1. Introduction
Neuromodulation for peripheral nerve stimulation (PNS) applications is increasingly being used to treat and manage chronic diseases (i.e., epilepsy, micturition, pain, etc. [
1,
2,
3,
4]). A major problem with chronic neurostimulation of peripheral nerve for purposes of neural interfacing is that of lead wires tugging on microelectrodes penetrating into the body of a nerve. This becomes a more severe problem when large numbers of wires are used for advanced multichannel neural interface systems needed for both sensory and motor control of prosthetics [
5]. Such systems require more channels than can be provided by most nerve cuff systems and need to contact or stimulate nerves lying deeper at the fascicular and subfasicular level. Penetrating needle electrode arrays such as the Utah array (USEA), flat interface nerve electrodes (FINE), transverse intrafascicular multi-channel electrodes (TIME), and longitudinal intrafascicular electrodes (LIFE) [
6,
7], are increasingly being used to make this connection but lead wire ribbon cables create differential inertia during sudden movement and the potential for damaging nerves during normal nerve movement with the limb. Wireless systems using RF, optical, heat, magnetic and ultrasound energy are increasingly being considered for neuromodulation [
8,
9,
10,
11,
12,
13,
14,
15]. The present work suggests the potential use of free-floating, stimulating, diode-electrode systems that are wholly implanted within the nerve and the use of strong electric field gradients produced by extraneural electrodes to achieve channel selection.
Excitable tissues of the body are not generally stimulated by short pulses of zero-mean, high frequency (>100 kHz) electric alternating currents (AC) at typically used amplitudes (10 µA–10’s of mA). In fact, classic strength-duration curves reflect that nerve excitation at lower durations (corresponding to high frequency) of stimulus require exponentially increasing monophasic current amplitudes for stimulation. Recent nerve stimulation studies using transdermal amplitude modulated signals (TAMS) using computational models and in vivo experiments indicate that sinusoidal carrier waves of frequencies >20 kHz (variable amplitudes) do not significantly enhance the activation of neurons [
16].
However, it is known that high-frequency, pulsed, monophasic (half wave-rectified) or partially rectified currents can stimulate a nerve and do so in ways that depend more on the envelope of the pulse rather than its carrier frequency [
17]. Such stimulation currents can be achieved by diodes that rectify high frequency AC currents driven in tissues by remote electrodes that behave according to induced field distributions of volume conductors. Diodes placed in tissue rectify the fraction of high-frequency currents that pass through them relative to that passing through the tissue and can cause local neural activation, as we demonstrate in this study. Different diode placements on the nerve elicited selective electromyogram (EMG) responses in different muscle groups. The differential motor responses suggest the potential for the employment of many very small diodes dispersed around and within nerve to achieve a multichannel configuration driven by combinations of remote electrodes.
This approach to using volume-conducted currents to power implanted diodes and other devices in tissue was first explored by Palti [
17] and others more recently [
18,
19,
20,
21] for direct stimulation of muscles [
18] and nerves [
19,
20,
21]. But a careful assessment of nerve activation using smaller microscale diodes as a function of the AC stimulation parameters such as frequency, peak-to-peak voltage amplitudes and diode parameters such as diode–dipole lengths, feature size and relative position of the diodes with respect to the stimulation electrodes has not yet been done. We find that non-stimulatory AC currents can be remotely driven in the conductivity of the nerve and a small diode with attached microelectrodes will allow intra-neural placement. In this situation, there is the potential for highly localized neurostimulation because of the short dipole spacing of the electrodes on the diode. Therefore, the key aspects of this design are the electric field gradient in tissue and the geometric factors of the diode and its electrodes.
The effect of diodes in a volume-conducted AC field has been modeled for various dipolar configurations in prior modeling studies [
22,
23]. This study models specific geometric relationships between the diode’s electrodes, the remote activation electrodes, their proximity, and orientation. There is particularly a strong dependence on the anode–cathode length of the diode and the distance of remote activation electrodes. In general, volume conduction of significant amounts of current is limited by the roughly cubic expanding region of reduced current density around the stimulation electrodes as a function of separation distance. Even so, relatively high amplitude pulsed AC currents at high frequencies are well tolerated by tissues and so offer a way to help overcome path losses. We investigate the potential for energy transfer within constraints acceptable for local power transfer from outside a nerve epineurium to inside the nerve. The focus of the study here is to partly understand the limitations on the scheme of placing very short, but untethered diode–dipoles within a nerve cuff and then using differences in volume conduction and electrode current path lengths to define diode activation. This is proposed in order to reduce the need for penetrating electrodes and their potential for damage by lead wires. It is this key aspect of ‘wirelessness’ at this point in the chain of electrical pulse generators and lead wires that offers an advantage to the development of advanced nerve interfaces. The concept we are testing is the potential to achieve multichannel stimulation of a compound nerve by inserting or placing multiple small diodes within or on the nerve such that each responds to specific combinations of remote electrodes providing different configurations of electrical fields. Such diodes will not be connected by wires. This concept is enabled for experimentation by the availability of commercially available Schottky diodes in formats such as the Skyworks CDC-7630 having a 1.5 mm length and unpackaged silicon diode die of 220 μm square, but we note that diodes are easily made by modern photolithography at much smaller sizes.
2. Materials and Methods
2.1. In Vivo Rodent Sciatic Nerve Model
All animal procedures were done with the approval of the Institute of Animal Care and Use Committee (IACUC) of Arizona State University and in accordance with the National Institute of Health (NIH) guidelines. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data. In all, 17 rats were used in total for all experiments.
Briefly, 300–600 g male Sprague–Dawley (Rattus norvegicus) rats (n = 17 rats total) were anesthetized (induction) using 50 mg/mL ketamine, 5 mg/mL xylazine, and 1 mg/mL acepromazine administered via intraperitoneal injection and maintained with 0.5–1% isoflurane. The left hind legs were shaved and residual hair was removed using hair removal cream. The animal was mounted on a stereotaxic frame and heart rate (~280–350 beats/min) and breathing (~60 breaths/min) were monitored using SurgiVet™ (Smith Medical Systems, Dublin, OH, USA). Aseptic techniques to disinfect the skin (i.e., application of isopropyl alcohol or betadiene) were used to ensure sterility. After skin incision and dissection of the muscle planes, the sciatic nerve was identified and isolated. Connective tissue surrounding the nerve was gently removed using iris microscissors at least 1 cm distal from the trifurcation point. The nerve cuff described previously was placed approximately 1 cm distal from the trifurcation point where the sural, peroneal and tibial bundles split. The cuff was placed such that the insulating silicone bottom under the rings as the only contact point with the rat body to ensure no contact with surrounding muscle groups to prevent potential off-target stimulation effects. A total of 10 (out of 17) animals were used for characterizing the performance of remote diodes with a stimulation threshold as a function of AC stimulation parameters (i.e., frequency, AC burst duration, measurements of diode current amplitudes based on relative position of remote diodes from stimulating electrodes, diode–dipole length and implantation depth).
