Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies
Abstract
:1. Introduction
2. Search
3. Radiofrequency Ablation (RFA)
4. Microwave Ablation (MWA)
5. Cryoablation
6. High-Intensity Focused Ultrasound (HIFU)
7. Stereotactic Body Radiotherapy (SBRT)
8. Iodine-125 Seed Implantation
9. Irreversible Electroporation (IRE)
10. Photodynamic Therapy (PDT)
11. Electrochemotherapy (ECT)
12. Discussion
13. Future Perspectives
14. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), 56–68. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, K.S.; Kumar, S.; Davidson, B.R.; Fusai, G. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Ritchey, J.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Validation of the 6th edition ajcc pancreatic cancer staging system: Report from the national cancer database. Cancer 2007, 110, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Ierardi, A.M.; Fontana, F.; Petrillo, M.; Floridi, C.; Lucchina, N.; Cuffari, S.; Dionigi, G.; Rotondo, A.; Fugazzola, C. Microwave ablation of pancreatic head cancer: Safety and efficacy. J. Vasc. Interv. Radiol. 2013, 24, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Arrigoni, F.; Zugaro, L.; Barile, A.; Masciocchi, C.; et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016, 33, 140. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-intensity focused ultrasound: Current potential and oncologic applications. AJR Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouche, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar] [PubMed]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Niu, L.; Mu, F.; Hu, Y. Cryosurgery in combination with brachytherapy of iodine-125 seeds for pancreatic cancer. Gland Surg. 2013, 2, 91–99. [Google Scholar] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Nielsen, K.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, R.A.; Kazemier, G.; Niessen, H.W.; Meijer, S.; van Kuijk, C.; van den Tol, M.P.; et al. Ablation of colorectal liver metastases by irreversible electroporation: Results of the COLDFIRE-I ablate-and-resect study. Eur. Radiol. 2014, 24, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Leongito, M.; Granata, V.; Barbieri, A.; Del Vecchio, V.; Falco, M.; Nasto, A.; Albino, V.; Piccirillo, M.; Palaia, R.; et al. Electrochemotherapy in pancreatic adenocarcinoma treatment: Pre-clinical and clinical studies. Radiol. Oncol. 2016, 50, 14–20. [Google Scholar] [PubMed]
- Cantore, M.; Girelli, R.; Mambrini, A.; Frigerio, I.; Boz, G.; Salvia, R.; Giardino, A.; Orlandi, M.; Auriemma, A.; Bassi, C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br. J. Surg. 2012, 99, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Girelli, R.; Frigerio, I.; Giardino, A.; Regi, P.; Gobbo, S.; Malleo, G.; Salvia, R.; Bassi, C. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch. Surg. 2013, 398, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Girelli, R.; Frigerio, I.; Salvia, R.; Barbi, E.; Tinazzi Martini, P.; Bassi, C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br. J. Surg. 2010, 97, 220–225. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Crosara, S.; De Robertis, R.; Butturini, G.; Salvia, R.; Paiella, S.; Bassi, C.; Mucelli, R.P. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol. Cancer Res. Treat. 2017, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, I.; Girelli, R.; Giardino, A.; Regi, P.; Salvia, R.; Bassi, C. