3D Printing Solutions for Microfluidic Chip-To-World Connections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stereolithography
2.2. Cell Line for Biocompatibility Test
2.3. Cell Line Preparation
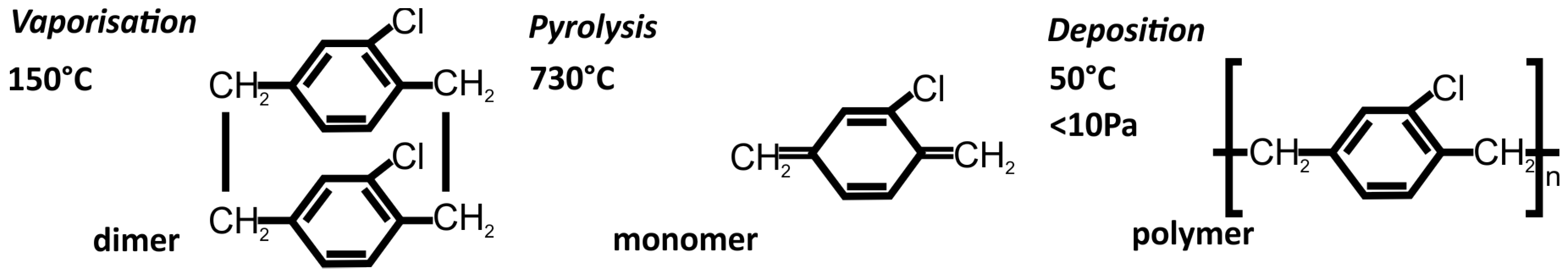
2.4. Coating of 3D-Printed Structures with Parylene-C
3. Results and Discussion
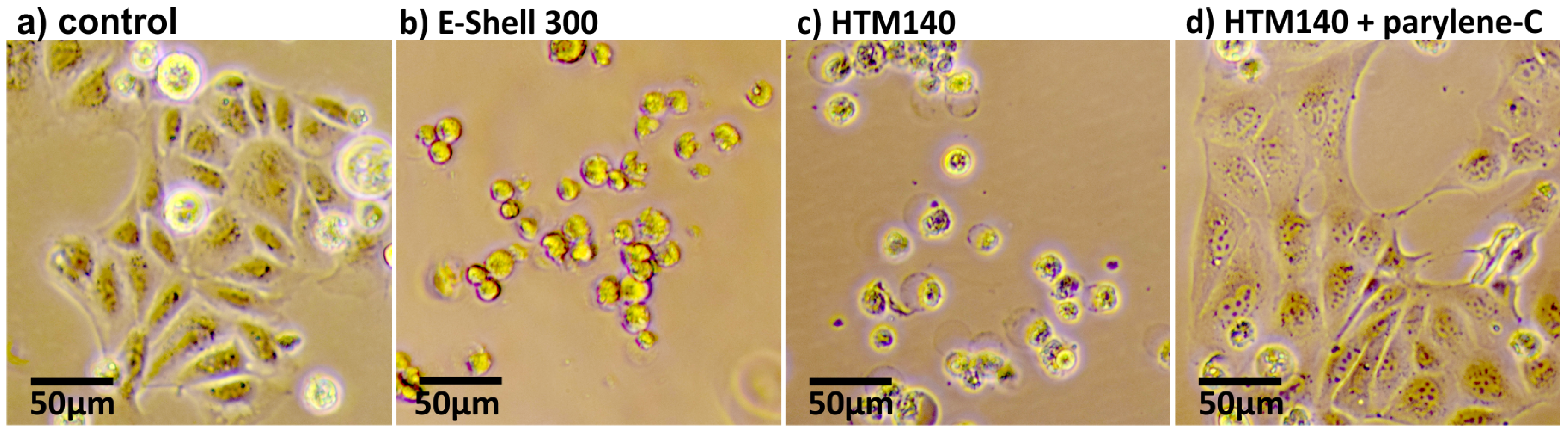
3.1. Cytotoxicity Tests of MDCK and HaCaT Cells Exposed to 3D-Printed Structures
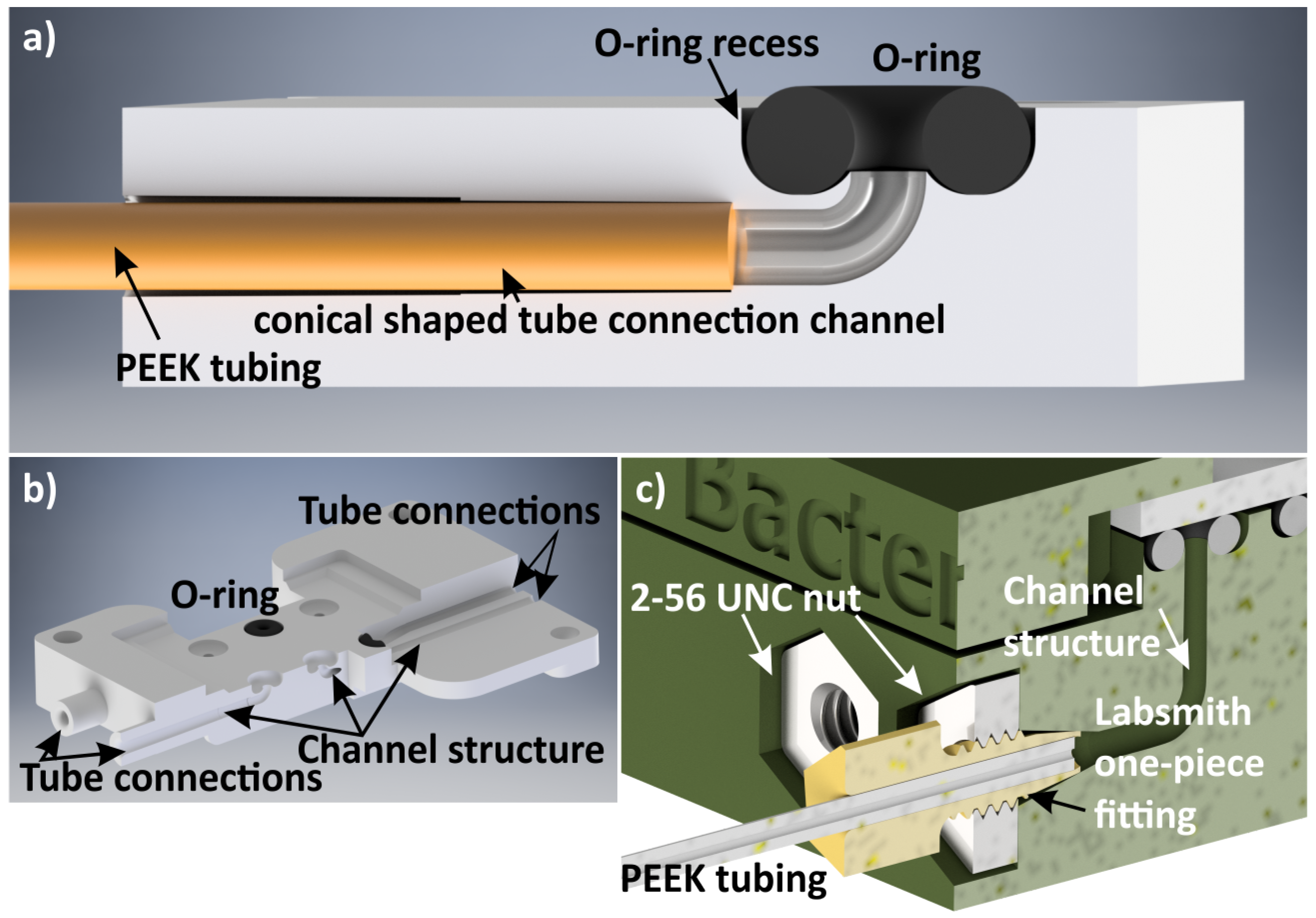
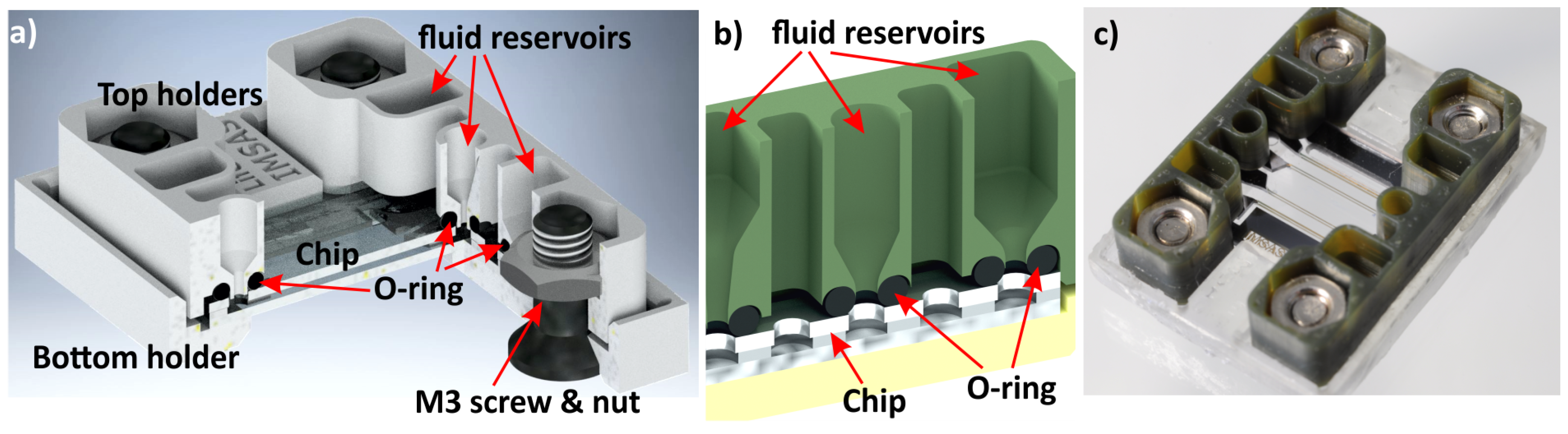
3.2. Fluidic Chip Holder Connections
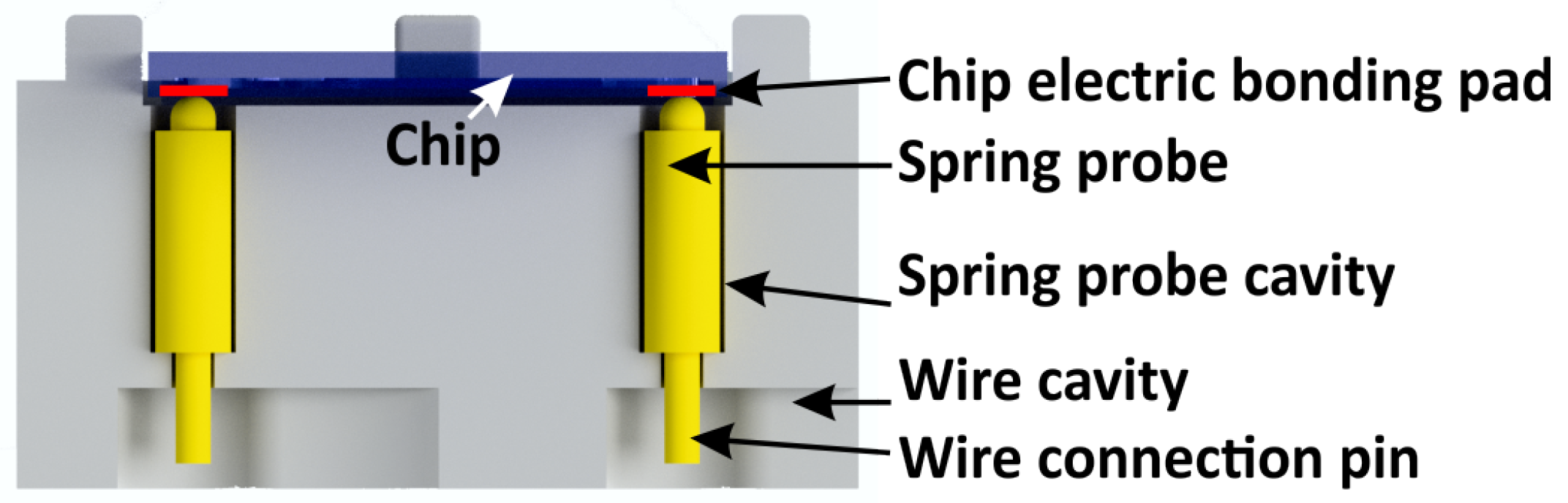
3.3. Electric Chip Holder Connections
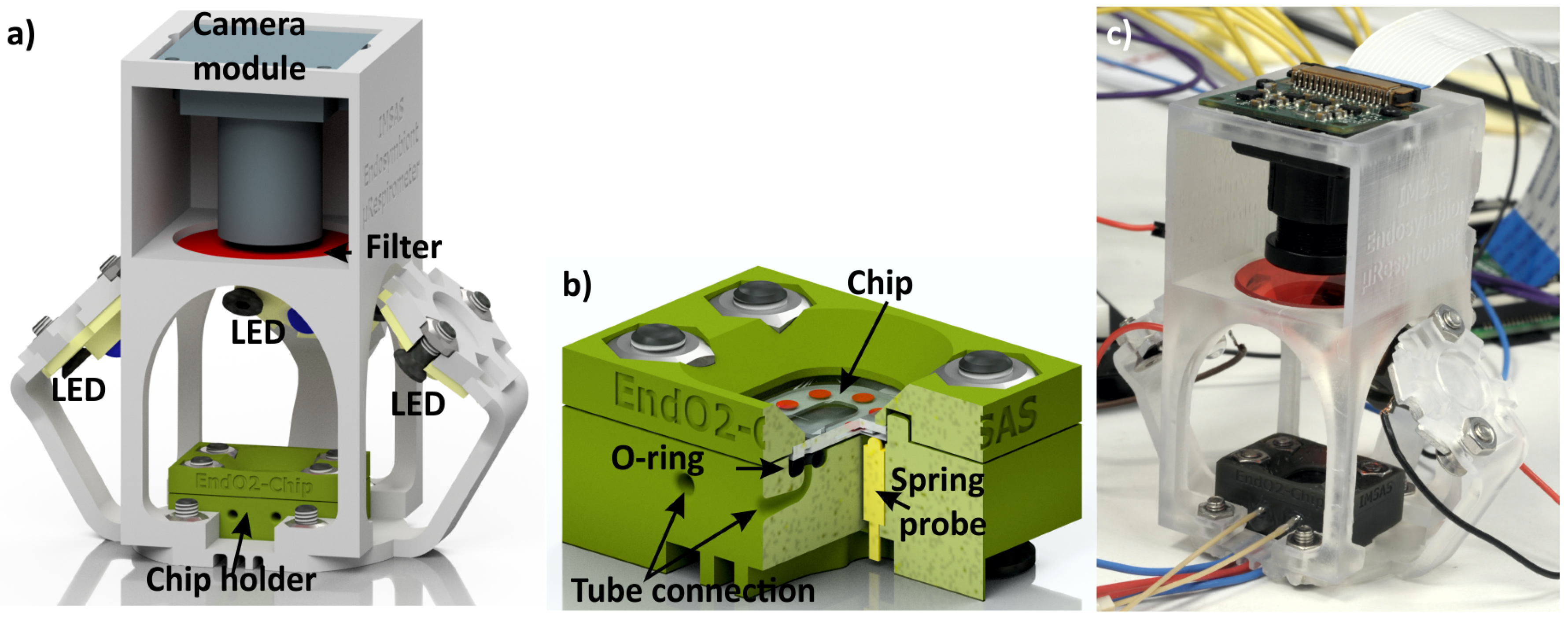
3.4. 3D-Printed Microfluidic Chip Holders for Cell-Growth and Cell-Analysis Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CLIP | Continuous Liquid Interface Production |
| DLP | Digital Light Processing |
| EPDM | Ethylene Propylene Diene Monomer |
| GMEM | Glasgow Minimum Essential Medium |
| MDCK | Madin-Darby Canine Kidney |
| PEEK | Polyether Ether Ketone |
| STL | Standard Tessellation Language |
References