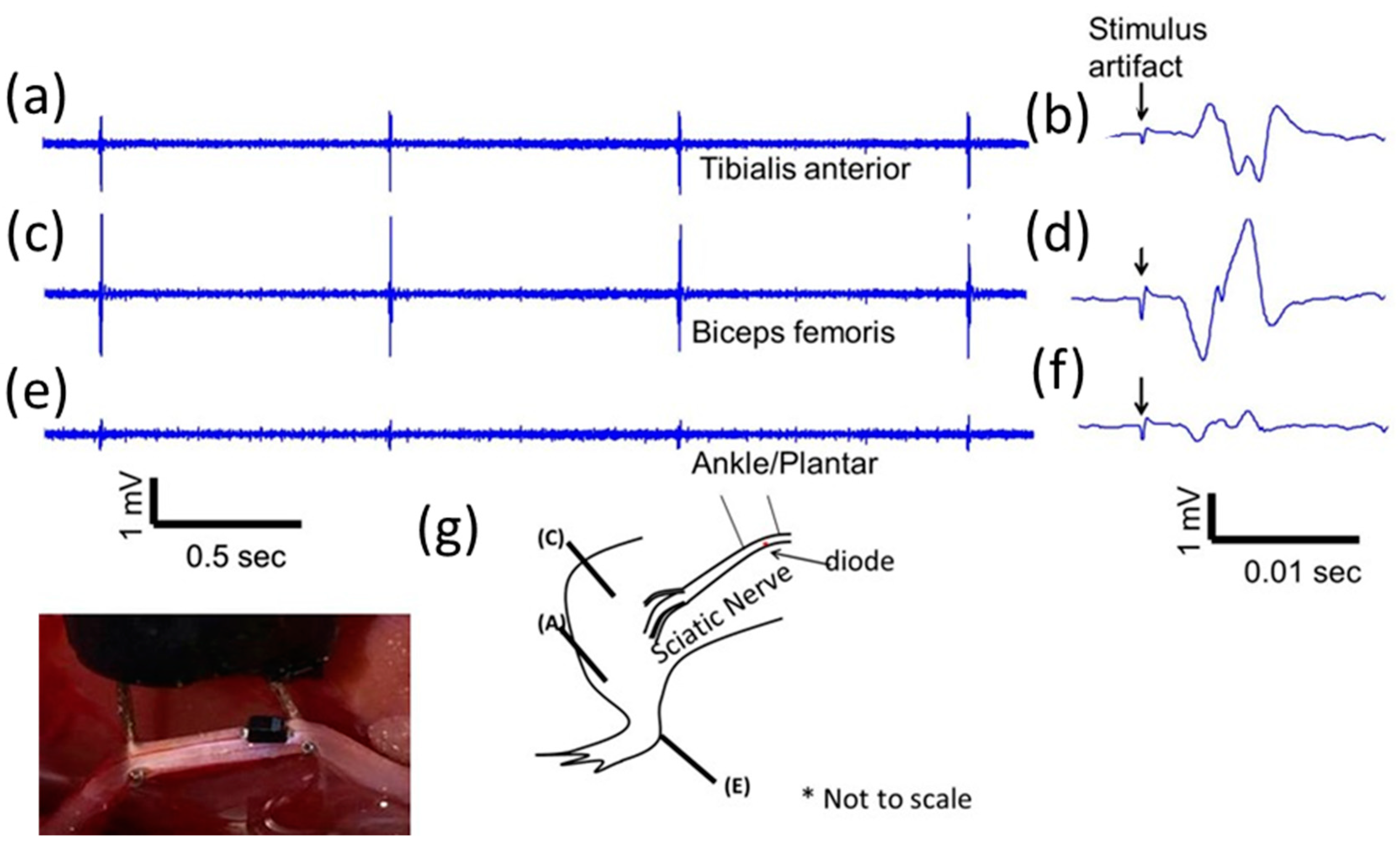
To demonstrate that modified, implanted mini-, and micro-diodes can stimulate the nerve, needle-based electromyography (EMG) was used. Disposable monopolar needle electrodes (Rhythmlink™, Columbia, SC, USA) were placed in digit 5 of the rat hind leg paw (either left or right) for nerve cuff based experiments. The animal was grounded with a needle electrode in the opposite hind leg. EMGs were recorded using Intan™ recording system (Intan, Los Angeles, CA, USA) and analyzed in MATLAB offline. The recordings were digitally filtered on the Intan™ system using a bandpass filter from 100–3000 Hz to remove motion artifacts. EMG recordings were analyzed for 10 repetition trials of each stimulation condition.
Large SC-79 package diodes were also used to test selectivity. Multiple EMG electrodes at different sites (ankle/plantar, biceps femoris, and tibalis anterior) were placed in 3 (out of the total of 17) additional animals to test for muscle selectivity using AC excitation at 300 kHz or 1 MHz. Muscle response was recorded using needle-based EMGs.
Mean ± standard deviation of the EMG amplitude was calculated and muscle recruitment curves were plotted for nerve stimulation using diodes placed subepineurally and diodes implanted in the sciatic nerve. A total of 4 additional animals (out of the total 17) were used for the in vivo validation (2 for subepineurial and 2 for deep nerve implants). For stimulus threshold voltage measurements, mean ± standard error (SE) was plotted and statistical analysis was performed using one-way analysis of variance (ANOVA) and if found significant, the maximum and minimum values were evaluated for significance (α = 0.01) using the Student’s t-test.
2.2. Diode Packaging and Modification
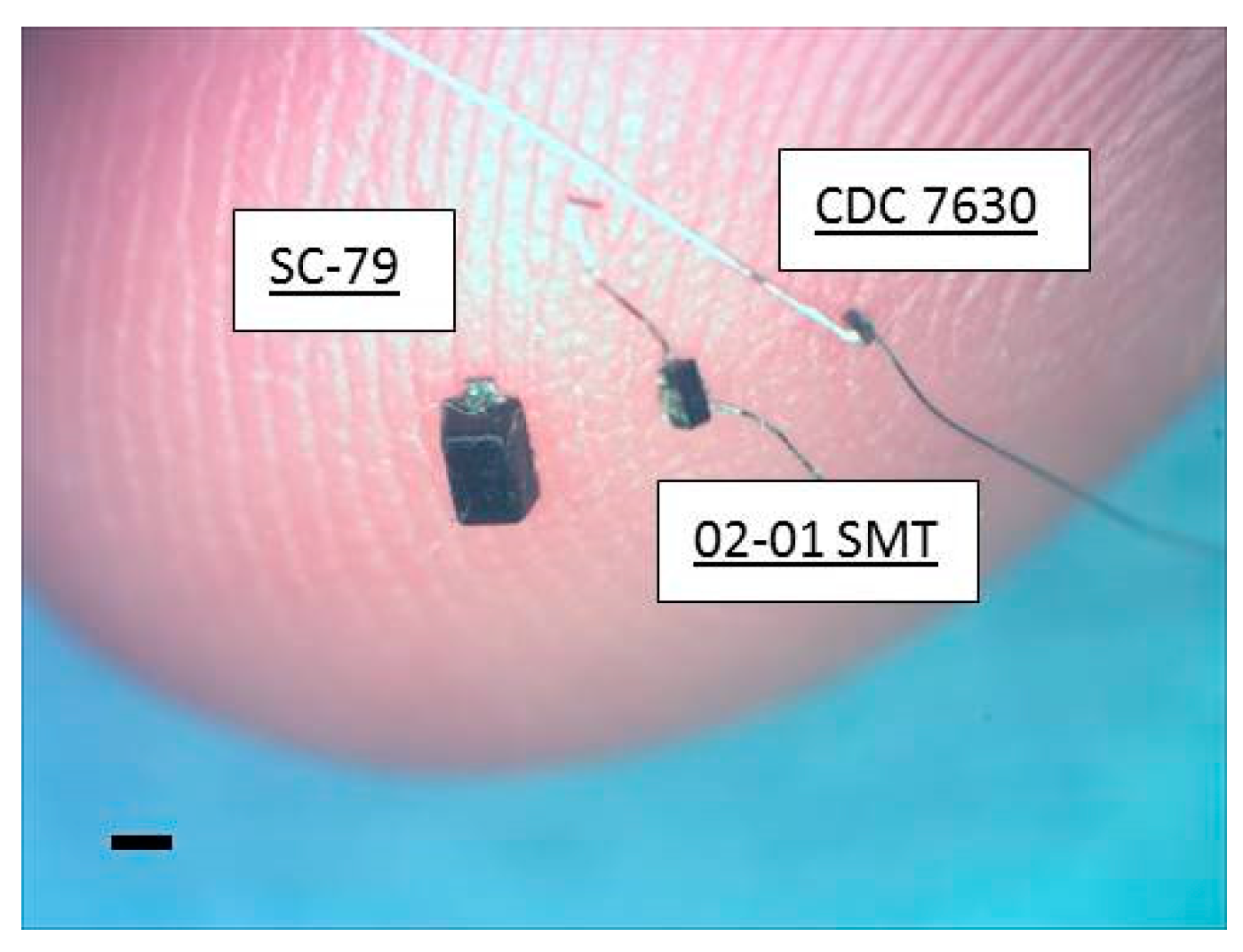
Ultra-small, commercially-available Schottky diodes (Skyworks 7630, Woburn, MA, USA) were purchased in three different packages (SC-79, 0201 SMT, and bare die CDC7630)). The diode lengths were 1.5 mm, 0.5 mm and 0.22 mm respectively, with the first diode capable of stimulation on or outside the nerve, while the second and third diode packages offered intraneural, implantable sizes. The implantable 0201 SMT and bare die (hereafter referred to as mini- and micro-diodes respectively) were connected with 50 µm diameter platinum leads for nerve tissue contact that could be trimmed to desired diode–dipole lengths. Mini- and micro-diodes were dipped in a fluorosilane-based coating (3M-Novec EGC-1720) for 2 min and dried at room temperature for insulating electrically sensitive portions of the device. An additional, ethyl-cyanoacrylate based layer (Gorilla™ impact tough super glue) was added to strengthen the bond between the platinum leads and the diode bond pads to withstand mechanical stresses from the animal for durable implantation. The three packaged diodes are shown against the tip of smallest finger of a human hand in
Figure 1.
