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Mellor, J.D.; Brown, M.P.; Irving, H.R.; Zalcberg, J.R.; Dobrovic, A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceylan, K.; Jahns, L.J.; Lode, B.N.; Ehlert, K.; Kietz, S.; Troschke-Meurer, S.; Siebert, N.; Lode, H.N. Inflammatory response and treatment tolerance of long-term infusion of the anti-GD2 antibody ch14.18/CHO in combination with interleukin-2 in patients with high-risk neuroblastoma. Pediatr. Blood Cancer 2018, 65, e26967. [Google Scholar] [CrossRef] [PubMed]
- Lode, H.N.; Schmidt, M.; Seidel, D.; Huebener, N.; Brackrock, D.; Bleeke, M.; Reker, D.; Brandt, S.; Mueller, H.P.; Helm, C.; et al. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD(2)-specific anti-neuroblastoma immune response. Cancer Immunol. Immunother. 2013, 62, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Baader, P.; Dopfer, R.; Klingebiel, T.; Reuland, P.; Treuner, J.; Reisfeld, R.A.; Niethammer, D. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol. Immunother. 1992, 35, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L.; Cunningham, J.E.; Brewer, H.; Mujoo, K.; Zukiwski, A.A.; Podoloff, D.A.; Kasi, L.P.; Bhadkamkar, V.; Fritsche, H.A.; Benjamin, R.S.; et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J. Clin. Oncol. 1994, 12, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Khazaeli, M.B.; Wheeler, R.H.; Dropcho, E.; Liu, T.; Urist, M.; Miller, D.M.; Lawson, S.; Dixon, P.; Russell, C.H.; et al. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992, 52, 4342–4347. [Google Scholar] [PubMed]
- Cheung, N.K.; Cheung, I.Y.; Canete, A.; Yeh, S.J.; Kushner, B.; Bonilla, M.A.; Heller, G.; Larson, S.M. Antibody response to murine anti-GD2 monoclonal antibodies: Correlation with patient survival. Cancer Res. 1994, 54, 2228–2233. [Google Scholar] [PubMed]
- Albert, D.; Dunham, J.; Khan, S.; Stansberry, J.; Kolasinski, S.; Tsai, D.; Pullman-Mooar, S.; Barnack, F.; Striebich, C.; Looney, R.J.; et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann. Rheum Dis 2008, 67, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Hernández, M.P.; López-Morales, C.A.; Ramírez-Ibáñez, N.D.; Piña-Lara, N.; Pérez, N.O.; Molina-Pérez, A.; Revilla-Beltri, J.; Flores-Ortiz, L.F.; Medina-Rivero, E. Assessment of Physicochemical Properties of Rituximab Related to Its Immunomodulatory Activity. J. Immunol. Res. 2015, 2015, 910763. [Google Scholar] [CrossRef] [PubMed]
- Pijpe, J.; van Imhoff, G.W.; Spijkervet, F.K.; Roodenburg, J.L.; Wolbink, G.J.; Mansour, K.; Vissink, A.; Kallenberg, C.G.; Bootsma, H. Rituximab treatment in patients with primary Sjogren’s syndrome: An open-label phase II study. Arthritis Rheum. 2005, 52, 2740–2750. [Google Scholar] [CrossRef]
- Yuki, N.; Yamada, M.; Tagawa, Y.; Takahashi, H.; Handa, S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J. Neurol. Sci. 1997, 149, 127–130. [Google Scholar] [CrossRef]
- Vriesendorp, F.J.; Quadri, S.M.; Flynn, R.E.; Malone, M.R.; Cromeens, D.M.; Stephens, L.C.; Vriesendorp, H.M. Preclinical analysis of radiolabeled anti-GD2 immunoglobulin G. Cancer 1997, 80, 2642–2649. [Google Scholar] [CrossRef]