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D printing microfluidics and applications in chemistry and biology: A review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The emerging frontiers and applications of high-resolution 3D printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Guo, X.; Kong, B.; Zhang, M.; Qian, X.; Mi, S.; Sun, W. The boom in 3D-printed sensor technology. Sensors 2017, 17, 1166. [Google Scholar] [CrossRef] [PubMed]
- Krujatz, F.; Lode, A.; Seidel, J.; Bley, T.; Gelinsky, M.; Steingroewer, J. Additive Biotech—Chances, challenges, and recent applications of additive manufacturing technologies in biotechnology. New Biotechnol. 2017, 39, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Niemöller, A.; Jakes, P.; Kayser, S.; Lin, Y.; Lehnert, W.; Granwehr, J. 3D printed sample holder for in-operando EPR spectroscopy on high temperature polymer electrolyte fuel cells. J. Magn. Reson. 2016, 269, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.T.; Kozek, K.M.; Hucknall, A.; Smith, D.R.; Chilkoti, A. Nanoparticle–film plasmon ruler interrogated with transmission visible spectroscopy. ACS Photonics 2014, 1, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Desmaison, A.; Lorenzo, C.; Rouquette, J.; Ducommun, B.; Lobjois, V. A versatile sample holder for single plane illumination microscopy. J. Microsc. 