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: Results from a single center. J. Hepatobiliary Pancreat. Sci. 2013, 20, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Malleo, G.; Cataldo, I.; Gasparini, C.; De Pastena, M.; De Marchi, G.; Marchegiani, G.; Rusev, B.; Scarpa, A.; Girelli, R.; et al. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbecks Arch. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.J.; Sharma, S.K.; Papastratis, P.; Zivanovic, V.; Kefalourous, H.; Koshariya, M.; Lintzeris, I.; Porfiris, T.; Koutsiouroumba, D. Microwave ablation in locally advanced pancreatic carcinoma—A new look. Hepatogastroenterology 2007, 54, 1305–1310. [Google Scholar] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Yang, H.; Wang, X.; Yuan, D.; Zeng, Y.; Wen, T.; Yan, L.; Li, B. Tumour cryoablation combined with palliative bypass surgery in the treatment of unresectable pancreatic cancer: A retrospective study of 142 patients. Postgrad. Med. J. 2011, 87, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.G.; Hao, J.H.; Gao, S.; Gao, C.T.; Tang, Y.; Liu, J.C. The outcome of cryoablation in treating advanced pancreatic cancer: A comparison with palliative bypass surgery alone. J. Dig. Dis. 2014, 15, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Wang, K.; Meng, Z.Q.; Chen, Z.; Lin, J.H.; Zhou, Z.H.; Wang, P.; Shi, W.D.; Sheng, Y.H. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology 2013, 60, 1906–1910. [Google Scholar] [PubMed]
- Li, Y.J.; Huang, G.L.; Sun, X.L.; Zhao, X.C.; Li, Z.G. The combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinoma. World J. Surg. Oncol. 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Sofuni, A.; Moriyasu, F.; Sano, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J. Gastroenterol. 2014, 20, 9570–9577. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.Y.; Jung, S.E.; Cho, S.H.; Zhou, K.; Han, J.Y.; Han, S.T.; Kim, J.I.; Kim, J.K.; Choi, J.Y.; Yoon, S.K.; et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011, 40, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Meng, Z.; Lin, J.; Zhou, Z.; Wang, P.; Chen, L.; Liu, L. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int. J. Hyperther. 2011, 27, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, G.; Wang, D.; Yu, X.; Zhang, Y.; Zhu, J.; Ji, Y.; Zhong, B.; Zhao, W.; Yang, Z.; et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010, 21, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.Y.; Cheng, C.S.; Xie, J.; Chen, Q.W.; Xu, L.T.; Zhuang, L.P.; Zhang, C.Y.; Song, L.B.; Shi, W.D.; Zhu, X.Y.; et al. A retrospective analysis of survival factors of high intensity focused ultrasound (HIFU) treatment for unresectable pancreatic cancer. Discov. Med. 2016, 21, 435–445. [Google Scholar] [PubMed]
- Shi, Y.; Ying, X.; Hu, X.; Shen, H. Pain management of pancreatic cancer patients with high-intensity focused ultrasound therapy. Pak. J. Pharm. Sci. 2017, 30, 303–307. [Google Scholar] [PubMed]
- Xiong, L.L.; Hwang, J.H.; Huang, X.B.; Yao, S.S.; He, C.J.; Ge, X.H.; Ge, H.Y.; Wang, X.F. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009, 10, 123–129. [Google Scholar] [PubMed]
