Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women
Abstract
:1. Introduction
2. Results
2.1. Clinical and Pathological Data
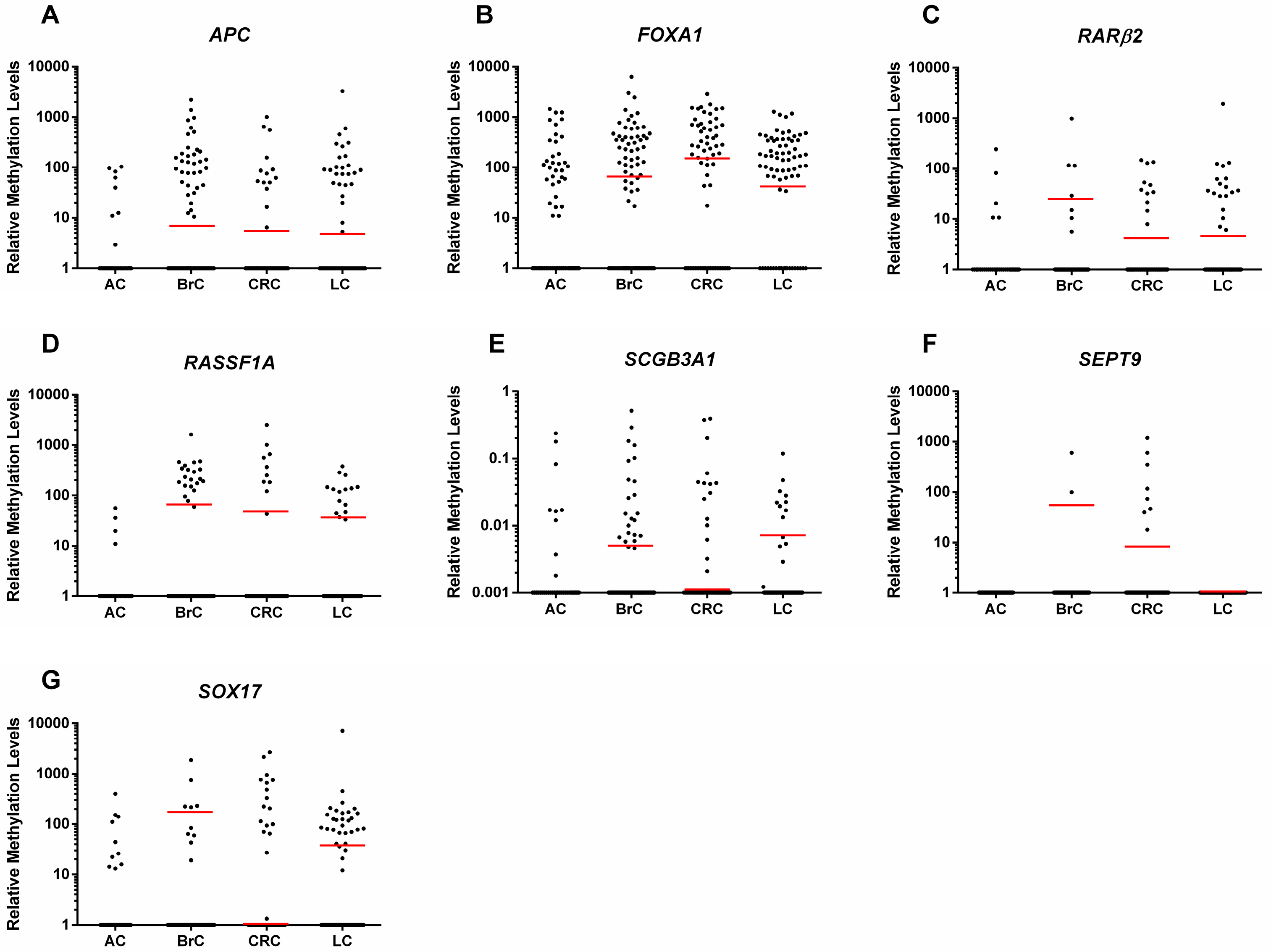
2.2. Gene Promoter Methylation Levels in ccfDNA
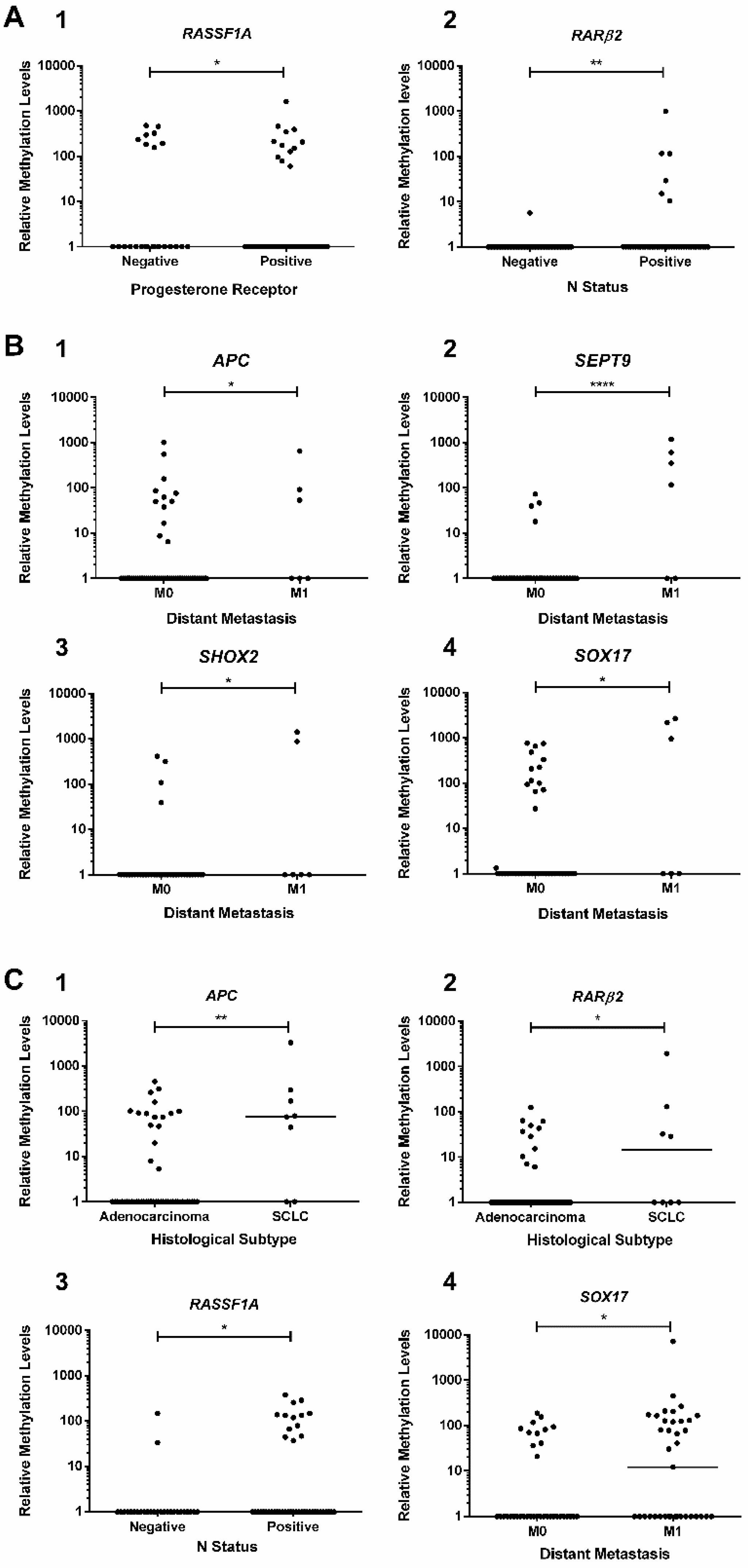
2.3. Association Between Promoters’ Methylation Levels and Clinicopathological Features
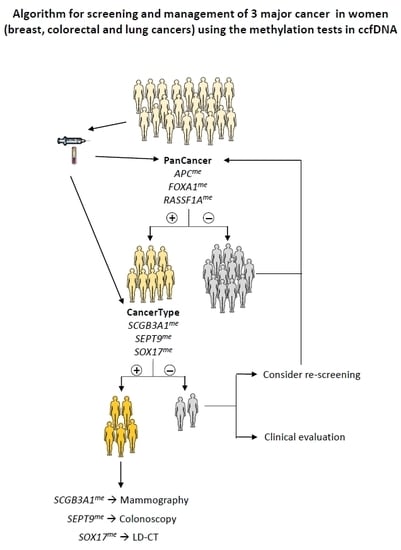
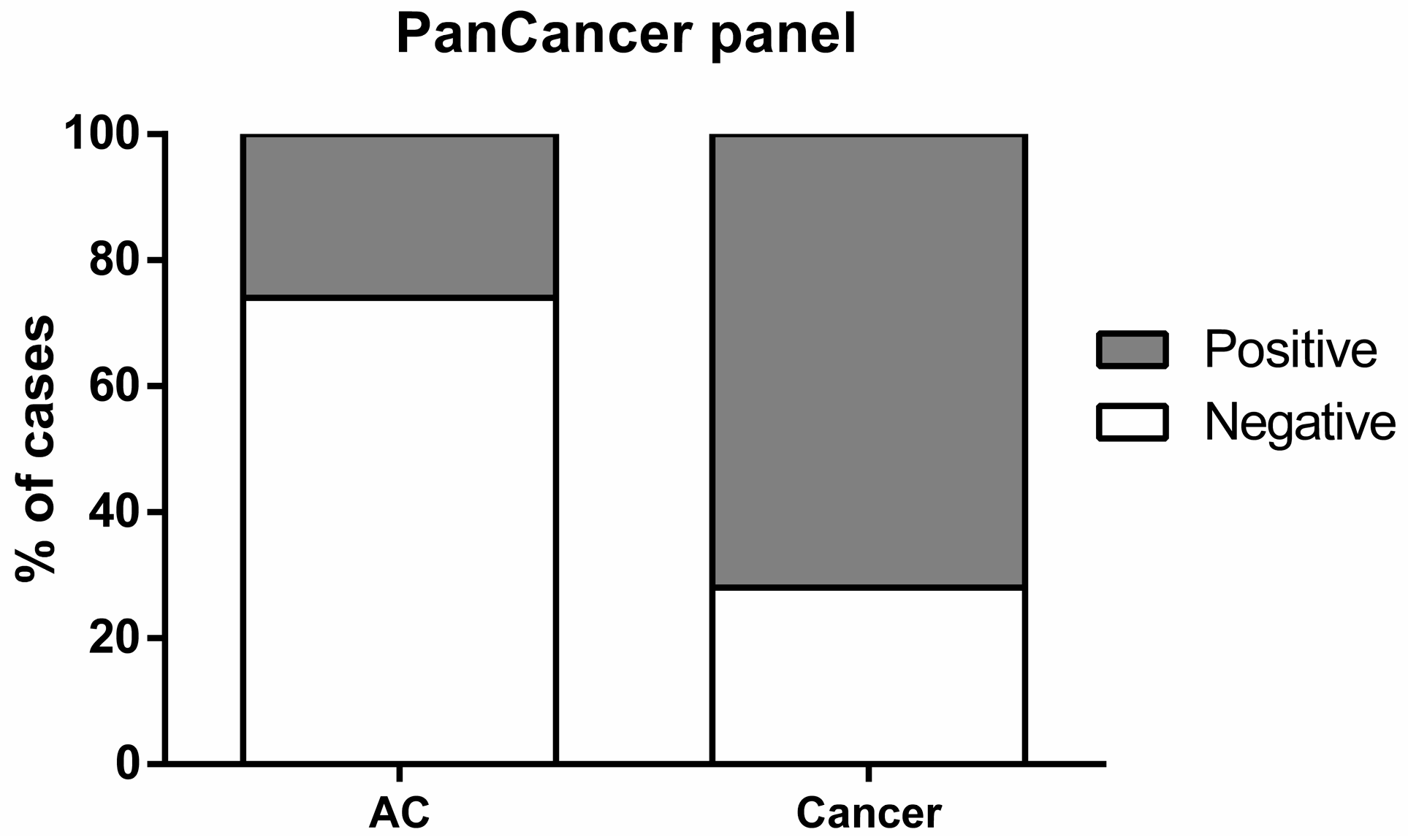
2.4. Biomarker Performance in ccfDNA
3. Discussion
4. Materials and Methods
4.1. Patients and Samples Collection
4.2. Ccf-DNA Extraction, Sodium-Bisulfite Modification and Whole Genome Amplification (WGA)
4.3. Multiplex qMSP
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A.; D’Orsi, C.J. How should screening breast US be audited? The BI-RADS perspective. Radiology 2014, 272, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Mravunac, M.; Hendriks, J.H.; Bekker, B.V. So-called interval cancers of the breast. Pathologic and radiologic analysis of sixty-four cases. Cancer 1982, 49, 2527–2533. [Google Scholar] [CrossRef]
- Tabar, L.; Vitak, B.; Chen, H.H.; Yen, M.F.; Duffy, S.W.; Smith, R.A. Beyond randomized controlled trials: Organized mammographic screening substantially reduces breast carcinoma mortality. Cancer 2001, 91, 1724–1731. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Pawa, N.; Arulampalam, T.; Norton, J.D. Screening for colorectal cancer: Established and emerging modalities. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumb, A.A.; Halligan, S. Colorectal cancer screening. Semin. Roentgenol. 2015, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Prosch, H.; Schaefer-Prokop, C. Screening for lung cancer. Curr. Opin. Oncol. 2014, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Cavuto, S.; Lutman, F.R.; Passera, E.; Chiarenza, M.; Chiesa, G.; Brambilla, G.; Angeli, E.; Aranzulla, G.; Chiti, A.; et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am. J. Respir. Crit. Care Med. 2015, 191, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, V.V.; Grady, W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Pinheiro, P.; Montezuma, D.; Henrique, R.; Jeronimo, C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics 2015, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J.; Sun, Y. Circulating tumor DNA as biomarkers for cancer detection. Genom. Proteom. Bioinf. 