2013, 251, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, A.; Busch, J.; van der Woerd, H.; Zielinski, O. SmartFluo: A method and affordable adapter to measure chlorophyll a fluorescence with smartphones. Sensors 2017, 17, 678. [Google Scholar] [CrossRef] [PubMed]
- Cevenini, L.; Calabretta, M.M.; Tarantino, G.; Michelini, E.; Roda, A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sens. Actuators B Chem. 2016, 225, 249–257. [Google Scholar] [CrossRef]
- Yafia, M.; Ahmadi, A.; Hoorfar, M.; Najjaran, H. Ultra-portable smartphone controlled integrated digital microfluidic system in a 3D-printed modular assembly. Micromachines 2015, 6, 1289–1305. [Google Scholar] [CrossRef]
- Lücking, T.H.; Sambale, F.; Beutel, S.; Scheper, T. 3D-printed individual labware in biosciences by rapid prototyping: A proof of principle. Eng. Life Sci. 2015, 15, 51–56. [Google Scholar] [CrossRef]
- Shemelya, C.; Cedillos, F.; Aguilera, E.; Espalin, D.; Muse, D.; Wicker, R.; MacDonald, E. Encapsulated copper wire and copper mesh capacitive sensing for 3-D printing applications. IEEE Sens. J. 2015, 15, 1280–1286. [Google Scholar] [CrossRef]
