Assessment of Sub-Micron Particles by Exploiting Charge Differences with Dielectrophoresis
Abstract
:1. Introduction
2. Theory
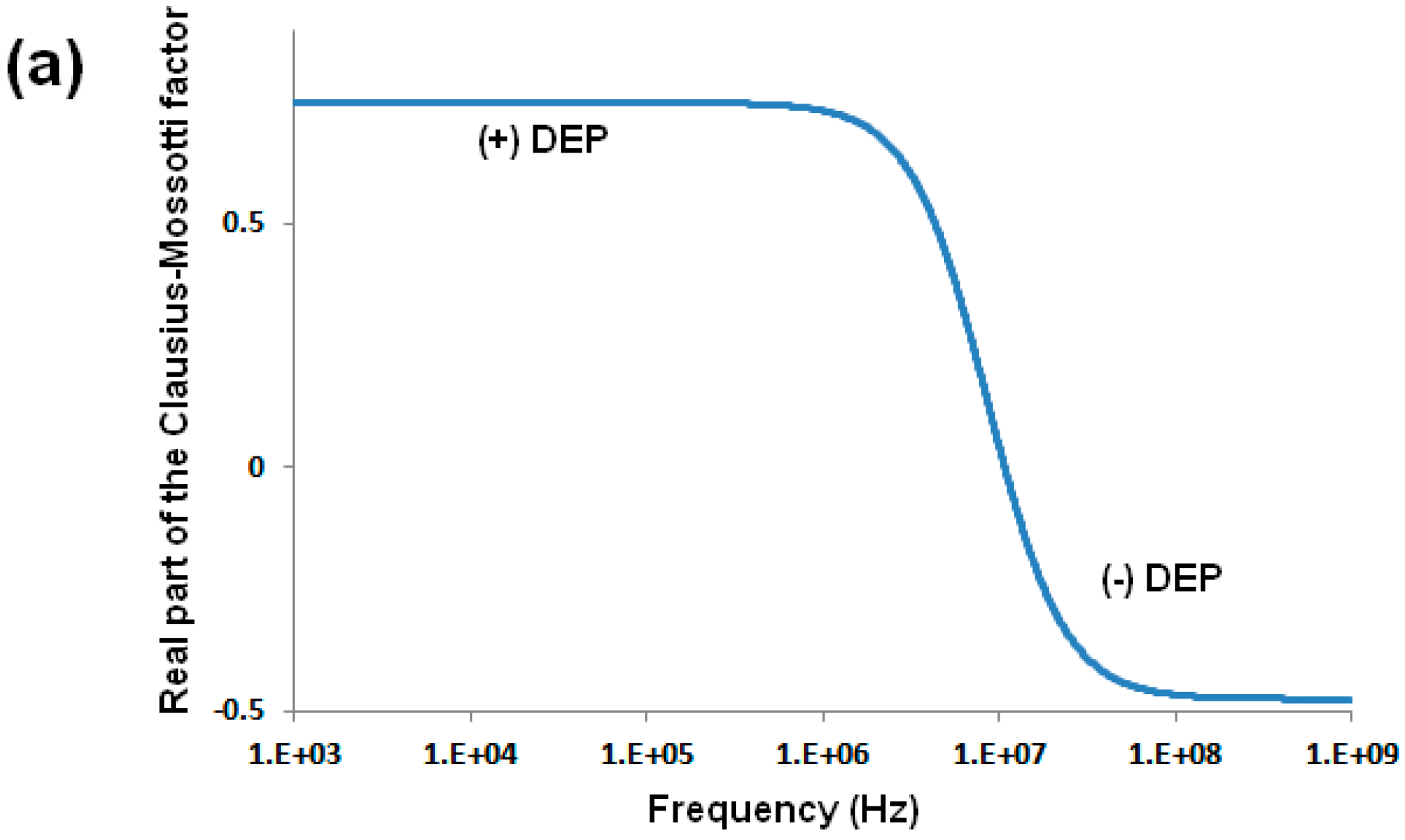
2.1. Dielectrophoretic Force
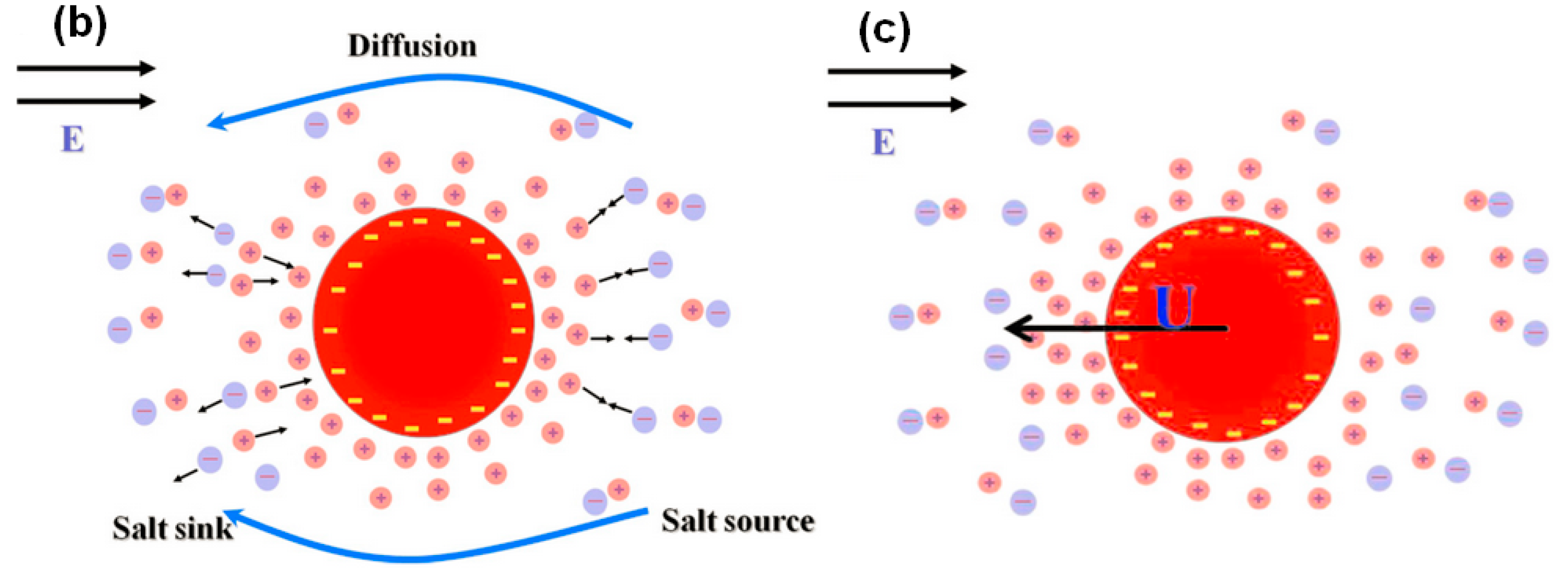
2.2. Particle Polarization and Dipole Moment
2.3. Particle Behavior in iDEP
3. Materials and Methods
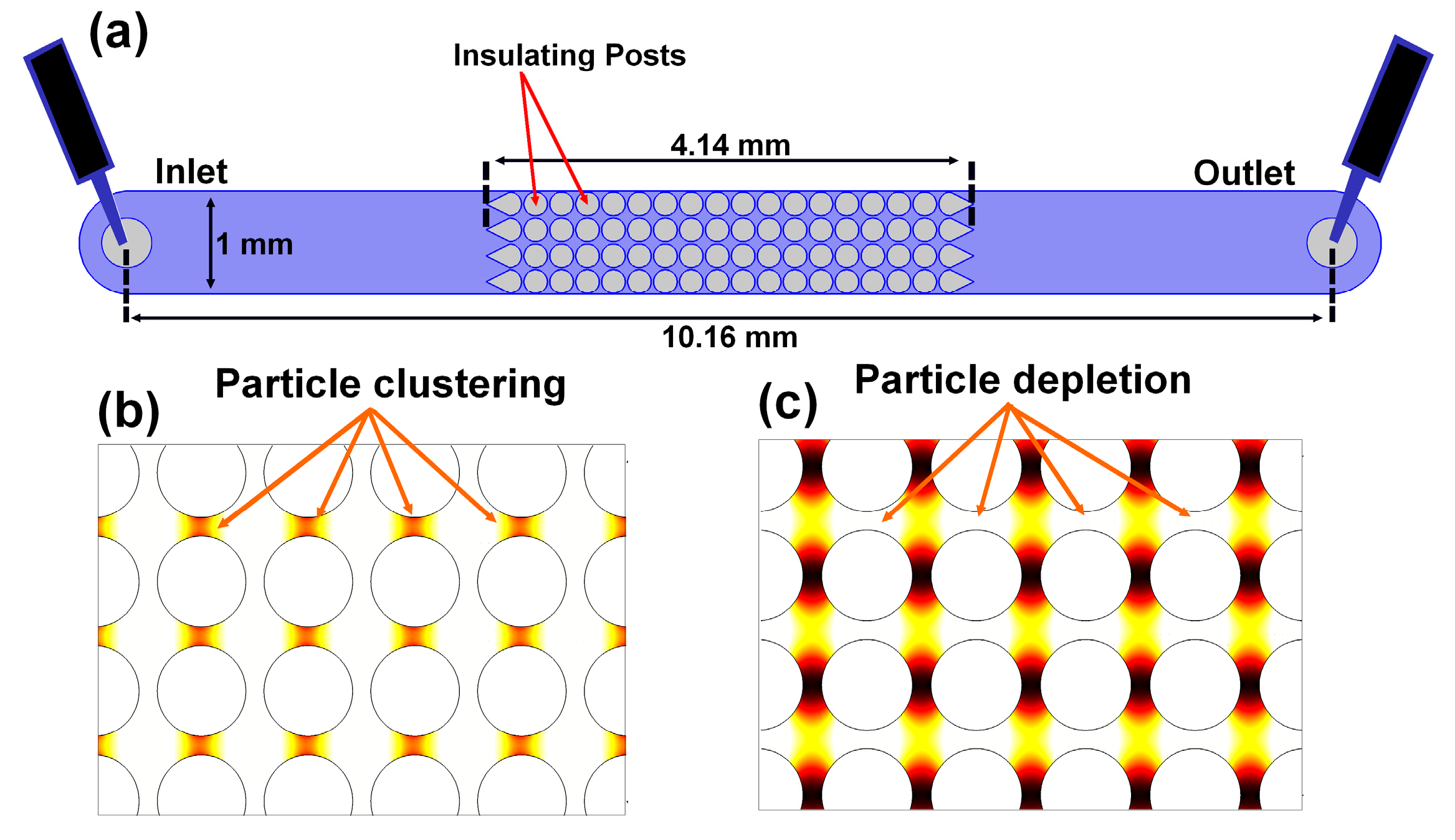
3.1. Microfluidic Devices
3.2. Particles and Suspending Media
3.3. Equipment and Software
3.4. Experimental Procedure
4. Results and Discussion
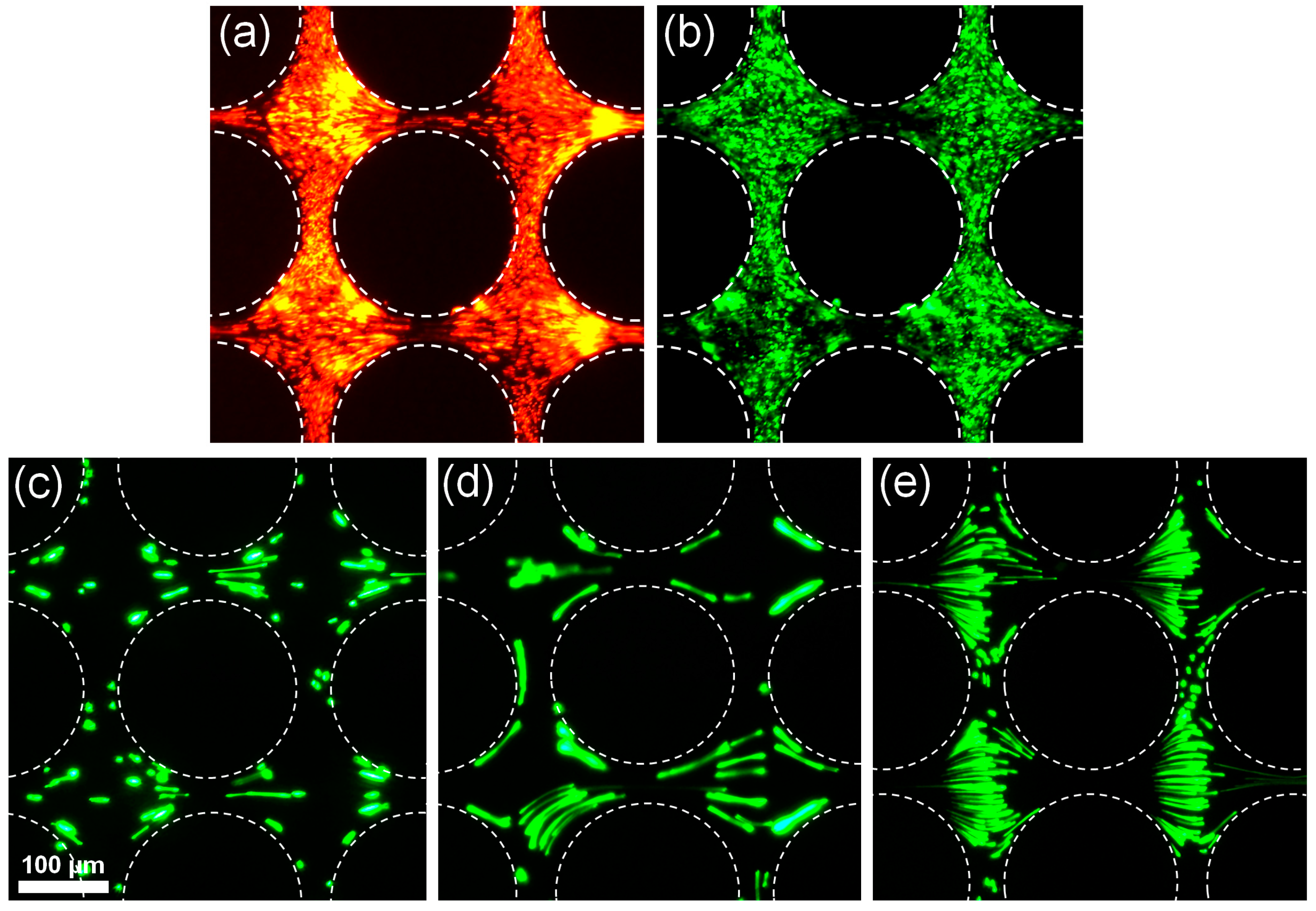
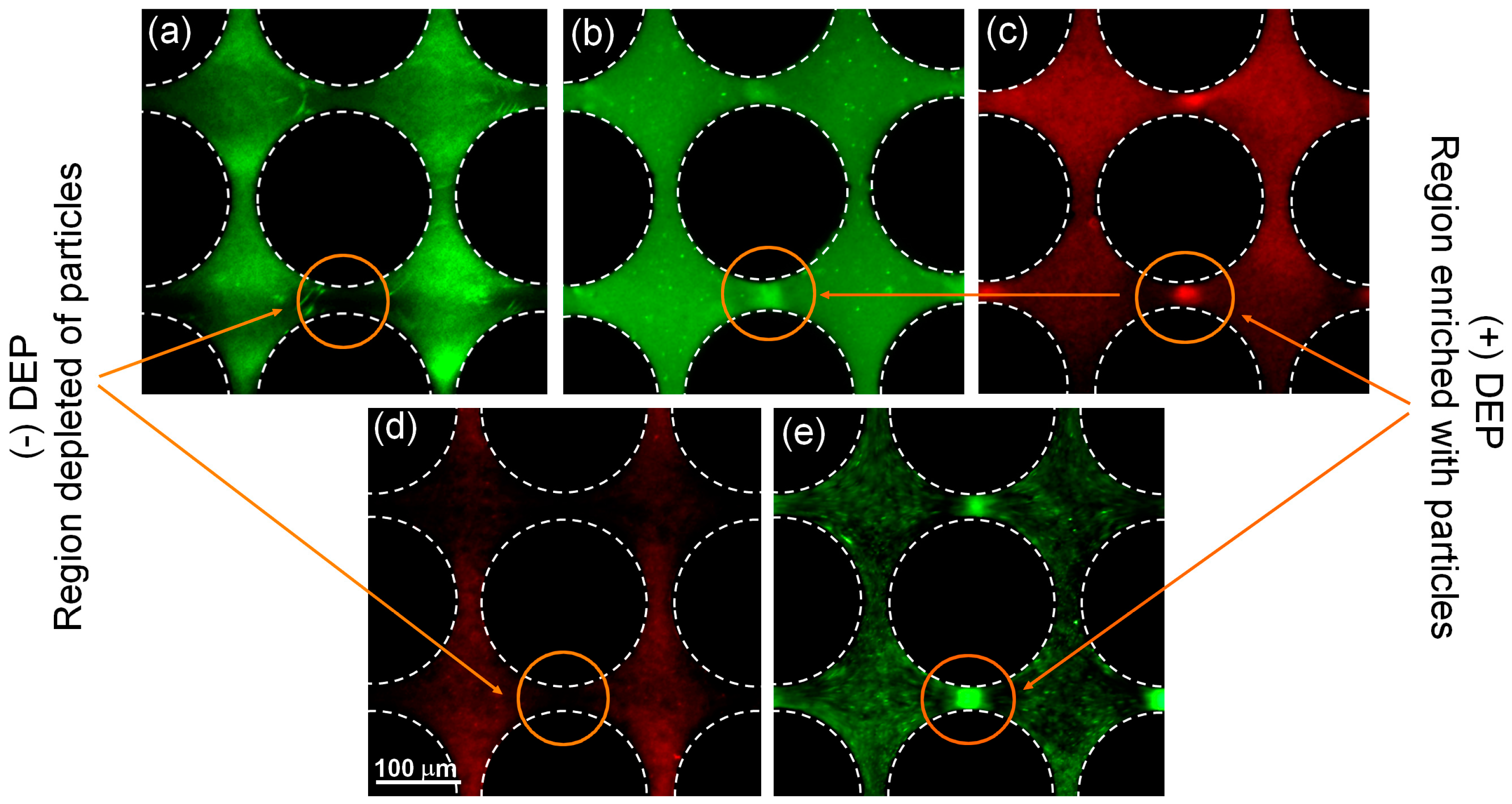
4.1. Effect of Particle Size on Polarization
4.2. Effect of Particle Charge Magnitude for Smaller Particles (dp < 500 nm)
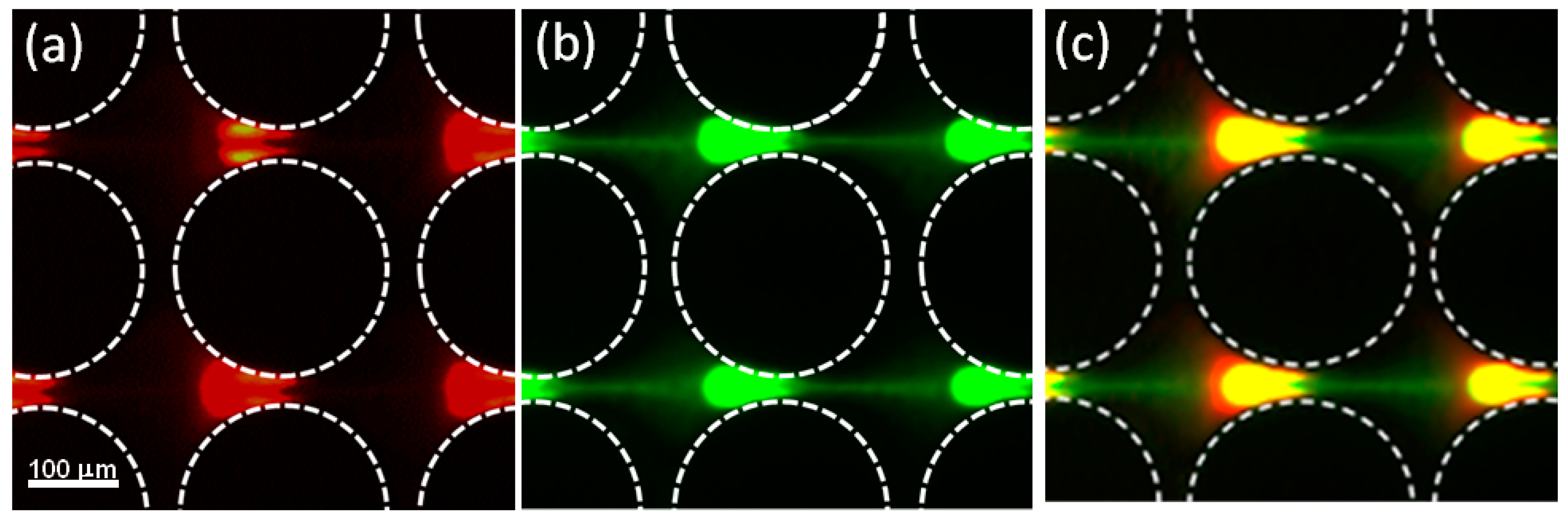
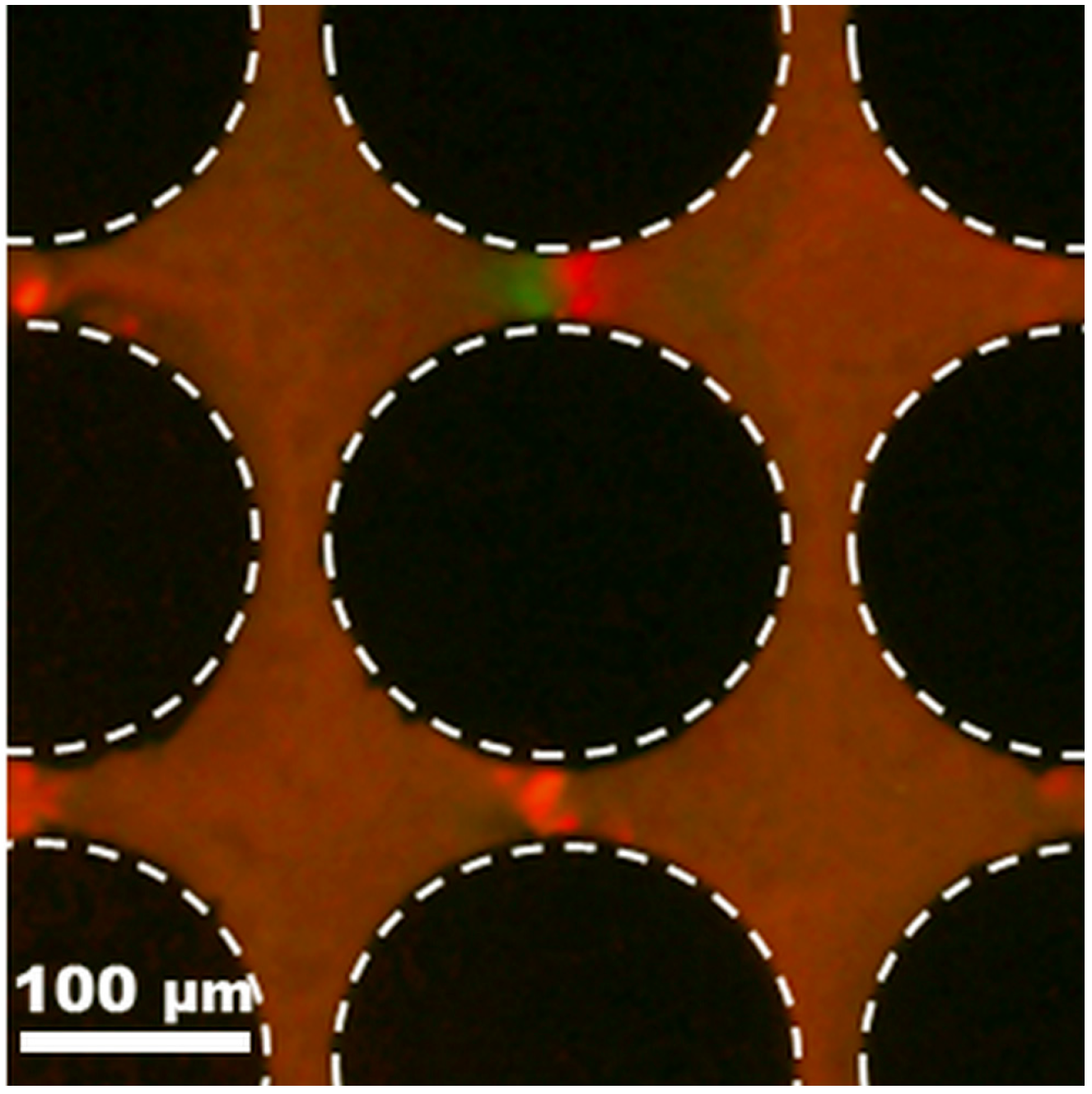
4.3. Application: Dielectrophoretic Differentiation of Sub-Micron Particles by Exploiting Charge Differences
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lapizco-Encinas, B.H. Chapter 7 applications of dielectrophoresis in microfluidics. In Microfluidics in Detection Science: Lab-on-a-Chip Technologies; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 192–223. [Google Scholar]
- Yan, Y.; Guo, D.; Wen, S. Joule heating effects on two-phase flows in dielectrophoresis microchips. BioChip J. 2017. [Google Scholar] [CrossRef]