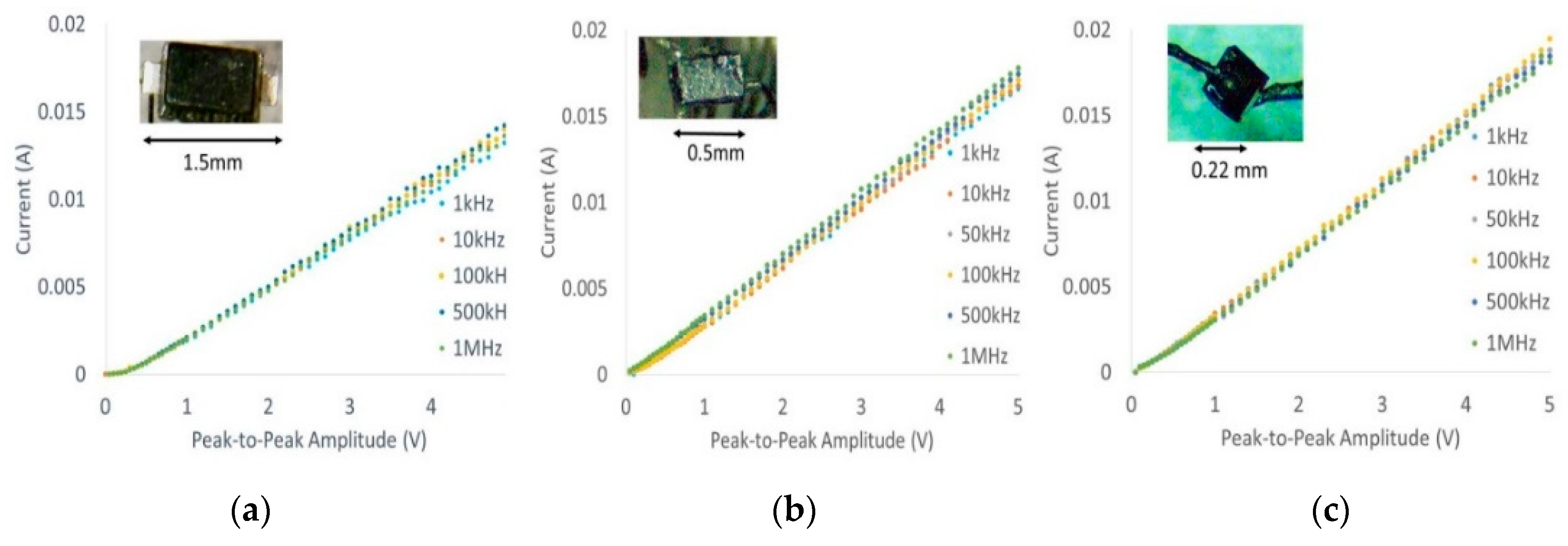
Current–voltage characteristic (I-V) curves of all three packages shown in
Figure 2 were generated (10 kHz–1 MHz) using a Siglent™ function generator and oscilloscope to ensure that post-modifications did not affect diode characteristics such as threshold voltage as a function of frequency. Typical thresholds ranged from 150–180 mV at different frequencies, which is comparable to manufacturer datasheets.
2.3. Nerve Cuff Testing Platform
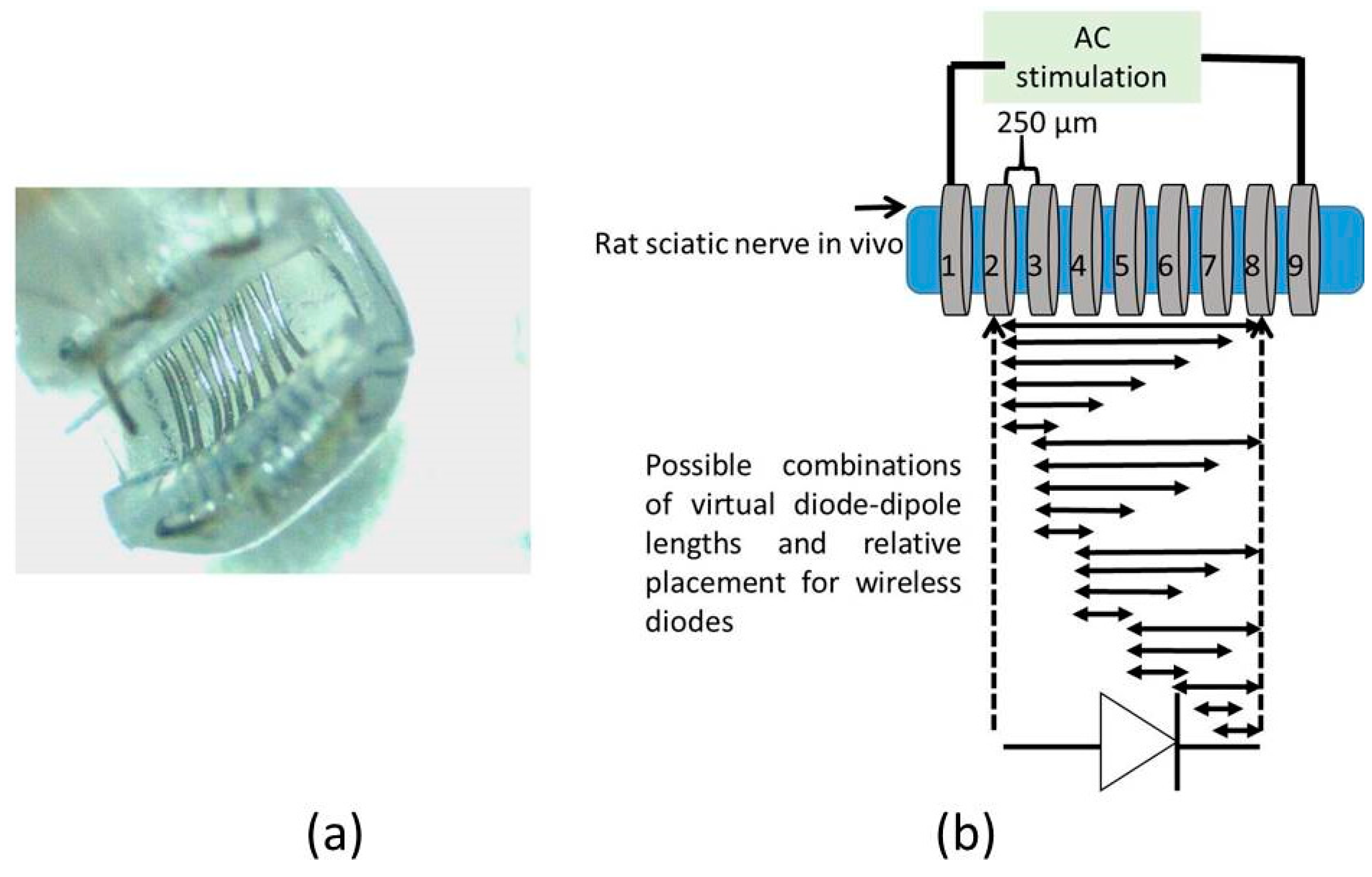
A cuff-electrode was used as a platform to generate AC fields in the sciatic nerve. A nerve cuff with 100 µm diameter platinum electrodes with 9 rings spaced 250 µm apart as shown in
Figure 3a was custom fabricated by Microprobes (Gaithersburg, MD, USA). The total distance between the inner edge of electrode rings ‘1’ and ‘9’ was 2.7 mm. Each ring on the cuff had an impedance of ~2 kΩ at 1 kHz. This set-up was used to measure currents through a diode that is superficially placed on the sciatic nerve between two electrodes with AC excitation.