- Plevniak, K.; Campbell, M.; Myers, T.; Hodges, A.; He, M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics 2016, 10, 054113. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Ermagan, R.; Thompson, B.; Friedman, A.; Malmstadt, N. Modular, discrete micromixer elements fabricated by 3D Printing. Micromachines 2017, 8, 137. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Bhattacharjee, N.; Horowitz, L.F.; Chang, T.C.; Folch, A. 3D-printed microfluidic automation. Lab Chip 2015, 15, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Van den Driesche, S.; Bunge, F.; Lucklum, F.; Vellekoop, M.J. 3D-Printing: An attractive tool to realize microfluidic chip holders. In Proceedings of the 3rd International Conference on MicroFluidic Handling Systems, Enschede, The Netherlands, 4–6 October 2017; pp. 94–97. [Google Scholar]
- Oomen, P.; Mulder, J.; Verpoorte, E.; Oleschuk, R. Controlled, synchronized actuation of microdroplets by gravity in a superhydrophobic, 3D-printed device. Anal. Chim. Acta 2017, 988, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.C.; Thompson, B.; Malmstadt, N. Discrete elements for 3D microfluidics. Proc. Natl. Acad. Sci. 2014, 111, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- Paydar, O.; Paredes, C.; Hwang, Y.; Paz, J.; Shah, N.; Candler, R. Characterization of 3D-printed microfluidic chip interconnects with integrated O-rings. Sens. Actuators A Phys. 2014, 205, 199–203. [Google Scholar] [CrossRef]