- Zhao, J.; Zhao, F.; Shi, Y.; Deng, Y.; Hu, X.; Shen, H. The efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, H.; Meng, Z.; Chen, Z.; Lin, J.; Shen, Y.; Gao, H. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie 2013, 36, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Perich, E.; Del Castillo, M.A. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason. Sonochem. 2015, 27, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Comito, T.; Cozzi, L.; Clerici, E.; Franzese, C.; Tozzi, A.; Iftode, C.; Navarria, P.; D’Agostino, G.; Rimassa, L.; Carnaghi, C.; et al. Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol. Cancer Res. Treat. 2017, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, A.S.; Chaudhry, M.; Leal, J.P.; Chang, D.T.; Raman, S.P.; Hacker-Prietz, A.; Su, Z.; Pai, J.; Oteiza, K.E.; Griffith, M.E.; et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Collins, S.P.; Slack, R.; Tse, G.; Charabaty, A.; Ley, L.; Berzcel, L.; Lei, S.; Suy, S.; Haddad, N.; et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat. Oncol. 2013, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S.; et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Koong, A.C.; Le, Q.T.; Ho, A.; Fong, B.; Fisher, G.; Cho, C.; Ford, J.; Poen, J.; Gibbs, I.C.; Mehta, V.K.; et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Polistina, F.; Costantin, G.; Casamassima, F.; Francescon, P.; Guglielmi, R.; Panizzoni, G.; Febbraro, A.; Ambrosino, G. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann. Surg. Oncol. 2010, 17, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, D.; Goodman, K.A.; Lee, F.; Chang, S.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Quon, A.; Desser, T.S.; Norton, J.; et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A.; et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; Comito, T.; Alongi, F.; Navarria, P.; Iftode, C.; Mancosu, P.; Reggiori, G.; Clerici, E.; Rimassa, L.; Zerbi, A.; et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat. Oncol. 2013, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, M.; Pollom, E.L.; von Eyben, R.; Kozak, M.M.; Aggarwal, S.; Poultsides, G.A.; Koong, A.C.; Chang, D.T. Albumin and Neutrophil-Lymphocyte Ratio (NLR) Predict Survival in Patients With Pancreatic Adenocarcinoma Treated With SBRT. Am. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.; Schellenberg, D.; Shen, J.; Kim, J.; Goodman, K.A.; Fisher, G.A.; Ford, J.M.; Desser, T.; Quon, A.; Koong, A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009, 115, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Springett, G.M.; Freilich, J.M.; Park, C.K.; Weber, J.M.; Mellon, E.A.; Hodul, P.J.; Malafa, M.P.; Meredith, K.L.; Hoffe, S.E.; et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Jain, S.; Goldstein, M.; Miksad, R.; Pleskow, D.; Sawhney, M.; Brennan, D.; Callery, M.; Vollmer, C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Miksad, R.; Goldstein, M.; Sullivan, R.; Bullock, A.; Buchbinder, E.; Pleskow, D.; Sawhney, M.; Kent, T.; Vollmer, C.; et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e615–e622. [Google Scholar] [CrossRef] [PubMed]
- Mellon, E.A.; Hoffe, S.E.; Springett, G.M.; Frakes, J.M.; Strom, T.J.; Hodul, P.J.; Malafa, M.P.; Chuong, M.D.; Shridhar, R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015, 54, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Dholakia, A.S.; Raman, S.P.; Blackford, A.; Cameron, J.L.; Le, D.T.; De Jesus-Acosta, A.M.; Hacker-Prietz, A.; Rosati, L.M.; Assadi, R.K.; et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann. Surg. Oncol. 2015, 22, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, J.C.; Parikh, S.D.; Heron, D.E.; Howell, M.; Zeh, H.; Moser, A.J.; Bahary, N.; Quinn, A.; Burton, S.A. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am. J. Clin. Oncol. 