Recent Progress in Flexible Organic Thermoelectrics
Abstract
1. Introduction
2. Polymer-Based Thermoelectric Materials
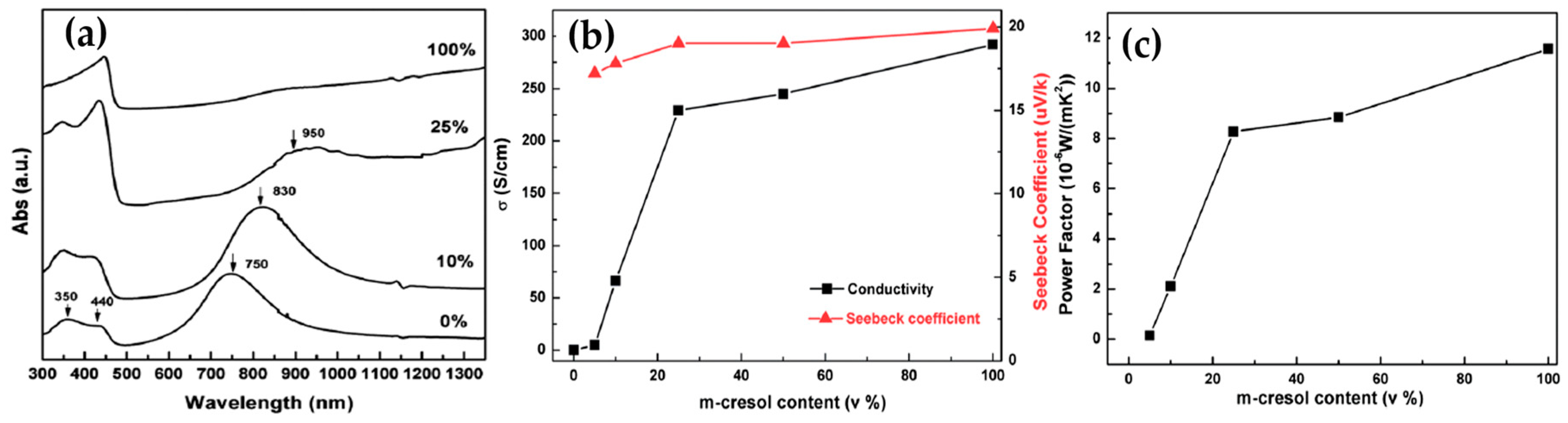
2.1. Polyaniline (PANi)
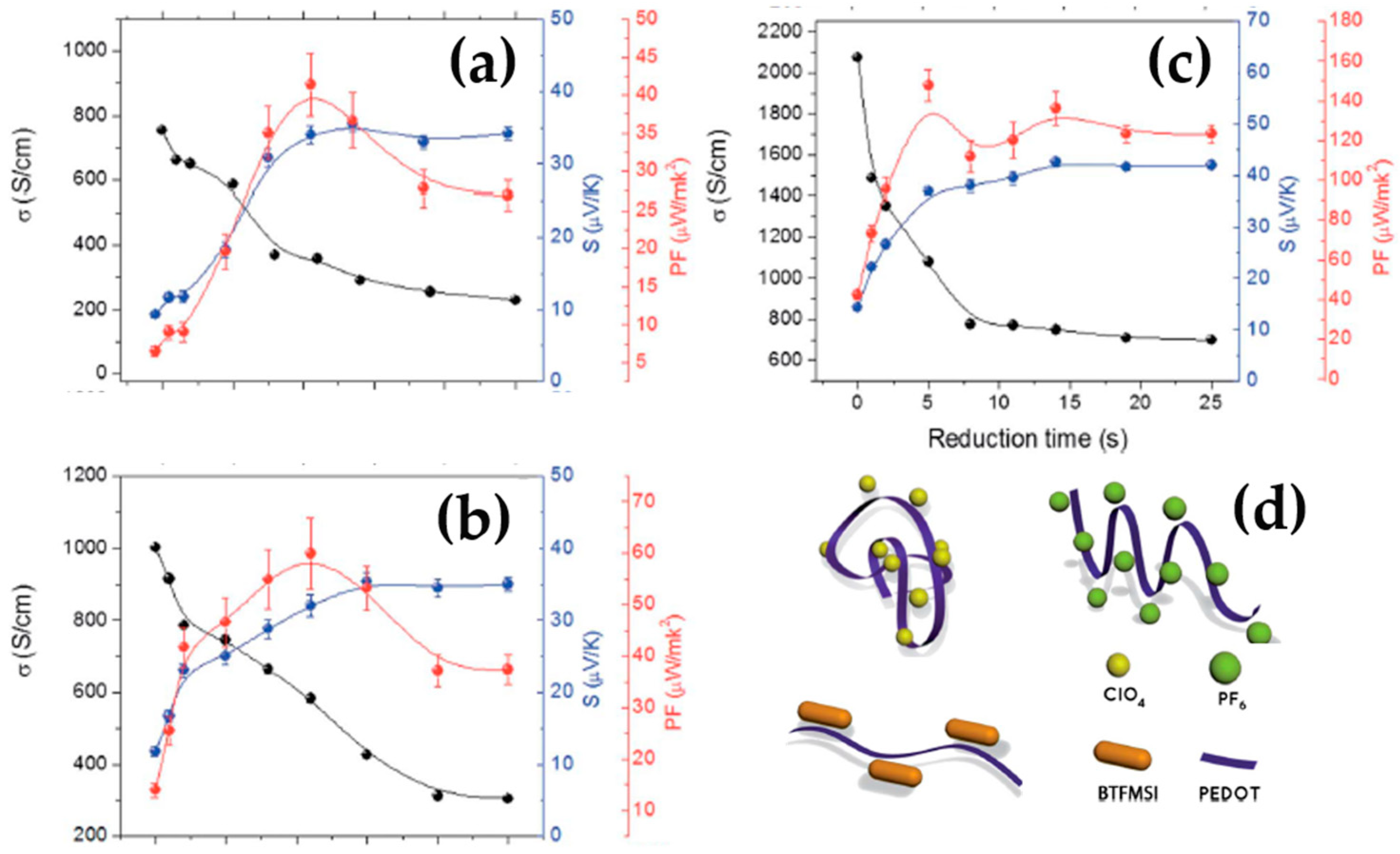
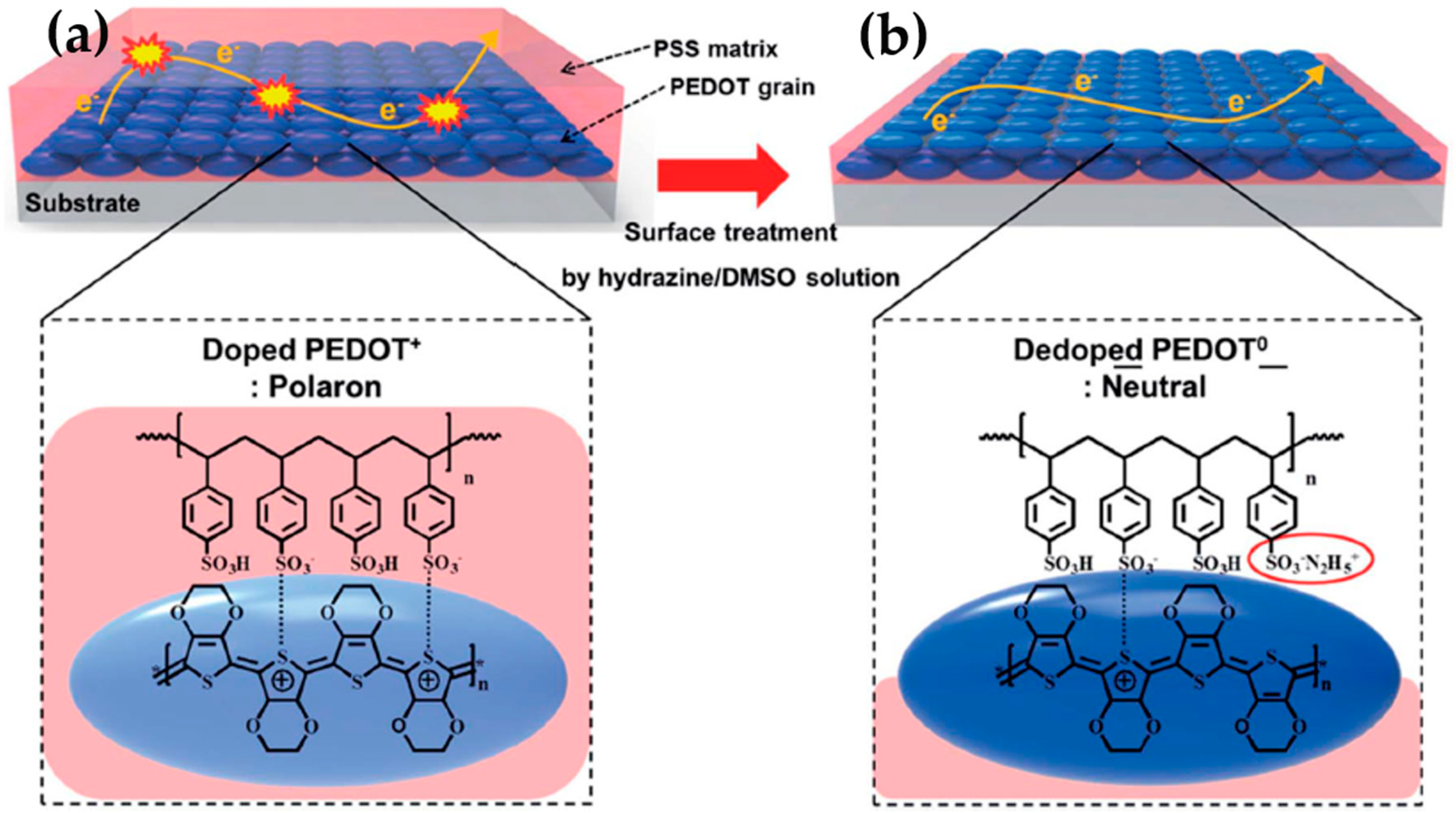
2.2. Poly(3,4-ethylenedioxythiophene) (PEDOT) and Their Derivatives
2.3. Poly(3-hexylthiophene) (P3HT)
2.4. Polymer/Polymer Thermoelectric Composites
3. Carbon-Based Polymer Nanocomposites
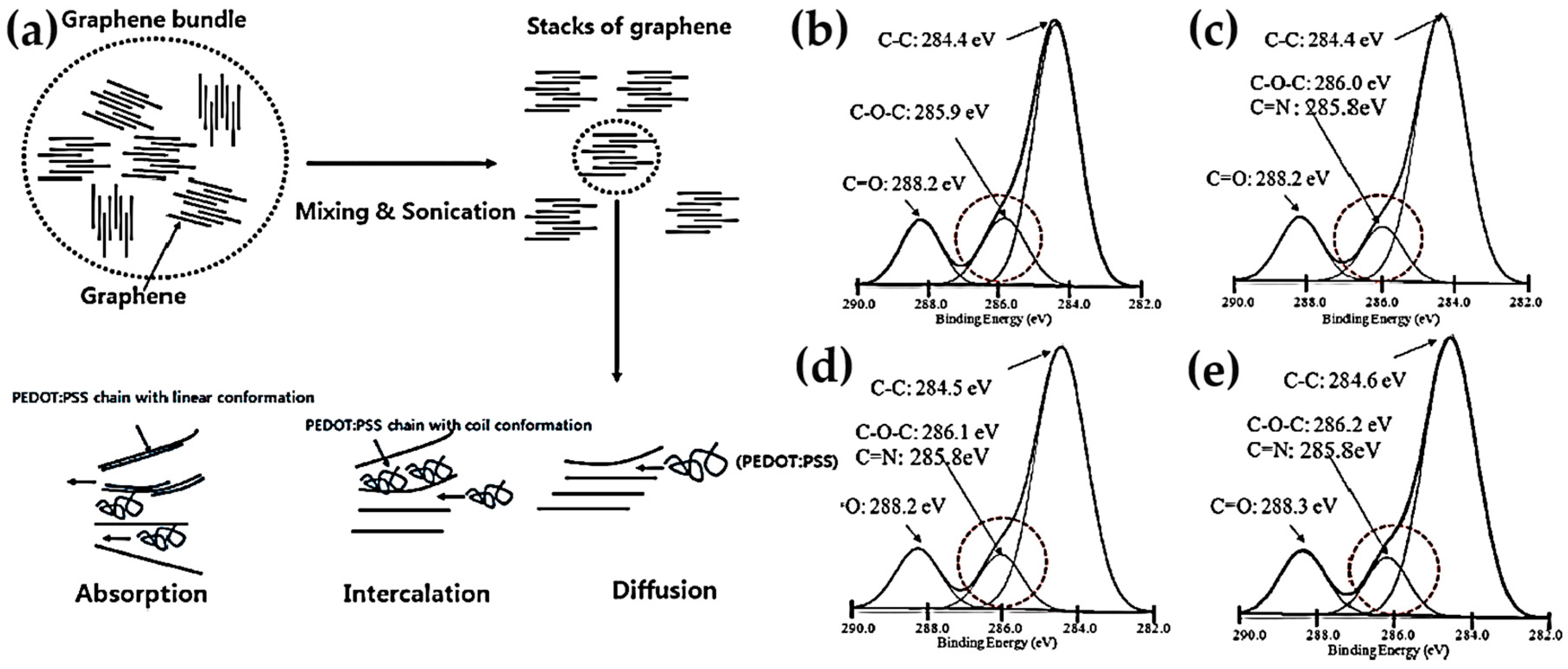
3.1. Graphene-Based Nanocomposites
3.1.1. PANi-Based Graphene Composites
3.1.2. Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS)-Based Graphene Composites
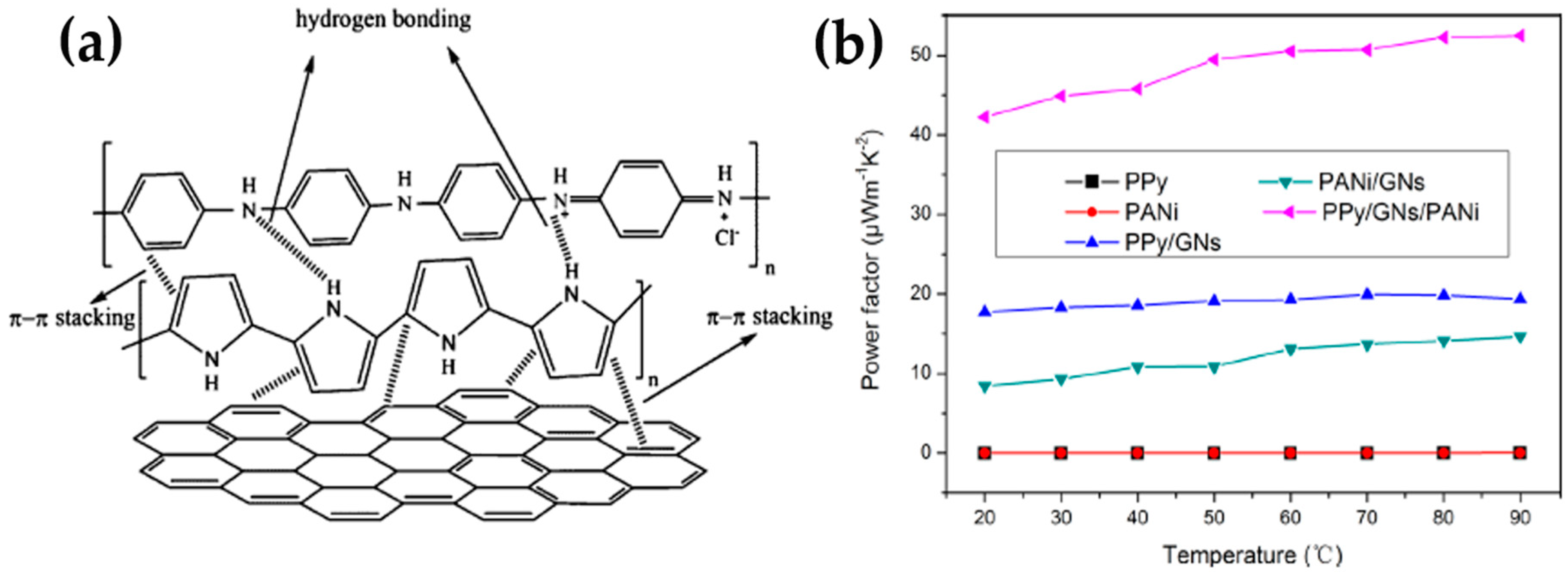
3.1.3. Other Conducting Polymers-Based Graphene Composites
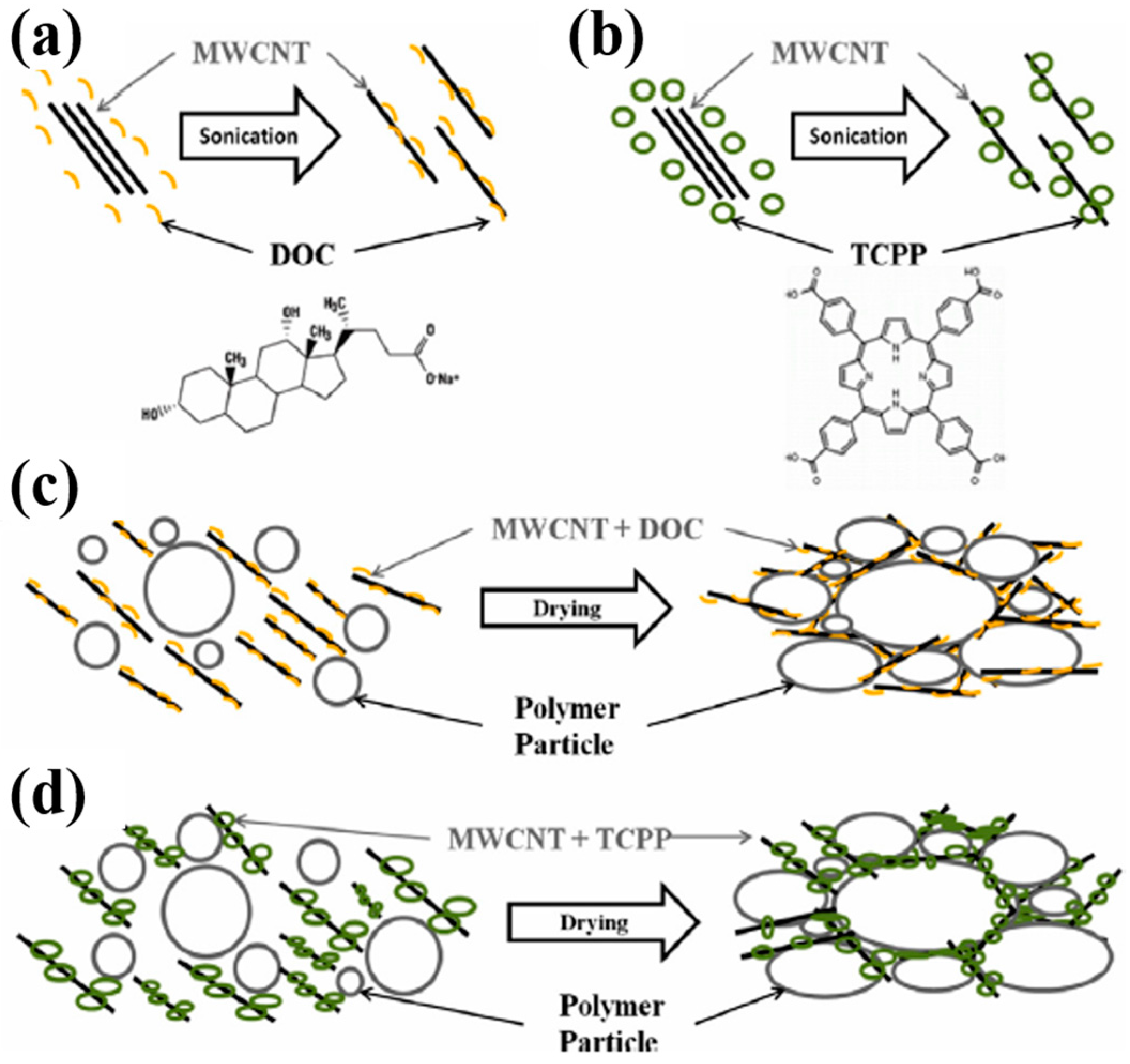
3.2. Carbon Nanotube-Based Nanocomposites
3.2.1. Non-Conductive Polymers-Based Carbon Nanotubes (CNTs) Composites
3.2.2. PANi-Based CNTs Composites
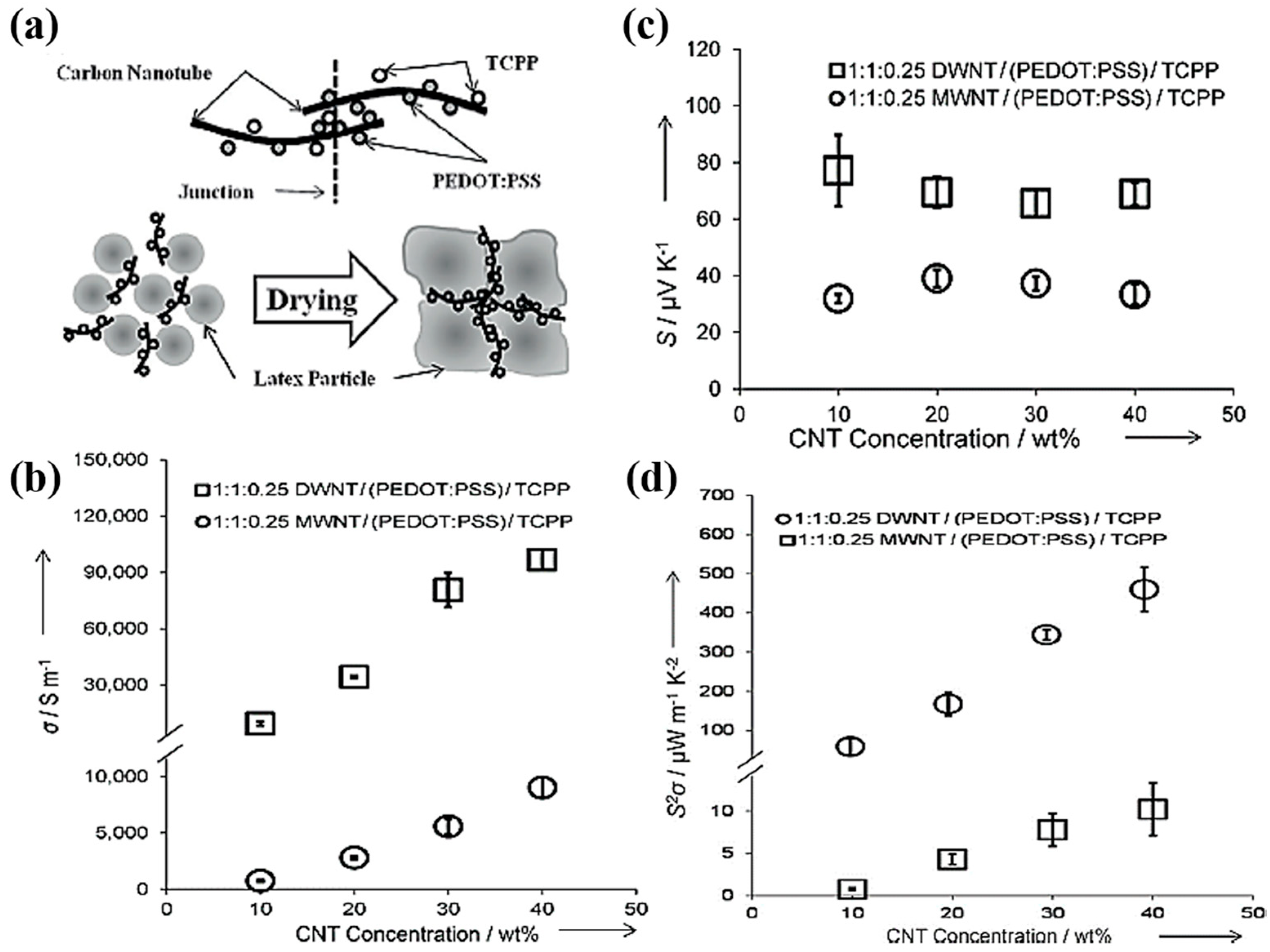
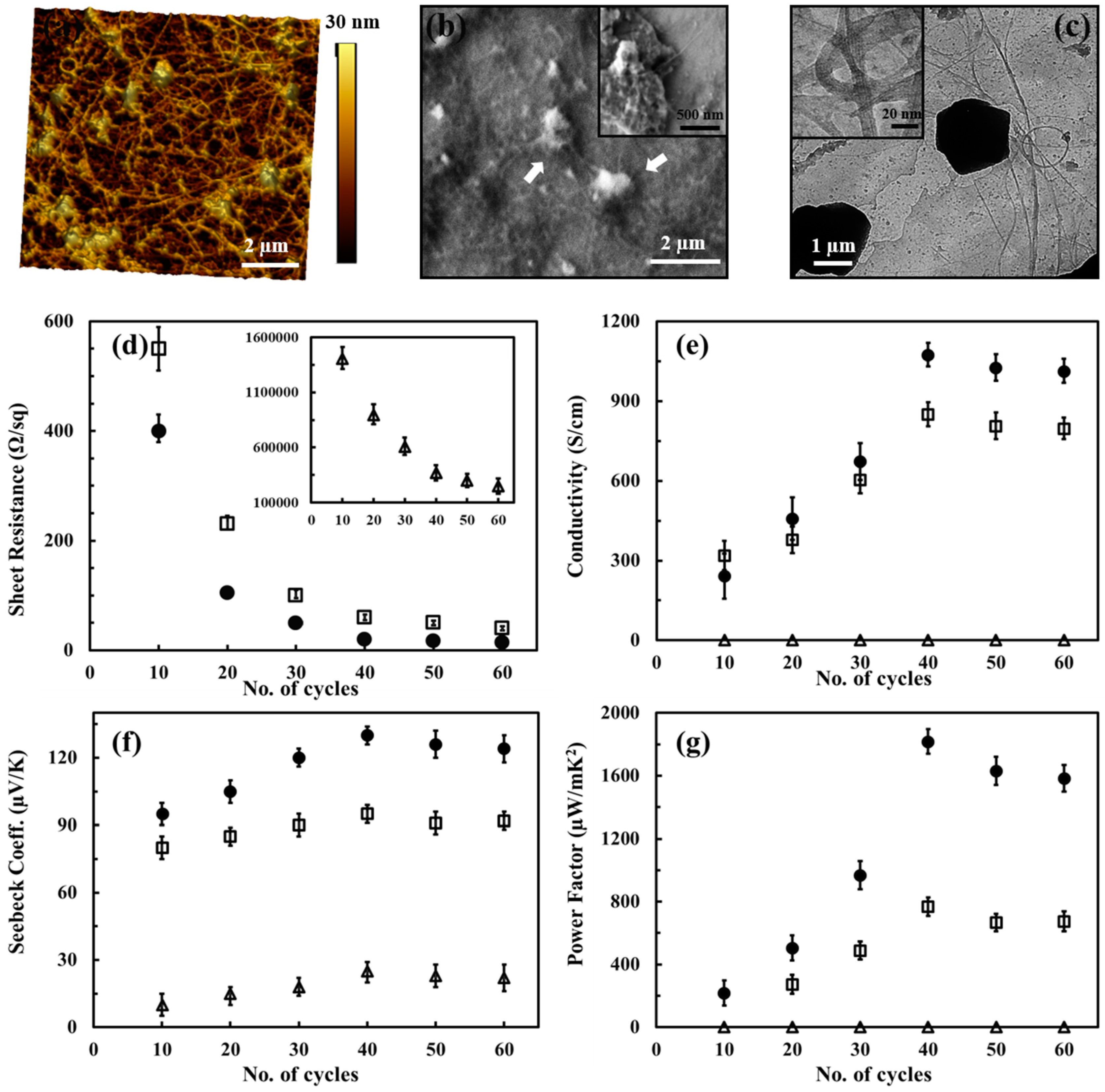
3.2.3. PEDOT:PSS-Based CNTs Composites
3.2.4. Other Conducting Polymers-Based CNTs Composites
3.3. Graphene/CNT-Based Nanocomposites
3.4. N-Type Thermoelectric Nanocomposites
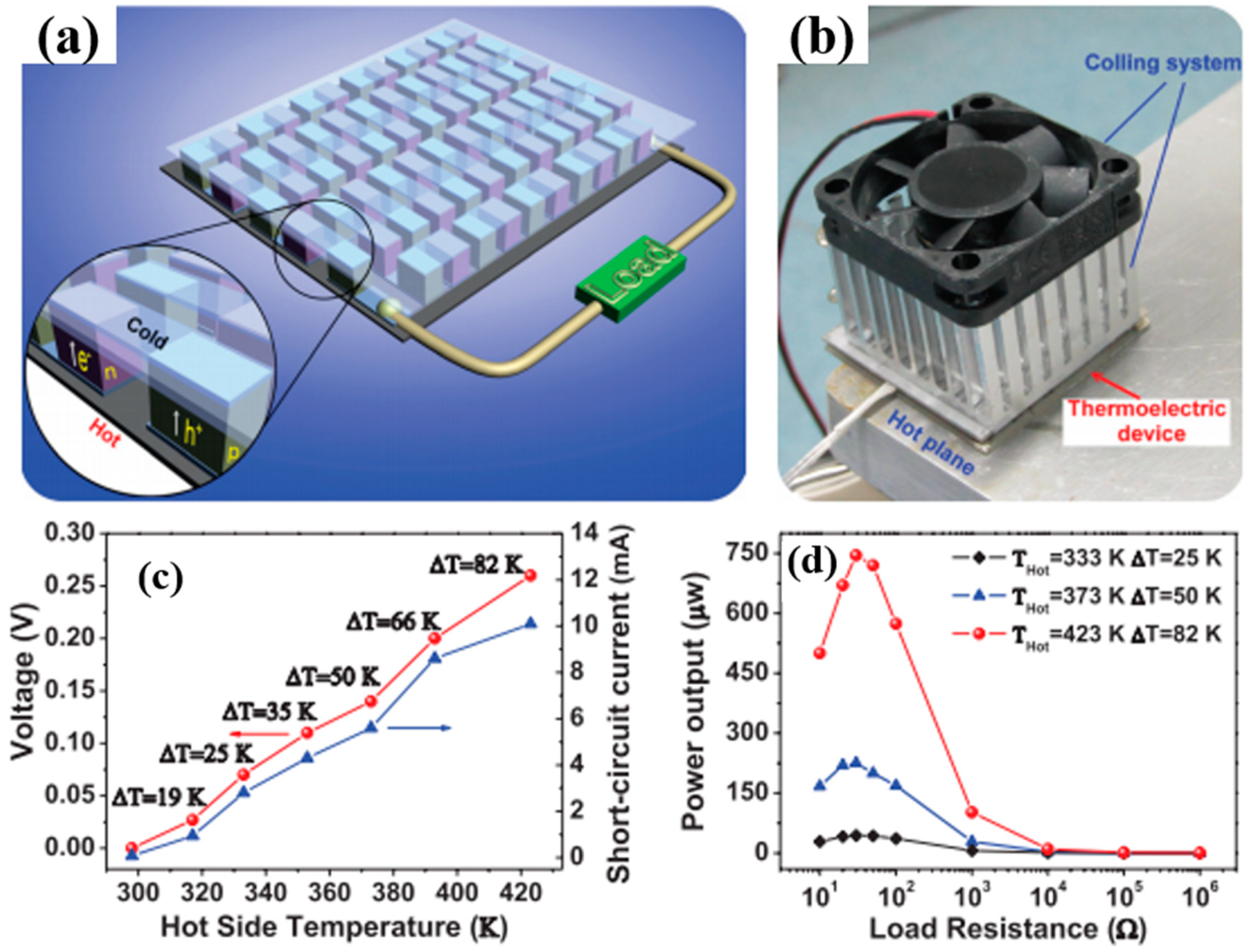
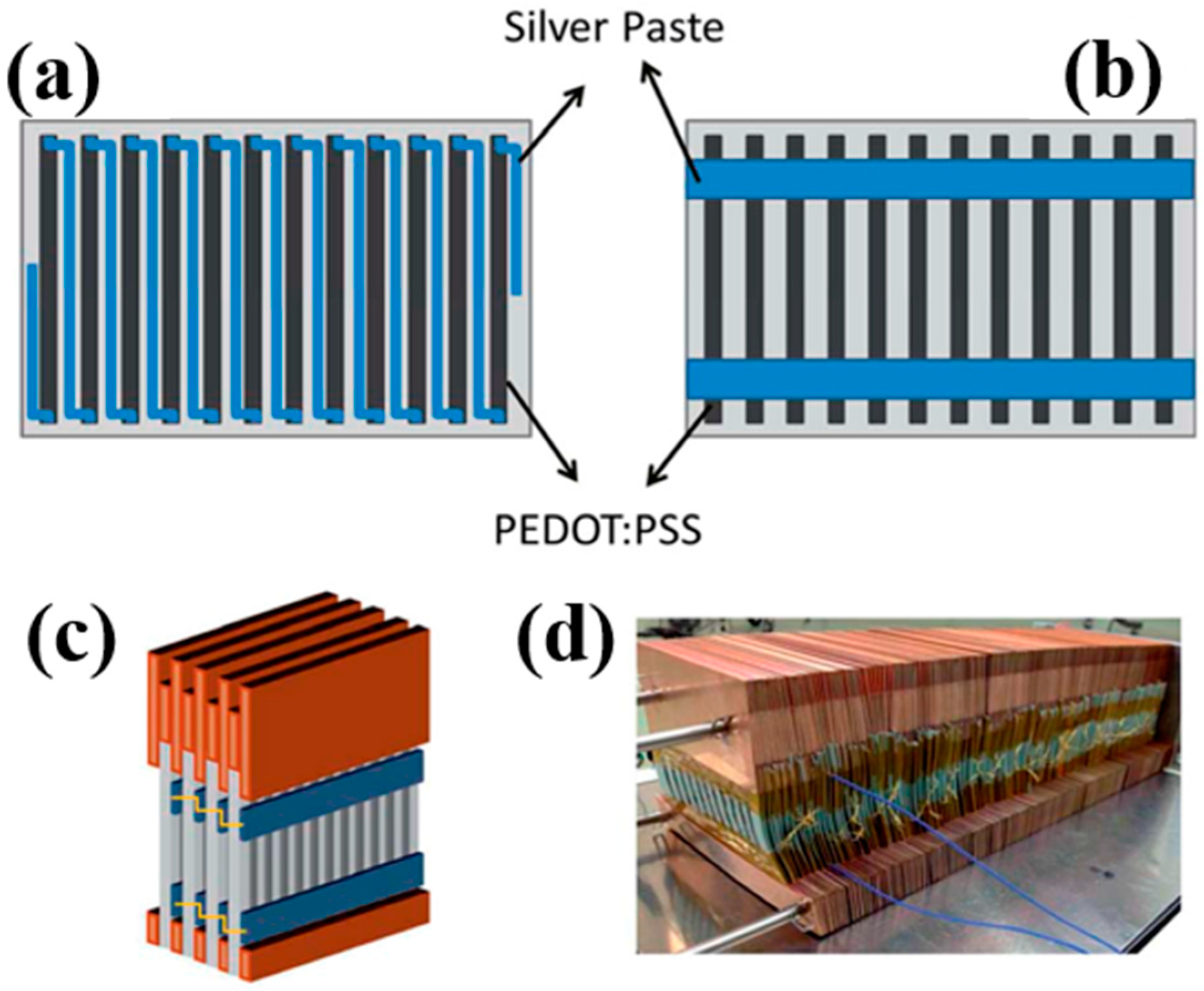
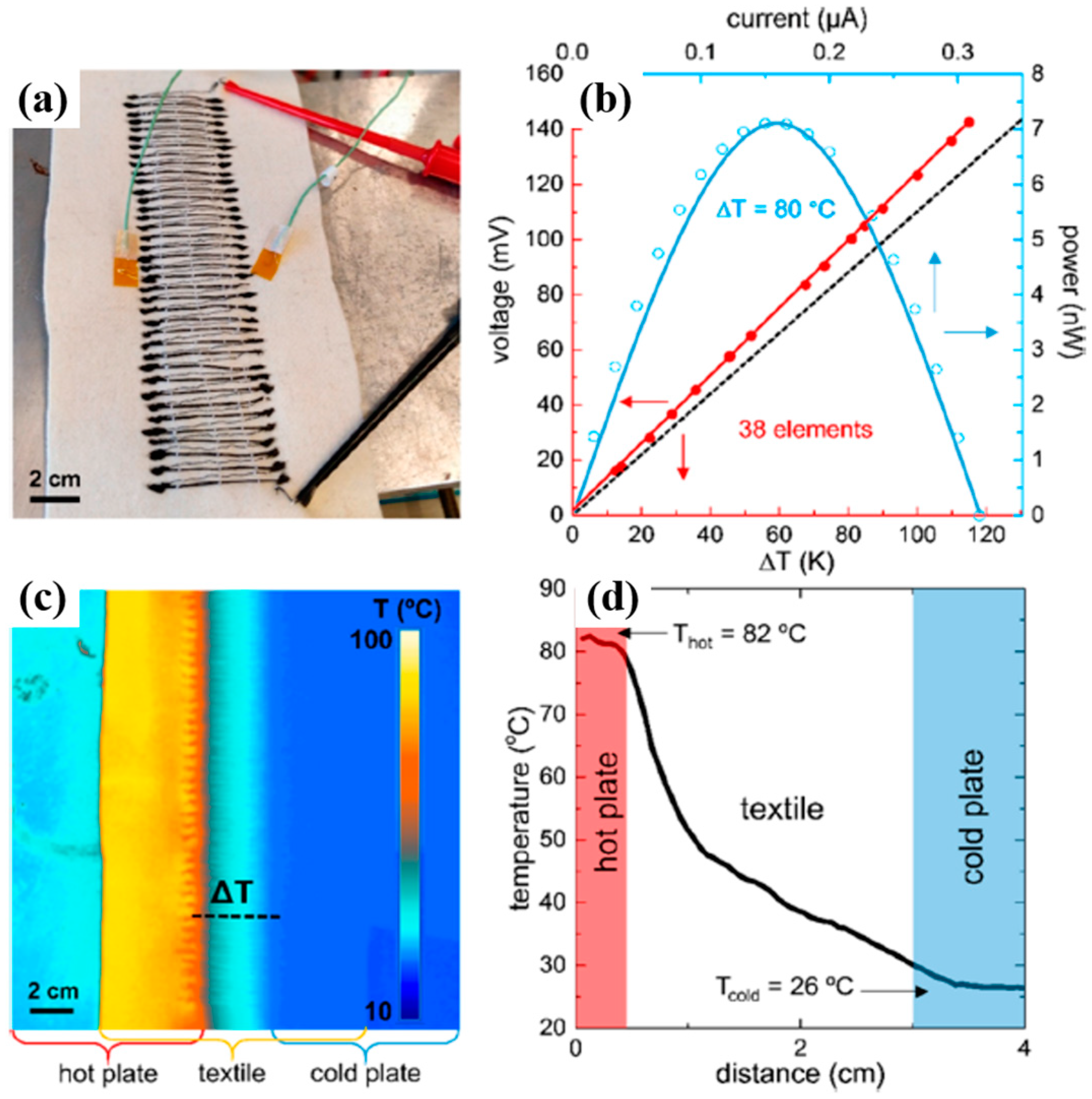
4. Flexible Organic Modules
5. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Seyboth, K.; Matschoss, P.; Kadner, S.; Zwickel, T.; Eickemeier, P.; Hansen, G.; Schlömer, S. IPCC special report on renewable energy sources and climate change mitigation. In Prepared By Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Panwar, N.; Kaushik, S.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Abolhosseini, S.; Heshmati, A.; Altmann, J. A Review of Renewable Energy Supply and Energy Efficiency Technologies; SSRN: Rochester, NY, USA, 2013. [Google Scholar]
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Theoretical efficiency limits for energy conversion devices. Energy 2010, 35, 2059–2069. [Google Scholar] [CrossRef]
- DoE, U. Waste Heat Recovery: Technology and Opportunities in US Industry; US Department of Energy Industrial Technologies Program: Washington, DC, USA, 2008. [Google Scholar]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Weathers, A.; Khan, Z.U.; Brooke, R.; Evans, D.; Pettes, M.T.; Andreasen, J.W.; Crispin, X.; Shi, L. Significant Electronic Thermal Transport in the Conducting Polymer Poly(3,4-ethylenedioxythiophene). Adv. Mater. 2015, 27, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Vashaee, D.; Shakouri, A. Improved thermoelectric power factor in metal-based superlattices. Phys. Rev. Lett. 2004, 92, 106103. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414. [Google Scholar] [CrossRef] [PubMed]
- Bahk, J.-H.; Bian, Z.; Shakouri, A. Electron energy filtering by a nonplanar potential to enhance the thermoelectric power factor in bulk materials. Phys. Rev. B 2013, 87, 075204. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.-D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Yazdani, S.; Pettes, M.T. Nanoscale self-assembly of thermoelectric materials: A review of chemistry-based approaches. Nanotechnology 2018, 29, 432001. [Google Scholar] [CrossRef] [PubMed]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- LeBlanc, S. Thermoelectric generators: Linking material properties and systems engineering for waste heat recovery applications. Sustain. Mater. Technol. 2014, 1, 26–35. [Google Scholar] [CrossRef]
- Yue, R.; Xu, J. Poly(3,4-ethylenedioxythiophene) as promising organic thermoelectric materials: A mini-review. Synth. Metals 2012, 162, 912–917. [Google Scholar] [CrossRef]