- Alharbi, N.; Wismeijer, D.; Osman, R. Additive manufacturing techniques in prosthodontics: Where do we currently stand? A critical review. Int. J. Prosthodont. 2017, 30, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bunge, F.; van den Driesche, S.; Vellekoop, M. Microfluidic platform for the long-term on-chip cultivation of mammalian cells for lab-on-a-chip applications. Sensors 2017, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Labsmith One-Piece Fitting. Available online: http://products.labsmith.com/one-piece-fitting (accessed on 25 October 2017).
- Buesink, W.; Heeren, H. Design Guideline for Microfluidic Side Connect. Available online: http://www.enablingmnt.co.uk/wp-content/uploads/2017/06/Design-Guidelines-for-Microfluidic-Side-Connect-version-1.0.pdf (accessed on 5 February 2018).
- Bullema, J.E.; Heeren, H.; Buesink, W. Guidelines for Packaging of Microfluidics: Electrical Interconnections. Available online: http://mf-manufacturing.eu/wp-content/uploads/Guidelinesfor-Electrical-Interconnections-to-microfluidic-devices-version-1.0.pdf (accessed on 5 February 2018).
- Heeren, H.; Atkins, T.; Verplanck, N.; Peponnet, C.; Hewkin, P.; Blom, M.; Buesink, W.; Bullema, J.E.; Dekker, S. Design Guideline for Microfluidic Device and Component Interfaces (Part 1). Available online: http://www.enablingmnt.co.uk/wp-content/uploads/2016/05/Design-for-Microfluidic-Interfacing-White-Paper-part-1-version-2.pdf (accessed on 5 February 2018).
- Heeren, H.; Atkins, T.; Verplanck, N.; Peponnet, C.; Hewkin, P.; Blom, M.; Buesink, W.; Bullema, J.E.; Dekker, S. Design Guideline for Microfluidic Device and Component Interfaces (Part 2). Available online: http://www.enablingmnt.co.uk/wp-content/uploads/2017/06/Design-for-Microfluidic-Interfacing-White-Paper-part-2-version-3.0.pdf (accessed on 5 February 2018).
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fang, N.; Wu, D.; Zhang, X. Projection micro-stereolithography using digital micro-mirror dynamic mask. Sens. Actuators A Phys. 2005, 121, 113–120. [Google Scholar] [CrossRef]
- EnvisionTec HTM140 Resin. Available online: https://envisiontec.com/wp-content/uploads/2016/09/2018-HTM-140-V2-1.pdf (accessed on 25 October 2017).
- EnvisionTec E-Shell 300 Resin. Available online: https://envisiontec.com/wp-content/uploads/2016/09/2018-E-Shell-300-Series.pdf (accessed on 26 January 2018).
