Demulsification of Kerosene/Water Emulsion in the Transparent Asymmetric Plate-Type Micro-Channel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Preparation of Kerosene-Water Emulsion
2.4. De-Emulsification Efficiency
3. Results and Discussion
3.1. Absorption and Coalescence of Oil Droplets on the Hydrophobic Wall of TAPM
3.2. Variation of Area Fractions of Oil Phase in TAPM
3.3. Photographs of De-Emulsified Emulsions with TAPM
3.4. Droplet Size Distributions of Emulsion I, II, and III
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| I | Emulsion I described in Table 2 |
| II | Emulsion II described in Table 2 |
| III | Emulsion III described in Table 2 |
| η | Demulsification efficiency |
| η(I − A) | Demulsification efficiency of emulsion I in TAPM A |
| η(II − A) | Demulsification efficiency of emulsion II in TAPM A |
| η(III − A) | Demulsification efficiency of emulsion III in TAPM A |
| ϕoil | Oil phase in the initial emulsion |
| ϕoil(I) | Oil phase in the initial Emulsion I |
| ϕoil(II) | Oil phase in the initial Emulsion II |
| ϕoil(III) | Oil phase in the initial Emulsion III |
| Ry | Fractions of yellow oil phase area of the total area of images in Figure 5 |
| TAPM A | Type A transparent asymmetric plate-type micro-channels in Table 1 |
| TAPM B | Type B transparent asymmetric plate-type micro-channels in Table 1 |
| V | Total volume of emulsion |
| Voil | Volume of oil phase separated |
| voil | Volume ratio of oil phases in emulsion |
| vwater | Volume ratio of waterphases in emulsion |
| PTFE | Polytetrafluoroethylene |
| SS | Stainless steel |
References
- Luo, G.S.; Wang, K.; Wang, Y.J.; Lv, Y.C.; Xu, J.H. Principles and applications of micro-structured chemical system. Chem. Ind. Eng. Prog. 2011, 30, 1637–1642. [Google Scholar] [CrossRef]
- Sun, C.; Pfeifer, P.; Dittmeyer, R. One-stage syngas-to-fuel in a micro-structured reactor: Investigation of integration pattern and operating conditions on the selectivity and productivity of liquid fuels. Chem. Eng. J. 2017, 326, 37–46. [Google Scholar] [CrossRef]