- Chen, G.; Xu, W.; Zhu, D. Recent advances in organic polymer thermoelectric composites. J. Mater. Chem. C 2017, 5, 4350–4360. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Li, D.; Coates, N.E.; Segalman, R.A.; Cahill, D.G. Thermal conductivity and elastic constants of PEDOT: PSS with high electrical conductivity. Macromolecules 2015, 48, 585–591. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, J.; Paul, B.; Eklund, P. Flexible thermoelectric materials and devices. Appl. Mater. Today 2018, 12, 366–388. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Pan, L.; Qiu, H.; Dou, C.; Li, Y.; Pu, L.; Xu, J.; Shi, Y. Conducting polymer nanostructures: Template synthesis and applications in energy storage. Int. J. Mol. Sci. 2010, 11, 2636–2657. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Hiroshige, Y.; Ookawa, M.; Toshima, N. High thermoelectric performance of poly(2,5-dimethoxyphenylenevinylene) and its derivatives. Synth. Metals 2006, 156, 1341–1347. [Google Scholar] [CrossRef]
- Yan, H.; Toshima, N. Thermoelectric properties of alternatively layered films of polyaniline and (±)-10-camphorsulfonic acid-doped polyaniline. Chem. Lett. 1999, 28, 1217–1218. [Google Scholar] [CrossRef]
- Yan, H.; Ohta, T.; Toshima, N. Stretched Polyaniline Films Doped by (±)-10-Camphorsulfonic Acid: Anisotropy and Improvement of Thermoelectric Properties. Macromol. Mater. Eng. 2001, 286, 139–142. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Wang, Y.; Sun, J.; Zeng, H.; Jin, Z.; Huang, X.; Chen, L. The synergic regulation of conductivity and Seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains. J. Mater. Chem. A 2014, 2, 2634–2640. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Li, H.; Yan, Y.; Zhang, Q. Synthesis and thermoelectric properties of hydrochloric acid-doped polyaniline. Synth. Metals 2010, 160, 1153–1158. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, D.A.; Chen, C.-H.; Boopathi, K.M.; Pranoto, F.W.; Li, L.-J.; Chu, C.-W. Enhanced thermoelectric performance of PEDOT: PSS flexible bulky papers by treatment with secondary dopants. ACS Appl. Mater. Interfaces 2014, 7, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, H.; Kim, S.; Son, W.; Cheong, I.W.; Kim, J.H. Transparent and flexible organic semiconductor nanofilms with enhanced thermoelectric efficiency. J. Mater. Chem. A 2014, 2, 7288–7294. [Google Scholar] [CrossRef]
- Wang, X.; Kyaw, A.K.K.; Yin, C.; Wang, F.; Zhu, Q.; Tang, T.; Yee, P.I.; Xu, J. Enhancement of thermoelectric performance of PEDOT: PSS films by post-treatment with a superacid. RSC Adv. 2018, 8, 18334–18340. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Song, H.; Xu, J.; Kong, F.; Wang, J. Thermoelectric performance of poly(3-hexylthiophene) films doped by iodine vapor with promising high seebeck coefficient. Electron. Mater. Lett. 2014, 10, 427–431. [Google Scholar] [CrossRef]
- Qu, S.; Yao, Q.; Wang, L.; Chen, Z.; Xu, K.; Zeng, H.; Shi, W.; Zhang, T.; Uher, C.; Chen, L. Highly anisotropic P3HT films with enhanced thermoelectric performance via organic small molecule epitaxy. NPG Asia Mater. 2016, 8, e292. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Xu, J.; Song, H.; Lu, B.; Jiang, F.; Zhou, W.; Zhang, G.; Jiang, Q. Facile fabrication of PEDOT: PSS/polythiophenes bilayered nanofilms on pure organic electrodes and their thermoelectric performance. ACS Appl. Mater. Interfaces 2013, 5, 12811–12819. [Google Scholar] [CrossRef] [PubMed]
- Lin-Chung, P.; Reinecke, T. Thermoelectric figure of merit of composite superlattice systems. Phys. Rev. B 1995, 51, 13244. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhu, G.; Li, J.; Pan, F. Thermoelectric properties of conducting polyaniline/graphite composites. Mater. Lett. 2011, 65, 1086–1088. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Yang, W.; Donelson, R.; Cai, K.; Casey, P.S. Simultaneous increase in conductivity and Seebeck coefficient in a polyaniline/graphene nanosheets thermoelectric nanocomposite. Synth. Metals 2012, 161, 2688–2692. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Bi, H.; Huang, F.; Wang, Q.; Chen, L. Large thermoelectric power factor in polyaniline/graphene nanocomposite films prepared by solution-assistant dispersing method. J. Mater. Chem. A 2014, 2, 11107–11113. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Bi, H.; Huang, F.; Wang, Q.; Chen, L. PANI/graphene nanocomposite films with high thermoelectric properties by enhanced molecular ordering. J. Mater. Chem. A 2015, 3, 7086–7092. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, G.-S.; Yu, Z.-Z.; Qi, J.