Combined Effect of Bortezomib and Menadione Sodium Bisulfite on Proteasomes of Tumor Cells: The Dramatic Decrease of Bortezomib Toxicity in a Preclinical Trial
Abstract
1. Introduction
2. Results and Discussion
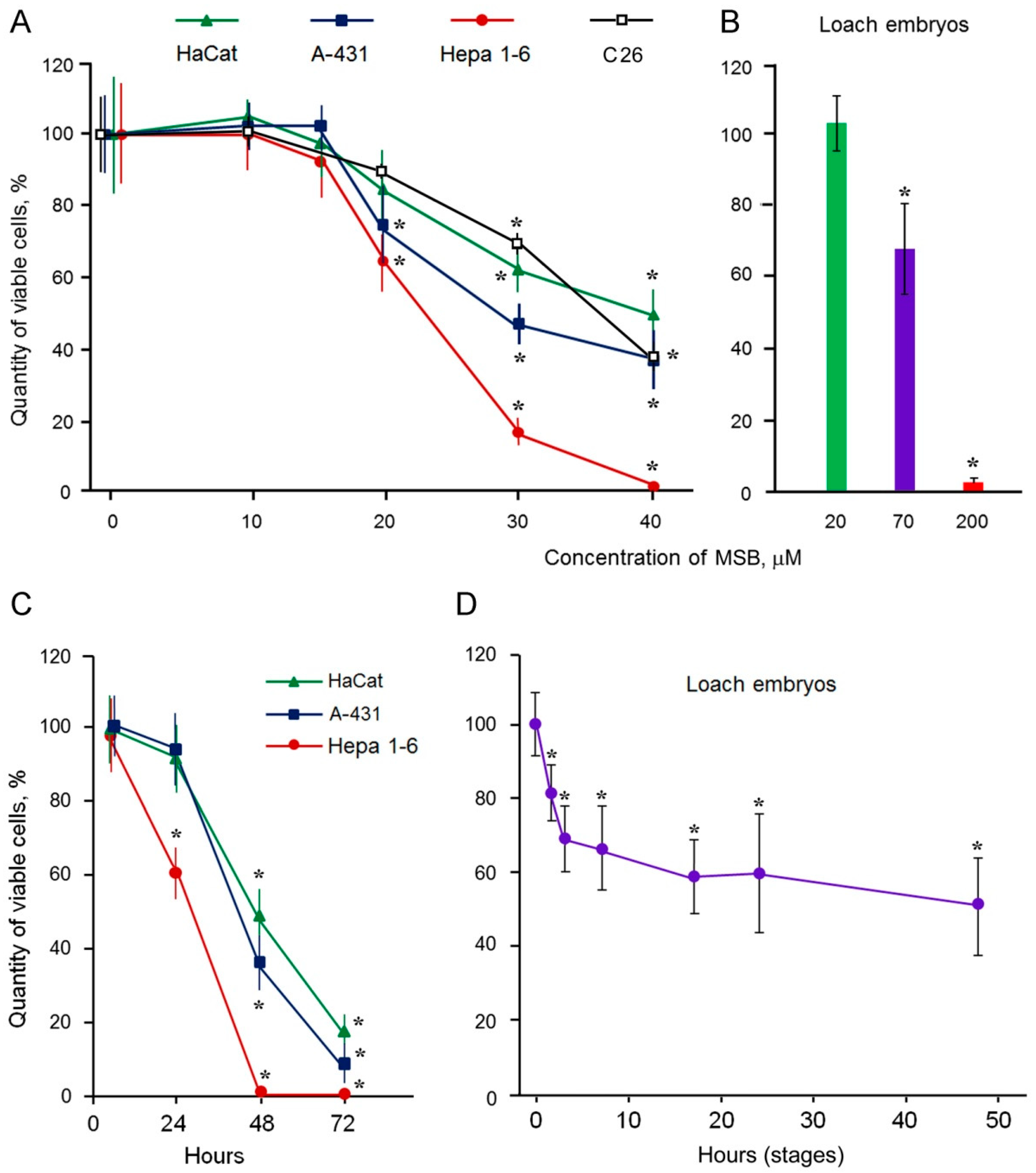
2.1. Cytotoxic Action of MSB
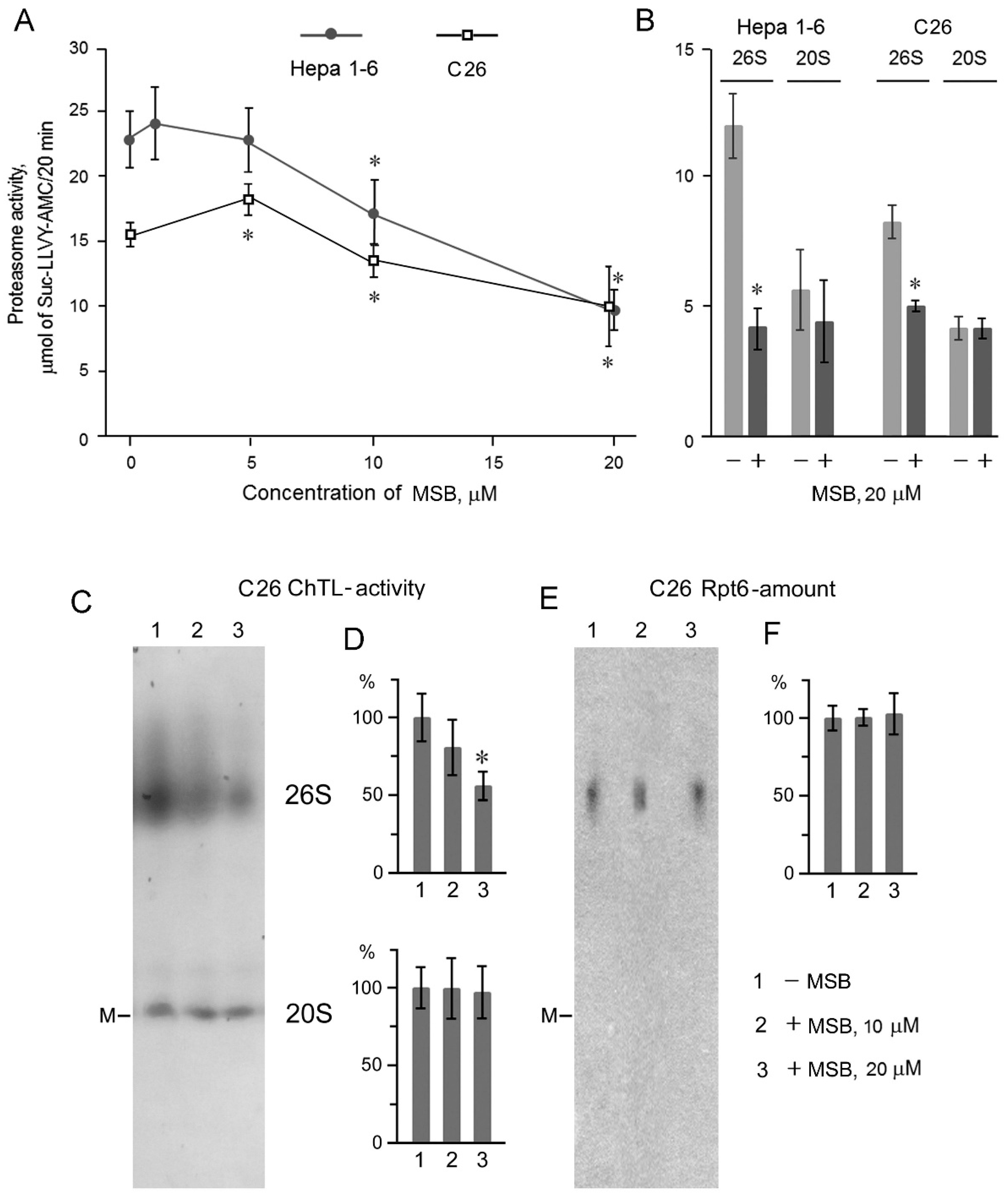
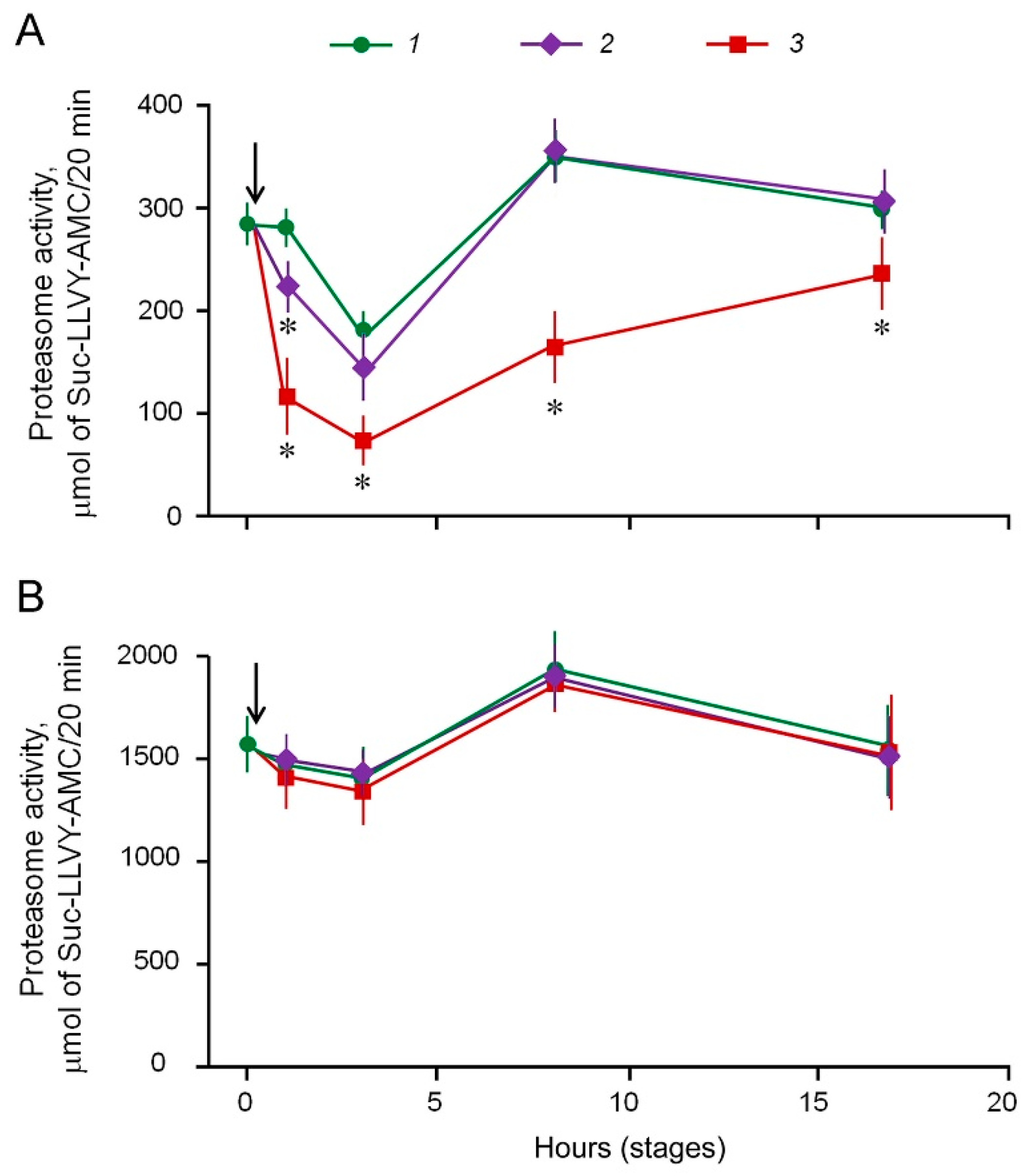
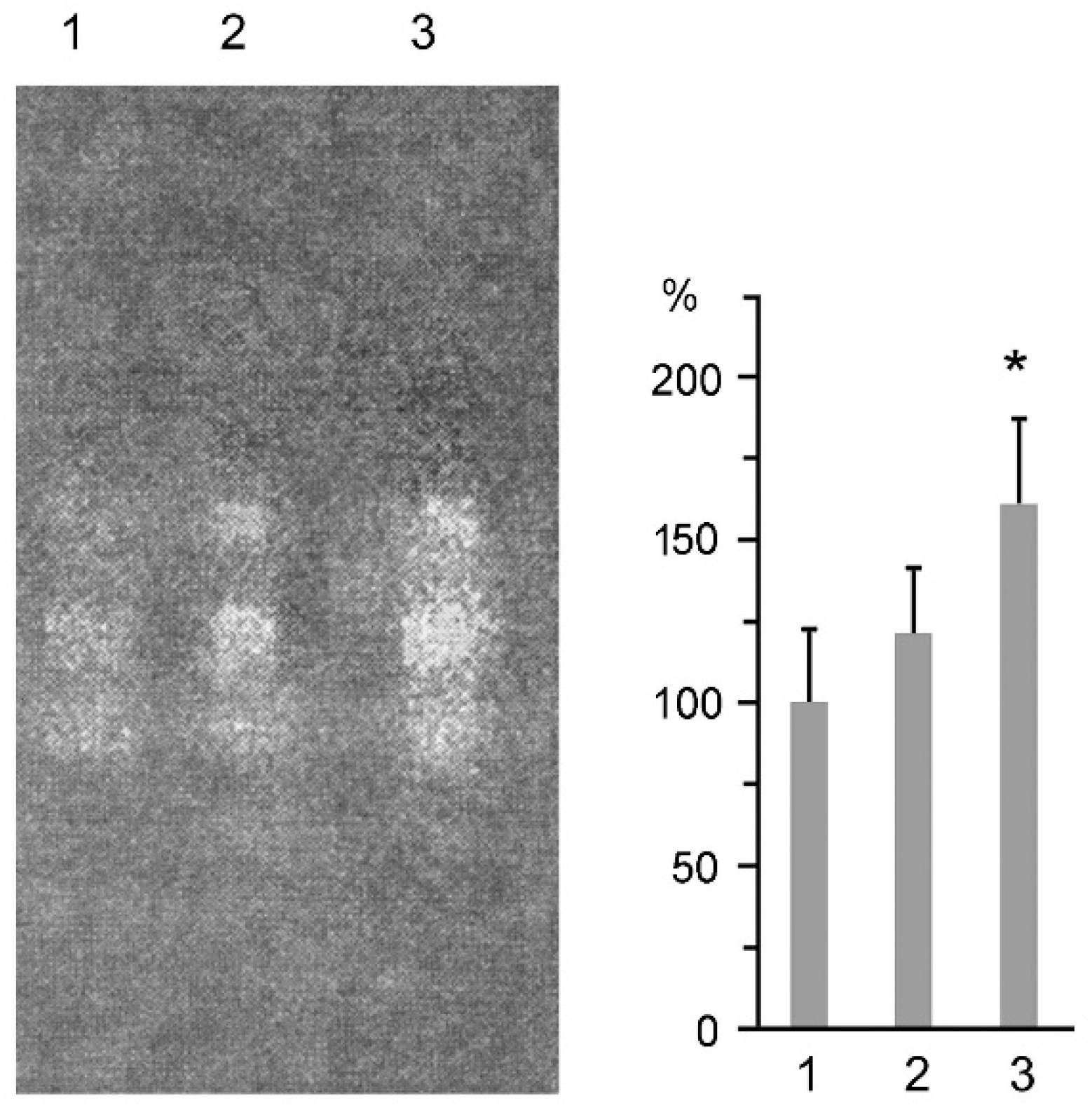
2.2. Suppression of Proteasome Activity by MSB In Cells
2.3. Content of Antitumor Compositions
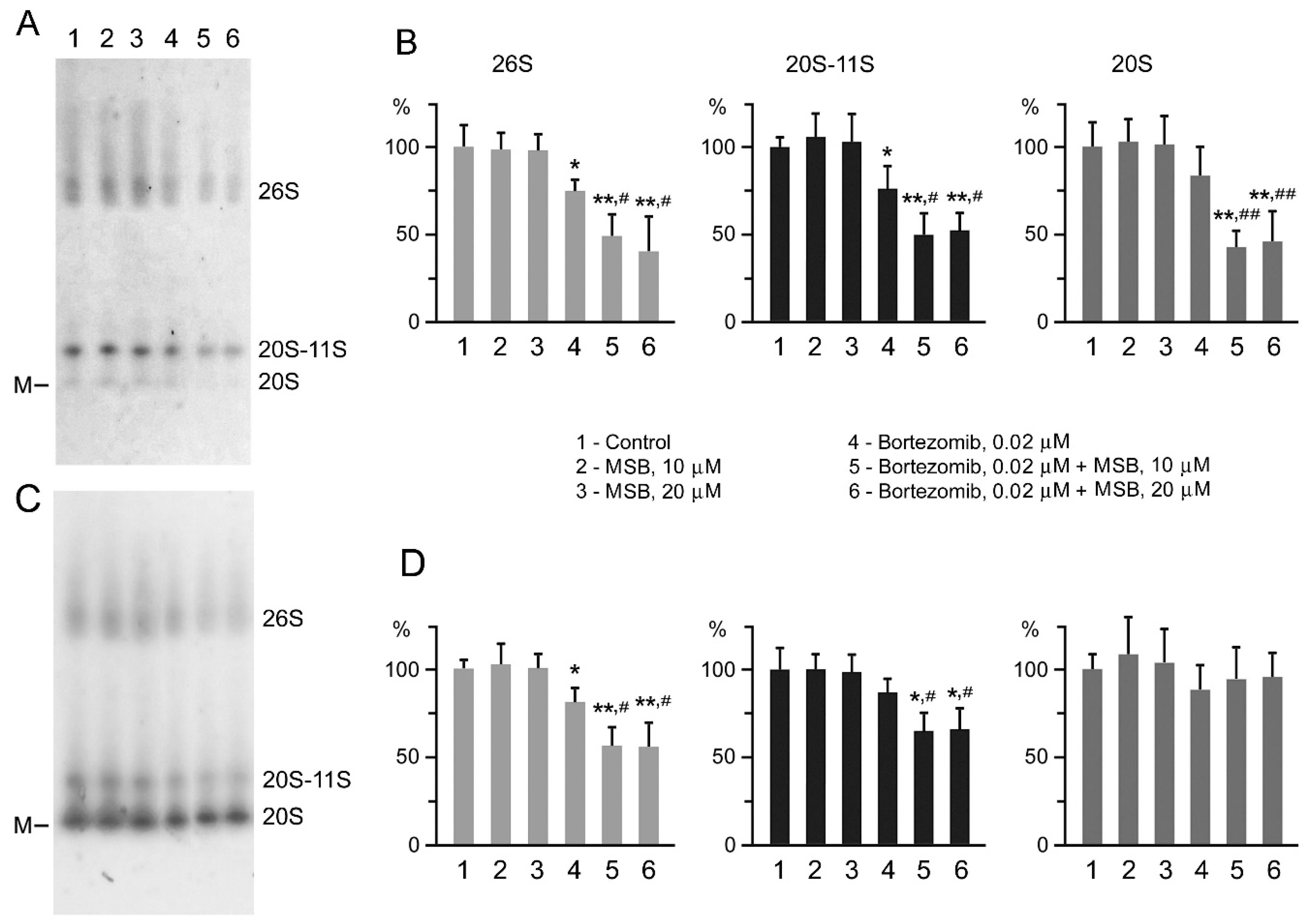
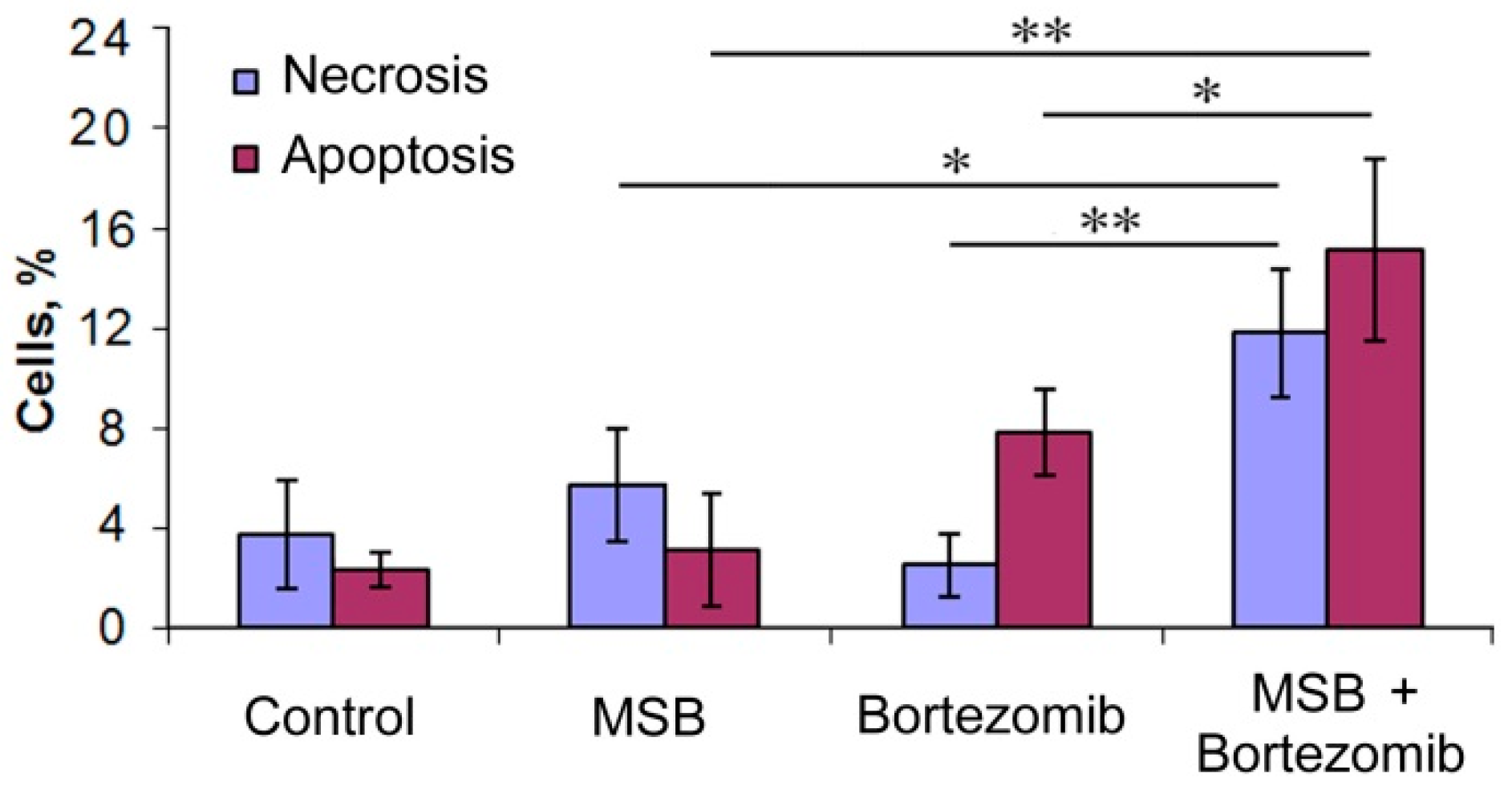
2.4. Effects of Bortezomib and MSB In Vitro and In Cellulo
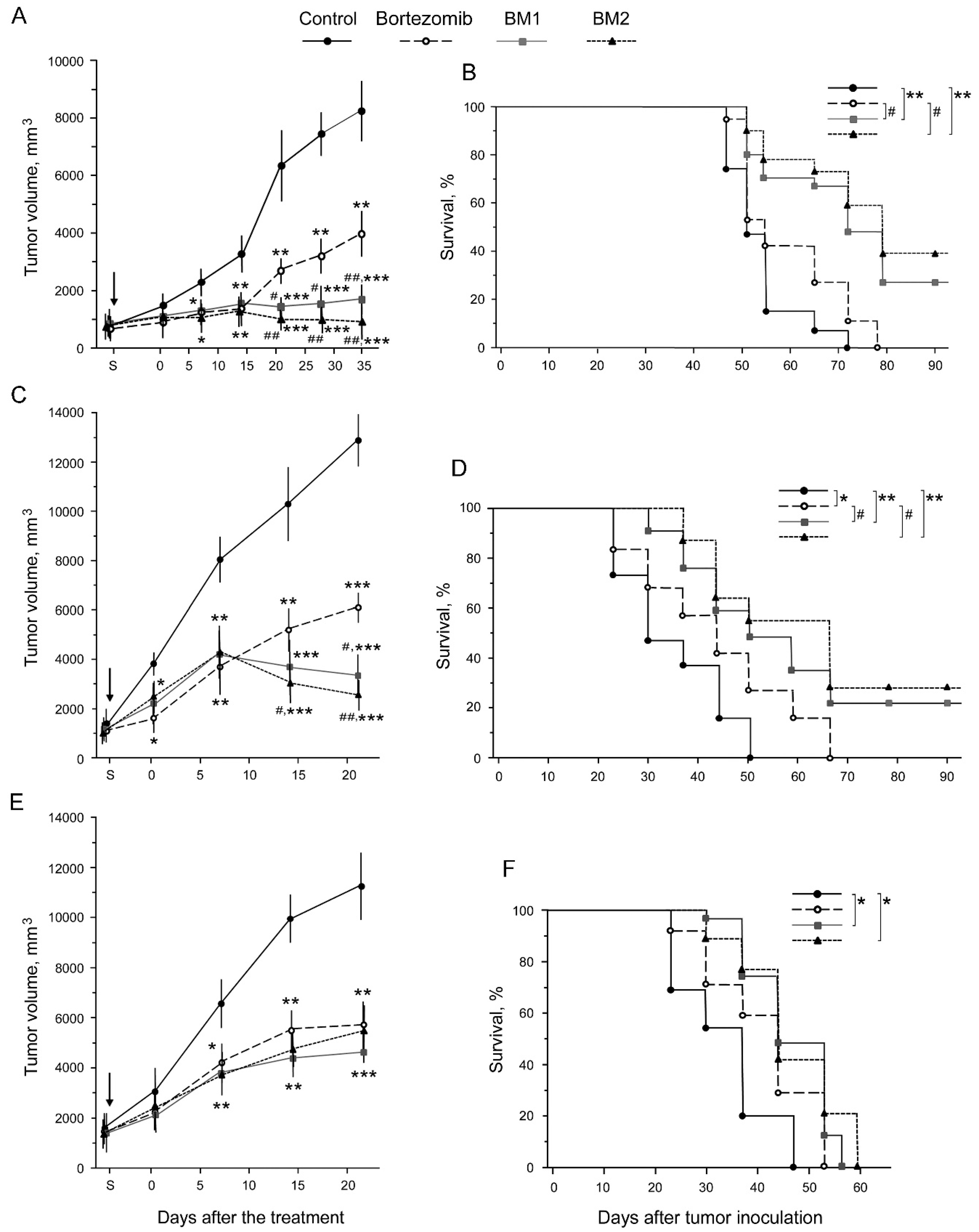
2.5. Toxicity of BM1 and BM2 In Vivo
2.6. Antitumor Effects of BM1 and BM2 In Vivo
3. Materials and Methods
3.1. Animals and Cell Lines
3.2. Chemicals
3.3. Treatment of Cells with MSB
3.4. Preparation of Proteasome Fractions
3.5. Determination of Proteasome ChTL-Activity and Superoxide Dismutase Activity
3.6. Western Blotting
3.7. Assay for Cytotoxic Effects
3.8. Detection of Apoptosis and Necrosis
3.9. Tumor Strain Maintaining
3.10. Inoculation of Tumor Cells
3.11. Evaluation of Antitumor Effects
3.12. MTD Detection
3.13. Evaluation of LD50