- Gallo-Villanueva, R.C.; Sano, M.B.; Lapizco-Encinas, B.H.; Davalos, R. Joule heating effects in multipart insulator-based dielectrophoretic microdevices. Electrophoresis 2014, 35, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Li, D. Effect of joule heating on electrokinetic transport. Electrophoresis 2008, 29, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, K. Electrophoresis. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: New York, NY, USA, 2008; pp. 580–588. [Google Scholar]
- Castro, E.R.; Manz, A. Present state of microchip electrophoresis: State of the art and routine applications. J. Chromatogr. A 2015, 1382, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Double-layer polarization of a non-conducting particle in an alternating current field with applications to dielectrophoresis. Electrophoresis 2011, 32, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Review article—Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811–022835. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Review—Where is dielectrophoresis (dep) going? J. Electrochem. Soc. 2017, 164, B3049–B3055. [Google Scholar] [CrossRef]
- Qian, C.; Huang, H.; Chen, L.; Li, X.; Ge, Z.; Chen, T.; Yang, Z.; Sun, L. Dielectrophoresis for bioparticle manipulation. Int. J. Mol. Sci. 2014, 15, 18281. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; García-Sánchez, P.; Morgan, H. Ac electrokinetics of conducting microparticles: A review. Curr. Opin. Colloid Interface Sci. 2016, 24, 79–90. [Google Scholar] [CrossRef]
