Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety
Abstract
:1. Introduction
2. Results
2.1. Survival Analysis
2.2. Acute Toxicity
2.3. Chronic Toxicity
3. Discussion
4. Materials and Methods
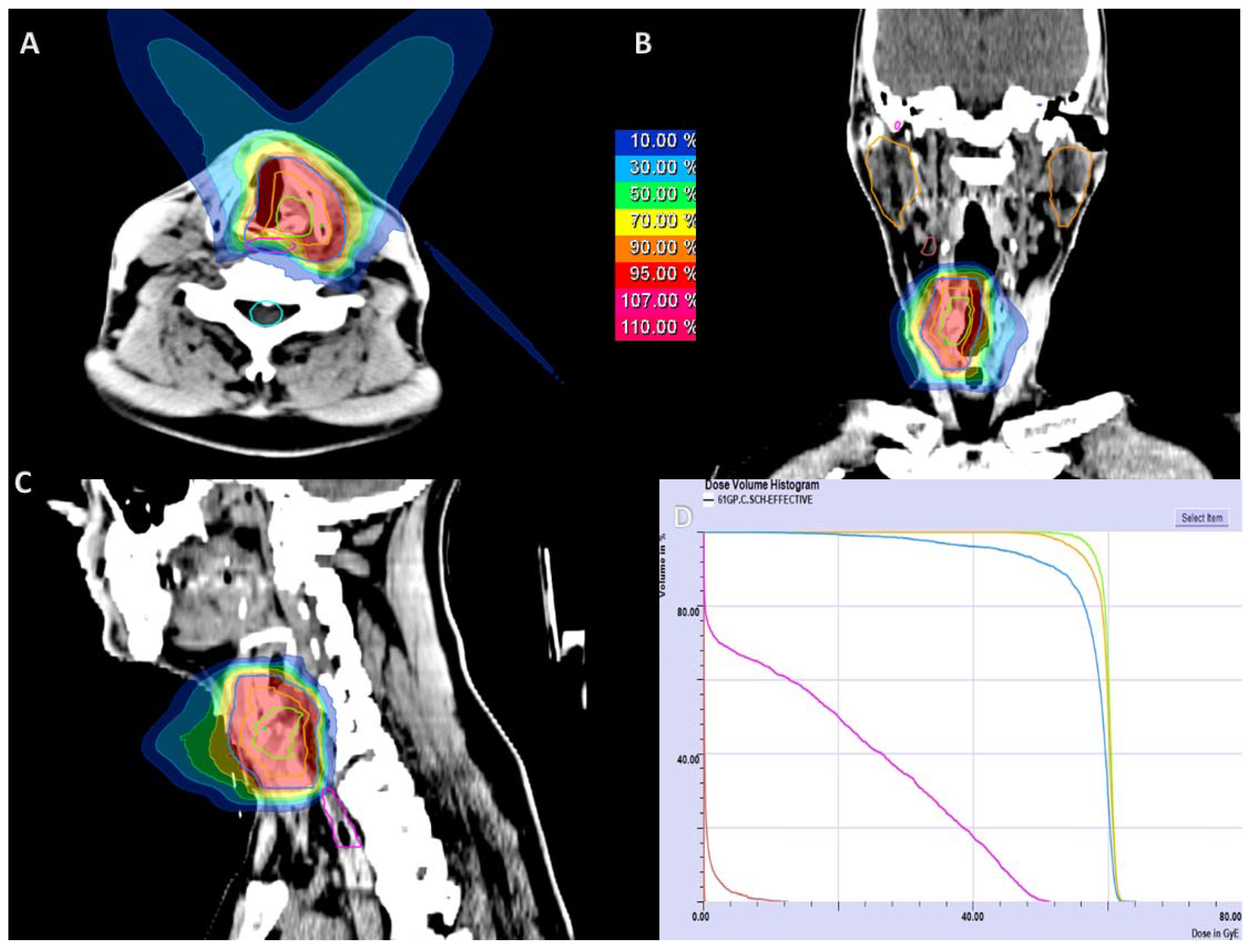
4.1. Treatment Planning
4.2. Follow-up
4.3. Analysis
4.4. Patient, Disease, and Treatment Characteristics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Budach, W.; Hehr, T.; Budach, V.; Belka, C.; Dietz, K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maitre, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Pignon, J.P.; Le Maitre, A.; Maillard, E.; Bourhis, J.; Group, M.-N.C. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.L.; Campbell, B.H.; Indresano, A.T.; Eaton, D.J.; Marbella, A.M.; Myers, K.B.; Layde, P.M. Dental status and quality of life in long-term head and neck cancer survivors. Laryngoscope 2005, 115, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hammerlid, E.; Silander, E.; Hornestam, L.; Sullivan, M. Health-related quality of life three years after diagnosis of head and neck cancer—A longitudinal study. Head Neck 2001, 23, 113–125. [Google Scholar] [CrossRef]
- Jensen, A.B.; Hansen, O.; Jorgensen, K.; Bastholt, L. Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol. 1994, 33, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Doornaert, P.; Verdonck-de Leeuw, I.M.; Leemans, C.R.; Aaronson, N.K.; Slotman, B.J. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J. Clin. Oncol. 2008, 26, 3770–3776. [Google Scholar] [CrossRef] [PubMed]
- List, M.A.; Bilir, S.P. Functional outcomes in head and neck cancer. Semin. Radiat. Oncol. 2004, 14, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Christianen, M.E.; Langendijk, J.A.; Westerlaan, H.E.; Van de Water, T.A.; Bijl, H.P. Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother. Oncol. 2011, 101, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, B.L.; Pijls-Johannesma, M.; Joore, M.A.; Van den Ende, P.; Langendijk, J.A.; Lambin, P.; Kessels, A.G.; Grutters, J.P. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat. Rev. 2011, 37, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nikoghosyan, A.V.; Poulakis, M.; Hoss, A.; Haberer, T.; Jakel, O.; Munter, M.W.; Schulz-Ertner, D.; Huber, P.E.; Debus, J. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer 2015, 121, 3001–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, A.D.; Nikoghosyan, A.V.; Lossner, K.; Haberer, T.; Jakel, O.; Munter, M.W.; Debus, J. COSMIC: A Regimen of Intensity Modulated Radiation Therapy Plus Dose-Escalated, Raster-Scanned Carbon Ion Boost for Malignant Salivary Gland Tumors: Results of the Prospective Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Hasegawa, A.; Jingu, K.; Takagi, R.; Bessyo, H.; Morikawa, T.; Tonoki, M.; Tsuji, H.; Kamada, T.; Tsujii, H.; et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. 