- Najafabadi, M.S.; Esfahany, M.N.; Wu, Z.; Sundén, B. Hydrodynamics and mass transfer in liquid-liquid non-circular microchannels: Comparison of two aspect ratios and three junction structures. Chem. Eng. J. 2017, 322, 328–338. [Google Scholar] [CrossRef]
- Ye, C.; Chen, G.; Yuan, Q. Process Characteristics of CO2 Absorption by Aqueous Monoethanola mine in a Microchannel Reactor. Chin. J. Chem. Eng. 2012, 20, 111–119. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Yang, J.; Qi, L.; Chen, Y.; Ma, H. Design and Fabrication of a Three Dimensional Spiral Micromixer. Chin. J. Chem. 2013, 31, 209–214. [Google Scholar] [CrossRef]
- Bally, F.; Serra, C.A.; Hessel, V.; Hadziioannou, G. Micromixer-assisted polymerization processes. Chem. Eng. Sci. 2011, 66, 1449–1462. [Google Scholar] [CrossRef]
- Wu, J.H.; Tang, Y.; Lu, L.S. Capillary force of a novel skew-grooved wick structure for micro heat pipes. J. Cent. South. Univ. Technol. 2011, 18, 2170–2175. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Chu, B.Z.; Cao, C.X.; Jin, Y.; Cheng, Y. Catalytic performance of Ni catalyst for steam methane reforming in a micro-channel reactor at high pressure. Chem. Eng. Prog. 2017, 118, 19–25. [Google Scholar] [CrossRef]