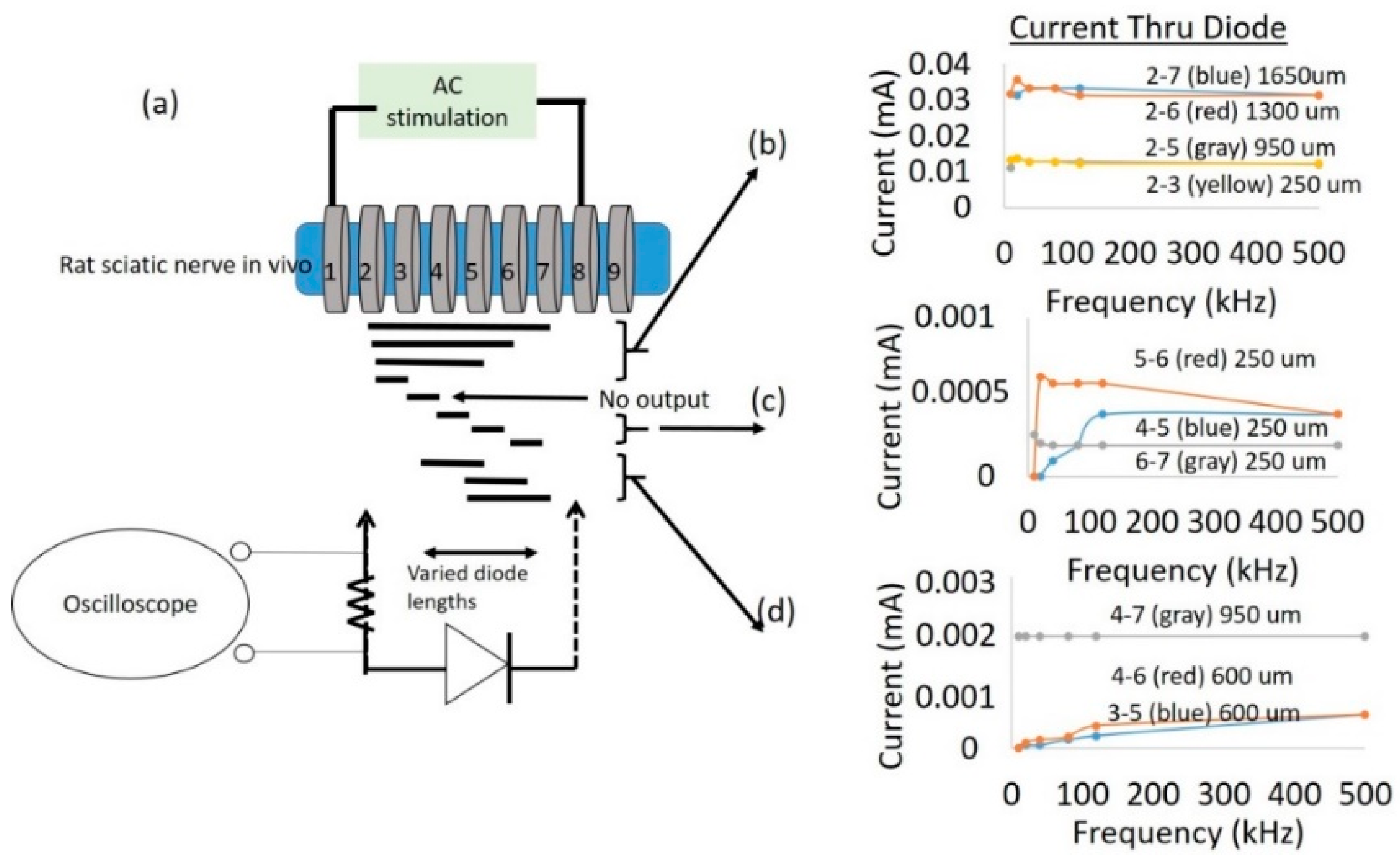
To study the impact of diode–dipole length and placement of the diodes relative to the excitation electrodes on nerve excitation, the outer rings ‘1’ and ‘9’ were used to deliver AC stimulation (peak-to-peak voltage of 0–20 V sine waveform) to the sciatic nerve in vivo. The mini- or micro-diode was wired to any 2 of the remaining rings (rings 2 through 8). Different combinations of the inner rings were used to test different diode–dipole lengths with the externally attached, mini- or micro-diode as illustrated in
Figure 3b. A 510 Ω resistor was placed in series with the diode for current measurements through the diode and in series with the function generator for current measurements through rings ‘1’ and ‘9’ in the cuff for comparison (
Figure 4a). The AC stimulation leads were electrically isolated using a custom-built transformer with a broad frequency range (10 kHz–2 MHz) to prevent ground loops. At 10 kHz, the output was slightly attenuated by 25% and was adjusted in current calculations.
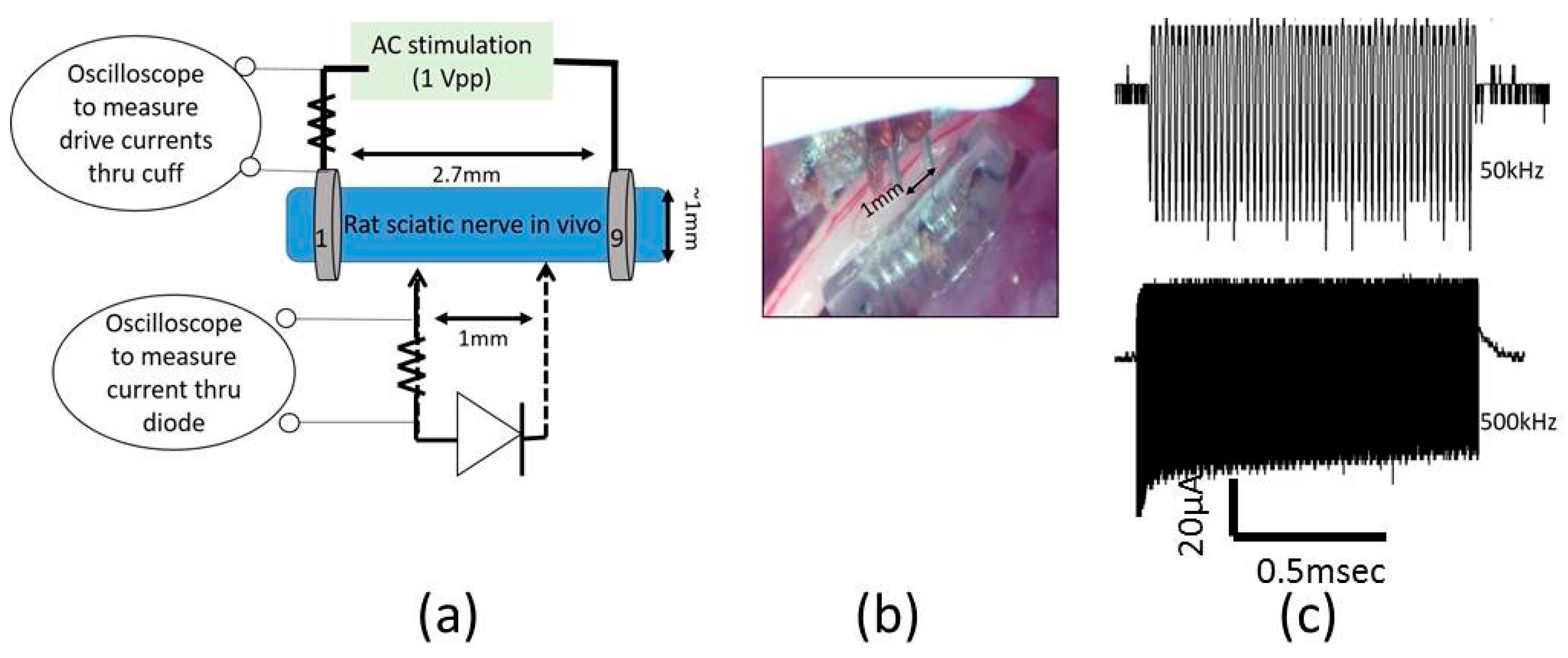
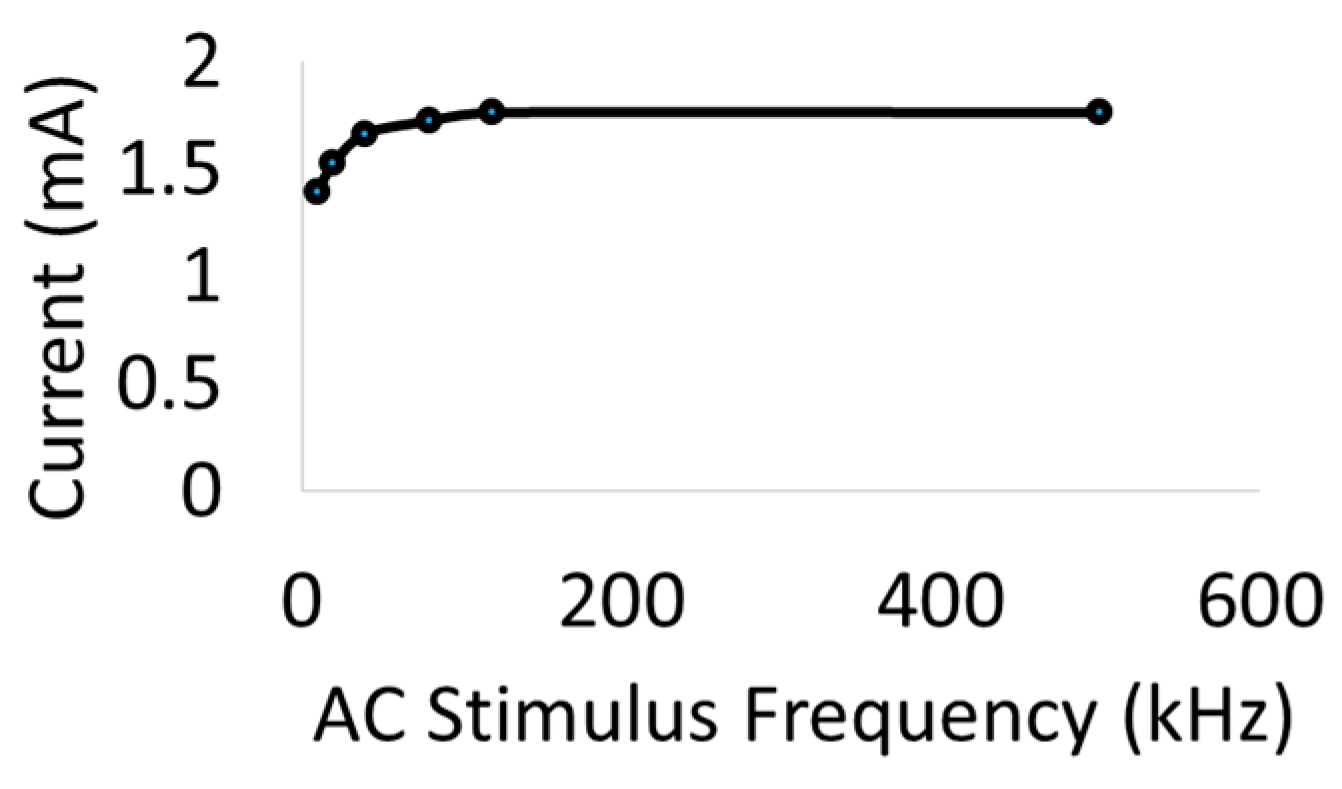