2012, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Demizu, Y.; Hashimoto, N.; Mima, M.; Terashima, K.; Fujii, O.; Otsuki, N.; Murakami, M.; Fuwa, N.; Nibu, K. Particle radiotherapy using protons or carbon ions for unresectable locally advanced head and neck cancers with skull base invasion. Jpn. J. Clin. Oncol. 2014, 44, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nikoghosyan, A.V.; Ecker, S.; Ellerbrock, M.; Debus, J.; Munter, M.W. Carbon ion therapy for advanced sinonasal malignancies: feasibility and acute toxicity. Radiat. Oncol. 2011, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Ertner, D.; Karger, C.P.; Feuerhake, A.; Nikoghosyan, A.; Combs, S.E.; Jakel, O.; Edler, L.; Scholz, M.; Debus, J. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Hof, H.; Didinger, B.; Combs, S.E.; Jakel, O.; Karger, C.P.; Edler, L.; Debus, J. Carbon ion radiotherapy of skull base chondrosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Poulakis, M.; Nikoghosyan, A.V.; Welzel, T.; Uhl, M.; Federspil, P.A.; Freier, K.; Krauss, J.; Hoss, A.; Haberer, T.; et al. High-LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years’ experience with raster-scanned carbon ion therapy. Radiother. Oncol. 2016, 118, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Poulakis, M.; Nikoghosyan, A.V.; Chaudhri, N.; Uhl, M.; Munter, M.W.; Herfarth, K.K.; Debus, J. Re-irradiation of adenoid cystic carcinoma: Analysis and evaluation of outcome in 52 consecutive patients treated with raster-scanned carbon ion therapy. Radiother. Oncol. 2015, 114, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Welzel, T.; Oelmann, J.; Habl, G.; Hauswald, H.; Jensen, A.; Ellerbrock, M.; Debus, J.; Herfarth, K. Active raster scanning with carbon ions: Reirradiation in patients with recurrent skull base chordomas and chondrosarcomas. Strahlenther. Onkol. 2014, 190, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Nemoto, K.; et al. Multicenter Study of Carbon-Ion Radiation Therapy for Mucosal Melanoma of the Head and Neck: Subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, J.I.; Koto, M.; Demizu, Y.; Suefuji, H.; Ohno, T.; Tsuji, H.; Okimoto, T.; Shioyama, Y.; Nemoto, K.; Nakano, T.; et al. A Multicenter Study of Carbon-Ion Radiation Therapy for Head and Neck Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Saitoh, J.I.; Musha, A.; Abe, T.; Kobayashi, D.; Takahashi, T.; Tamaki, T.; Kawamura, H.; Takayasu, Y.; Shino, M.; et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. 2017, 108, 2039–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, N.S.; Demizu, Y.; Koto, M.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Ohno, T.; Shioyama, Y.; Okimoto, T.; Daimon, T.; et al. Multicenter Study of Carbon-Ion Radiation Therapy for Adenoid Cystic Carcinoma of the Head and Neck: Subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Mattke, M.; Welzel, T.; Oelmann, J.; Habl, G.; Jensen, A.D.; Ellerbrock, M.; Haberer, T.; Herfarth, K.K.; Debus, J. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: First report of long-term results. Cancer 2014, 120, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munter, M.W.; Schulz-Ertner, D.; Hof, H.; Nikoghosyan, A.; Jensen, A.; Nill, S.; Huber, P.; Debus, J. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the treatment of incompletely and completely resected adenoid cystic carcinomas of the head and neck: Initial clinical results and toxicity of treatment. Radiat. Oncol. 2006, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Christianen, M.E.; Schilstra, C.; Beetz, I.; Muijs, C.T.; Chouvalova, O.; Burlage, F.R.; Doornaert, P.; Koken, P.W.; Leemans, C.R.; Rinkel, R.N.; et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: Results of a prospective observational study. Radiother. Oncol. 2012, 105, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, F.Y.; Kim, H.M.; Lyden, T.H.; Haxer, M.J.; Worden, F.P.; Feng, M.; Moyer, J.S.; Prince, M.E.; Carey, T.E.; Wolf, G.T.; et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. J. Clin. Oncol. 2010, 28, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Levendag, P.C.; Teguh, D.N.; Voet, P.; Van der Est, H.; Noever, I.; De Kruijf, W.J.; Kolkman-Deurloo, I.K.; Prevost, J.B.; Poll, J.; Schmitz, P.I.; et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiother. Oncol. 2007, 85, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Schwartz, M.; Rasch, C.; Vineberg, K.; Damen, E.; Van As, C.J.; Marsh, R.; Pameijer, F.A.; Balm, A.J. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nikoghosyan, A.V.; Ecker, S.; Ellerbrock, M.; Debus, J.; Herfarth, K.K.; Munter, M.W. Raster-scanned carbon ion therapy for malignant salivary gland tumors: Acute toxicity and initial treatment response. Radiat. Oncol. 2011, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Bhide, S.A.; Newbold, K.L.; Harrington, K.J.; Nutting, C.M. Clinical evaluation of intensity-modulated radiotherapy for head and neck cancers. Br. J. Radiol. 2012, 85, 487–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thariat, J.; Bolle, S.; Demizu, Y.; Marcy, P.Y.; Hu, Y.; Santini, J.; Bourhis, J.; Pommier, P. New techniques in radiation therapy for head and neck cancer: IMRT, CyberKnife, protons, and carbon ions. Improved effectiveness and safety? Impact on survival? Anticancer Drugs 2011, 22, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Spiotto, M.T.; Weichselbaum, R.R. Comparison of 3D confromal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PLoS ONE 2014, 9, e94456. [Google Scholar] [CrossRef] [PubMed]
- Pauloski, B.R.; Rademaker, A.W.; Logemann, J.A.; Discekici-Harris, M.; Mittal, B.B. Comparison of swallowing function after intensity-modulated radiation therapy and conventional radiotherapy for head and neck cancer. Head Neck 2015, 37, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Batth, S.S.; Caudell, J.J.; Chen, A.M. Practical considerations in reducing swallowing dysfunction following concurrent chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer. Head Neck 2014, 36, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Mehilal, R.; Van Rooij, P.H.; Tans, L.; Sewnaik, A.; Levendag, P.C. Toxicity, quality