- Nakano, A.; Ros, A. Protein dielectrophoresis: Advances, challenges, and applications. Electrophoresis 2013, 34, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications—A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Gencoglu, A.; Minerick, A. Dc insulator dielectrophoretic applications in microdevice technology: A review. Anal. Bioanal. Chem. 2010, 399, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Marciniak, J.Y.; McCanna, J.; Krishnan, R.; Rassenti, L.; Kipps, T.J.; Heller, M.J. Dielectrophoretic isolation and detection of cfc-DNA nanoparticulate biomarkers and virus from blood. Electrophoresis 2013, 34, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R.; Camacho-Alanis, F.; Renaud, P.; Ros, A. Dielectrophoresis of lambda-DNA using 3D carbon electrodes. Electrophoresis 2013, 34, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gomez, M.A.; Perez-Gonzalez, V.H.; Gallo-Villanueva, R.C.; Gonzalez-Valdez, J.; Rito-Palomares, M.; Martinez-Chapa, S.O. Modelling of electrokinetic phenomena for capture of pegylated ribonuclease a in a microdevice with insulating structures. Biomicrofluidics 2016, 10, 033106. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.G.; Chao, T.-C.; Kupitz, C.; Fromme, P.; Ros, A. Dielectrophoretic sorting of membrane protein nanocrystals. ACS Nano 2013, 7, 9129–9137. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Maruyama, H.; Honda, A.; Arai, F. Virus enrichment for single virus infection by using 3D insulator based dielectrophoresis. PLoS ONE 2014, 9, e94083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, D. Continuous separation of nanoparticles by type via localized DC-dielectrophoresis using asymmetric nano-orifice in pressure-driven flow. Sens. Actuator B Chem. 2017, 250, 274–284. [Google Scholar] [CrossRef]
- Zhao, H.; Bau, H.H. The polarization of a nanoparticle surrounded by a thick electric double layer. J. Colloid Interface Sci. 2009, 333, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Espinosa, M.A.; Rauch, M.M.; LaLonde, A.; Lapizco-Encinas, B.H. Polarization behavior of polystyrene particles under direct current and low-frequency (<1 kHz) electric fields in dielectrophoretic systems. Electrophoresis 2016, 37, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Basuray, S.; Chang, H.-C. Induced dipoles and dielectrophoresis of nanocolloids in electrolytes. Phys. Rev. E 2007, 75, 060501. [Google Scholar] [CrossRef] [PubMed]
- Green, N.G.; Morgan, H. Dielectrophoretic separation of nano-particles. J. Phys. D Appl. Phys. 1997, 30, L41–L44. [Google Scholar] [CrossRef]
- Green, N.G.; Morgan, H. Dielectrophoresis of submicrometer latex spheres. 1. Experimental results. J. Phys. Chem. B 1999, 103, 41–50. [Google Scholar] [CrossRef]
