Cell Density Detector Based on Light Beam Focusing
Abstract
:1. Introduction
2. Optical Sensors Design
2.1. Initial Motive and Design
2.2. Input Unit and Collimating Unit
2.3. Focusing Unit
2.4. Spot Optimization
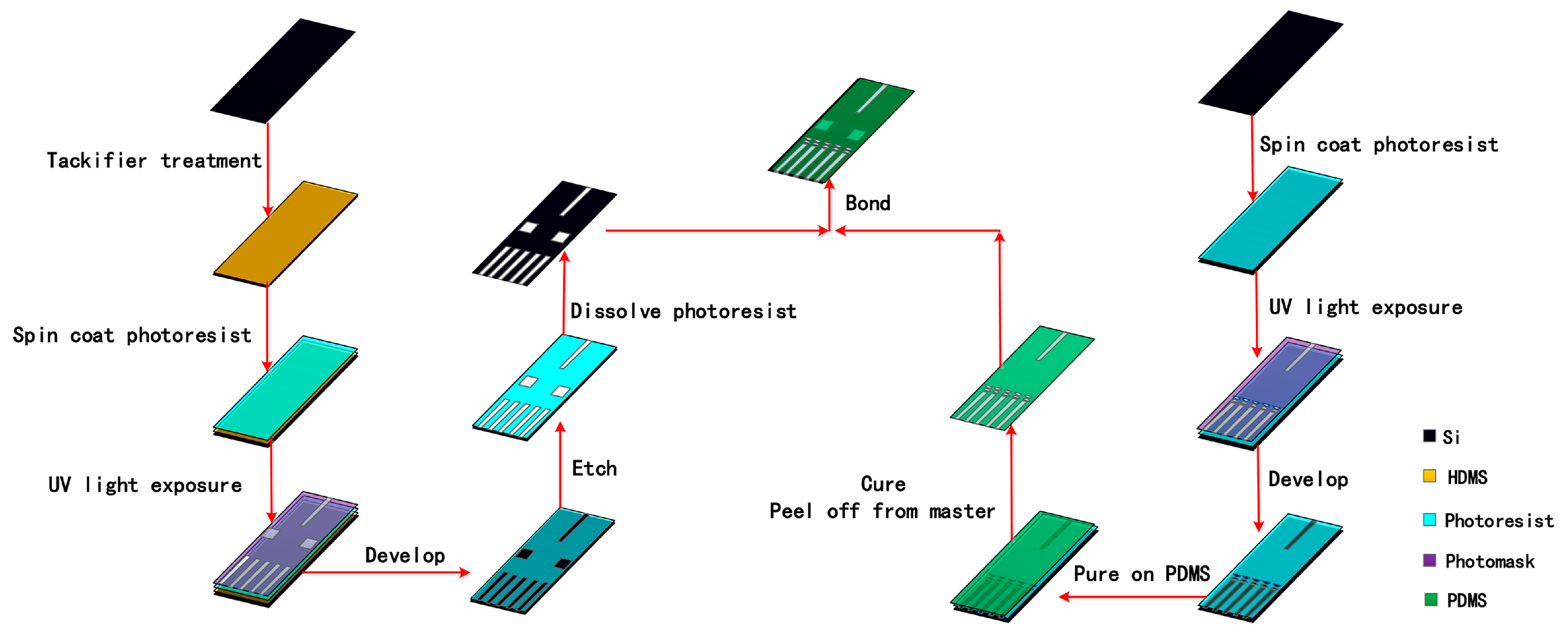
3. Fabrication
3.1. Process of the Optical Sensor
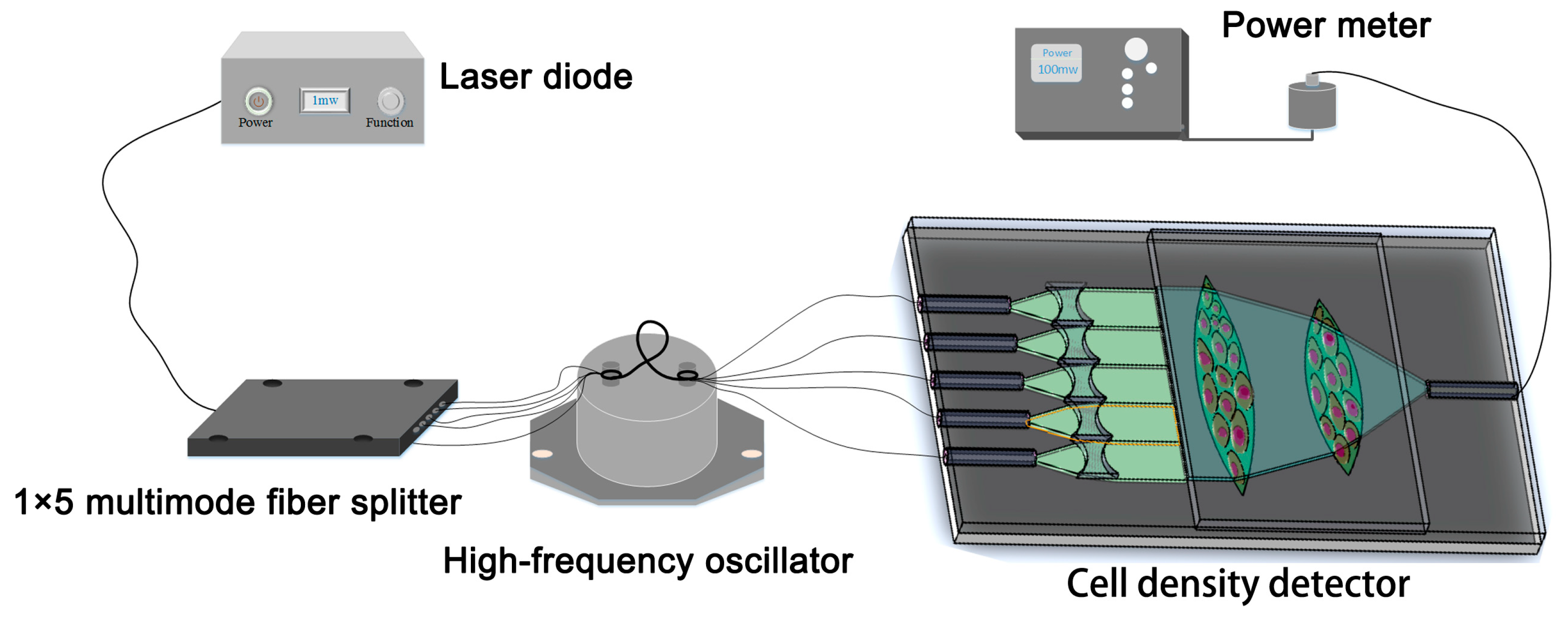
3.2. Cell Density Detection System
4. Results and Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Byun, J.; Verardo, M.R.; Sumengen, B.; Lewis, G.P.; Manjunath, B.S.; Fisher, S.K. Automated tool for the detection of cell nuclei in digital microscopic images: Application to retinal images. Mol. Vis. 2006, 12, 949–960. [Google Scholar] [PubMed]
- Chan, L.L.; Laverty, D.J.; Smith, T.; Nejad, P.; Hei, H.; Gandhi, R.; Kuksin, D.; Qiu, J. Accurate measurement of peripheral blood mononuclear cell concentration using image cytometry to eliminate RBC-induced counting error. J. Immunol. Methods 2013, 388, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Simonson, L.W.; Ganz, J.; Melancon, E.; Eisen, J.S. Characterization of Enteric Neurons in Wild-Type and Mutant Zebrafish Using Semi-Automated Cell Counting and Co-Expression Analysis. Zebrafish 2013, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Bertucco, L.; Nunnari, G.; Randieri, C.; Rizza, V. A cellular neural network based system for cell counting in culture of biological cells. In Proceedings of the IEEE International Conference on Control Applications, Trieste, Italy, 1–4 September 1998; Volume 1, pp. 341–345. [Google Scholar]
- Brinkmann, M.; Lütkemeyer, D.; Gudermann, F.; Lehmann, J. New technologies for automated cell counting based on optical image analysis ‘The Cellscreen’. Cytotechnology 2002, 38, 119. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Wang, H.T.; Hsu, J.T. Relationship of somatic cell count, physical, chemical and enzymatic properties to the bacterial standard plate count in dairy goat milk. Livest. Prod. Sci. 2002, 74, 63–77. [Google Scholar] [CrossRef]