of life, and functional outcomes of 176 hypopharyngeal cancer patients treated by (chemo)radiation: the impact of treatment modality and radiation technique. Laryngoscope 2012, 122, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.C.; Adelstein, D.J.; Bhateja, P.; Nwizu, T.I.; Scharpf, J.; Houston, N.; Lamarre, E.D.; Lorenz, R.; Burkey, B.B.; Greskovich, J.F.; et al. Severe late dysphagia and cause of death after concurrent chemoradiation for larynx cancer in patients eligible for RTOG 91-11. Oral Oncol. 2016, 57, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Caudell, J.J.; Carroll, W.R.; Spencer, S.A.; Bonner, J.A. Examination of laryngoesophageal dysfunction-free survival as an endpoint in nonsurgical treatment of squamous cell carcinomas of the larynx and hypopharynx. Cancer 2011, 117, 4447–4451. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Hsu, S.; Meshman, J.; Chin, R.; Beron, P.; Abemayor, E.; St John, M. Effect of daily fraction size on laryngoesophageal dysfunction after chemoradiation for squamous cell carcinomas of the larynx and hypopharynx. Head Neck 2017, 39, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Thames, H.D., Jr.; Withers, H.R.; Peters, L.J.; Fletcher, G.H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 219–226. [Google Scholar] [CrossRef]
- Thames, H.D.; Bentzen, S.M.; Turesson, I.; Overgaard, M.; Van den Bogaert, W. Fractionation parameters for human tissues and tumors. Int. J. Radiat. Biol. 1989, 56, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Acute Toxicity, No. (%) | Chronic Toxicity, No. (%) | ||||
|---|---|---|---|---|---|---|
| (n = 15) | C12 Alone (n = 7) | IMRT + C12 (n = 8) | Total (n = 15) | C12 Alone (n = 7) | IMRT + C12 (n = 8) | Total (n = 15) |
| overall toxicity | ||||||
| grade 1 | 3 (43) | 2 (25) | 5 (33) | 2 (29) | 3 (38) | 5 (33) |
| grade 2 | 2 (29) | 4 (50) | 6 (40) | 2 (29) | 1 (13) | 3 (20) |
| grade 3 | 2 (29) | 2 (25) | 4 (27) | 1 (14) | 0 | 1 (7) |
| grade 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| dysphagia | ||||||
| grade 1 | 3 (43) | 3 (38) | 6 (40) | 3 (43) | 0 | n = 3 (20) |
| grade 2 | 1 (14) | 2 (25) | 3 (20) | 1 (14) | 0 | n = 1 (7) |
| grade 3 | 1 (14) | 1 (13) | 2 (13) | 0 | 0 | 0 |
| odynophagia | ||||||
| grade 1 | 4 (57) | 3 (38) | 7 (53) | 0 | 0 | 0 |
| grade 2 | 1 (14) | 1 (13) | 2 (13) | 0 | 0 | 0 |
| grade 3 | 2 (29) | 1 (13) | 3 (20) | 0 | 0 | 0 |
| mucositis | ||||||
| grade 1 | 2 (29) | 0 | 2 (13) | 0 | 0 | 0 |
| grade 2 | 1 (14) | 3 (38) | 2 (13) | 0 | 0 | 0 |
| dermatitis | ||||||
| grade 1 | 3 (43) | 3 38 () | 6 (40) | 0 | 0 | 0 |
| grade 2 | 0 | 3 (38) | 3 (20) | 0 | 0 | 0 |
| xerostomia | ||||||
| grade 1 | 3 (43) | 4 (50) | 7 (53) | 5 (71) | 3 (38) | 8 (53) |
| grade 2 | 1 (14) | 0 | 1 (7) | 0 | 3 (38) | 3 (20) |
| hoarseness | ||||||
| grade 1 | 2 (29) | 3 (38) | 5 (33) | 2 (29) | 3 (38) | 5 (33) |
| grade 2 | 2 (29) | 1 (13) | 3 (20) | 2 (29) | 1 (13) | 3 (20) |
| grade 3 | 0 | 0 | 0 | 1 (14) | 0 | 1 (7) |