2011, 34, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yuan, Z.; Li, F.; Dong, Y.; Zhuang, H.; Wang, J.; Chen, H.; Wang, P. Analysis of clinical efficacy of CyberKnife® treatment for locally advanced pancreatic cancer. Onco Targets Ther. 2015, 8, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, F.; Ju, X.; Cao, F.; Cao, Y.; Fang, F.; Qing, S.; Shen, Y.; Jia, Z.; Zhang, H. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017, 6, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Li, J.; Tian, S.; Ran, W.; Xiu, D. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Jiang, Y.; Li, J.; Tian, S.; Ran, W.; Xiu, D.; Gao, Y. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J. Exp. Clin. Cancer Res. 2013, 32, 106. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.C.; Niu, L.Z.; Hu, Y.Z.; He, W.B.; He, Y.S.; Li, Y.F.; Zuo, J.S. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J. Gastroenterol. 2008, 14, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.C.; Niu, L.Z.; Hu, Y.Z.; He, W.B.; He, Y.S.; Zuo, J.S. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J. Dig. Dis. 2008, 9, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.P.; Li, W.M.; Zheng, F.; Li, F.C.; Huang, H.; Du, J.D.; Liu, H.R. Intraoperative radiofrequency ablation combined with 125 iodine seed implantation for unresectable pancreatic cancer. World J. Gastroenterol. 2010, 16, 5104–5110. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.D.; Epelboym, I.; Schrope, B.A.; Mahendraraj, K.; Hecht, E.M.; Susman, J.; Weintraub, J.L.; Chabot, J.A. Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann. Surg. Oncol. 2016, 23, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015, 262, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible electroporation in locally advanced pancreatic cancer: Potential improved overall survival. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S443–S449. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, G.; Belfiore, M.P.; Reginelli, A.; Capasso, R.; Romano, F.; Ianniello, G.P.; Cappabianca, S.; Brunese, L. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med. Oncol. 2017, 34, 38. [Google Scholar] [CrossRef] [PubMed]
- Dunki-Jacobs, E.M.; Philips, P.; Martin, R.C. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J. Am. Coll. Surg. 2014, 218, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Horejs, J.; Krska, Z.; Hoskovec, D.; Petruzelka, L.; Krechler, T.; Kriz, P.; Briza, J. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: General hospital cancer center experience. Neoplasma 2016, 63, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Mansson, C.; Brahmstaedt, R.; Nilsson, A.; Nygren, P.; Karlson, B.M. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. Eur. J. Surg. Oncol. 2016, 42, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Hosein, P.J.; Beulaygue, I.C.; Froud, T.; Scheffer, H.J.; Venkat, S.R.; Echenique, A.M.; Hevert, E.C.; Livingstone, A.S.; Rocha-Lima, C.M.; et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J. Vasc. Interv. Radiol. 2017, 28, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Butturini, G.; Frigerio, I.; Salvia, R.; Armatura, G.; Bacchion, M.; Fontana, M.; D’Onofrio, M.; Martone, E.; Bassi, C. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: Results of a prospective study. Dig. Surg. 2015, 32, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Vroomen, L.G.; de Jong, M.C.; Melenhorst, M.C.; Zonderhuis, B.M.; Daams, F.; Vogel, J.A.; Besselink, M.G.; van Kuijk, C.; Witvliet, J.; et al. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology 2017, 282, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.A.; Rombouts, S.J.; de Rooij, T.; van Delden, O.M.; Dijkgraaf, M.G.; van Gulik, T.M.; van Hooft, J.E.; van Laarhoven, H.W.; Martin, R.C.; Schoorlemmer, A.; et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann. Surg. Oncol. 2017, 24, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, Y.L.; Su, M.; Liu, T.; Xu, K.; Liang, F.; Gu, W.Q.; Lu, S.C. A Single-institution Experience with Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin. Med. J. Engl. 2016, 129, 2920–2925. [Google Scholar] [PubMed]
- Zhang, Y.; Shi, J.; Zeng, J.; Alnagger, M.; Zhou, L.; Fang, G.; Long, X.; Pan, Z.; Li, Y.; Chen, J.; et al. Percutaneous Irreversible Electroporation for Ablation of Locally Advanced Pancreatic Cancer: Experience From a Chinese Institution. Pancreas 2017, 46, e12–e14. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Ronza, F.M.; Romano, F.; Ianniello, G.P.; De Lucia, G.; Gallo, C.; Marsicano, C.; Di Gennaro, T.L.; Belfiore, G. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience. Int. J. Surg. 2015, 21 (Suppl. S1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A.; Hatfield, A.W. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B.; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1028–1061. [Google Scholar] [CrossRef] [PubMed]
- Giardino, A.; Girelli, R.; Frigerio, I.; Regi, P.; Cantore, M.; Alessandra, A.; Lusenti, A.; Salvia, R.; Bassi, C.; Pederzoli, P. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB Oxford 2013, 15, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- de Geus, S.W.L.; Eskander, M.F.; Kasumova, G.G.; Ng, S.C.; Kent, T.S.; Mancias, J.D.; Callery, M.P.; Mahadevan, A.; Tseng, J.F. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Comito, T.; Ghidini, A.; Torri, V.; Scorsetti, M.; Barni, S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bohoudi, O.; Bruynzeel, A.M.E.; Senan, S.; Cuijpers, J.P.; Slotman, B.J.; Lagerwaard, F.J.; Palacios, M.A. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Strunk, H.M.; Henseler, J.; Rauch, M.; Mucke, M.; Kukuk, G.; Cuhls, H.; Radbruch, L.; Zhang, L.; Schild, H.H.; Marinova, M. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. Rofo 2016, 188, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Vroomen, L.; Petre, E.N.; Cornelis, F.H.; Solomon, S.B.; Srimathveeravalli, G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagn. Interv. Imaging 2017, 98, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Kristoffersson, S.; Andersson, R.; Bergenfeldt, M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: A systematic review of safety and efficacy. Scand. J. Gastroenterol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; Durham, A.N.; Besselink, M.G.; Iannitti, D.; Weiss, M.J.; Wolfgang, C.L.; Huang, K.W. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J. Surg. Oncol. 2016, 114, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Giardino, A.; Innamorati, G.; Ugel, S.; Perbellini, O.; Girelli, R.; Frigerio, I.; Regi, P.; Scopelliti, F.; Butturini, G.; Paiella, S.; et al. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology 2017. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Chen, J.; He, L.; Liao, M.; Yuan, Y.; Zeng, J.; Li, J.; Zuo, J.; Xu, K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 2013, 42, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Rovere-Querini, P.; Manfredi, A.A. Tumor destruction and in situ delivery of antigen presenting cells promote anti-neoplastic immune responses: Implications for the immunotherapy of pancreatic cancer. JOP 2004, 5, 308–314. [Google Scholar] [PubMed]