Figure 4a shows the setup for drive current measurements with a 510 Ω resistor placed in series with AC input. As seen in
Figure 5, the current through the nerve was between 1–2 mA and fairly stable across frequencies. At lower frequencies (10–20 kHz), a marginal dependence on frequency was observed.
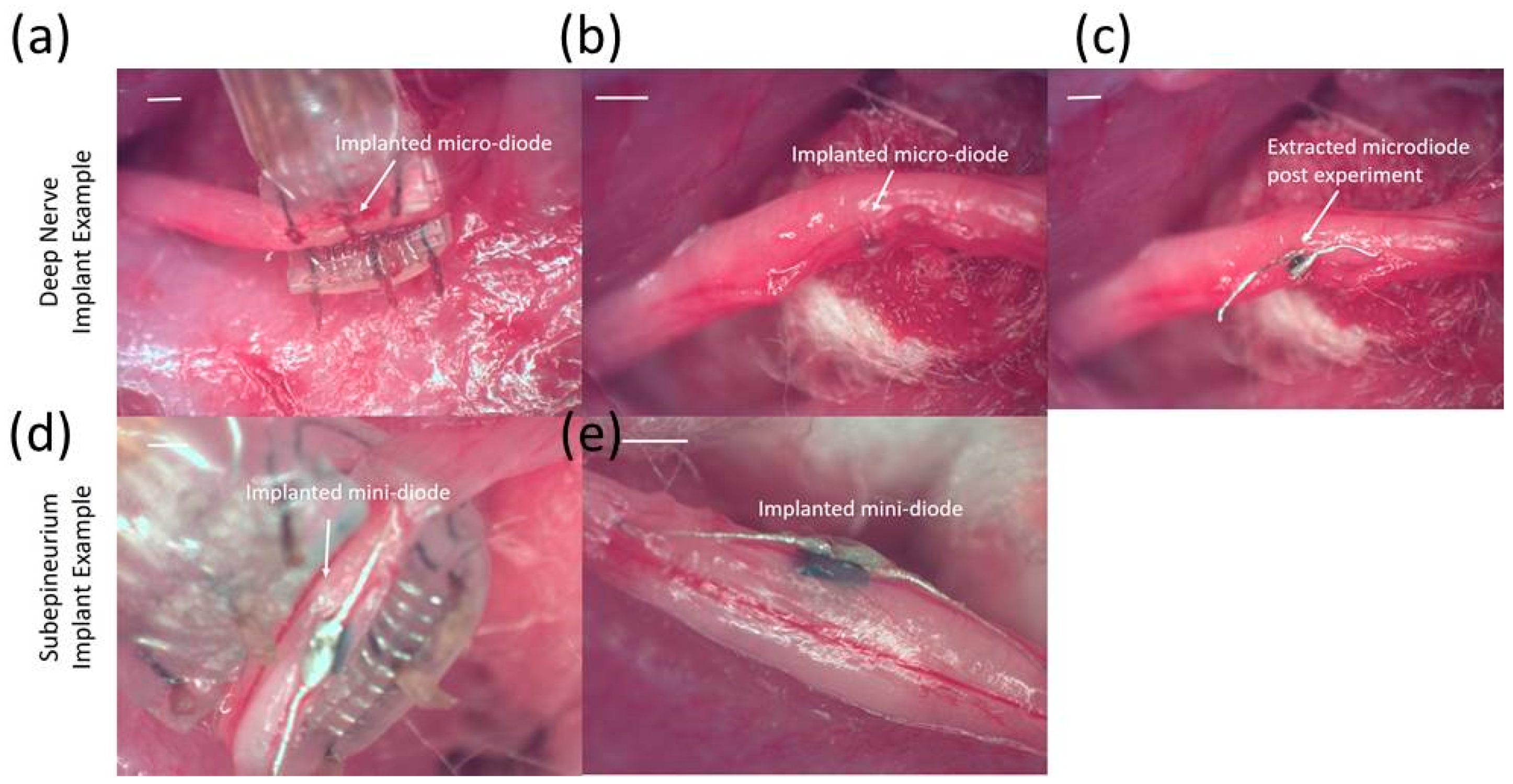
To measure typical currents through a microdiode that is implanted in a nerve, the anode and the cathode of the diodes were connected to Teflon-insulated platinum wires (~110 µm diameter, A–M Systems) spaced 1 mm apart as shown in
Figure 4a,b. The diode was then mounted on a micromanipulator and the ends of the platinum wires were then used as probes to measure current through different depths in the nerve, namely (a) on the surface of the epineurium, (b) subepineurial placement (c) ~500 µm deep in the sciatic nerve, and (d) ~1 mm deep inside the nerve. Two different diode positions—‘edge’ which is ~250 μm away from the stimulating electrode, and ‘center’ which is 1 mm away from the stimulating electrode)—were assessed for current flow. Examples of partially rectified output for a 1 Vpp (peak-to-peak amplitude) input AC burst at 50 kHz and 500 kHz are shown in
Figure 4c.