- Abdollahi, A.; Sharma, R.N.; Vatani, A. Fluid flow and heat transfer of liquid-liquid two phase flow in microchannels: A review. Int. Commun. Heat. Mass. 2017, 84, 66–74. [Google Scholar] [CrossRef]
- Li, M.; Li, D. Separation of Janus droplets, oil droplets in microchannels by wall-induced dielectrophoresis. J. Chromatogr. A 2017, 1501, 151–160. [Google Scholar] [CrossRef]
- Okubo, Y.; Toma, M.; Ueda, H.; Maki, T.; Mae, K. Microchannel devices for the coalescence of dispersed droplets produced for use in rapid extraction processes. Chem. Eng. J. 2004, 101, 39–48. [Google Scholar] [CrossRef]
- Kolehmainen, E.; Turunen, I. Micro-scale liquid–liquid separation in a plate-type coalesce. Chem. Eng. Prog. 2007, 46, 834–839. [Google Scholar] [CrossRef]
- Chen, X.; Lu, H.F.; Jiang, W.; Chu, L.Y.; Liang, B. De-emulsification of Kerosene/Water Emulsions with Plate-Type Microchannels. Ind. Eng. Chem. Res. 2010, 49, 9279–9288. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Marchal, P.J.; Portha, F.; Marchal, P.; Falk, L. Influence of the plate-type continuous micro-separator dimensions on the efficiency of demulsification of oil-in-water emulsion. Chem. Eng. Res. Des. 2014, 92, 2758–2769. [Google Scholar] [CrossRef]
- Ookawara, S.; Ishikawa, T.; Ogawa, K. Applicability of a Miniaturized Micro-Separator/Classifier to Oil-Water Separation. Chem. Eng. Technol. 2007, 30, 316–321. [Google Scholar] [CrossRef]
- Pu, Y.D.; Ruan, D.; Diliyaer, H.; Zhao, Z.G.; Chen, X. Demulsification of W/O emulsion with three-dimensional electric spiral plate-type microchannel. CIESC J. 2017, 68, 2790–2797. [Google Scholar] [CrossRef]
- Zhao, C.X.; Middelberg, A.P.J. Two-phase microfluidic flows. Chem. Eng. Sci. 2011, 66, 1394–1411. [Google Scholar] [CrossRef]
- Kanazawa, S.; Takahashi, Y.; Nomoto, Y. Emulsification and Demulsification Processes in Liquid–Liquid System by Electrostatic Atomization Technique. IEEE Trans. Ind. Appl. 2008, 44, 1084–1089. [Google Scholar] [CrossRef]