-S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Mitra, M.; Kulsi, C.; Chatterjee, K.; Kargupta, K.; Ganguly, S.; Banerjee, D.; Goswami, S. Reduced graphene oxide-polyaniline composites—Synthesis, characterization and optimization for thermoelectric applications. RSC Adv. 2015, 5, 31039–31048. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Yan, S.; Miao, T.; Shi, S.; Xu, Z.; Zhang, X.; Gao, C. Chemically doped macroscopic graphene fibers with significantly enhanced thermoelectric properties. Nano Res. 2018, 11, 741–750. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene): Poly(4-styrenesulfonate)(PEDOT: PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhang, X.; Zhang, Y.; Cui, Y.; Qiu, J. Thermoelectric performance of p-type nanohybrids filled polymer composites. Nano Energy 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.; Shen, S.; Casey, P. Preparation and characterization of graphene nanosheets/poly (3-hexylthiophene) thermoelectric composite materials. Synth. Metals 2012, 162, 2102–2106. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jin, C.; Zhang, T.; Yin, Q. Preparation of polypyrrole/graphene nanosheets composites with enhanced thermoelectric properties. RSC Adv. 2014, 4, 46187–46193. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Wang, H.; Zhai, W. Enhanced thermoelectric property by the construction of a nanocomposite 3D interconnected architecture consisting of graphene nanolayers sandwiched by polypyrrole nanowires. J. Mater. Chem. C 2015, 3, 1649–1654. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, L.; Du, K.; Yin, Q.; Yin, Q. Polypyrrole/graphene/polyaniline ternary nanocomposite with high thermoelectric power factor. ACS Appl. Mater. Interfaces 2017, 9, 20124–20131. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Park, S.-G.; Kim, Y.-G.; Sung, B.J.; Jang, S.-Y.; Kim, W. Effect of the carbon nanotube type on the thermoelectric properties of CNT/Nafion nanocomposites. Org. Electron. 2011, 12, 2120–2125. [Google Scholar] [CrossRef]
- Yu, C.; Kim, Y.S.; Kim, D.; Grunlan, J.C. Thermoelectric behavior of segregated-network polymer nanocomposites. Nano Lett. 2008, 8, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, D.; Martin, K.J.; Yu, C.; Grunlan, J.C. Influence of Stabilizer Concentration on Transport Behavior and Thermopower of CNT-Filled Latex-Based Composites. Macromol. Mater. Eng. 2010, 295, 431–436. [Google Scholar] [CrossRef]
- Moriarty, G.P.; Wheeler, J.N.; Yu, C.; Grunlan, J.C. Increasing the thermoelectric power factor of polymer composites using a semiconducting stabilizer for carbon nanotubes. Carbon 2012, 50, 885–895. [Google Scholar] [CrossRef]
- Hicks, L.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 16631. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. A promising approach to enhanced thermoelectric properties using carbon nanotube networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, W.; Li, J.; Zhu, J.; Wang, L.; Zhu, M.; Jiang, W. Preparation and thermoelectric properties of multi-walled carbon nanotube/polyaniline hybrid nanocomposites. J. Mater. Chem. A 2013, 1, 12109–12114. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Gao, L. Flexible single-walled carbon nanotubes/polyaniline composite films and their enhanced thermoelectric properties. Nanoscale 2011, 3, 3616–3619. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced thermoelectric performance of single-walled carbon nanotubes/polyaniline hybrid nanocomposites. Acs Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, Q.; Xiao, J.; Zeng, K.; Qu, S.; Shi, W.; Wang, Q.; Chen, L. Engineered Molecular Chain Ordering in Single-Walled Carbon Nanotubes/Polyaniline Composite Films for High-Performance Organic Thermoelectric Materials. Chemistry 2016, 11, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Choi, K.; Grunlan, J.C.; Yu, C. Improved thermoelectric behavior of nanotube-filled polymer composites with poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate). ACS Nano 2009, 4, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J.C. Light-weight flexible carbon nanotube based organic composites with large thermoelectric power factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, G.P.; Briggs, K.; Stevens, B.; Yu, C.; Grunlan, J.C. Fully Organic Nanocomposites with High Thermoelectric Power Factors by using a Dual-Stabilizer Preparation. Energy Technol. 2013, 1, 265–272. [Google Scholar] [CrossRef]