4. Discussion
The primary goal of this study was to determine the set of stimulation parameters, diode dimensions and placement that would enable microscale, implantable diodes to function as wireless neuromodulators. The working principle was to use the volume conduction properties of tissue as a method of transferring power from non-contacting but nearby electrodes to free-floating diodes placed on or inside the nerves. The initial concept of using a rectifier (germanium diodes with silver leads (1–3 cm long) to stimulate external organs was demonstrated by Palti in 1966 [
17]. Recent work reiterated the concept by placing leads from a full-wave bridge rectifying circuit prototype (eAxon) into selected muscle fibers that are stimulated using a 1 MHz sinusoidal input [
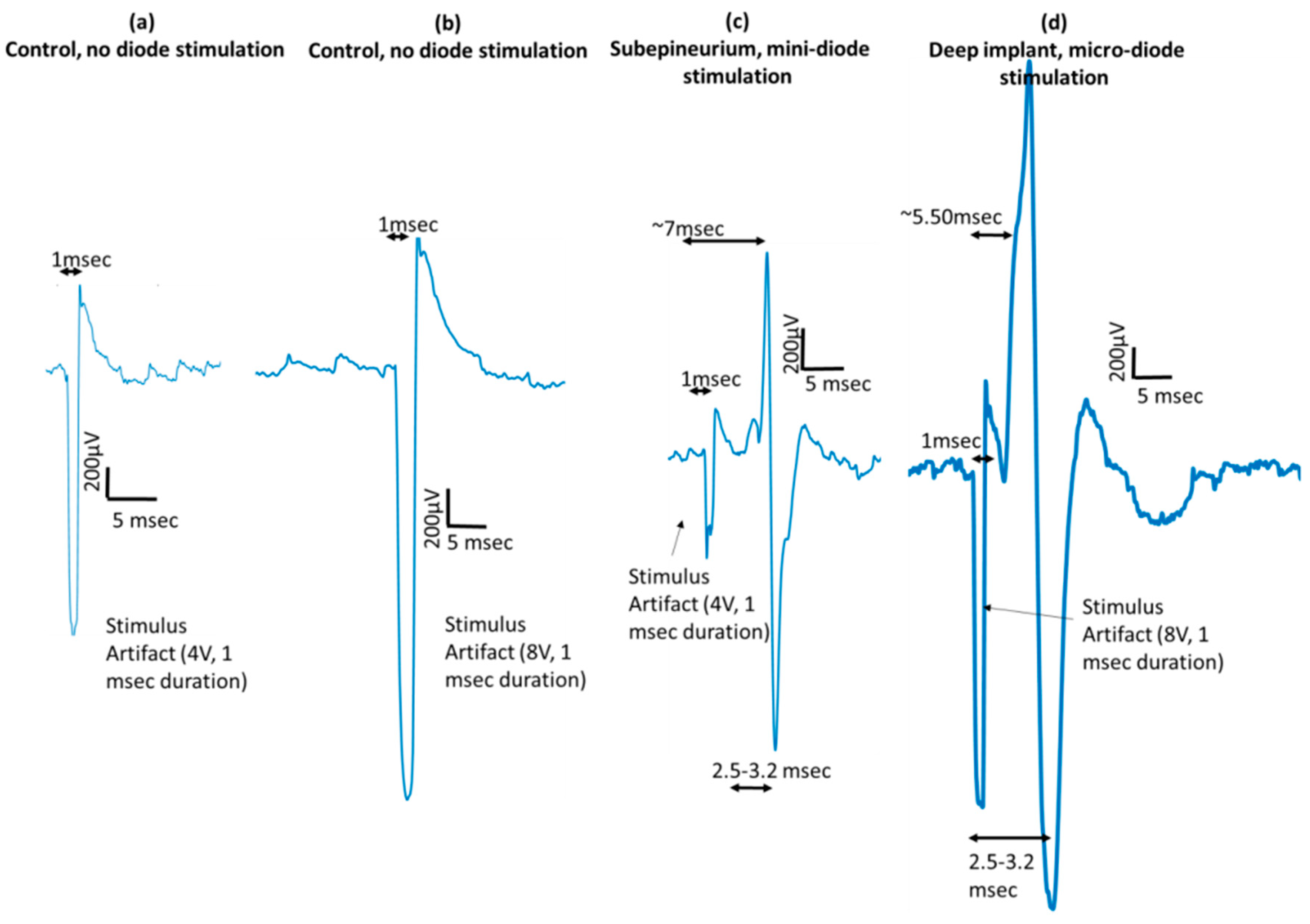
18]. In this study, we demonstrated remote neurostimulation using microscale, silicon diodes directly implanted in the nerve in at least 13 animals (
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 12 and
Figure 13). This approach allowed neurostimulation without wires traversing the epineurium to contact electrodes. Thus, there is a wireless bridging of the last millimeter of distance between the local environment outside of the nerve body and its interior. Using different feature sizes (1.5 mm, 0.5 mm, and 0.22 mm) off-the-shelf, commercially-available, Schottky diodes (Skyworks 7630), we assessed the parameters of the external stimulating AC signal (such as frequency 10–500 kHz, drive voltages, and currents), diode dimensions and relative position of the diode with respect to the external AC stimulating electrodes that would be required to achieve wireless neurostimulation for microdiodes implanted in the sciatic nerve. We found AC stimulating frequency and diode length to be major factors in diode performance in vivo, followed by proximity of the diode to the stimulating electrodes and implantation depth.
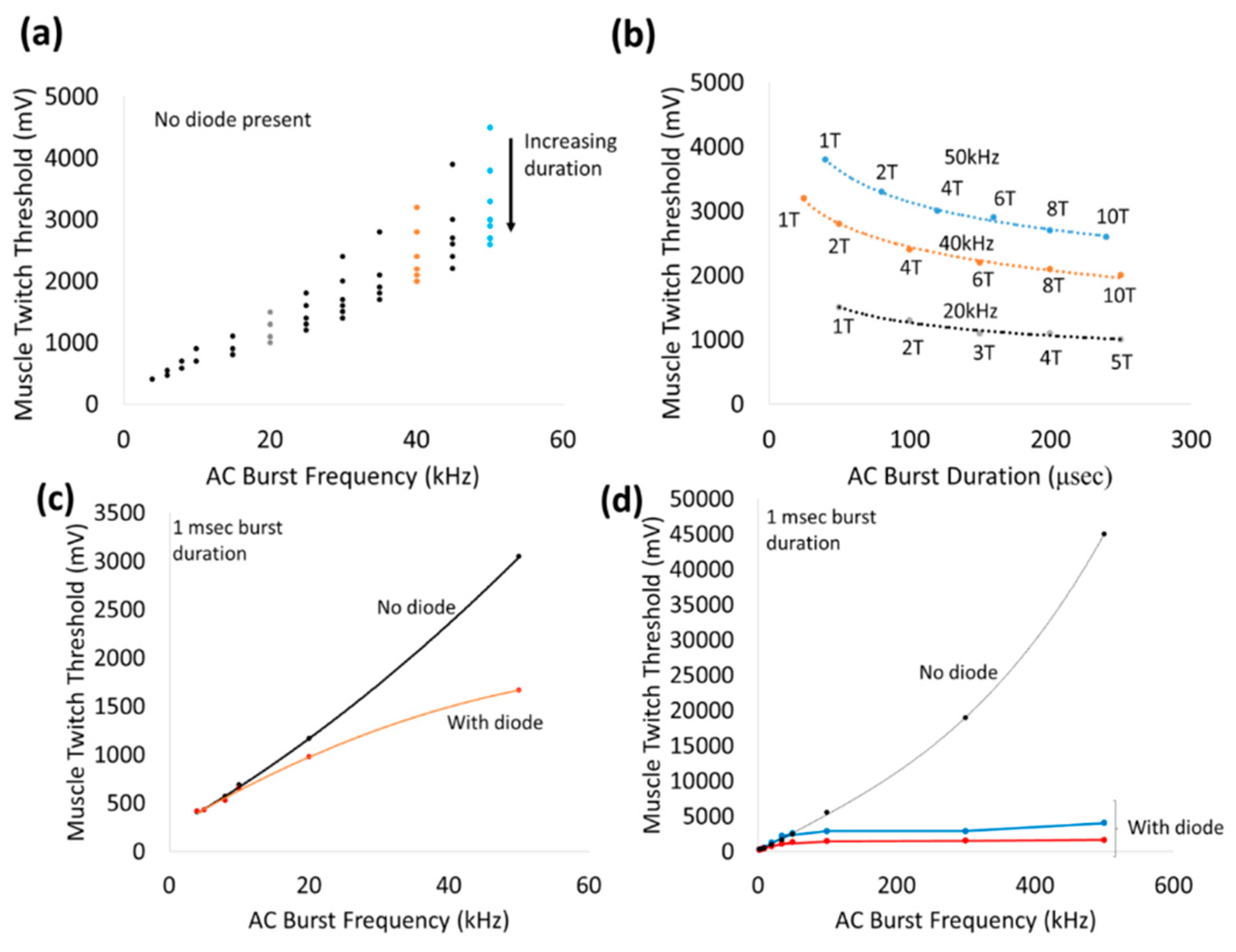
Application of 1 msec bursts of zero-offset, sinusoidal AC waveforms via a nerve cuff platform by itself stimulated the sciatic nerve in a frequency-dependent manner from 10–500 kHz (
Figure 8). The non-linearly increasing thresholds required to achieve a visible muscle twitch at higher frequencies can perhaps be explained by earlier observations of classic strength–duration relationships for nerve stimulation. Such strength–duration curves for nerves have demonstrated a non-linear, hyperbolic relationship between strength and duration required to achieve threshold. High frequencies correspond to lower durations and hence threshold for AC stimulation of nerves can be expected to increase non-linearly with frequency. In addition, high frequency AC stimulation that exceed the kinetics of the ion channels in the cell membrane, will result in significantly higher voltage thresholds (such as ~45 Vpp at 500 kHz). In fact, pure sinusoidal AC continuous waveforms up to 40 kHz have been used effectively in nerve conduction block applications, such as relief from phantom limb pain [
26,
27,
28].