- Green, N.G.; Morgan, H. Dielectrophoretic investigations of sub-micrometre latex spheres. J. Phys. D Appl. Phys. 1997, 30, 2626–2633. [Google Scholar] [CrossRef]
- Hughes, M.P.; Morgan, H. Dielectrophoretic characterization and separation of antibody-coated submicrometer latex spheres. Anal. Chem. 1999, 71, 3441–3445. [Google Scholar] [CrossRef]
- Hughes, M.P. Dielectrophoretic behavior of latex nanospheres: Low-frequency dispersion. J. Colloid Interface Sci. 2002, 250, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, M.P.; Morgan, H.; Flynn, M.F. The dielectrophoretic behavior of submicron latex spheres: Influence of surface conductance. J. Colloid Interface Sci. 1999, 220, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.B.; Singh, A.K. Dielectrophoresis in microchips containing arrays of insulating posts: Theoretical and experimental results. Anal. Chem. 2003, 75, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.B. Streaming dielectrophoresis for continuous-flow microfluidic devices. IEEE Eng. Med. Biol. Mag. 2003, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.V.; Hayes, M.A. Development of the resolution theory for gradient insulator-based dielectrophoresis. Electrophoresis 2015, 36, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Abdallah, B.G.; Wolken, G.G.; Arriaga, E.A.; Ros, A. Insulator-based dielectrophoresis of mitochondria. Biomicrofluidics 2014, 8, 021801. [Google Scholar] [CrossRef] [PubMed]