3.14. Statistics
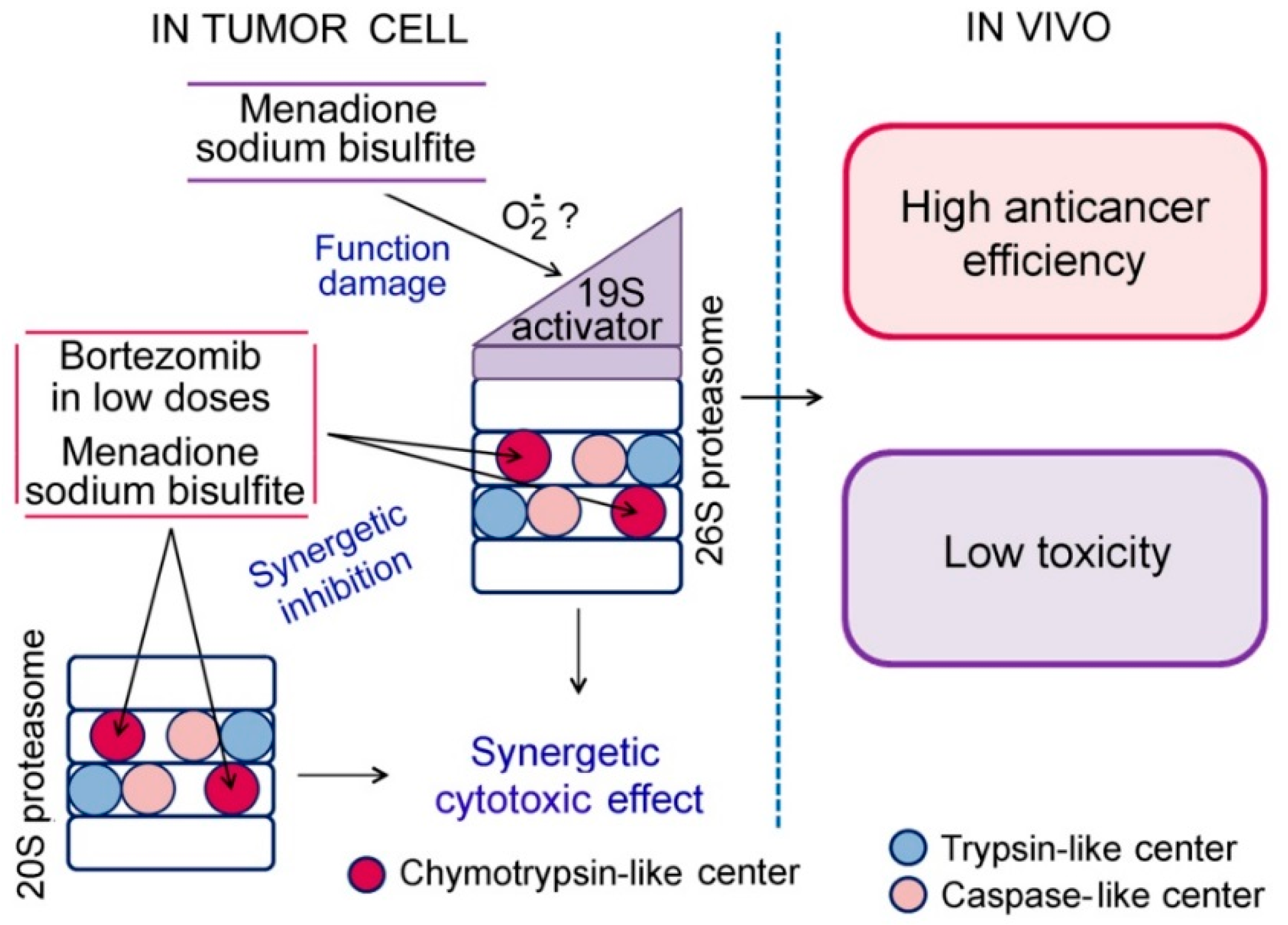
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abramova, E.B.; Sharova, N.P.; Karpov, V.L. The proteasome: Destroy to live. Mol. Biol. 2002, 36, 613–624. [Google Scholar] [CrossRef]
- Spirina, L.V.; Kondakova, I.V.; Choynzonov, E.L.; Chigevskaya, S.Y.; Shishkin, D.A.; Kulbakin, D.Y. Expression of vascular endothelial growth factor and transcription factors HIF-1, NF-κB in squamous cell carcinoma of head and neck; association with proteasome and calpain activities. J. Cancer Res. Clin. Oncol. 2013, 139, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.R.; Wu, H.; Wang, B.; Abuetabh, Y.; Sergi, C.; Leng, R.P. The regulation of tumor suppressor p63 by the ubiquitin-proteasome system. Int. J. Mol. Sci. 2016, 17, 2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Madura, K. Increased proteasome activity, ubiquitinconjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005, 65, 5599–5606. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhou, H.; Xiong, R.; Lu, Y.; Yan, D.; Xing, T.; Dong, L.; Tang, E.; Yang, H. Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res. Treat. 