- Borneman, D.L.; Ingham, S. Correlation between standard plate count and somatic cell count milk quality results for Wisconsin dairy producers. J. Dairy Sci. 2014, 97, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Borowski, M.; Giovino-Doherty, M.; Ji, L.; Shi, M.J.; Smith, K.P.; Cambridge, S.B. Basic Pluripotent Stem Cell Culture Protocols; Harvard Stem Cell Institute: Cambridge, MA, USA, 2012. [Google Scholar]
- Tee, D.E.H. Culture of Animal Cells: A Manual of Basic Technique. Yale J. Biol. Med. 1984, 57, 247–248. [Google Scholar]
- Coulter, W.H.; Hogg, W.R. Apparatus and Method for Measuring Particle Concentration of a Suspension Passing through a Sensing Zone. U.S. Patent 3,733,548, 15 May 1973. [Google Scholar]
- Bull, B.S.; Schneiderman, M.A.; George, B. Platelet Counts with the Coulter Counter. Am. J. Clin. Pathol. 1965, 44, 678. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Evans, A.G.R.; Brunnschweiler, A. Design and fabrication of a micromachined Coulter counter. J. Micromech. Microeng. 1999, 9, 159–161. [Google Scholar] [CrossRef]
- Zhe, J.; Jagtiani, A.; Dutta, P.; Hu, J.; Carletta, J. A micromachined high throughput Coulter counter for bioparticle detection and counting. J. Micromech. Microeng. 2007, 17, 304–313. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Stein, D.; Mui, T.; Mack, R.; Hubbard, M.; Denny, T. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin. Diagn. Lab. Immunol. 1997, 4, 309–313. [Google Scholar] [PubMed]
- Hur, S.C.; Tse, H.T.K.; Di Carlo, D. Sheathless inertial cell ordering for extreme throughput flow cytometry. Lab Chip 2010, 10, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Cordelli, E.; Eleuteri, P.; Leter, G.; Rescia, M.; Spanò, M. Flow cytometry applications in the evaluation of sperm quality: Semen analysis, sperm function and DNA integrity. Contraception 2005, 72, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Schauer, U.; Heusser, C.; Neumann, C.; Rieger, C. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 1993, 159, 197–207. [Google Scholar] [CrossRef]
- Blair, O.C. Practical Flow Cytometry; Alan R. Liss: New York, NY, USA, 1988; p. 759. [Google Scholar]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Valdivia, R.H.; Falkow, S. Bacterial genetics by flow cytometry: Rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 2010, 22, 367–378. [Google Scholar] [CrossRef]