- Song, H.; Liu, C.; Xu, J.; Jiang, Q.; Shi, H. Fabrication of a layered nanostructure PEDOT: PSS/SWCNTs composite and its thermoelectric performance. RSC Adv. 2013, 3, 22065–22071. [Google Scholar] [CrossRef]
- Lee, W.; Kang, Y.H.; Lee, J.Y.; Jang, K.-S.; Cho, S.Y. Improving the thermoelectric power factor of CNT/PEDOT: PSS nanocomposite films by ethylene glycol treatment. RSC Adv. 2016, 6, 53339–53344. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Wang, D.; Zhu, G.; Li, J. Preparation and thermoelectric properties of polythiophene/multiwalled carbon nanotube composites. Synth. Metals 2013, 181, 79–85. [Google Scholar] [CrossRef]
- Lee, W.; Hong, C.T.; Kwon, O.H.; Yoo, Y.; Kang, Y.H.; Lee, J.Y.; Cho, S.Y.; Jang, K.-S. Enhanced thermoelectric performance of bar-coated SWCNT/P3HT thin films. ACS Appl. Mater. Interfaces 2015, 7, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Martín-González, M.S.; Goni, A.R.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Hong, C.T.; Lee, W.; Kang, Y.H.; Yoo, Y.; Ryu, J.; Cho, S.Y.; Jang, K.-S. Effective doping by spin-coating and enhanced thermoelectric power factors in SWCNT/P3HT hybrid films. J. Mater. Chem. A 2015, 3, 12314–12319. [Google Scholar] [CrossRef]
- Hong, C.T.; Kang, Y.H.; Ryu, J.; Cho, S.Y.; Jang, K.-S. Spray-printed CNT/P3HT organic thermoelectric films and power generators. J. Mater. Chem. A 2015, 3, 21428–21433. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.; Shen, S.; Yin, J. Preparation and thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Metals 2014, 195, 132–136. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Enhanced thermoelectric performance by self-assembled layered morphology of polypyrrole nanowire/single-walled carbon nanotube composites. Compos. Sci. Technol. 2016, 129, 130–136. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Lee, S.H.; Cho, W.; Choi, H.H.; Kim, F.S.; Kim, J.H. Effects of one-and two-dimensional carbon hybridization of PEDOT: PSS on the power factor of polymer thermoelectric energy conversion devices. J. Mater. Chem. A 2015, 3, 6526–6533. [Google Scholar] [CrossRef]
- Li, X.; Liang, L.; Yang, M.; Chen, G.; Guo, C.-Y. Poly(3,4-ethylenedioxythiophene)/graphene/carbon nanotube ternary composites with improved thermoelectric performance. Org. Electron. 2016, 38, 200–204. [Google Scholar] [CrossRef]
- Cho, C.; Stevens, B.; Hsu, J.H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C. Completely organic multilayer thin film with thermoelectric power factor rivaling inorganic tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Outstanding low temperature thermoelectric power factor from completely organic thin films enabled by multidimensional conjugated nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, P.; Di, C.; Jiao, F.; Xu, W.; Qiu, D.; Zhu, D. Organic Thermoelectric Materials and Devices Based on p-and n-Type Poly(metal 1, 1, 2, 2-ethenetetrathiolate)s. Adv. Mater. 2012, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Fukumaru, T.; Fujigaya, T.; Nakashima, N. Development of n-type cobaltocene-encapsulated carbon nanotubes with remarkable thermoelectric property. Sci. Rep. 2015, 5, 7951. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Murali, A.; Choi, K.; Ryu, Y. Air-stable fabric thermoelectric modules made of N-and P-type carbon nanotubes. Energy Environ. Sci. 2012, 5, 9481–9486. [Google Scholar] [CrossRef]
- Cho, C.; Culebras, M.; Wallace, K.L.; Song, Y.; Holder, K.; Hsu, J.-H.; Yu, C.; Grunlan, J.C. Stable n-type thermoelectric multilayer thin films with high power factor from carbonaceous nanofillers. Nano Energy 2016, 28, 426–432. [Google Scholar] [CrossRef]
- Luo, J.; Cerretti, G.; Krause, B.; Zhang, L.; Otto, T.; Jenschke, W.; Ullrich, M.; Tremel, W.; Voit, B.; Pötschke, P. Polypropylene-based melt mixed composites with singlewalled carbon nanotubes for thermoelectric applications: Switching from p-type to n-type by the addition of polyethylene glycol. Polymer 2017, 108, 513–520. [Google Scholar] [CrossRef]