2007, 104, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Astakhova, T.M.; Delone, G.V.; Lupina, Y.V.; Abramova, E.B.; Uryvaeva, I.V.; Sharova, N.P. Changes in the proteasome pool during malignant transformation of mouse liver cells. Acta Naturae 2010, 2, 102–108. [Google Scholar] [PubMed]
- Sharova, N.P.; Astakhova, T.M.; Karpova, Y.D.; Lyupina, Y.V.; Alekhin, A.I.; Goncharov, N.G.; Sumedi, I.R.; Cherner, V.A.; Rodoman, G.V.; Kuznetsov, N.A.; et al. Changes in proteasome pool in human papillary thyroid carcinoma development. Cent. Eur. J. Biol. 2011, 6, 486–496. [Google Scholar] [CrossRef]
- Shashova, E.E.; Lyupina, Y.V.; Glushchenko, S.A.; Slonimskaya, E.M.; Savenkova, O.V.; Kulikov, A.M.; Gornostaev, N.G.; Kondakova, I.V.; Sharova, N.P. Proteasome functioning in breast cancer: Connection with clinical-pathological factors. PLoS ONE 2014, 9, e109933. [Google Scholar] [CrossRef]
- Zakharova, L.A.; Khegai, I.I.; Sharova, N.P.; Melnikova, V.I.; Karpova, Y.D.; Astakhova, T.M.; Popova, N.A.; Ivanova, L.N. Pattern of MHC class I and immune proteasome expression in Walker 256 tumor during growth and regression in Brattleboro rats with the hereditary defect of arginine-vasopressin synthesis. Cell. Immunol. 2011, 271, 385–391. [Google Scholar] [CrossRef]