2017, 15, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.O.; Feng, Q.; Toure, P.; Dem, A.; Critchlow, C.W.; Hawes, S.E.; Wood, T.; Jeronimo, C.; Rosenbaum, E.; Stern, J.; et al. Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. J. Clin. Oncol. 2006, 24, 4262–4269. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Ferreira, F.; Carvalho, S.; Silva, F.; Lopes, P.; Antunes, L.; Salta, S.; Diniz, F.; Santos, L.L.; Videira, J.F.; et al. A novel DNA methylation panel accurately detects colorectal cancer independently of molecular pathway. J. Transl. Med. 2018, 16, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, S.; Brait, M.; Dasgupta, S.; Ostrow, K.L.; Zahurak, M.; Carvalho, A.L.; Califano, J.A.; Goodman, S.N.; Westra, W.H.; Hoque, M.O.; et al. An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin. Cancer Res. 2011, 17, 4494–4503. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, C.; Costa, I.; Martins, M.C.; Monteiro, P.; Lisboa, S.; Palmeira, C.; Henrique, R.; Teixeira, M.R.; Lopes, C. Detection of gene promoter hypermethylation in fine needle washings from breast lesions. Clin. Cancer Res. 2003, 9, 3413–3417. [Google Scholar] [PubMed]
- Weiss, G.; Schlegel, A.; Kottwitz, D.; Konig, T.; Tetzner, R. Validation of the SHOX2/PTGER4 DNA Methylation Marker Panel for Plasma-Based Discrimination between Patients with Malignant and Nonmalignant Lung Disease. J. Thorac. Oncol. 2017, 12, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.T.; Hurban, P.; White, M.N.; Whitlock, K.D.; Lofton-Day, C.E.; Tetzner, R.; Koenig, T.; Quigley, N.B.; Weiss, G. Validation of a Real-Time PCR–Based Qualitative Assay for the Detection of Methylated SEPT9 DNA in Human Plasma. Clin. Chem. 2014, 60, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Toung, J.M.; Jassowicz, A.F.; Vijayaraghavan, R.; Kang, H.; Zhang, R.; Kruglyak, K.M.; Huang, H.J.; Hinoue, T.; Shen, H.; et al. Targeted methylation sequencing of plasma cell-free DNA for cancer detection and classification. Ann. Oncol. 