- An, C.J.; Kang, Y.H.; Song, H.; Jeong, Y.; Cho, S.Y. High-performance flexible thermoelectric generator by control of electronic structure of directly spun carbon nanotube webs with various molecular dopants. J. Mater. Chem. A 2017, 5, 15631–15639. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.; Xu, X.; Wang, C. The high thermoelectric properties of conducting polyaniline with special submicron-fibre structure. Chem. Lett. 2005, 34, 522–523. [Google Scholar] [CrossRef]
- Cao, Y.; Smith, P. Liquid-crystalline solutions of electrically conducting polyaniline. Polymer 1993, 34, 3139–3143. [Google Scholar] [CrossRef]
- De Albuquerque, J.; Melo, W.; Faria, R. Determination of physical parameters of conducting polymers by photothermal spectroscopies. Rev. Sci. Instrum. 2003, 74, 306–308. [Google Scholar] [CrossRef]
- Möller, S.; Perlov, C.; Jackson, W.; Taussig, C.; Forrest, S.R. A polymer/semiconductor write-once read-many-times memory. Nature 2003, 426, 166. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Park, C.; Kim, B.; Shin, H.; Kim, E. Flexible PEDOT electrodes with large thermoelectric power factors to generate electricity by the touch of fingertips. Energy Environ. Sci. 2013, 6, 788–792. [Google Scholar] [CrossRef]
- Scholdt, M.; Do, H.; Lang, J.; Gall, A.; Colsmann, A.; Lemmer, U.; Koenig, J.D.; Winkler, M.; Boettner, H. Organic semiconductors for thermoelectric applications. J. Electr. Mater. 2010, 39, 1589–1592. [Google Scholar] [CrossRef]
- Liu, C.; Lu, B.; Yan, J.; Xu, J.; Yue, R.; Zhu, Z.; Zhou, S.; Hu, X.; Zhang, Z.; Chen, P. Highly conducting free-standing poly(3,4-ethylenedioxythiophene)/poly (styrenesulfonate) films with improved thermoelectric performances. Synth. Metals 2010, 160, 2481–2485. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Wang, S. Thermoelectric properties of PEDOT nanowire/PEDOT hybrids. Nanoscale 2016, 8, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT: PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Lee, D.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Metals 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Culebras, M.; Gómez, C.; Cantarero, A. Enhanced thermoelectric performance of PEDOT with different counter-ions optimized by chemical reduction. J. Mater. Chem. A 2014, 2, 10109–10115. [Google Scholar] [CrossRef]
- Wang, G.; Huang, W.; Eastham, N.D.; Fabiano, S.; Manley, E.F.; Zengg, L.; Wang, B.; Zhang, X.; Chend, Z.; Lib, R.; et al. Aggregation control in natural brush-printed conjugated polymer films and implications for enhancing charge transport. Proc. Natl. Acad. Sci. USA 2017, 114, E10066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, C.; Shi, H.; Jiang, Q.; Xu, J.; Jiang, F.; Xiong, J.; Liu, E. An effective approach to enhanced thermoelectric properties of PEDOT: PSS films by a DES post-treatment. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 885–892. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Fréchet, J.M.; Toney, M.F. Dependence of regioregular poly (3-hexylthiophene) film morphology and field-effect mobility on molecular weight. Macromolecules 2005, 38, 3312–3319. [Google Scholar] [CrossRef]
- Teehan, S.; Efstathiadis, H.; Haldar, P. Thermoelectric power factor enhancement of AZO/In-AZO quantum well multilayer structures as compared to bulk films. J. Alloys Compounds 2012, 539, 129–136. [Google Scholar] [CrossRef]
- Lee, H.J.; Anoop, G.; Lee, H.J.; Kim, C.; Park, J.-W.; Choi, J.; Kim, H.; Kim, Y.-J.; Lee, E.; Lee, S.-G. Enhanced thermoelectric performance of PEDOT: PSS/PANI–CSA polymer multilayer structures. Energy Environ. Sci. 2016, 9, 2806–2811. [Google Scholar] [CrossRef]
- McGrail, B.T.; Sehirlioglu, A.; Pentzer, E. Polymer composites for thermoelectric applications. Angew. Chem. Int. Ed. 2015, 54, 1710–1723. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bajpai, O.P.; Sikder, A.K.; Chattopadhyay, S.; Khan, M.A.S. Recent advances in CNT/graphene based thermoelectric polymer nanocomposite: A proficient move towards waste energy harvesting. Renew. Sustain. Energy Rev. 2016, 53, 653–671. [Google Scholar] [CrossRef]