- Chester, A.K. The moldeling of coalescence process in fluid dispersions: A review of current understanding. Chem. Eng. Res. Des. 1991, 69, 259–270. [Google Scholar]
- Belkad, A.; Tarlet, D.; Montillet, A.; Bellettre, J.; Massoli, P. Optical diagnostics for W/O emulsification within impinging flow and right angle mini-channel. La HouilleBlanche 2013, 2, 52–59. [Google Scholar] [CrossRef]
- Cao, G.; Pan, M.Y.; Zhao, Z.G.; Chen, X. Hydrophobic modification and micro-nano structure characterization of silicate glass. J. Sichuan Univ. 2014, 51, 804–808. [Google Scholar]
- Lü, L.; Wu, K.J.; Tang, Y.; Tang, S.Y.; Liang, B. De-emulsification of 2-ethyl-1-hexanol/water emulsion using oil-wet narrow channel combined with low-speed rotation. Chin. J. Chem. Eng. 2018, 26, 2048–2054. [Google Scholar] [CrossRef]
- Tesfai, J.T.; Perry, R.N.; Jablonski, E.L. Water-in-oil emulsion separation within a milli-fluidic device. J. Colloid Interface Sci. 2011, 354, 895–899. [Google Scholar] [CrossRef]
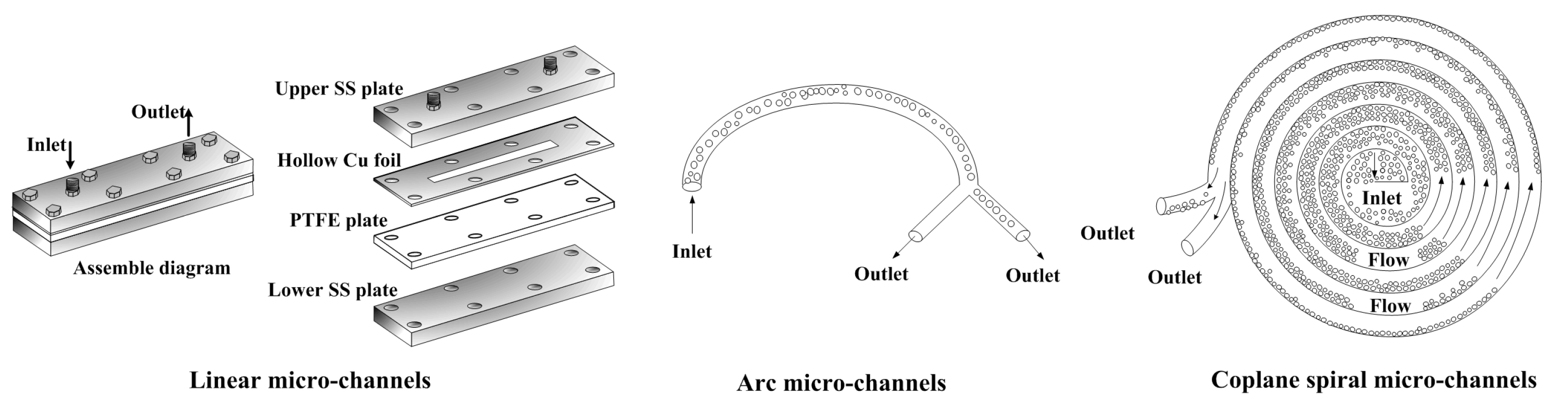
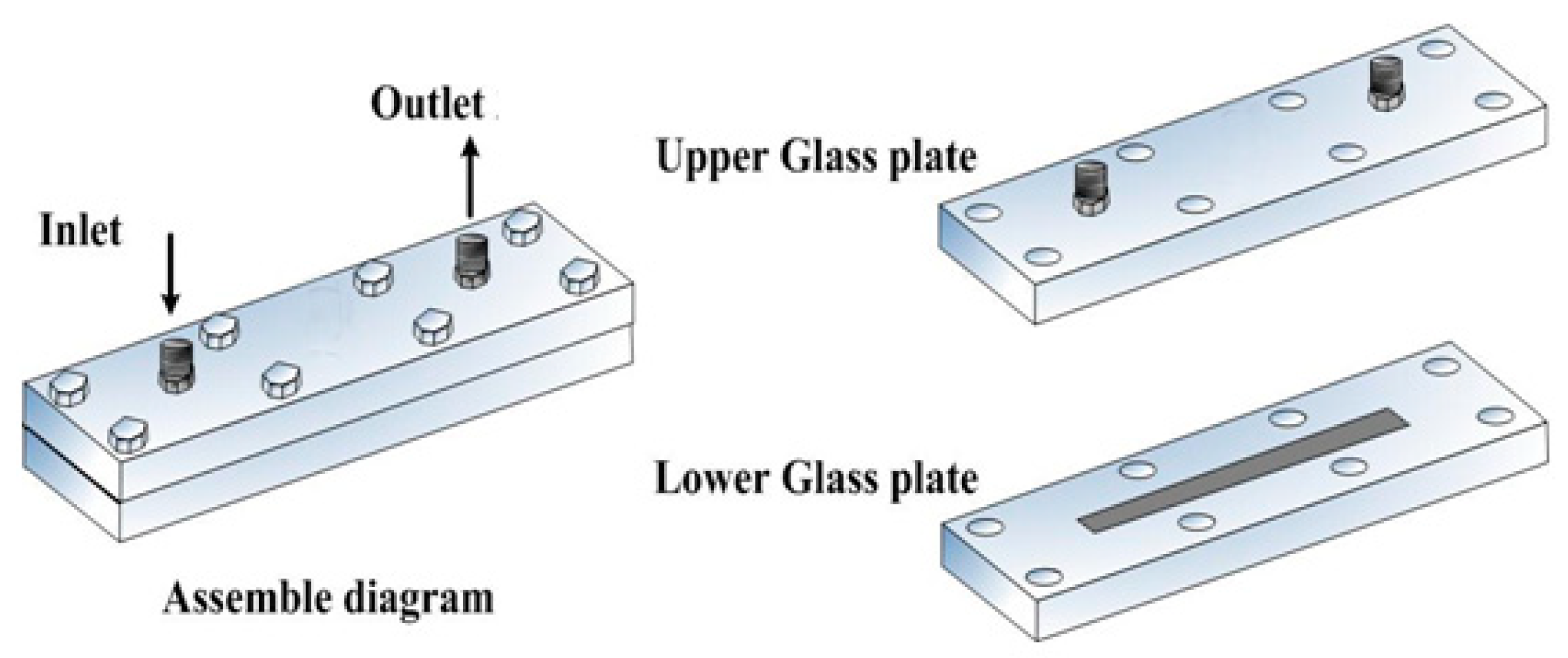
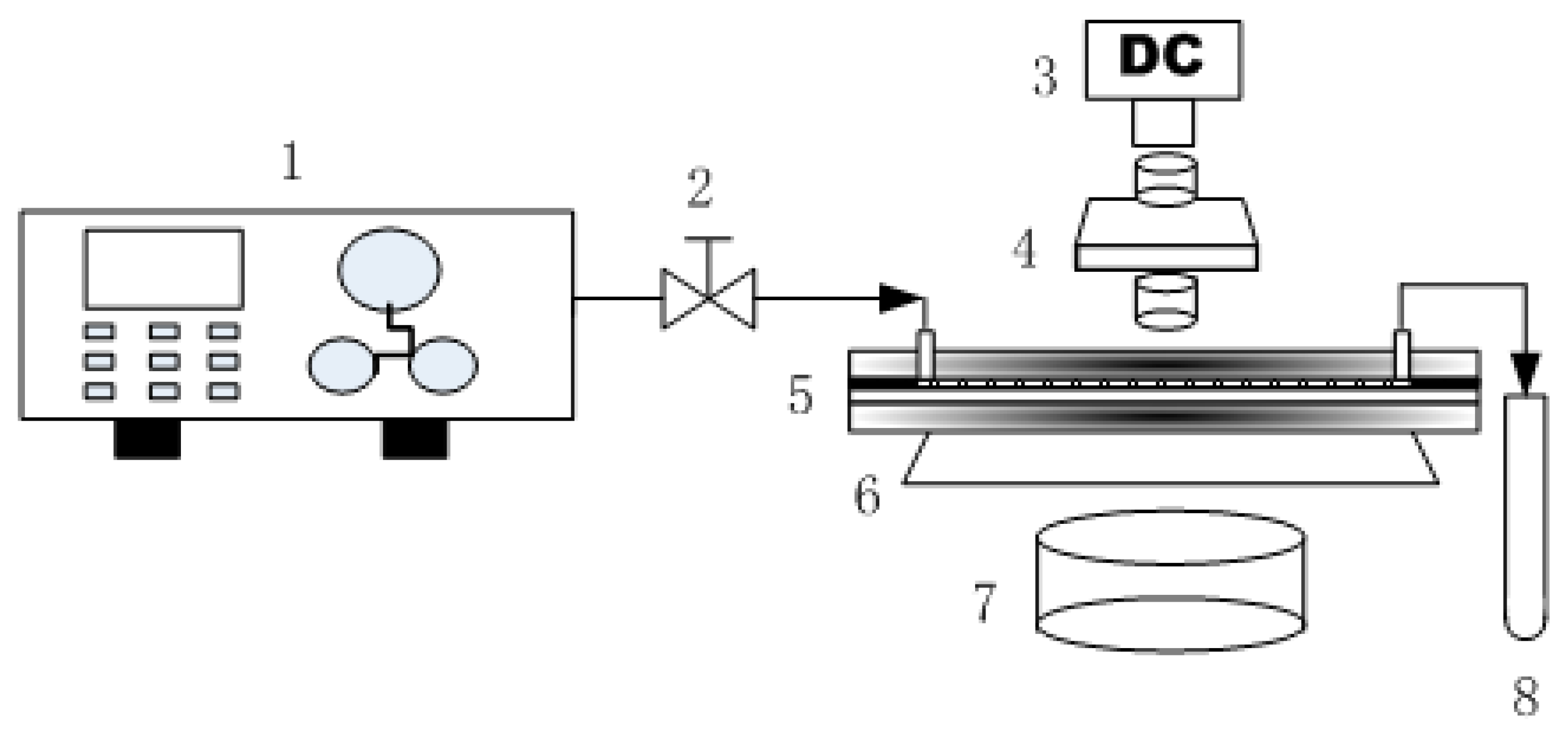