2018, 29, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Warton, K.; Samimi, G. Methylation of cell-free circulating DNA in the diagnosis of cancer. Front. Mol. Biosci. 2015, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, M.H.; Kweon, S.S.; Park, M.H.; Yoon, J.H.; Lee, J.S.; Choi, C.; Fackler, M.J.; Sukumar, S. Evaluation of promoter hypermethylation detection in serum as a diagnostic tool for breast carcinoma in Korean women. Gynecol. Oncol. 2010, 118, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yin, H.; Li, J.; Li, X.; Wang, D.; Su, Y.; Niu, M.; Zhong, Z.; Wang, J.; Zhang, X.; et al. Detection of aberrant methylation of a six-gene panel in serum DNA for diagnosis of breast cancer. Oncotarget 2016, 7, 18485–18494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Chen, H.Y.; Bai, E.Q.; Luo, Y.X.; Fu, R.J.; He, Y.S.; Jiang, J.; Wang, H.Q. Development of a multiplex MethyLight assay for the detection of multigene methylation in human colorectal cancer. Cancer Genet. Cytogenet. 2010, 202, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roperch, J.P.; Incitti, R.; Forbin, S.; Bard, F.; Mansour, H.; Mesli, F.; Baumgaertner, I.; Brunetti, F.; Sobhani, I. Aberrant methylation of NPY, PENK, and WIF1 as a promising marker for blood-based diagnosis of colorectal cancer. BMC Cancer 2013, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Lee, E.J.; Jung, E.H.; Chun, H.K.; Chang, D.K.; Song, S.Y.; Park, J.; Kim, D.H. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin. Cancer Res. 2009, 15, 6185–6191. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.; Jusue-Torres, I.; Stark, A.; Chen, C.; Rodgers, K.; Lee, B.; Griffin, C.; Yang, A.; Huang, P.; Wrangle, J.; et al. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and Sputum. Clin. Cancer Res. 2017, 23, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, K.L.; Hoque, M.O.; Loyo, M.; Brait, M.; Greenberg, A.; Siegfried, J.M.; Grandis, J.R.; Gaither Davis, A.; Bigbee, W.L.; Rom, W.; et al. Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative methylation-specific PCR. Clin. Cancer Res. 2010, 16, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Aggarwal, A.; Imperiale, T.F. Colorectal Cancer Screening: Stool DNA and Other Noninvasive Modalities. Gut Liver 2016, 10, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Crino, L.; Weder, W.; Van Meerbeeck, J.; Felip, E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v103–v115. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Galan, J.; Torres, B.; Del Moral, R.; Munoz-Gamez, J.A.; Martin-Oliva, D.; Villalobos, M.; Nunez, M.I.; Luna Jde, D.; Oliver, F.J.; Ruiz de Almodovar, J.M. Quantitative detection of methylated ESR1 and 14-3-3-sigma gene promoters in serum as candidate biomarkers for diagnosis of breast cancer and evaluation of treatment efficacy. Cancer Biol. Ther. 2008, 7, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Fackler, M.J.; McVeigh, M.; Mehrotra, J.; Blum, M.A.; Lange, J.; Lapides, A.; Garrett, E.; Argani, P.; Sukumar, S. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004, 64, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nakayama, T.; Kajita, M.; Miyake, T.; Iwamoto, T.; Kim, S.J.; Sakai, A.; Ishihara, H.; Tamaki, Y.; Noguchi, S. Detection of aberrant promoter methylation of GSTP1, RASSF1A, and RARbeta2 in serum DNA of patients with breast cancer by a newly established one-step methylation-specific PCR assay. Breast Cancer Res. 2012, 132, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chimonidou, M.; Strati, A.; Malamos, N.; Georgoulias, V.; Lianidou, E.S. SOX17 promoter methylation in circulating tumor cells and matched cell-free DNA isolated from plasma of patients with breast cancer. Clin. Chem. 2013, 59, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Miladi-Abdennadher, I.; Abdelmaksoud-Damak, R.; Ayadi, L.; Khabir, A.; Frikha, F.; Kallel, L.; Amouri, A.; Frikha, M.; Sellami-Boudawara, T.; Gargouri, A.; et al. Hypermethylation of RARbeta2 correlates with high COX-2 expression and poor prognosis in patients with colorectal carcinoma. Tumour Biol. 2010, 31, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Roemen, G.M.; Brink, M.; Pachen, M.M.; Weijenberg, M.P.; de Bruine, A.P.; Arends, J.W.; van den Brandt, P.A.; de Goeij, A.F.; Herman, J.G. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene 2002, 21, 3792–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.L.; Krarup, H.B.; Sunesen, K.G.; Johansen, M.B.; Stender, M.T.; Pedersen, I.S.; Madsen, P.H.; Thorlacius-Ussing, O. Hypermethylated DNA, a circulating biomarker for colorectal cancer detection. PLoS ONE 2017, 12, e0180809. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Dawson, S.N.; Arends, M.J.; Guttula, K.; Hall, N.; Cameron, E.A.; Huang, T.H.; Brenton, J.D.; Tavare, S.; Bienz, M.; et al. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer 2014, 14, 891. [Google Scholar] [CrossRef] [PubMed]
- Balgkouranidou, I.; Chimonidou, M.; Milaki, G.; Tsaroucha, E.; Kakolyris, S.; Georgoulias, V.; Lianidou, E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin. Chem. Lab. Med. 2016, 54, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Kneip, C.; Schmidt, B.; Seegebarth, A.; Weickmann, S.; Fleischhacker, M.; Liebenberg, V.; Field, J.K.; Dietrich, D. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer in plasma. J. Thorac. Oncol. 2011, 6, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.P.; Lin, J.; Liles, E.; Beil, T.; Fu, R.; O’Connor, E.; Thompson, R.N.; Cardenas, T. Screening for Colorectal Cancer: An Updated Systematic Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Kajabova, V.; Smolkova, B.; Zmetakova, I.; Sebova, K.; Krivulcik, T.; Bella, V.; Kajo, K.; Machalekova, K.; Fridrichova, I. RASSF1A Promoter Methylation Levels Positively Correlate with Estrogen Receptor Expression in Breast Cancer Patients. Transl. Oncol. 2013, 6, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Hoon, D.S.; Giuliano, A.E.; Hansen, N.M.; Wang, H.J.; Turner, R.; Taback, B. Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin. Cancer Res. 2005, 11, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Orlandi, R.; Zhao, N.; Carcangiu, M.L.; Tagliabue, E.; Xu, J.; Bast, R.C., Jr.; Yu, Y. Tumor suppressor genes are frequently methylated in lymph node metastases of breast cancers. BMC Cancer 