- Xiang, J.; Drzal, L.T. Templated growth of polyaniline on exfoliated graphene nanoplatelets (GNP) and its thermoelectric properties. Polymer 2012, 53, 4202–4210. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mitra, M.; Kargupta, K.; Ganguly, S.; Banerjee, D. Synthesis, characterization and enhanced thermoelectric performance of structurally ordered cable-like novel polyaniline–bismuth telluride nanocomposite. Nanotechnology 2013, 24, 215703. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Hwang, D.H.; Woo, S.I. Thermoelectric properties of nanocomposite thin films prepared with poly(3, 4-ethylenedioxythiophene) poly(styrenesulfonate) and graphene. Phys. Chem. Chem. Phys. 2012, 14, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Vavro, J.; Llaguno, M.C.; Satishkumar, B.; Luzzi, D.E.; Fischer, J.E. Electrical and thermal properties of C 60-filled single-wall carbon nanotubes. Appl. Phys. Lett. 2002, 80, 1450–1452. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci. Rep. 2013, 3, 3448. [Google Scholar] [CrossRef] [PubMed]
- Paloheimo, J.; Isotalo, H.; Kastner, J.; Kuzmany, H. Conduction mechanisms in undoped thin films of C60 and C60/70. Synth. Metals 1993, 56, 3185–3190. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Y.; Wang, F. Thermoelectric properties of graphene nanosheets-modified polyaniline hybrid nanocomposites by an in situ chemical polymerization. Mater. Chem. Phys. 2013, 138, 238–244. [Google Scholar] [CrossRef]
- Han, S.; Zhai, W.; Chen, G.; Wang, X. Morphology and thermoelectric properties of graphene nanosheets enwrapped with polypyrrole. RSC Adv. 2014, 4, 29281–29285. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Hofmann, M. Other one-dimensional systems and thermal properties. J. Vacuum Sci. Technol. B Microelectron. Nanometer Struct. Proc. Meas. Phenom. 2008, 26, 1613–1618. [Google Scholar] [CrossRef]
- Botiz, I.; Stingelin, N. Influence of molecular conformations and microstructure on the optoelectronic properties of conjugated polymers. Materials 2014, 7, 2273–2300. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Feser, J.P.; Chen, C.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Water-processable polymer− nanocrystal hybrids for thermoelectrics. Nano Lett. 2010, 10, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, G.; Wang, H.; Li, X. Template-Directed In Situ Polymerization Preparation of Nanocomposites of PEDOT: PSS-Coated Multi-Walled Carbon Nanotubes with Enhanced Thermoelectric Property. Chemistry 2015, 10, 149–153. [Google Scholar]
- Lin, Y.-J.; Ni, W.-S.; Lee, J.-Y. Effect of incorporation of ethylene glycol into PEDOT: PSS on electron phonon coupling and conductivity. J. Appl. Phys. 2015, 117, 215501. [Google Scholar]
- Rivadulla, F.; Mateo-Mateo, C.; Correa-Duarte, M. Layer-by-layer polymer coating of carbon nanotubes: Tuning of electrical conductivity in random networks. J. Am. Chem. Soc. 2010, 132, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, L.; Portale, G.; Koopmans, M.; Ten Brink, G.; Hummelen, J.C.; Koster, L.J.A. N-Type Organic Thermoelectrics: Improved Power Factor by Tailoring Host–Dopant Miscibility. Adv. Mater. 2017, 29, 1701641. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Potscavage, W.J.; Yang, Y.S.; Park, I.S.; Matsushima, T.; Adachi, C. Solution-processed organic thermoelectric materials exhibiting doping-concentration-dependent polarity. Phys. Chem. Chem. Phys. 2016, 18, 29199–29207. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, D.A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Gu, X.; Dang, F.; Itoh, T.; Wang, Y.; Sasaki, H.; Kondo, M.; Koga, K.; Yabuki, K.; Snyder, G.J. Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2. Nat. Mater. 2015, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.C.; Mureau, N.; Tang, Z.; Miyajima, Y.; Carey, J.D.; Silva, S.R.P. The importance of oxygen-containing defects on carbon nanotubes for the detection of polar and non-polar vapours through hydrogen bond formation. Nanotechnology 2007, 18, 175701. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.J.; Park, C.; Choi, H.H.; Kim, J.-H. N-type organic thermoelectric materials based on polyaniline doped with the aprotic ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate. RSC Adv. 2016, 6, 37130–37135. [Google Scholar] [CrossRef]