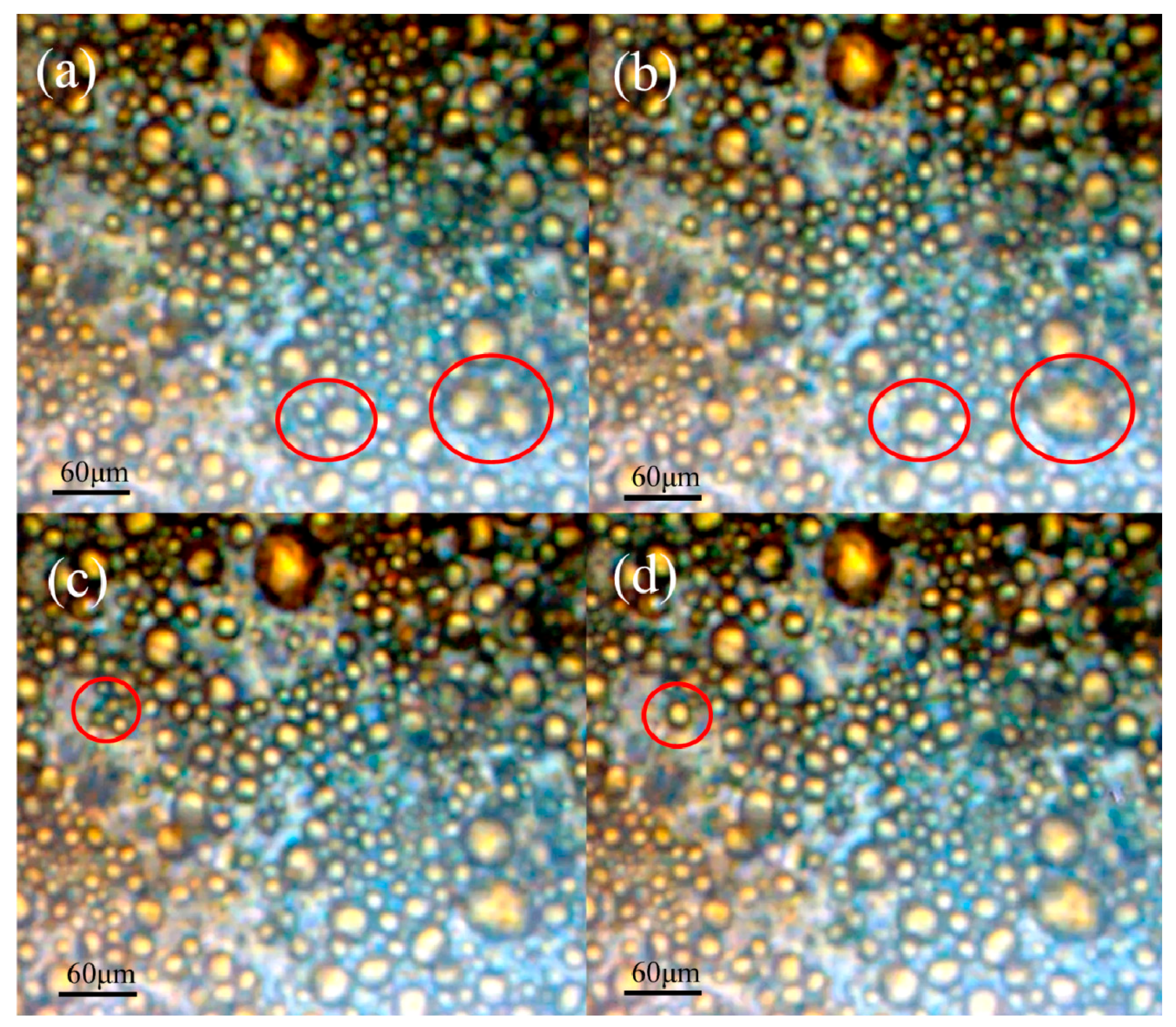

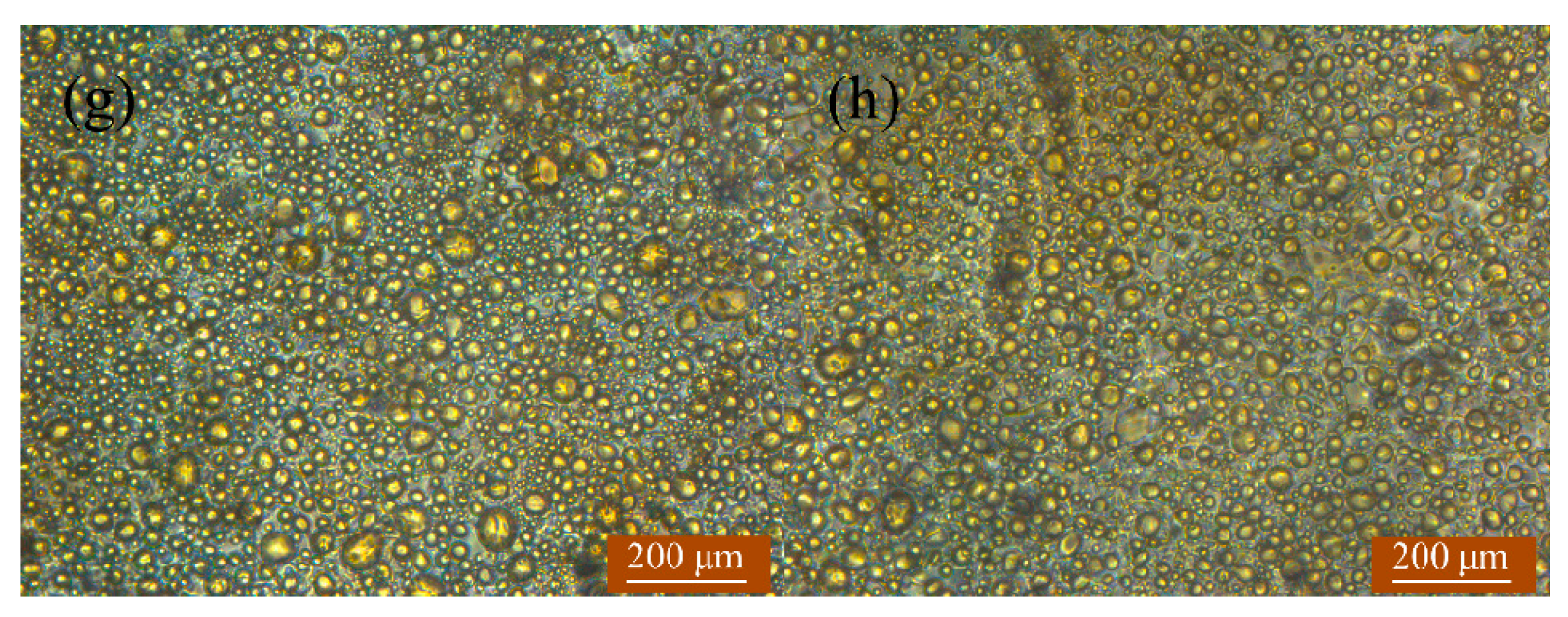
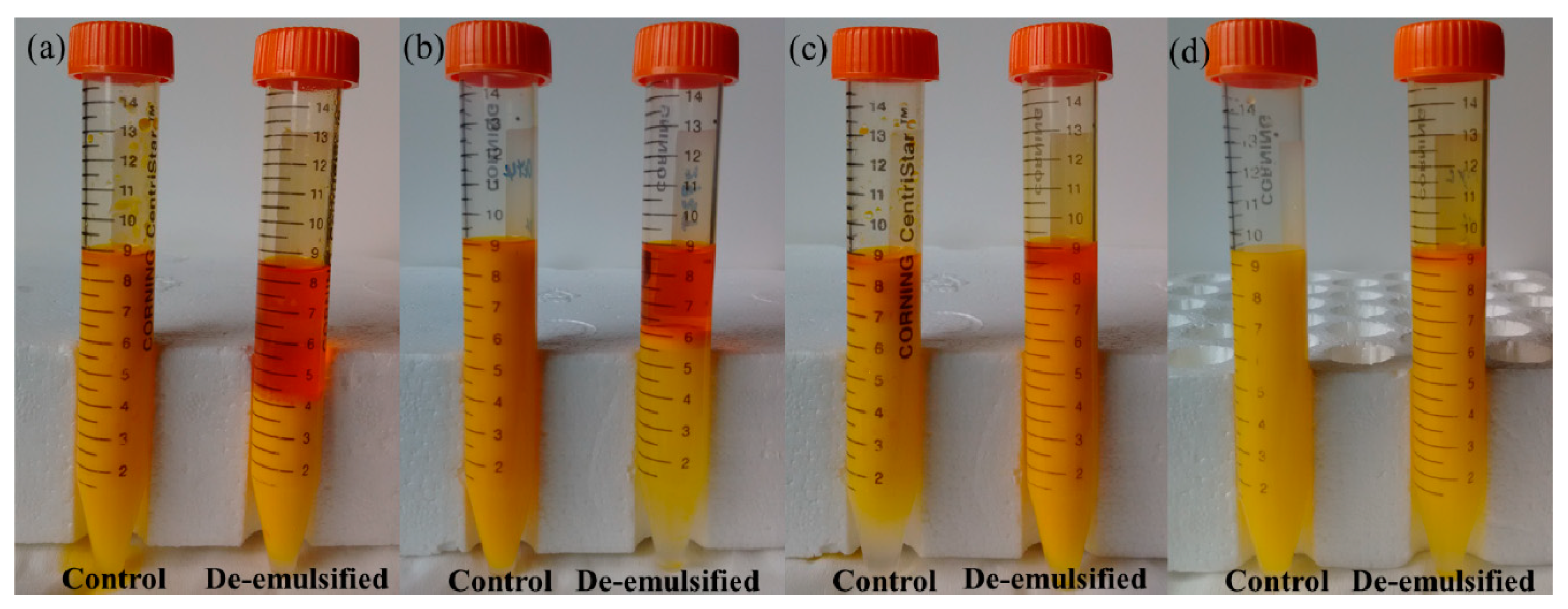
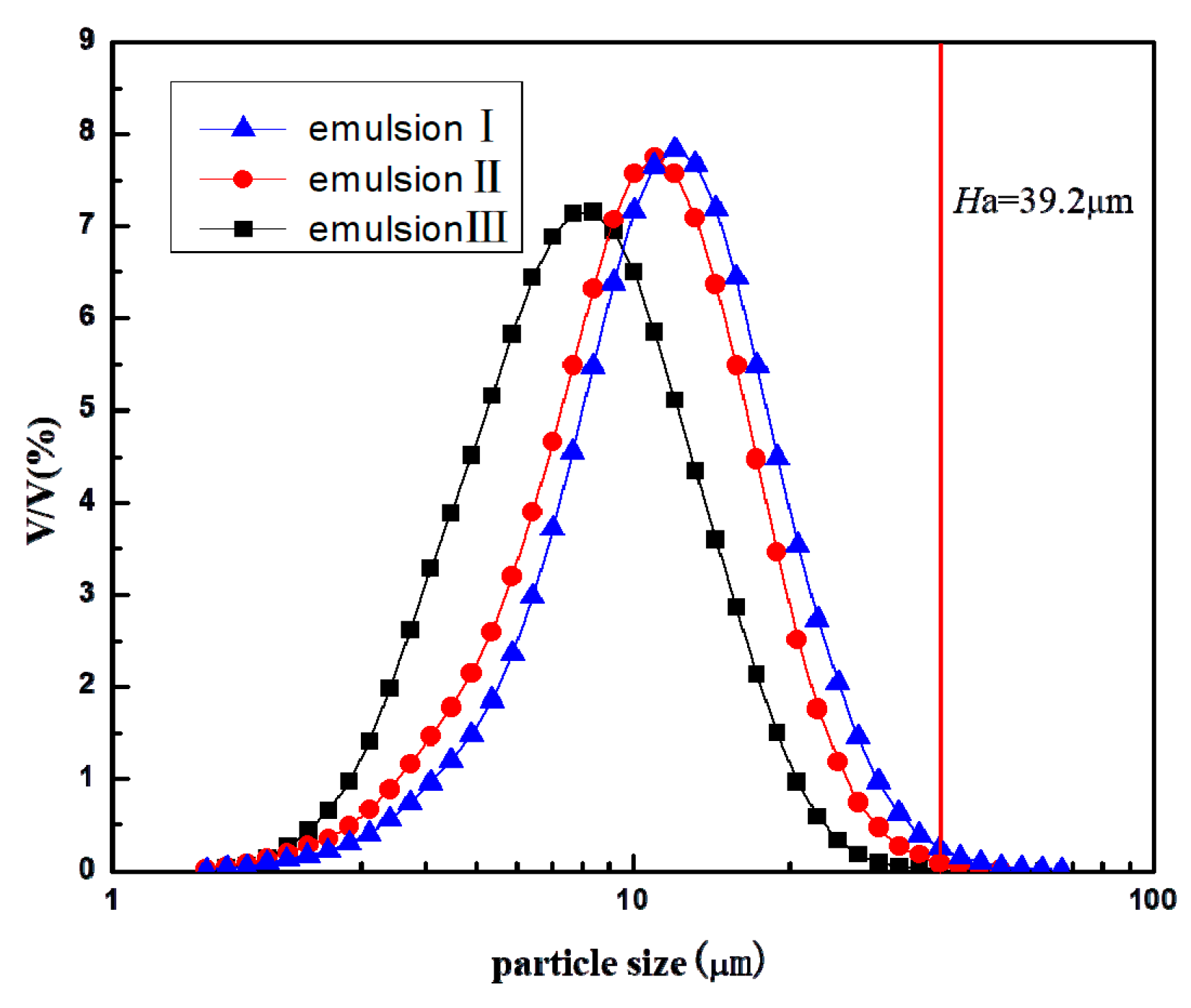
| No. | Length/mm | Width/mm | Depth/μm |
|---|---|---|---|
| TAPM A | 98.00 | 10.60 | 39.2 |
| TAPM B | 108.22 | 10.10 | 159.5 |
| No. | voil:vwater | Hydrophilic Lypophilic Balance (HLB) of Mixing Emulsifier | Content of Emulsifier /wt% | Average Droplet Size (D4,3)/μm |
|---|---|---|---|---|
| Emulsion I | 1:1 | 12 | 0.03 | 12.3 |
| Emulsion II | 3:7 | 12 | 0.01 | 10.8 |
| Emulsion III | 1:9 | 12 | 0.005 | 8.4 |
| Emulsion | TAPM | Capturing Position | Ry |
|---|---|---|---|
| Emulsion I | TAPM A | 37 mm | 22.4% |
| Emulsion I | TAPM A | 65 mm | 96.5% |
| Emulsion II | TAPM A | 37 mm | 25.7% |
| Emulsion II | TAPM A | 65 mm | 77.0% |
| Emulsion III | TAPM A | 37 mm | 38.6% |
| Emulsion III | TAPM A | 65 mm | 68.0% |
| Emulsion II | TAPM B | 37 mm | 24.3% |
| Emulsion II | TAPM B | 65 mm | 52.7% |
| Emulsion | TAPM | η |
|---|---|---|
| Emulsion I | TAPM A | 95.2% |