2010, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, J.; Semaan, A.; Gevensleben, H.; Groening, S.; Knoblich, A.; Dietrich, J.; Weber, J.; Kalff, J.C.; Bootz, F.; Kristiansen, G.; et al. Potential of quantitative SEPT9 and SHOX2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: A prospective observational cohort study. Br. J. Cancer 2018, 118, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Q.; Liu, P.-P.; Zhang, C.-H. Correlation between the methylation of APC gene promoter and colon cancer. Oncol. Lett. 2017, 14, 2315–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Rocken, C.; Lofton-Day, C.; Schulz, H.U.; Muller, O.; Kutzner, N.; Malfertheiner, P.; Ebert, M.P. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis 2005, 26, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, S.; Lithwick-Yanai, G.; Barshack, I.; Benjamin, S.; Krivitsky, I.; Edmonston, T.B.; Bibbo, M.; Thurm, C.; Horowitz, L.; Huang, Y.; et al. Classification of the four main types of lung cancer using a microRNA-based diagnostic assay. J. Mol. Diagn. 2012, 14, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Shin, A.Y.; Yeo, C.D.; Kim, J.S.; Kim, Y.H.; Kim, J.W.; Lee, S.H. A lower level of forced expiratory volume in one second predicts the poor prognosis of small cell lung cancer. J. Thorac. Dis. 2018, 10, 2179–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomizawa, Y.; Kohno, T.; Kondo, H.; Otsuka, A.; Nishioka, M.; Niki, T.; Yamada, T.; Maeshima, A.; Yoshimura, K.; Saito, R.; et al. Clinicopathological significance of epigenetic inactivation of RASSF1A at 3p21.3 in stage I lung adenocarcinoma. Clin. Cancer Res. 2002, 8, 2362–2368. [Google Scholar] [PubMed]
- Yanagawa, N.; Tamura, G.; Oizumi, H.; Kanauchi, N.; Endoh, M.; Sadahiro, M.; Motoyama, T. Promoter hypermethylation of RASSF1A and RUNX3 genes as an independent prognostic prediction marker in surgically resected non-small cell lung cancers. Lung Cancer 2007, 58, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bundo, M.; Sunaga, F.; Ueda, J.; Kasai, K.; Kato, T.; Iwamoto, K. A systematic evaluation of whole genome amplification of bisulfite-modified DNA. Clin. Epigenet. 