- Zuo, G.; Li, Z.; Wang, E.; Kemerink, M. High Seebeck Coefficient and Power Factor in n-Type Organic Thermoelectrics. Adv. Electron. Mater. 2018, 4, 1700501. [Google Scholar] [CrossRef]
- Hewitt, C.A.; Kaiser, A.B.; Roth, S.; Craps, M.; Czerw, R.; Carroll, D.L. Multilayered carbon nanotube/polymer composite based thermoelectric fabrics. Nano Lett. 2012, 12, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Nonoguchi, Y.; Nakano, M.; Murayama, T.; Hagino, H.; Hama, S.; Miyazaki, K.; Matsubara, R.; Nakamura, M.; Kawai, T. Simple Salt-Coordinated n-Type Nanocarbon Materials Stable in Air. Adv. Funct. Mater. 2016, 26, 3021–3028. [Google Scholar] [CrossRef]
- Ryu, Y.; Freeman, D.; Yu, C. High electrical conductivity and n-type thermopower from double-/single-wall carbon nanotubes by manipulating charge interactions between nanotubes and organic/inorganic nanomaterials. Carbon 2011, 49, 4745–4751. [Google Scholar] [CrossRef]
- Freeman, D.D.; Choi, K.; Yu, C. N-type thermoelectric performance of functionalized carbon nanotube-filled polymer composites. PLoS ONE 2012, 7, e47822. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials For Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 101–110. [Google Scholar]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.; Priya, S. A Review on Low-Grade Thermal Energy Harvesting: Materials, Methods and Devices. Materials 2018, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Polymer thermoelectric modules screen-printed on paper. RSC Adv. 2014, 4, 28802–28806. [Google Scholar] [CrossRef]
- Dörling, B.; Ryan, J.D.; Craddock, J.D.; Sorrentino, A.; Basaty, A.E.; Gomez, A.; Garriga, M.; Pereiro, E.; Anthony, J.E.; Weisenberger, M.C. Photoinduced p-to n-type Switching in Thermoelectric Polymer-Carbon Nanotube Composites. Adv. Mater. 2016, 28, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gao, C.; Chen, G.; Wang, X.; Wang, H. High-performance organic thermoelectric modules based on flexible films of a novel n-type single-walled carbon nanotube. J. Mater. Chem. A 2016, 4, 14187–14193. [Google Scholar] [CrossRef]
- An, C.J.; Kang, Y.H.; Lee, A.-Y.; Jang, K.-S.; Jeong, Y.; Cho, S.Y. Foldable thermoelectric materials: Improvement of the thermoelectric performance of directly spun CNT webs by individual control of electrical and thermal conductivity. ACS Appl. Mater. Interfaces 2016, 8, 22142–22150. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Cho, C.; Krecker, M.; Smith, R.; Song, Y.; Gómez, C.M.; Cantarero, A.S.; Grunlan, J.C. High thermoelectric power factor organic thin films through combination of nanotube multilayer assembly and electrochemical polymerization. ACS Appl. Mater. Interfaces 2017, 9, 6306–6313. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, J. Waterborne polyurethane based thermoelectric composites and their application potential in wearable thermoelectric textiles. Compo. Part B Eng. 2016, 107, 59–66. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.; Chen, S.; Wang, H.; Shen, S.Z.; Donelson, R.; Lin, T. Thermoelectric fabrics: Toward power generating clothing. Sci. Rep. 2015, 5, 6411. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Lund, A.; Hofmann, A.I.; Kroon, R.; Sarabia-Riquelme, R.; Weisenberger, M.C.; Müller, C. All-Organic Textile Thermoelectrics with Carbon-Nanotube-Coated n-Type Yarns. ACS Appl. Energy Mater. 2018. [Google Scholar] [CrossRef] [PubMed]

| Nonconducting Polymers | Abbreviation | Polymer Structure | Conducting Polymers | Abbreviation | Polymer Structure |
|---|---|---|---|---|---|
| Polypropylene | PP | 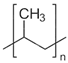 | polypyrrole | PPy | 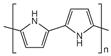 |
| polyvinylpyrrolidone | PVP | 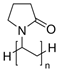 | polythiophene | PTh |  |
| poly(vinyl acetate) | PVAc | 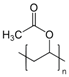 | poly(3,4-ethylenedioxythiophene) | PEDOT |  |