- Braff, W.A.; Pignier, A.; Buie, C.R. High sensitivity three-dimensional insulator-based dielectrophoresis. Lab Chip 2012, 12, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Regtmeier, J.; Eichhorn, R.; Viefhues, M.; Bogunovic, L.; Anselmetti, D. Electrodeless dielectrophoresis for bioanalysis: Theory, devices and applications. Electrophoresis 2011, 32, 2253–2273. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, A.; Olney, D.; LaLonde, A.; Koppula, K.S.; Lapizco-Encinas, B.H. Dynamic microparticle manipulation with an electroosmotic flow gradient with low frequency alternating current dielectrophoresis. Electrophoresis 2014, 35, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, A.; Olney, D.; LaLonde, A.; Koppula, K.S.; Lapizco-Encinas, B.H. Particle manipulation in insulator based dielectrophoretic devices. J. Nanotechnol. Eng. Med. 2013, 4, 021002. [Google Scholar] [CrossRef]
- LaLonde, A.; Romero-Creel, M.F.; Saucedo-Espinosa, M.A.; Lapizco-Encinas, B.H. Isolation and enrichment of low abundant particles with insulator-based dielectrophoresis. Biomicrofluidics 2015, 9, 064113. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: New York, NY, USA, 1995; p. 265. [Google Scholar]
- Markx, G.H.; Dyda, P.A.; Pethig, R. Dielectrophoretic separation of bacteria using a conductivity gradient. J. Biotechnol. 1996, 51, 175–180. [Google Scholar] [CrossRef]