- Kip, S.N.; Strehler, E.E. Characterization of PMCA isoforms and their contribution to transcellular Ca2+ flux in MDCK cells. Am. J. Physiol. Ren. Physiol. 2003, 284, F122–F132. [Google Scholar] [CrossRef] [PubMed]
- Rehders, M.; Grosshäuser, B.B.; Smarandache, A.; Sadhukhan, A.; Mirastschijski, U.; Kempf, J.; Dünne, M.; Slenzka, K.; Brix, K. Effects of lunar and mars dust simulants on HaCaT keratinocytes and CHO-K1 fibroblasts. Adv. Space Res. 2011, 47, 1200–1213. [Google Scholar] [CrossRef]
- Parylene Coating. Available online: https://www.plasmaparylene.de/en/parylene-coating/parylenerosenheim.php (accessed on 5 February 2018).
- ISO 10993-6:2016, Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. Available online: https://www.iso.org/standard/61089.html (accessed on 5 February 2018).
- Chang, T.Y.; Yadav, V.G.; De Leo, S.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and protein compatibility of parylene-C surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, E.; Over, P.; Gridelli, B.; Gerlach, J.C. Response of primary human bone marrow mesenchymal stromal cells and dermal keratinocytes to thermal printer materials in vitro. J. Med. Biol. Eng. 2016, 36, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Stratasys MED610 Resin. Available online: http://www.stratasys.com/de/materials/search/biocompatible (accessed on 29 January 2018).
- Akhtar, M.; van den Driesche, S.; Bödecker, A.; Vellekoop, M.J. Long-term storage of droplets on a chip by Parylene AF4 coating of channels. Sens. Actuators B Chem. 2018, 255, 3576–3584. [Google Scholar] [CrossRef]
- Van den Driesche, S.; Bunge, F.; Tepner, S.; Kotitschke, M.; Vellekoop, M.J. Travelling-wave dielectrophoresis allowing flexible microchannel design for suspended cell handling. Proc. SPIE 2017, 10247, 102470H. [Google Scholar]
- Bunge, F.; van den Driesche, S.; Waite, A.; Mirastschijski, U.; Vellekoop, M.J. µRespirometer to determine the oxygen consumption rate of mammalian cells in a microfluidic cell culture. In Proceedings of the 2017 IEEE 30th International Conference onMicro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 414–417. [Google Scholar]
- TNO–Improved Stereolithography. Available online: https://www.tno.nl/en/focus-areas/industry/roadmaps/flexible-free-form-products/additive-manufacturing/improved-micro-stereolithography/ (accessed on 5 February 2018).
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Sandström, C.G. The non-disruptive emergence of an ecosystem for 3D Printing—Insights from the hearing aid industry’s transition 1989–2008. Technol. Forecast. Soc. Chang. 2016, 102, 160–168. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Larson, R. Footwear Assembly Method with 3D Printing. U.S. Patent 9,005,710, 14 April 2015. [Google Scholar]
- Weller, C.; Kleer, R.; Piller, F.T. Economic implications of 3D printing: Market structure models in light of additive manufacturing revisited. Int. J. Prod. Econ. 2015, 164, 43–56. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Driesche, S.; Lucklum, F.; Bunge, F.; Vellekoop, M.J. 3D Printing Solutions for Microfluidic Chip-To-World Connections. Micromachines 2018, 9, 71. https://doi.org/10.3390/mi9020071
Van den Driesche S, Lucklum F, Bunge F, Vellekoop MJ. 3D Printing Solutions for Microfluidic Chip-To-World Connections. Micromachines. 2018; 9(2):71. https://doi.org/10.3390/mi9020071
Chicago/Turabian StyleVan den Driesche, Sander, Frieder Lucklum, Frank Bunge, and Michael J. Vellekoop. 2018. "3D Printing Solutions for Microfluidic Chip-To-World Connections" Micromachines 9, no. 2: 71. https://doi.org/10.3390/mi9020071
APA StyleVan den Driesche, S., Lucklum, F., Bunge, F., & Vellekoop, M. J. (2018). 3D Printing Solutions for Microfluidic Chip-To-World Connections. Micromachines, 9(2), 71. https://doi.org/10.3390/mi9020071