| Emulsion II | TAPM A | 56.8% |
| Emulsion III | TAPM A | 4.3% |
| Emulsion II | TAPM B | 33.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, D.; Hamiti, D.; Ma, Z.-D.; Pu, Y.-D.; Chen, X. Demulsification of Kerosene/Water Emulsion in the Transparent Asymmetric Plate-Type Micro-Channel. Micromachines 2018, 9, 680. https://doi.org/10.3390/mi9120680
Ruan D, Hamiti D, Ma Z-D, Pu Y-D, Chen X. Demulsification of Kerosene/Water Emulsion in the Transparent Asymmetric Plate-Type Micro-Channel. Micromachines. 2018; 9(12):680. https://doi.org/10.3390/mi9120680
Chicago/Turabian StyleRuan, Da, Diliyaer Hamiti, Zheng-Dong Ma, Ya-Dong Pu, and Xiao Chen. 2018. "Demulsification of Kerosene/Water Emulsion in the Transparent Asymmetric Plate-Type Micro-Channel" Micromachines 9, no. 12: 680. https://doi.org/10.3390/mi9120680
APA StyleRuan, D., Hamiti, D., Ma, Z.-D., Pu, Y.-D., & Chen, X. (2018). Demulsification of Kerosene/Water Emulsion in the Transparent Asymmetric Plate-Type Micro-Channel. Micromachines, 9(12), 680. https://doi.org/10.3390/mi9120680