- Goldberg, A.L. Development of proteasome inhibitors as research tools and cancer drugs. J. Cell Biol. 2012, 199, 583–588. [Google Scholar] [CrossRef]
- Mohan, M.; Matin, A.; Davies, F.E. Update on the optimal use of bortezomib in the treatment of multiple myeloma. Cancer Manag. Res. 2017, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Baiz, D.; Pozzato, G.; Dapas, B.; Farra, R.; Scaggiante, B.; Grassi, M.; Uxa, L.; Giansante, C.; Zennaro, C.; Guarnieri, G.; et al. Bortezomib arrests the proliferation of hepatocellular carcinoma cells HepG2 and JHH6 by differentially affecting E2F1, p21 and p27 levels. Biochimie 2009, 91, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, W.; Zhong, Y.; Hou, X.; Fang, S.; Liu, C.Y.; Wang, G.; Yu, T.; Huang, Y.Y.; Ouyang, X.; et al. Nuclear export of ubiquitinated proteins determines the sensitivity of colorectal cancer to proteasome inhibitor. Mol. Cancer Ther. 2017, 16, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.S.; Torcun, C.C.; Grune, T.; Ozer, N.K.; Karademir, B. Proteasome inhibitors in cancer therapy: Treatment regimen and peripheral neuropathy as a side effect. Free Radic. Biol. Med. 2017, 103, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Gong, Y.; Szady, A.; Sun, Q.; Pepine, C.J.; Langaee, T.; Lucas, A.R.; Moreb, J.S. Unanticipated cardiotoxicity associated with targeted anticancer therapy in patients with hematologic malignancies: Natural history and risk factors. Cardiovasc. Toxicol. 2018, 18, 184–191. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Orlowski, R.Z. The immunoproteasome as a target in hematologic malignancies. Semin. Hematol. 2012, 49, 258–262. [Google Scholar] [CrossRef]
- Powis, G. Free radical formation by antitumor quinones. Free Radic. Biol. Med. 1989, 6, 63–101. [Google Scholar] [CrossRef]
- Yamashoji, S. Different characteristics between menadione and menadione sodium bisulfite as redox mediator in yeast cell suspension. Biochem. Biophys. Rep. 2016, 6, 88–93. [Google Scholar] [CrossRef]
- Sharova, N.P.; Stroeva, O.G.; Erokhov, P.A.; Dmitrieva, S.B.; Usova, T.A. Changes in the activity of proteasomes in vivo induced by oxidative stress or para-aminobenzoic acid in loach embryos. Dokl. Biochem. Biophys. 2007, 415, 197–199. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, Y.K.; Sohn, D.S.; Lee, C.S. Menadione induces the formation of reactive oxygen species and depletion of GSH-mediated apoptosis and inhibits the FAK-mediated cell invasion. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 799–809. [Google Scholar] [CrossRef]