Placement of a wireless, remote diode in AC electric field is expected to fully or partially rectify the input sinusoidal wave and generate a DC component that is proportional to input Vrms and large enough to stimulate a nerve. Above 20 kHz, the addition of a wireless diode between the stimulus electrodes achieved increasing reductions in the stimulus voltage threshold. For instance, the stimulation voltage thresholds at 20 kHz and 50 kHz were ~700 mV and ~1.5 V with a wireless diode compared to ~900 mV, ~3 V without diode, respectively. Beyond 100 kHz, the stimulus voltage threshold plateaued (<5 V) and was fairly independent of frequency. We speculate that between 20–100 kHz, the rectified signals of the diode augmented the neurostimulation of the applied external AC signal in achieving the threshold. Below 20 kHz, the augmentation effect of the diode presence was not significant, suggesting the neurostimulation was dominated primarily by the external AC stimulation. Frequency-dependent effects are not expected from diodes since current-voltage (I-V) characterization of modified diodes show similar diode threshold values and rectification properties across a range of frequencies (10 kHz–1 MHz,
Figure 2).
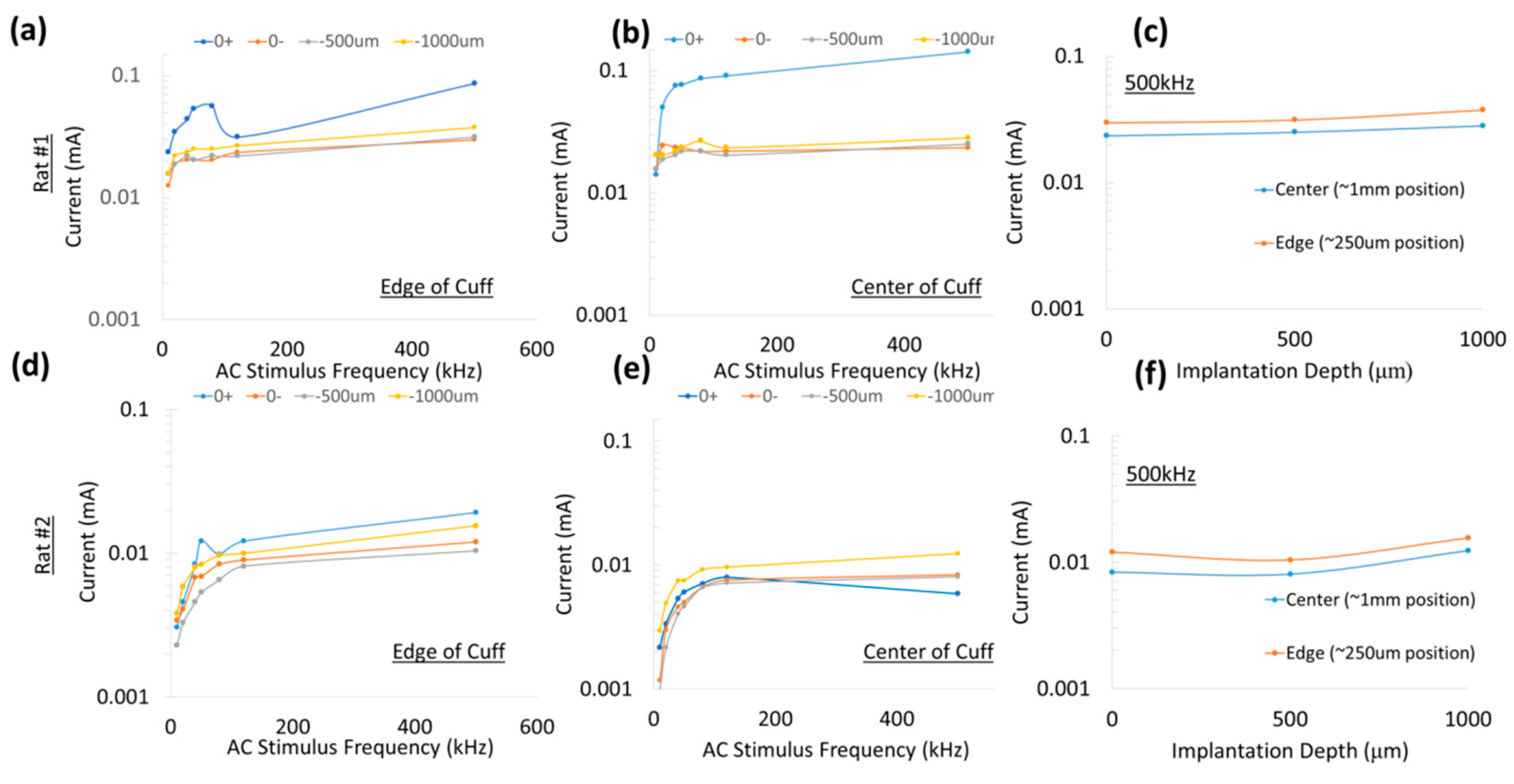
In this study, the measured currents through the diode were at least 100-fold less (
Figure 10) than the currents through the rings ‘1’ and ‘9’ of the cuff electrode (
Figure 5), suggesting high levels of volume conduction loss. Increasing the diode–dipole length from 250 µm to 2 mm reduced the threshold by ~10–20% and ~55% at 20 kHz and 50 kHz, respectively, as shown in
Figure 9. Significant improvements in stimulus voltage thresholds were seen for diode–dipole lengths >1 mm at 50 kHz, while the effect of diode length was only marginal at 20 kHz. The effects of diode–dipole lengths were more pronounced for 500 kHz stimulation frequency. The stimulus voltage threshold lowered 10-fold at 500 kHz as the diode–dipole length changed increased from 250 µm to 2 mm. The smaller diode–dipole lengths have relatively lower energy transfer efficiency due to less volume-conducted currents being intercepted by diode–electrodes. At high frequencies, only the current through the diode that is rectified would be useful for neurostimulation. The results are in agreement with [
23], who theorized that diode designs with long, thin geometry that maximize separation distance with short electrode leads would have maximum energy transfer efficiency. Sahin et al. [
22] showed that separation distance of remote electrodes more than two times the diode anode–cathode separation distances (or diode–dipole length) entails high volume conductor losses. The stimulating electrode in the cuff were separated by 2.7 mm and, indeed, at 500 kHz where the augmentation of the diode would be most dominant, a diode–dipole length of >1 mm resulted in the lowest voltage thresholds.