| fatigue | ||||||
| grade 1 | 0 | 2 (25) | 2 (13) | 2 (29) | 1 (13) | 3 (20) |
| grade 2 | 4 (57) | 0 | 4 (27) | 1 (14) | 0 | 1 (7) |
| grade 3 | 0 | 1 (13) | 1 (7) | 0 | 0 | 0 |
| dysgeusia | ||||||
| grade 1 | 0 | 2 (25) | 2 (13) | 1 (14) | 1 (13) | 2 (13) |
| grade 2 | 0 | 2 (25) | 2 (13) | 0 | 0 | 0 |
| alopecia | ||||||
| grade 1 | 3 (43) | 2 (25) | 5 (33) | 0 | 0 | 0 |
| dry cough | ||||||
| grade 1 | 1 (14) | 1 (13) | 2 (13) | 0 | 0 | 0 |
| Characteristic | C12 Alone, n = 7 No. (%) | C12 + IMRT, n = 8 No. (%) | Total, n = 15 No. (%) |
|---|---|---|---|
| median age | 61 years (55–73 years) | 58 years (21–68 years) | 61 years (21–73 years) |
| median follow-up | 22 months (9–48 months) | 24 months (8–61 months) | 24 months (8–61 months) |
| gender | |||
| male | 3 (43) | 4 (50) | 7 (47) |
| female | 4 (57) | 4 (50) | 8 (53) |
| WHO performance status | |||
| 0 | 4 (57) | 4 50 () | 8 (53) |
| 1 | 3 (43) | 3 (38) | 6 (40) |
| 2 | 0 | 1 (12) | 1 (7) |
| tumor type | |||
| ACC | 0 | 8 (100) | 8 (53) |
| CS | 7 (100) | 0 | 7 (47) |
| tumor site | |||
| glottic larynx | 2 (29) | 4 (50) | 7 (47) |
| subglottic larynx | 1 (14) | 0 | 1 (7) |
| supraglottic larynx | 4 (57) | 4 (50) | 7 (47) |
| initial T stage | |||
| T1 | 2 (29) | 1 (12) | 3 (20) |
| T2 | 1 (14) | 1 (12) | 2 (13) |
| T3 | 2 (29) | 0 | 2 (13) |
| T4a | 1 (14) | 5 (63) | 6 (40) |
| T4b | 1 (14) | 1 (12) | 2 (13) |
| initial N stage | |||
| N0 | 7 (100) | 8 (100) | 15 (100) |
| N+ | 0 | 0 | 0 |
| initial M stage | |||
| M1 | 7 (100) | 8 (100) | 15 (100) |
| M0 | 0 | 0 | 0 |
| treatment | |||
| RT only | 3 (43) | 6 (75) | 9 (60) |
| postop. RT | 4 (57) | 2 (25) | 6 (40) |
| total laryngectomy | 0 | 1 (12) | 1 (7) |
| laser surgical resection | 4 (57) | 1 (12) | 5 (33) |
| concomitant cetuximab | 0 | 1 (12) | 1 (7) |
| median EQD2 in Gy | 75 Gy (75–75 Gy) | 76.25 Gy (72.5–80 Gy) | 75 Gy (72.5–80 Gy) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbaba, S.; Lang, K.; Held, T.; Bulut, O.C.; Mattke, M.; Uhl, M.; Jensen, A.; Plinkert, P.; Rieken, S.; Herfarth, K.; et al. Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety. Cancers 2018, 10, 388. https://doi.org/10.3390/cancers10100388
Akbaba S, Lang K, Held T, Bulut OC, Mattke M, Uhl M, Jensen A, Plinkert P, Rieken S, Herfarth K, et al. Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety. Cancers. 2018; 10(10):388. https://doi.org/10.3390/cancers10100388
Chicago/Turabian StyleAkbaba, Sati, Kristin Lang, Thomas Held, Olcay Cem Bulut, Matthias Mattke, Matthias Uhl, Alexandra Jensen, Peter Plinkert, Stefan Rieken, Klaus Herfarth, and et al. 2018. "Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety" Cancers 10, no. 10: 388. https://doi.org/10.3390/cancers10100388
APA StyleAkbaba, S., Lang, K., Held, T., Bulut, O. C., Mattke, M., Uhl, M., Jensen, A., Plinkert, P., Rieken, S., Herfarth, K., Debus, J., & Adeberg, S. (2018). Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety. Cancers, 10(10), 388. https://doi.org/10.3390/cancers10100388