- Peskin, A.V.; Sharova, N.P.; Dimitrova, D.D.; Stolyarov, S.D.; Filatova, L.S. Effect of oxidative stress on DNA polymerases in the embryogenesis of the loach Misgurnus fossilis L. Doclady Biochem. 1997, 355, 72–75. [Google Scholar]
- Sharova, N.; Zakharova, L. Multiple forms of proteasomes and their role in tumor fate. Recent Pat. Endocr. Metab. Immune Drug Discov. 2008, 2, 152–161. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; He, L.; Aittokallio, T.; Tang, J. Synergy Finder: A web application for analyzing drug combination dose–response matrix data. Bioinformatics 2017, 33, 2413–2415. [Google Scholar] [CrossRef] [PubMed]
- Erokhov, P.A.; Lyupina, Y.V.; Radchenko, A.S.; Kolacheva, A.A.; Nikishina, Y.O.; Sharova, N.P. Detection of active proteasome structures in brain extracts: Proteasome features of August rat brain with violations in monoamine metabolism. Oncotarget 2017, 8, 70941–70957. [Google Scholar] [CrossRef] [PubMed]
- Abramova, E.B.; Astakhova, T.M.; Erokhov, P.A.; Sharova, N.P. Multiple forms of proteasomes and approaches to their separation. Biol. Bull. 2004, 31, 115–120. [Google Scholar] [CrossRef]