- Rebeiz, G.M.; Dussopt, L.; Rebeiz, G.M. MEMS Varactors and Tunable Oscillators; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 327–358. [Google Scholar]
- Lorenz, H.; Despont, M.; Fahrni, N.; La Bianca, N.; Renaud, P.; Vettiger, P. SU-8: A low-cost negative resist for MEMS. J. Micromech. Microeng. 1997, 7, 121–124. [Google Scholar] [CrossRef]
- CookChennault, K.A.; Thambi, N.; Sastry, A.M. TOPICAL REVIEW: Powering MEMS portable devices—A review of non-regenerative and regenerative power supply systems with special emphasis on piezoelectric energy harvesting systems. Smart Mater. Struct. 2008, 17, 1240–1246. [Google Scholar]
- Kretzmer, G. Influence of Stress on Adherent Cells; Springer: Berlin/Heidelberg, Germany, 2000; pp. 123–137. [Google Scholar]
- Brodbeck, W.G.; Shive, M.S.; Colton, E.; Nakayama, Y.; Matsuda, T.; Anderson, J.M. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 55, 661–668. [Google Scholar] [CrossRef]
- Russer, P. The Transmission Line Matrix Method; Springer: Berlin/Heidelberg, Germany, 2000; pp. 243–269. [Google Scholar]
- Ha, W.; Jung, Y.; Kim, J.; Oh, K. Speckle reduction in multimode fiber with a piezoelectric transducer in radial vibration for fiber laser marking and display applications. In Proceedings of the SPIE 6873, Fiber Lasers V: Technology, Systems, and Applications, San Jose, CA, USA, 19–24 January 2008; p. 68731V. [Google Scholar]
- Paxton, A.H. Reduction of speckle contrast in multimode fibers using piezoelectric vibrator. Proc. SPIE Int. Soc. Opt. Eng. 2014, 8960, 271–283. [Google Scholar]
- Zhao, X.-M.; Xia, Y.; Whitesides, G.M. Soft lithographic methods for nano-fabrication. J. Mater. Chem. 1997, 7, 1069–1074. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A review. BioChip J. 2008, 2, 1–11. [Google Scholar]
- Gourley, P.L. Biocavity laser for high-speed cell and tumour biology. J. Phys. D Appl. Phys. 2003, 36, R228–R239. [Google Scholar] [CrossRef]