- Vatner, R.E.; Cooper, B.T.; Vanpouille-Box, C.; Demaria, S.; Formenti, S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014, 4, 325. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Adsay, N.V.; Argani, P.; Iacobuzio-Donahue, C.; De Marzo, A.; Cameron, J.L.; Yeo, C.J.; Hruban, R.H. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod. Pathol. 2003, 16, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized phase 2 trial of the oncolytic virus pelareorep (reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Sze, D.Y.; Reid, T.R.; Rose, S.C. Oncolytic virotherapy. J. Vasc. Interv. Radiol. 2013, 24, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
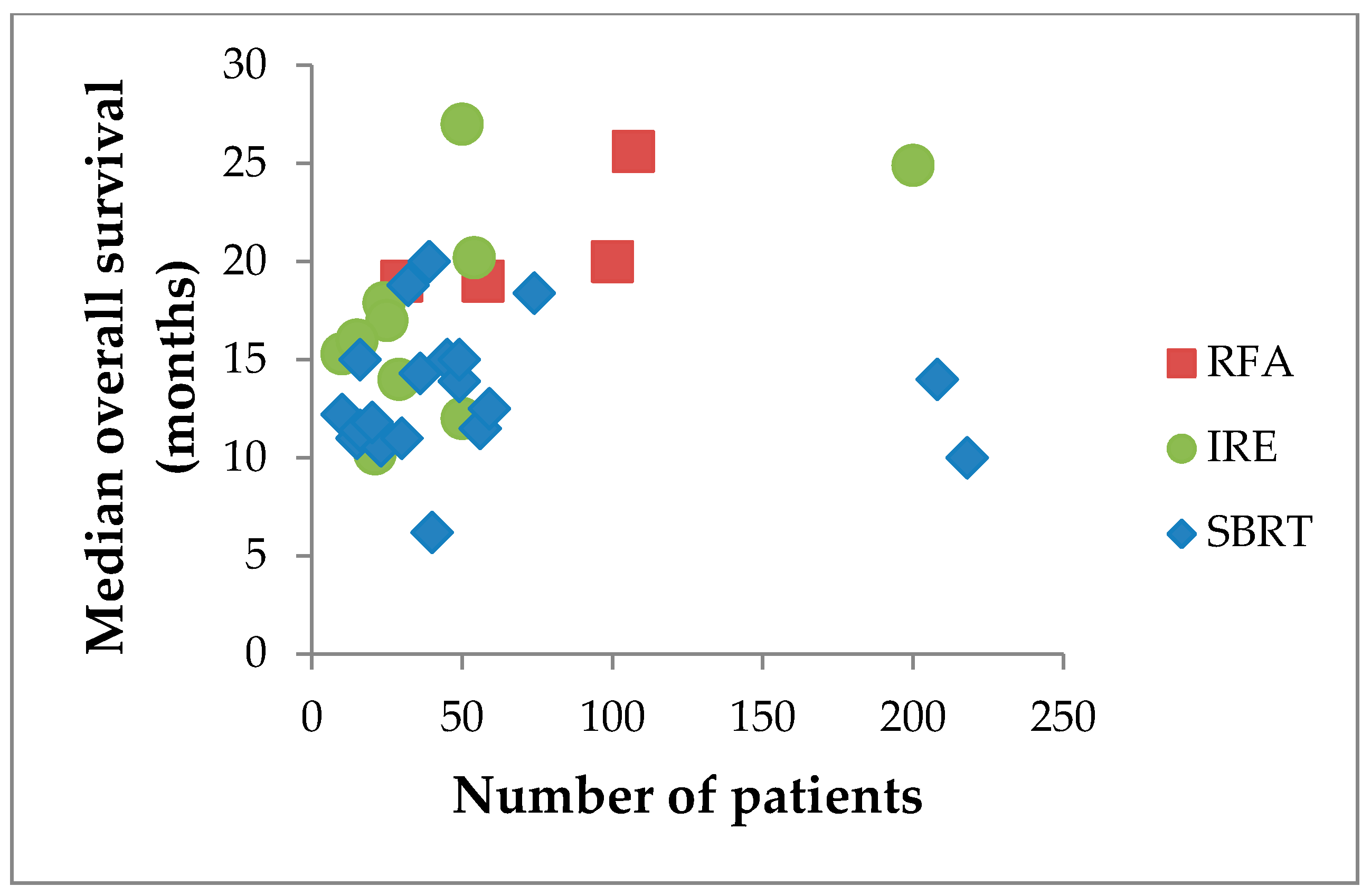
| Reference | Design | # pts | Age, yrs | Size, mm | Morbidity | 30-day Mortality | Median FU | Median OS |
|---|---|---|---|---|---|---|---|---|
| Cantore [21] ** | Prospective | 107 | N.S. | N.S. | 28.0% (n = 30) | 1.9% (n = 2) | N.R. | 25.6 months |
| D’Onofrio [24] ** | Prospective | 18 | mean 62.4 | mean 48.1 (25–86) | 0% | 0% | N.R. | N.R. |
| Frigerio [25] ** | Retrospective | 57 | med 63 | N.R. | 14% (n = 8) | 0% | N.R. | 19 months |
| Girelli [23] ** | Prospective | 50 | med 64.5 | med 40 (IQR 30–50) | 24% (n = 12) | 2% (n = 1) | 8 months | N.R. |
| Girelli [22] ** | Prospective | 100 | mean 64 | med 36 (IQR 30–45) | 26% (n = 26) | 3% (n = 3) | 12 months | 20 months |
| Paiella [26] ** | Retrospective | 30 | N.R. | N.R. | N.R. | 0% | 15 months | 19 months * |
| Reference | # pts | Age, yrs | RECIST | Median OS | Morbidity | Pain Relief |
|---|---|---|---|---|---|---|
| Gao [31] | 39 | med 58 (42–79) | CR 0 PR 5 (12.8%) SD 25 (64.1%) PD 9 (23.1%) | 11.0 months | 12.8% | Total 31 (79.5%) Complete 9 (23.1%) Partial 22 (56.4%) † |
| Li [32] | 16 | mean 62 (49–72) | CR 0% * PR 43.7% SD 25% PD 31.3% | 14.0 months (from treatment) | 12.5% | Mean pre-VAS 5.1 Mean post-VAS 3.3 Median PRT 5.6 months |
| Ning [37] | 100 | N.S. | N.R. | 8.3 months | 23.2% | N.R. |
| Shi [38] | 71 | N.R. | N.R. | N.R. | N.R. | Pre-HIFU 70.42% painless Post-HIFU 92.96% painless |
| Sofuni [33] | 30 16 III | N.S. | N.S. | N.R. | 10% | 66.7% (N.S.) ‡ |
| Sung [34] | 46 18 III | N.S. | N.R. | N.S. | N.R. | Pre-VAS 4.9 Post-VAS 2.1 p < 0.001 (N.S.) |
| Wang [35] | 40 13 III | N.S. | N.S. | 10 months | 0% | Total 35 (87.5%) (N.S.) Complete 9 (22.5%) Partial 26 (65%) Median PRT 10 weeks |
| Xiong [39] | 89 39 III | N.S. | N.S. | 11.2 months | 11.2% | Total 54 (80.6%) (N.S.) Complete 21 (31.3%) Partial 33 (49.3%) † |
| Zhao H. [36] | 39 31 III | N.S. | N.S. | N.S. | N.R. | Total 22 (78.6%) (N.S.) Complete 9 (32.1%) Partial 13 (46.4%) † |
| Zhao J. [40] | 38 | med 75 (62–80) | N.R. | 6.0 months vs. 10.3 months ~ | N.R. | N.R. |
| Reference | Design | # pts | Age, yrs | Median Dose | Fractions | Median FU | Local Control | Median OS | Downstage |
|---|---|---|---|---|---|---|---|---|---|
| Alagappan [52] | Retrospective | 208 * | med 75.2 (IQR 65.9–86.1) | 25 Gy (103 pts) 33 Gy (105 pts) | 1 5 | 7.5 months | 87% | 14.0 months (OSd) | N.R. |
| Chang [53] | Retrospective | 77 56 III | N.S. | 25 Gy (61 pts) 25 Gy + EBRT | 1 1 + 25 | 6 months | 87% (N.S.) | 6.7 months (OSt) ‡ 11.5 months (OSd) ‡ | 1 pt (N.S.) |
| Chuong [54] | Retrospective | 73 16 III | N.S. | 30 Gy | 5 | 7.8 months | 1-year LC = 81% (N.S.) | 15 months (OSd) ‡ | 0 |