| polyethylenimine | PEI | 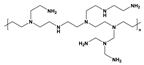 | Polyaniline leucoemeraldine(x=1, y=0) emeraldine(x=y=0.5) pernigraniline(x=0, y=1) | PANI |  |
| Nafion | Nafion | 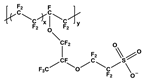 | Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) | PEDOT:PSS |  |
| Materials | Systems | Electrical Conductivity (S∙cm−1) | Seebeck Coefficient (μV∙K−1) | Power Factor (μW∙m−1∙K−2) | Thermal Conductivity (W·m−1∙K−1) | ZT | Ref. |
|---|---|---|---|---|---|---|---|
| Non-Conducting Polymers | PVP | 5 × 10−4 | −9.5 | 5 × 10−6 | [25] | ||
| PEI | 5.5 × 10−4 | −5.5 | 2 × 10−6 | [25] | |||
| Conducting polymers | PANi | 173 | 14 | 3.5 | [26] | ||
| 67 | 12.8 | 1.1 | [27] | ||||
| 270 | 20 | 10.8 | [28] | ||||
| 600 | 0.276 | 2.7 × 10−4 (423 K) | [29] | ||||
| PEDOT:PSS | 800 | 65 | 400 | 0.52 | 0.42 (300 K) | [30] | |
| 1900 | 20.6 | 80.63 | 0.2 | 0.32 | [31] | ||
| 318.4 | 0.3 | 0.31 | [32] | ||||
| 2980 | 21.9 | 142 | [33] | ||||
| P3HT | 0.47 | 386 | 7 | [34] | |||
| 320 | 269 | 62.4 | [35] | ||||
| PPy | 4.6 | 9.4 | 0.04 | [16] | |||
| All-Polymer Composites | PEDOT/PEDOT:Tos | 446.6 | 0.44 (300 K) | [31] | |||
| PEDOT:PSS/P3HT | 200 | 17 | 5.79 | [36] | |||
| PEDOT:PSS/PANi-CSA | 56 | [37] | |||||
| Graphene-based Composites | PANi/Graphene | 1.2 × 104 | 18.66 | 4.2 | 1.2 | 1.37 × 10−3 (393 K) | [38] |
| 5.6 | [39] | ||||||
| 856 | 19 | 30.9 | [40] | ||||
| 814 | 26 | 55 | [41] | ||||
| 750 | 28 | 58.8 | 4.84 × 10−4 | [42] | |||
| PEDOT:PSS/Graphene | 11 | 2.1 × 10−2 | [43] | ||||
| 624 | [44] | ||||||
| 637 | 26.8 | 45.7 | [45] | ||||
| 83.2 | 0.1 | [46] | |||||
| P3HT/Graphene | 1.2 | 35.5 | 0.16 | [47] | |||
| PPy/Graphene | 10.2 | 0.84 | 2.8 × 10−3 | [48] | |||
| 75 | 34 | 8.6 | [49] | ||||
| Ppy/Graphene/PANi | 500 | 32 | 52.5 | [50] | |||
| CNT-based Composites | Nafion/CNTs | 13 | 28 | 1 | [51] | ||
| PVAc/CNTs | 48 | 0.34 | 0.006 | [52] | |||
| PVAc/SWNT-GA | 90.4 | 40 | 14.5 | 0.25 | [53] | ||
| PVAc/MWNT-TCPP | 1.28 | 28 | 0.1 | [54] | |||
| PVAc/DWNT-TCPP | 71 | 78 | 42.8 | [55] | |||
| PANi/CNT | 61 | 28.6 | 5 | [56] | |||
| 1.6 | 26 | 1.1 | 7.6 × 10−5 | [57] | |||
| 16 | 10 | 0.16 | [58] | ||||
| 31.5 | 45.4 | 6 | [59] | ||||
| 125 | 40 | 20 | 1.5 | 0.004 | [60] | ||
| 1440 | 39 | 217 | [61] | ||||
| PEDOT:PSS/CNT | 400 | 25 | 25 | 0.4 | 0.02 (300 K) | [62] | |
| 1350 | 41 | 160 | [63] | ||||
| 960 | 70 | 500 | [64] | ||||
| 241 | 38.9 | 21.1 | [65] | ||||
| 780 | 43.7 | 150 | [66] | ||||
| P3HT/MWNT | 30 | 28 | 2.4 | 0.59 | 8.7 × 10−4 | [67] | |
| P3HT/SWNT | 105 | [68] | |||||
| 1000 | 32.5 | 107 | [69] | ||||
| 2760 | 31 | 308 | [70] | ||||
| P3HT/CNT | 348 | 97 | 325 | [71] | |||
| PPy/MWNT | 72 | 17 | 2 | [72] | |||
| PPy/SWNT | 21.7 | [73] | |||||
| Graphene/CNT-based Composites | PEDOT:PSS/Graphene/MWNT | 690 | 23 | 37 | 0.36 | 0.31 (300 K) | [74] |
| PEDT/rGO/SWNT | 208 | 21 | 9 | [75] | |||
| PANi/Graphene/PANi/DWNT | 1050 | 132 | 1825 | [76] | |||
| PANi/Graphene-PEDOT:PSS/PANi/DWNTPEDOT:PSS | 1900 | 120 | 2710 | [77] | |||
| n-type TE Materials | poly[Kx (Ni-ett)] | 66 | 0.2 | [78] | |||
| PVAc/PEI/CNT-SDBS | 15 | −100 | 15 | [79] | |||
| PEI/CNT-NaBH4 | 240 | −80 | 153.6 | [80] | |||
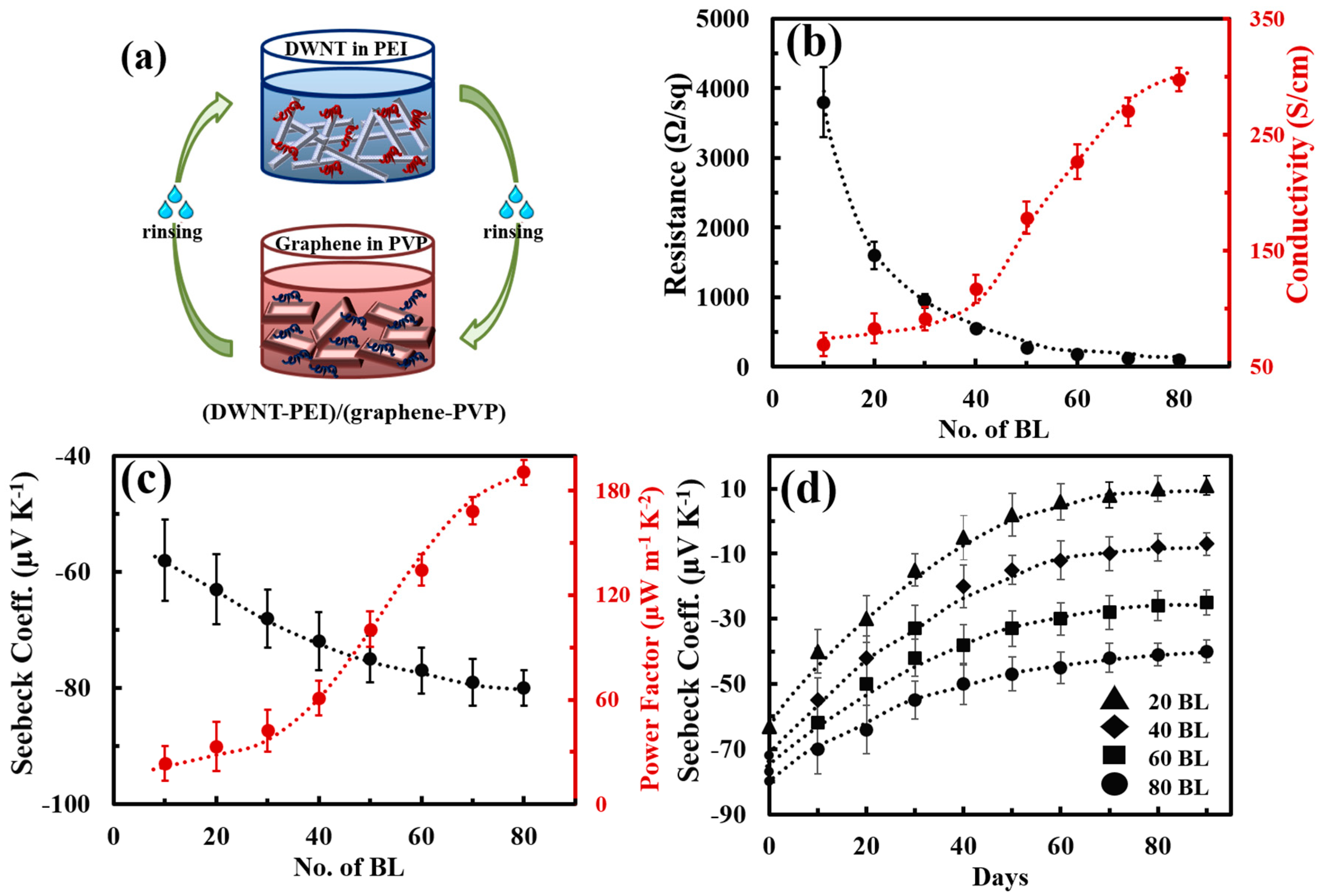
| DWNT-PEI/Graphene-PVP | 300 | −80 | 190 | [81] | |||
| PP/SWNT/CuO/PEG | 0.024 | −57 | 0.008 | [82] | |||
| BV/CNT | 2220 | −116 | 3100 | [83] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culebras, M.; Choi, K.; Cho, C. Recent Progress in Flexible Organic Thermoelectrics. Micromachines 2018, 9, 638. https://doi.org/10.3390/mi9120638
Culebras M, Choi K, Cho C. Recent Progress in Flexible Organic Thermoelectrics. Micromachines. 2018; 9(12):638. https://doi.org/10.3390/mi9120638
Chicago/Turabian StyleCulebras, Mario, Kyungwho Choi, and Chungyeon Cho. 2018. "Recent Progress in Flexible Organic Thermoelectrics" Micromachines 9, no. 12: 638. https://doi.org/10.3390/mi9120638
APA StyleCulebras, M., Choi, K., & Cho, C. (2018). Recent Progress in Flexible Organic Thermoelectrics. Micromachines, 9(12), 638. https://doi.org/10.3390/mi9120638