- Bin Mat Yunus, W.M.; bin Abdul Rahman, A. Refractive index of solutions at high concentrations. Appl. Opt. 1988, 27, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Zhernovaya, O.; Sydoruk, O.; Tuchin, V.; Douplik, A. The refractive index of human hemoglobin in the visible range. Phys. Med. Biol. 2011, 56, 4013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Jin, W.F.; Wen, T.D.; Zhu, L.Q.; Zhang, J.L. Study on the Relational Expression of Solution concentration and Its Refractive Index. J. North Univ. China 2009, 30, 281–285. (In Chinese) [Google Scholar]
- Ciminelli, C.; Campanella, C.M.; Dell’Olio, F.; Campanella, C.E.; Armenise, M.N. Label-free optical resonant sensors for biochemical applications. Prog. Quantum Electron. 2013, 37, 51–107. [Google Scholar] [CrossRef]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express 2008, 16, 1020–1028. [Google Scholar] [CrossRef] [PubMed]












| Parameters | Symbol | Values |
|---|---|---|
| The radius of the collimating lens | R | 403.55 μm |
| The spacing of the lens | L | 200 μm |
| The thickness of the lens | T | 125 μm |
| Refractive index (RI) of polydimethylsiloxane (PDMS) | nPDMS | 1.406 |
| RI of air | nair | 1 |
| Parameter | Symbol | Values (mm) | |
|---|---|---|---|
| The primary lens | Radius | R1 | 4 |
| Width | D1 | 1.05 | |
| The secondary lens | Radius | R2 | 2.5 |
| Width | D2 | 1.05 | |
| The distance between two lenses | - | B | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, A.; Li, H.; Zhang, Y.; Duan, Q.; Zhang, Q.; Sang, S. Cell Density Detector Based on Light Beam Focusing. Micromachines 2018, 9, 592. https://doi.org/10.3390/mi9110592
Jian A, Li H, Zhang Y, Duan Q, Zhang Q, Sang S. Cell Density Detector Based on Light Beam Focusing. Micromachines. 2018; 9(11):592. https://doi.org/10.3390/mi9110592
Chicago/Turabian StyleJian, Aoqun, Huiming Li, Yixia Zhang, Qianqian Duan, Qianwu Zhang, and Shengbo Sang. 2018. "Cell Density Detector Based on Light Beam Focusing" Micromachines 9, no. 11: 592. https://doi.org/10.3390/mi9110592
APA StyleJian, A., Li, H., Zhang, Y., Duan, Q., Zhang, Q., & Sang, S. (2018). Cell Density Detector Based on Light Beam Focusing. Micromachines, 9(11), 592. https://doi.org/10.3390/mi9110592