- O’Konski, C.T. Electric properties of macromolecules. V. Theory of ionic polarization in polyelectrolytes. J. Phys. Chem. 1960, 64, 605–619. [Google Scholar] [CrossRef]
- Ermolina, I.; Morgan, H. The electrokinetic properties of latex particles: Comparison of electrophoresis and dielectrophoresis. J. Colloid Interface Sci. 2005, 285, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. On the effect of hydrodynamic slip on the polarization of a nonconducting spherical particle in an alternating electric field. Phys. Fluids 2010, 22, 072004. [Google Scholar] [CrossRef]
- Khair, A.S.; Squires, T.M. The influence of hydrodynamic slip on the electrophoretic mobility of a spherical colloidal particle. Phys. Fluids 2009, 21, 042001. [Google Scholar] [CrossRef]
- Saucedo-Espinosa, M.A.; Lapizco-Encinas, B.H. Experimental and theoretical study of dielectrophoretic particle trapping in arrays of insulating structures: Effect of particle size and shape. Electrophoresis 2015, 36, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
| Diameter (μm) | Fluorescence (Ex/Em) | Provider | Charge (meq/g) | Surface Functionalization | Concentration (Particles/mL) |
|---|---|---|---|---|---|
| 0.1 | Green (480/501) | Magsphere | Not Available | Amine | 9.09 × 1011 |
| 0.1 | Green (480/501) | Magsphere | 0.0680 | Carboxyl | 9.09 × 1011 |
| 0.1 | Red (580/605) | Invitrogen | 0.3850 | Carboxyl | 9.09 × 1011 |
| 0.2 | Green (505/515) | Invitrogen | 0.5810 | Carboxyl | 5.68 × 1010 |