- Mlynarczuk-Bialy, I.; Doeppner, T.R.; Golab, J.; Nowis, D.; Wilczynski, G.M.; Parobczak, K.; Wigand, M.E.; Hajdamowicz, M.; Biały, L.P.; Aniolek, O.; et al. Biodistribution and efficacy studies of the proteasome Inhibitor BSc2118 in a mouse melanoma model. Transl. Oncol. 2014, 7, 570–579. [Google Scholar] [CrossRef]
- Sharova, N.P.; Eliseeva, E.D.; Mikhailov, V.S. Changes in the activity of two forms of DNA polymerase α during embryogenesis of teleost fish Misgurnus fossilis. Biochemistry 1992, 57, 644–649. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mitkevich, V.; Petrushanko, I.; Spirin, P.; Fedorova, T.; Kretova, O.; Tchurikov, N.; Prassolov, V.; Ilinskaya, O.; Makarov, A. Sensitivity of acute myeloid leukemia Kasumi-1 cells to binase toxic action depends on the expression of KIT and AML1-ETO oncogenes. Cell Cycle 2011, 10, 4090–4097. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astakhova, T.M.; Morozov, A.V.; Erokhov, P.A.; Mikhailovskaya, M.I.; Akopov, S.B.; Chupikova, N.I.; Safarov, R.R.; Sharova, N.P. Combined Effect of Bortezomib and Menadione Sodium Bisulfite on Proteasomes of Tumor Cells: The Dramatic Decrease of Bortezomib Toxicity in a Preclinical Trial. Cancers 2018, 10, 351. https://doi.org/10.3390/cancers10100351