- Twining, C.M.; Sloane, E.M.; Schoeniger, D.K.; Milligan, E.D.; Martin, D.; Marsh, H.; Maier, S.F.; Watkins, L.R. Activation of the spinal cord complement cascade might contribute to mechanical allodynia induced by three animal models of spinal sensitization. J. Pain 2005, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Sondel, P.M.; Barfield, R.; Shulkin, B.L.; Kaufman, R.A.; Allay, J.A.; Gan, J.; Hutson, P.; Seo, S.; Kim, K.; et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 2014, 32, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.G.; Duffy, S.S.; Moalem-Taylor, G. Immunotherapy targeting cytokines in neuropathic pain. Front. Pharmacol. 2013, 4, 142. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, L.S.; Yu, A.L.; Junger, H.; Doom, C.M. Antibody directed against GD(2) produces mechanical allodynia, but not thermal hyperalgesia when administered systemically or intrathecally despite its dependence on capsaicin sensitive afferents. Brain Res. 2002, 930, 67–74. [Google Scholar] [CrossRef]
- Milligan, E.D.; O’Connor, K.A.; Nguyen, K.T.; Armstrong, C.B.; Twining, C.; Gaykema, R.P.; Holguin, A.; Martin, D.; Maier, S.F.; Watkins, L.R. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001, 21, 2808–2819. [Google Scholar] [CrossRef] [PubMed]
- Schoeniger-Skinner, D.K.; Ledeboer, A.; Frank, M.G.; Milligan, E.D.; Poole, S.; Martin, D.; Maier, S.F.; Watkins, L.R. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav. Immun. 2007, 21, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.F.; Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 2011, 12, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Buttner, W.; Breitkopf, L.; Miele, B.; Finke, W. Initial results of the reliability and validity of a German-language scale for the quantitative measurement of postoperative pain in young children. Anaesthesist 1990, 39, 593–602. [Google Scholar] [PubMed]
- Ramsay, M.A.; Savege, T.M.; Simpson, B.R.; Goodwin, R. Controlled sedation with alphaxalone-alphadolone. Br. Med. J. 1974, 2, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.A.; Doyle, E. Validation of three paediatric pain scores for use by parents. Anaesthesia 1996, 51, 1005–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernikow, B.; Hechler, T. Pain therapy in children and adolescents. Dtsch. Arztebl. Int. 2008, 105, 511–522. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebert, N.; Troschke-Meurer, S.; Marx, M.; Zumpe, M.; Ehlert, K.; Gray, J.; Garaventa, A.; Manzitti, C.; Ash, S.; Klingebiel, T.; et al. Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial. Cancers 2018, 10, 387. https://doi.org/10.3390/cancers10100387
Siebert N, Troschke-Meurer S, Marx M, Zumpe M, Ehlert K, Gray J, Garaventa A, Manzitti C, Ash S, Klingebiel T, et al. Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial. Cancers. 2018; 10(10):387. https://doi.org/10.3390/cancers10100387
Chicago/Turabian StyleSiebert, Nikolai, Sascha Troschke-Meurer, Madlen Marx, Maxi Zumpe, Karoline Ehlert, Juliet Gray, Alberto Garaventa, Carla Manzitti, Shifra Ash, Thomas Klingebiel, and et al. 2018. "Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial" Cancers 10, no. 10: 387. https://doi.org/10.3390/cancers10100387
APA StyleSiebert, N., Troschke-Meurer, S., Marx, M., Zumpe, M., Ehlert, K., Gray, J., Garaventa, A., Manzitti, C., Ash, S., Klingebiel, T., Beck, J., Castel, V., Valteau-Couanet, D., Loibner, H., Ladenstein, R., & Lode, H. N. (2018). Impact of HACA on Immunomodulation and Treatment Toxicity Following ch14.18/CHO Long-Term Infusion with Interleukin-2: Results from a SIOPEN Phase 2 Trial. Cancers, 10(10), 387. https://doi.org/10.3390/cancers10100387