For a fixed diode–dipole length, increasing the diode proximity to the stimulating electrodes reduced the stimulus voltage threshold value by ~10% compared to positions more central between the stimulating electrodes (
Figure 9d,e). Measured currents through a diode reduced 20–60 times when the diode was placed >250 µm away from the stimulating electrode (
Figure 10). A remote diode with a smaller contact surface area also reduced by up to 2-fold toward the central position between two stimulating ring electrodes and toward ~500 µm implantation depth (
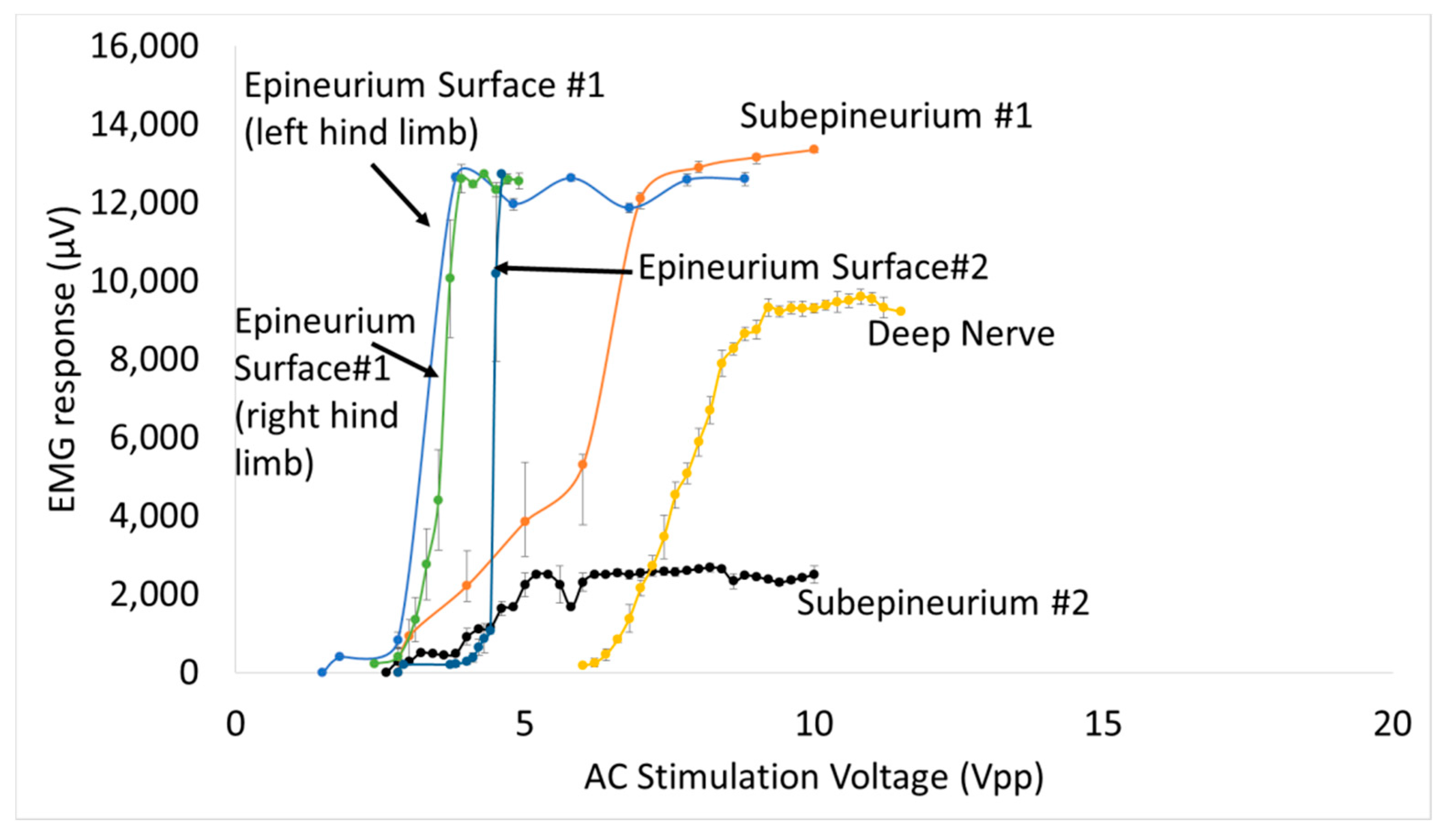
Figure 11). In the case of implanted microdiodes, a large contact surface area between the anode/cathode and the tissue is attained via an additional 1 mm extension of bare, uninsulated platinum wire (total length of the microdiode and wire extension ~3 mm). The larger contact surface area allowed for a lower interfacial impedance and hence a more conducive current path compared to the typically higher tissue impedance surrounding the implant. The feasibility of obtaining recruitment curves from implanted microdiode stimulators (
Figure 14) confirmed that having lower contact impedances is an important design parameter in addition to diode–dipole length and placement in the AC field.
An interesting point was that diodes placed on the nerve and diodes embedded just underneath the epineurium had similar threshold values, suggesting the epineurium did not impede in the energy transfer between the stimulating electrode and the diode. It is well known that at high frequencies (such as 500 kHz) the impedance of electrodes placed above the epineurium and those implanted just beneath in the nerve (sub-epineurium) would converge, essentially eliminating any impedance mismatches for energy transfer. However, implants placed deeper in the peripheral nerve tissue would be expected to have a higher threshold due to higher tissue path impedance. Indeed when comparing EMG recruitment curves of diodes implanted deep in the nerve and that of subepineurial diodes for similar diode lengths at 500 kHz input frequency (
Figure 14), the stimulus voltage threshold increased by 2–3 fold from 2.8–3 V to 6 V. In fact, one diode implanted deep in the nerve required a stimulus voltage threshold of 20 V, suggesting steep recruitment curves (data not shown). It should be noted that the minimum current needed to achieve the threshold were similar for a monophasic, square pulse with a 1 msec duration (17 µA) compared to the minimum current through a diode at the threshold (19.6 µA). Therefore, deep implants require more drive to achieve similar performance. It should be emphasized the repeatability and robustness of the diode placement would be an important experimental variable in potential application of this kind of neurostimulation strategy. The repeatability/robustness of the nerve responses from the diodes from trial to trial is governed partly by surgical technique and animal-to-animal variations in electrophysiological response. The primary focus of this study was not to characterize known biological responses to pulsed monophasic but rather the ability to stimulate them remotely by electric field manipulation. Strategies to optimize positioning and manipulation of diode lengths will be needed in the future for modulation of deep fibers. Volume conduction models that assume homogenous and isotropic tissue properties with uniform conduction would predict the highest threshold to be at the midpoint. However, the data in
Figure 8d–f suggested that while stimulus thresholds trended higher in the region between the edges of the stimulus electrodes, there was a large variance in the exact position where least current and higher thresholds occur. Considering that tissue properties have in reality more inhomogeneous composition [
29], and the nerve itself has a non-spherical, oblong geometry, better mapping of conduction properties inside the nerve would be needed in the future for the optimal placement of diodes. It should be noted that variance in current properties outside the nerve epineurium (
Figure 10) was possibly due to the presence and effective concentrations of body fluids over the time course of the experiment. It should be made clear that the study is not proposing the placing of commercial diodes in nerves, since even at the smallest feature size of 220 µm, they may cause significant tissue damage due to relative tissue motion. However, assessment of the biocompatibility of the current remote diode–dipole chips is beyond the scope of this work since the primary motivation here was to investigate the possibility of inter-neurally, implantable microstimulators to be remotely activated. The potential advantages of this approach are selective targeting and decoupling of the physical wire connectivity to mitigate the relative motion between implanted devices and the nerve tissue. The disadvantages are relatively higher currents applied to nerve cuffs (currents in the cuffs are in the order of a few mAs to 10’s of mA which are ~100 times higher than currents through the remote diodes) and limitations in placement of multiple diodes due to the need for diode electrode lengths in the order of 1 mm.
This often affects the performance of tethered implants in terms of energy usage, targeting precision and optimal performance since it may require frequent recalibration. This work did not examine issues of nerve damage due to diode placement. However, we note that to mitigate the tissue damage due to diode implant itself, design modifications such the use of materials that are mechanically matched to the tissue, flexible designs and miniaturization may be used to improve chronic functional performance.