| 0.2 | Red (538/584) | Magsphere | Not Available | Amine | 5.68 × 1010 |
| 0.5 | Green (480/501) | Magsphere | Not Available | Amine | 1.46 × 109 |
| 0.5 | Red (580/605) | Invitrogen | 0.3160 | Carboxyl | 1.46 × 109 |
| 1.0 | Green (505/515) | Invitrogen | 0.0227 | Amine | 2.18 × 108 |
| 1.0 | Green (505/515) | Invitrogen | 0.1826 | Carboxyl | 2.18 × 108 |
| 1.0 | Green (505/515) | Invitrogen | 0.0170 | Sulfate | 2.18 × 108 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Creel, M.F.; Goodrich, E.; Polniak, D.V.; Lapizco-Encinas, B.H. Assessment of Sub-Micron Particles by Exploiting Charge Differences with Dielectrophoresis. Micromachines 2017, 8, 239. https://doi.org/10.3390/mi8080239
Romero-Creel MF, Goodrich E, Polniak DV, Lapizco-Encinas BH. Assessment of Sub-Micron Particles by Exploiting Charge Differences with Dielectrophoresis. Micromachines. 2017; 8(8):239. https://doi.org/10.3390/mi8080239
Chicago/Turabian StyleRomero-Creel, Maria F., Eric Goodrich, Danielle V. Polniak, and Blanca H. Lapizco-Encinas. 2017. "Assessment of Sub-Micron Particles by Exploiting Charge Differences with Dielectrophoresis" Micromachines 8, no. 8: 239. https://doi.org/10.3390/mi8080239
APA StyleRomero-Creel, M. F., Goodrich, E., Polniak, D. V., & Lapizco-Encinas, B. H. (2017). Assessment of Sub-Micron Particles by Exploiting Charge Differences with Dielectrophoresis. Micromachines, 8(8), 239. https://doi.org/10.3390/mi8080239