2012, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Mill, J.; Yazdanpanah, S.; Gückel, E.; Ziegler, S.; Kaminsky, Z.; Petronis, A. Whole genome amplification of sodium bisulfite-treated DNA allows the accurate estimate of methylated cytosine density in limited DNA resources. Biotechniques 2006, 41, 603–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olkhov-Mitsel, E.; Zdravic, D.; Kron, K.; van der Kwast, T.; Fleshner, N.; Bapat, B. Novel multiplex MethyLight protocol for detection of DNA methylation in patient tissues and bodily fluids. Sci. Rep. 2014, 4, 4432. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G.; Kramer, B.S. Identifying genes that contribute most to good classification in microarrays. BMC Bioinf. 2006, 7, 407. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological Features | AC | Cancer Patients |
|---|---|---|
| Number | 103 | 253 |
| Age median (range) | 52 (45–65) | 63 (29–93) |
| Breast Cancer | ||
| Histological Type | n.a. | |
| Invasive Carcinoma, no special type (NST) | 80 | |
| Invasive lobular carcinoma | 12 | |
| Ductal carcinoma in situ | 7 | |
| Other invasive carcinoma subtypes a | 9 | |
| Primary Tumor (T) | n.a. | |
| Tis | 7 | |
| T1&T2 | 95 | |
| T3&T4 | 6 | |
| Regional lymph node (N) | n.a. | |
| N0 | 65 | |
| N+ | 43 | |
| Distant metastasis (M) | n.a. | |
| M0 | 105 | |
| M1 | 3 | |
| Clinical Stage | n.a. | |
| 0 | 7 | |
| I/II | 88 | |
| III/IV | 13 | |
| Colorectal Cancer | ||
| Histological Type | n.a. | |
| Premalignant Lesions b | 3 | |
| Adenocarcinoma (all subtypes) | 68 | |
| Neuroendocrine carcinoma | 1 | |
| Tumor location | ||
| Proximal colon | n.a. | 23 |
| Distal colon | 30 | |
| Rectum | 19 | |
| Primary tumor (T) c | n.a. | |
| Tis | 3 | |
| T1&T2 | 18 | |
| T3&T4 | 49 | |
| Regional lymph node (N) c | n.a. | |
| N0 | 37 | |
| N+ | 33 | |
| Distant metastasis (M) | n.a. | |
| M0 | 66 | |
| M1 | 6 | |
| Clinical Stage | n.a. | |
| 0 | 3 | |
| I/II | 34 | |
| III/IV | 35 | |
| Lung Cancer | ||
| Histological Type | n.a. | |
| Non-small cell lung carcinoma (NSCLC) | ||
| Adenocarcinoma | 56 | |
| Other NSCLC subtypes d | 8 | |
| Small-cell lung carcinoma (SCLC) | 8 | |