| Comito [43] | Prospective | 45 | mean 68 (40–87) | 45 Gy | 6 | 13.5 months | 89% | 15 months (OSd) | 3 pts |
| Dholakia [44] | Prospective | 32 | N.R. | 33 Gy | 5 | 13.4 months | 72% | 18.8 months (OSd) | N.R. |
| Gurka [45] | Prospective | 10 | mean 62.5 (50–79) | 25 Gy | 5 | N.R. | 40% | 12.2 months (N.S.) | 0 |
| Herman [46] | Prospective | 49 | med 67 (35–87) | 33 Gy | 5 | 13.9 months | max. 78% | 13.9 months (OSd) | 5 pts |
| Koong [47] | Prospective | 15 | med 62 (43–82) | 20 Gy | 1 | 5 months | max. 80% | 11 months (OSd) | N.R. |
| Mahadevan 2011 [56] | Retrospective | 39 | med 67 (44–88) | 24.92 Gy | 3 | 21 months | 85% | 20 months (OSd) | N.R. |
| Mahadevan 2010 [55] | Retrospective | 36 | med 65 (43–88) | 29.33 Gy | 3 | 24 months | 78% | 14.3 months (OSd) | N.R. |
| Mellon [57] | Retrospective | 159 49 III | med 67.2 (47–85) | 40 Gy | 5 | 14.0 months | 1-year LC = 78% (N.S.) | 15.0 months (OSd) ‡ | 5 pts |
| Moningi [58] | Retrospective | 88 74 III | N.S. | 33 Gy | 5 | 14.6 months | N.R. | 18.4 months (OSd) ‡ | 15 pts |
| Polistina [48] | Prospective | 23 | med 68 (44–75) | 30 Gy | 3 | 9 months | 82% | 10.6 months (OSd) | 2 pts |
| Rwigema [59] | Retrospective | 71 40 III | N.S. | 24 Gy | 1–3 | 6.0 months | 53% | 6.2 months (OSt) ‡ | N.R. |
| Schellenberg 2008 [49] | Prospective | 16 | med 69 (39–87) | 25 Gy | 1 | 9.1 months | 81% | 11.4 months (OSd) | 0 |
| Schellenberg 2011 [50] | Prospective | 20 | med 63 (45–85) | 25 Gy | 1 | N.R. | 75% | 11.8 months (OSd) | 0 |
| Song [60] | Retrospective | 59 | med 62 (28–86) | 45 Gy | 5 (3–8) | 10.9 months | N.R. | 12.5 months (OSt) | N.R. |
| Tozzi [51] | Prospective | 30 | mean 67 (43–87) | 45 Gy | 6 | 11 months | 86% | 11 months (OSt) | N.R. |
| Zhu [61] | Retrospective | 417 218 III | N.S. | 30–46.8 Gy | 5–8 | 11 months | N.R. | 10.0 months (OSd) ‡ | N.R. |
| Reference | # pts | Age, yrs | Size, mm | Approach | Treatment | Median FU | Median OS | Local Failure | Down-Stage | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Belfiore [71] | 29 | med 68.5 (55–81) | N.R. | Perc | Local | 29 months | 14 months (OSt) | 3% | N = 3 | N.R. |
| Dunki–Jacobs [72] | 65 | N.R. | med 35 | Perc 12 Open 53 | Local | 23 months | N.R. | 26% | N.R. | N.R. |
| Kluger [67] | 50 | med 66.5 (IQR 60.2–72.0) | med 30 (IQR 17–50) | N.R. | Margin 24 Local 29 | 8.69 months | 12.03 months (OSt) | 11% | N.R. | 6% (n = 3) * |
| Lambert [73] | 21 | 68.2 | 39 (21–65) | Perc 2 Open 19 | Local | N.R. | 10.2 months (OSt) | N.R. | N.R. | 0 |
| Mansson [74] | 24 | med 65 (42–77) | med 35 (15–45) | Perc | Local | N.R. | 17.9 months (OSd) 7.0 months (OSt) | 58.3% | N = 2 | 4% (n = 1) |
| Martin 2012 [69] | 27 | med 61 (45–82) | med 30 | Perc 1 Open 26 | Margin 8 Local 19 | 90 days | N.R. | 0% | N.R. | 4% (n = 1) |
| Martin 2013 [70] | 54 | med 61 (45–80) | N.R. | Open 52 Lap 2 | Margin 19 Local 35 | 15 months | 20.2 months (OSd) | 27.8% | N.R. | 2% (n = 1) |
| Martin 2015 [68] | 200 | med 62 (27–88) | med 28 | Open | Margin 50 Local 150 | 29 months | 24.9 months (OSd) | 6% | N.R. | 2% (n = 3) |
| Narayanan [75] | 50 | med 62.5 (46–91) | mean 32 (15–80) | Perc | Local | N.R. | 27.0 months (OSd) 14.2 months (OSt) | 18% | N = 3 | 6% (n = 3) |
| Paiella [76] | 10 | med 66 | med 30 (25–39) | Open | Local | 7.6 months | 15.3 months (OSd) | N.R. | N.R. | 0 |
| Scheffer [77] | 25 | med 61 (41–78) | med 40 (33–50) | Perc | Local | 12 months | 17 months (OSd) 11 months (OSt) | N.R. | N.R. | 0 |
| Vogel [78] | 15 | N.R. | N.R. | Open | Local | 24 months | 16 months (OSd) | N.R. | N.R. | 13% (n = 2) |