Astakhova TM, Morozov AV, Erokhov PA, Mikhailovskaya MI, Akopov SB, Chupikova NI, Safarov RR, Sharova NP. Combined Effect of Bortezomib and Menadione Sodium Bisulfite on Proteasomes of Tumor Cells: The Dramatic Decrease of Bortezomib Toxicity in a Preclinical Trial. Cancers. 2018; 10(10):351. https://doi.org/10.3390/cancers10100351
Chicago/Turabian StyleAstakhova, Tatiana M., Alexey V. Morozov, Pavel A. Erokhov, Maria I. Mikhailovskaya, Sergey B. Akopov, Natalia I. Chupikova, Ruslan R. Safarov, and Natalia P. Sharova. 2018. "Combined Effect of Bortezomib and Menadione Sodium Bisulfite on Proteasomes of Tumor Cells: The Dramatic Decrease of Bortezomib Toxicity in a Preclinical Trial" Cancers 10, no. 10: 351. https://doi.org/10.3390/cancers10100351
APA StyleAstakhova, T. M., Morozov, A. V., Erokhov, P. A., Mikhailovskaya, M. I., Akopov, S. B., Chupikova, N. I., Safarov, R. R., & Sharova, N. P. (2018). Combined Effect of Bortezomib and Menadione Sodium Bisulfite on Proteasomes of Tumor Cells: The Dramatic Decrease of Bortezomib Toxicity in a Preclinical Trial. Cancers, 10(10), 351. https://doi.org/10.3390/cancers10100351