| Carcinoid tumor | 1 | |
| Primary Tumor (T) e | n.a. | |
| T1 | 18 | |
| T2/T3/T4 | 51 | |
| Regional lymph node (N) f | ||
| N0 | n.a. | 27 |
| N+ | 45 | |
| Distant metastasis (M) | n.a. | |
| M0 | 36 | |
| M1 | 37 | |
| Clinical StageI/II | n.a. | |
| 21 | ||
| III/IV | 52 |
| Validity Estimates | PanCancer |
|---|---|
| Sensitivity % | 72.4 |
| Specificity % | 73.5 |
| Positive Predictive Value % | 87.1 |
| Negative Predictive Value % | 52.1 |
| Accuracy % | 72.8 |
| Gene | BrC | CRC | LC |
|---|---|---|---|
| SCGB3A1 | + | − | − |
| SEPT9 | − | + | − |
| SOX17 | − | − | + |
| Gene | Sensitivity % | Specificity % | Accuracy % |
|---|---|---|---|
| BrC | |||
| SCGB3A1 | 16.8 | 80.0 | 53.0 |
| SEPT9 | - | - | - |
| SOX17 | - | - | - |
| CRC | |||
| SCGB3A1 | - | - | - |
| SEPT9 | 11.1 | 98.9 | 73.9 |
| SOX17 | - | - | - |
| LC | |||
| SCGB3A1 | - | - | - |
| SEPT9 | - | - | - |
| SOX17 | 39.4 | 85.1 | 71.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, S.P.; Moreira-Barbosa, C.; Salta, S.; Palma de Sousa, S.; Pousa, I.; Oliveira, J.; Soares, M.; Rego, L.; Dias, T.; Rodrigues, J.; et al. Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women. Cancers 2018, 10, 357. https://doi.org/10.3390/cancers10100357
Nunes SP, Moreira-Barbosa C, Salta S, Palma de Sousa S, Pousa I, Oliveira J, Soares M, Rego L, Dias T, Rodrigues J, et al. Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women. Cancers. 2018; 10(10):357. https://doi.org/10.3390/cancers10100357
Chicago/Turabian StyleNunes, Sandra P., Catarina Moreira-Barbosa, Sofia Salta, Susana Palma de Sousa, Inês Pousa, Júlio Oliveira, Marta Soares, Licínio Rego, Teresa Dias, Jéssica Rodrigues, and et al. 2018. "Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women" Cancers 10, no. 10: 357. https://doi.org/10.3390/cancers10100357
APA StyleNunes, S. P., Moreira-Barbosa, C., Salta, S., Palma de Sousa, S., Pousa, I., Oliveira, J., Soares, M., Rego, L., Dias, T., Rodrigues, J., Antunes, L., Henrique, R., & Jerónimo, C. (2018). Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women. Cancers, 10(10), 357. https://doi.org/10.3390/cancers10100357