| Yan [79] | 25 | med 58 (49–80) | med 42 (28–49) | Open | Local | N.R. | N.R. | N.R. | N.R. | 4% (n = 1) |
| Zhang [80] | 21 | N.R. | med 35 (20–67) | Perc | Local | 1 month | N.R. | 0 | N.R. | 0 |
| Technique | Advantage | Disadvantage |
|---|---|---|
| RFA | Easily applicable; superior availability; low costs. Open approach allows for exploration of peritoneal cavity; percutaneous approach seems less invasive, however limited data (one study) for LAPC. Indication of RFA-based immunomodulation: general activation of adaptive immune response along with a decrease of immunosuppression [96]. | Tumor debulking, since a safety margin is required to prevent thermal damage to critical structures such as large blood vessels and bile ducts. All available literature from one single center. Heat-sink effect, decreasing treatment efficacy of tumors surrounding large vessels. 30-day mortality (0–3%); relatively high complication rate: 0–28%. |
| MWA | Limited data for pancreatic cancer | |
| Cryoablation | Presumed abscopal effect, especially when combined with immunotherapy [97]. | Cryoshock syndrome. Hemorrhage induced by ice-ball cracking. Probe-size demands open approach. No survival benefit for cryoablation with palliative bypass surgery versus bypass surgery alone. |
| HIFU | No needles required. Effective technique for pain relief. | Limited survival data. Complication rate: 0–23.2%. Risk of second and third degree skin burns and subcutaneous fat sclerosis. |
| SBRT | Noninvasive, except for the implantation of the fiducials (though, very low complication rate). Treatment in the outpatient setting. | No uniform data with regard to radiation doses used, making comparisons difficult. Retreatment often impossible. Lower dose at the border of the tumor due to organs at risk (OARs). Risk of late complications (i.e., >3 months after SBRT): 0–13% (≥grade 3); acute complication rate: 0–28.4% (≥grade 3). |
| Iodine-125 seeds | Limited data for pancreatic cancer. Implantation demands open approach. | |
| IRE | Deployable as primary tumor control or margin accentuation after resection. Treatment is repeatable. Preservation of critical structures, such as biliary ducts and large blood vessels. Not susceptible to heat-sink effect. Open approach allows for exploration of peritoneal cavity; percutaneous approach is less invasive. | No uniform protocol. High learning curve. 90-day mortality (0–13%); relatively high complication rate: 0–30% (≥grade 3). |
| PDT | Preservation of connective tissues, maintaining the mechanical integrity of critical structures, such as intestines and blood vessels. | Limited data for pancreatic cancer. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruarus, A.; Vroomen, L.; Puijk, R.; Scheffer, H.; Meijerink, M. Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers 2018, 10, 16. https://doi.org/10.3390/cancers10010016
Ruarus A, Vroomen L, Puijk R, Scheffer H, Meijerink M. Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers. 2018; 10(1):16. https://doi.org/10.3390/cancers10010016
Chicago/Turabian StyleRuarus, Alette, Laurien Vroomen, Robbert Puijk, Hester Scheffer, and Martijn Meijerink. 2018. "Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies" Cancers 10, no. 1: 16. https://doi.org/10.3390/cancers10010016
APA StyleRuarus, A., Vroomen, L., Puijk, R., Scheffer, H., & Meijerink, M. (2018). Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers, 10(1), 16. https://doi.org/10.3390/cancers10010016






