High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip
Abstract
:1. Introduction
2. Results
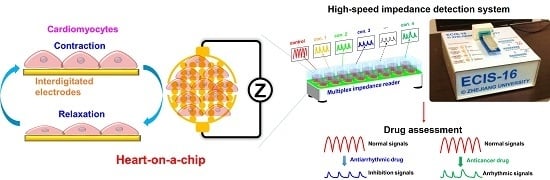
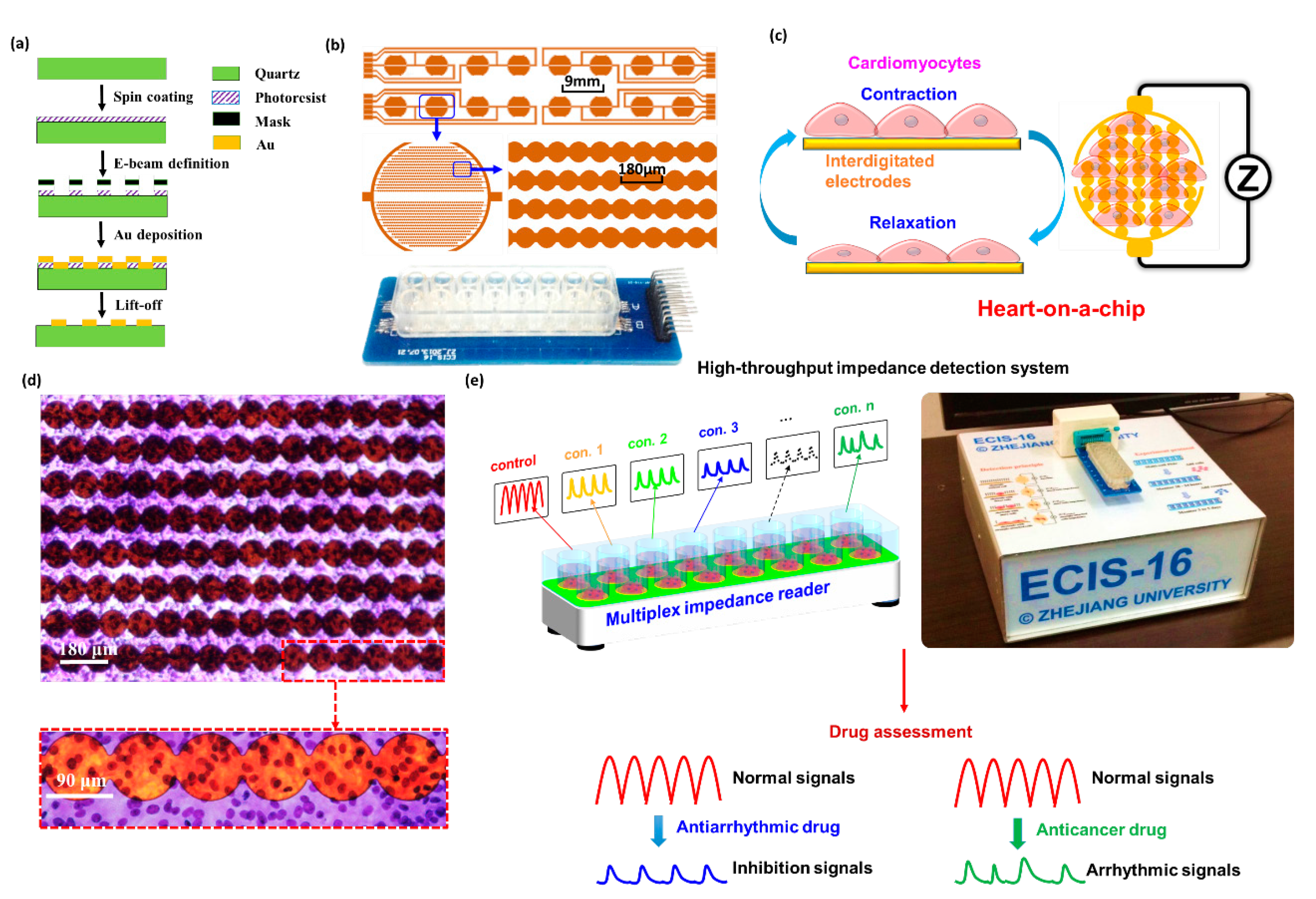
2.1. Establishment of Heart-on-a-Chip
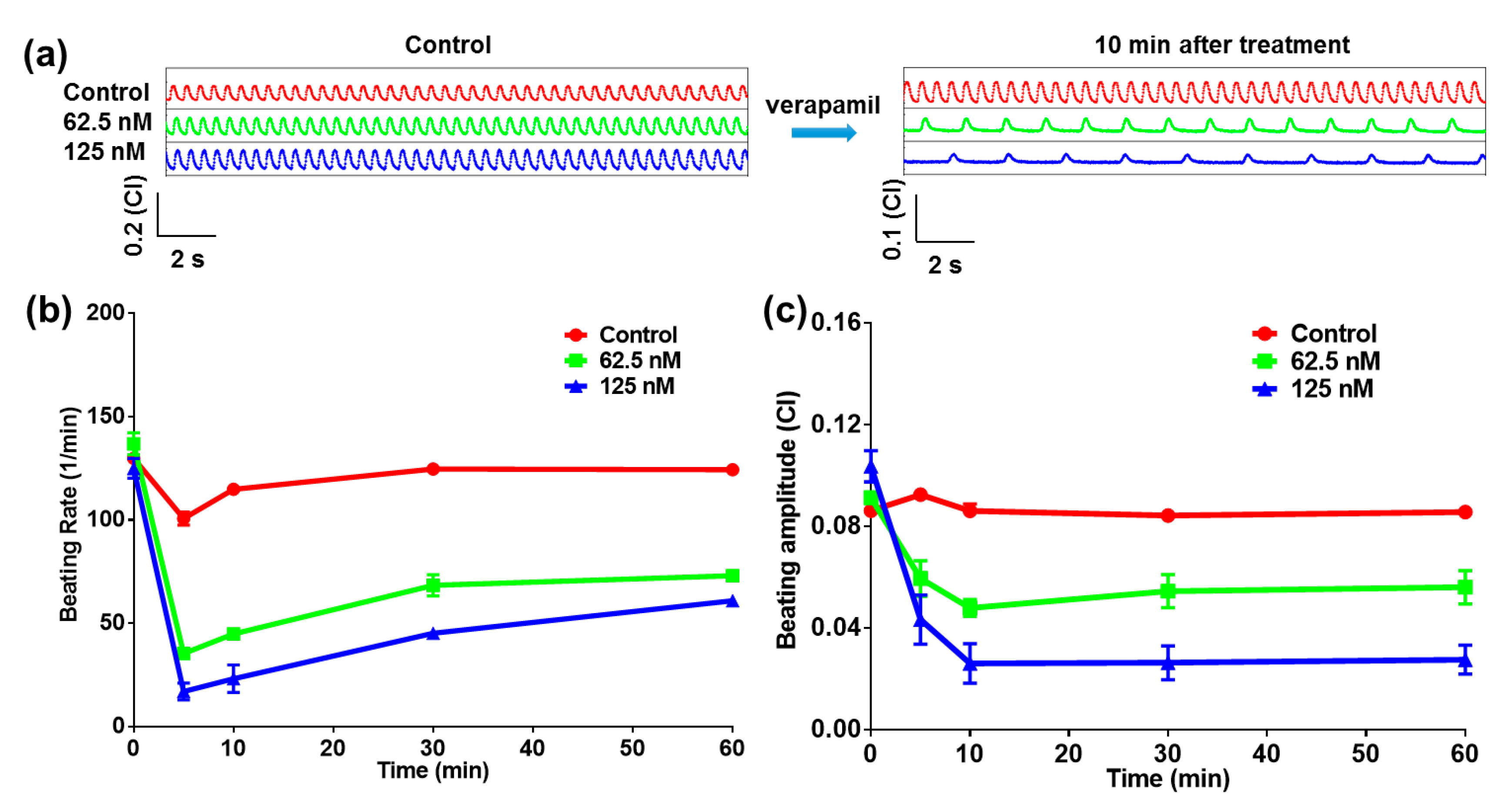
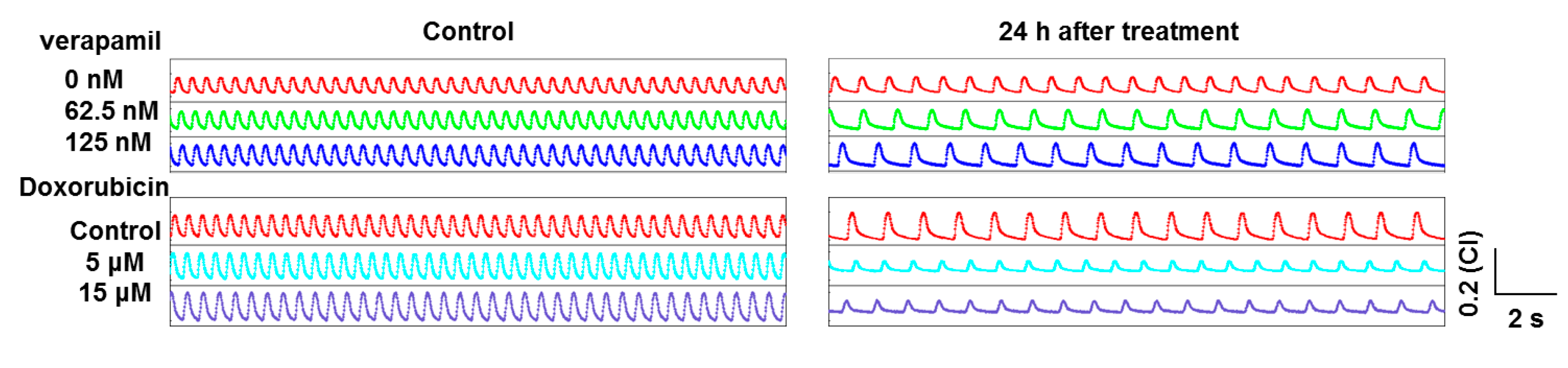
2.2. Heart-on-a-Chip for Antiarrhythmic Drug Test
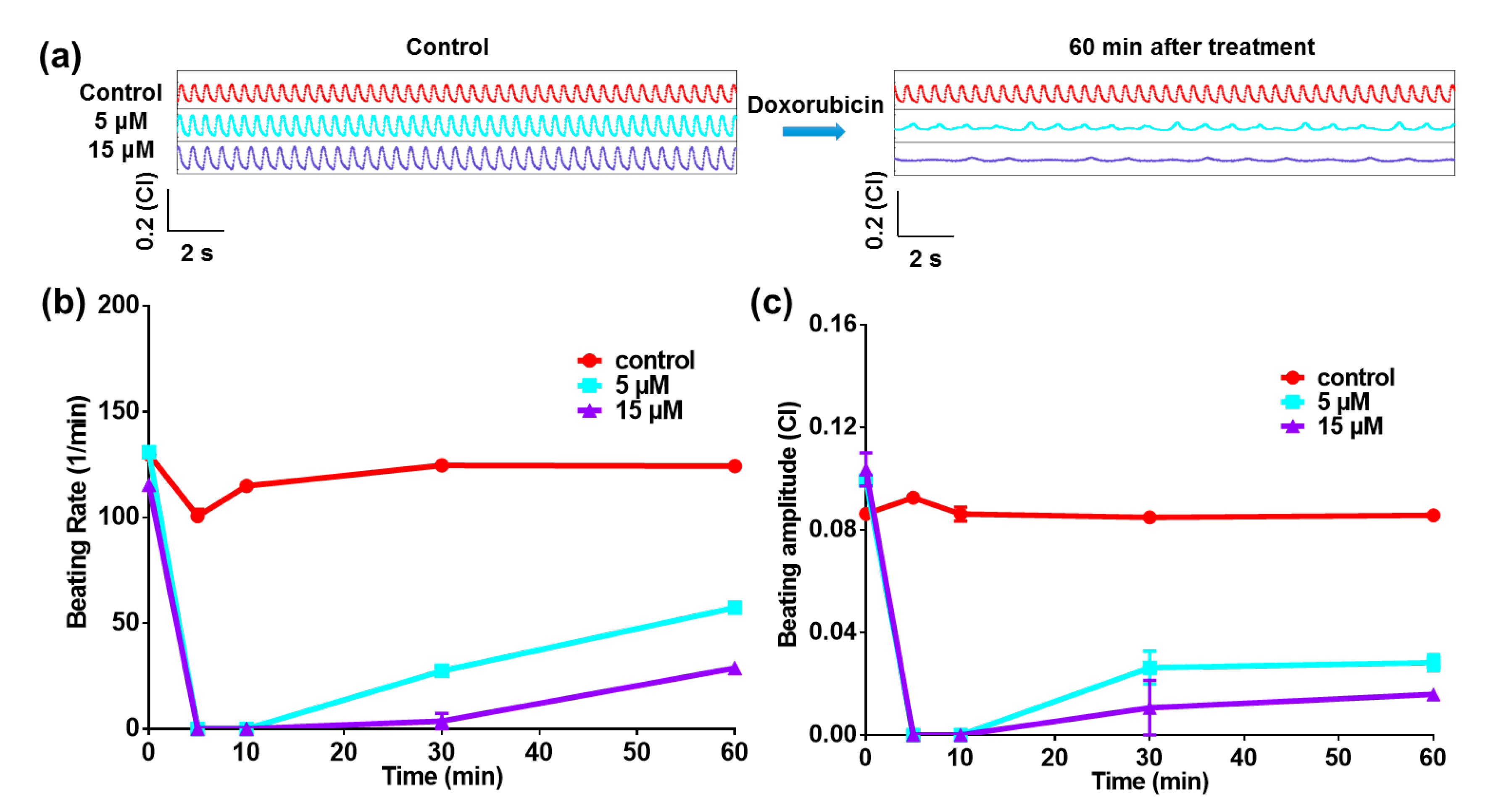
2.3. Heart-on-a-Chip for Anticancer Drug Test
2.4. Cell Viability Test by Impedance Detection Method
3. Materials and Methods
3.1. Device Fabrication
3.2. Cell Culture
3.3. Impedance Measurement
3.4. Drug Treatment
3.5. Statistical Analysis
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gintant, G.; Sager, P.T.; Stockbridge, N. Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 2016, 15, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing safety-related drug attrition: The use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Sakmann, B.; Neher, E. Single-Channel Recording, 2nd ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Hu, N.; Fang, J.; Li, H.; Su, K.; Wang, P. Dual-Function Microelectrode Array System for Simultaneously Monitoring Electromechanical Integration Status of Cardiomyocytes. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers '15), Anchorage, AK, USA, 21–25 June 2015; pp. 1519–1522.
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, V.; Simmons, C.; Gong, T.; Sharma, A.; Burridge, P.W.; Patlolla, B.; Lee, A.S. Screening Drug-Induced Arrhythmia Using Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes and Low-Impedance Microelectrode Arrays. Circulation 2013, 128, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Arlock, P.; Arner, A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am. J. Physiol. Cell Physiol. 2007, 293, C1148–C1153. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Lozinsky, I.T.; Sosunov, E.A.; Anyukhovsky, E.P.; Rosen, M.R.; Balke, C.W.; Efimov, I.R. Application of blebbistatin as an excitation–contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 2007, 4, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Kovács, M.; Tóth, J.; Hetényi, C.; Málnási-Csizmadia, A.; Sellers, J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip. Lab Chip 2011, 11, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Morishima, K.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. Demonstration of a PDMS-based bio-microactuator using cultured cardiomyocytes to drive polymer micropillars. Lab Chip 2006, 6, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Morishima, K.; Tanaka, Y.; Ebara, M.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. Demonstration of a bio-microactuator powered by cultured cardiomyocytes coupled to hydrogel micropillars. Sens. Actuators B Chem. 2006, 119, 345–350. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Enginsu, M.; Dumoulin, J.; Pieters, M.; Bras, M.; Evers, J.; Geraedts, J. Evaluation of human sperm morphology using strict criteria after Diff-Quik staining: Correlation of morphology with fertilization in vitro. Hum. Reprod. 1991, 6, 854–858. [Google Scholar] [PubMed]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Arola, O.J.; Saraste, A.; Pulkki, K.; Kallajoki, M.; Parvinen, M.; Voipio-Pulkki, L.-M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000, 60, 1789–1792. [Google Scholar] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, Q.; Tong, M.; Li, H.; Wang, J.; Hu, N.; Wang, P. Detection of diarrhetic shellfish poisoning toxins using high-sensitivity human cancer cell-based impedance biosensor. Sens. Actuators B Chem. 2016, 222, 205–212. [Google Scholar] [CrossRef]
- Kimura, S.; Bassett, A.L.; Xi, H.; Myerburg, R.J. Verapamil diminishes action potential changes during metabolic inhibition by blocking ATP-regulated potassium currents. Circ. Res. 1992, 71, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hu, Z.; Zhang, W.; Wu, C.; Yu, H.; Wang, P. Evaluation of doxorubicin toxicity on cardiomyocytes using a dual functional extracellular biochip. Biosens. Bioelectron. 2010, 26, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Zou, Q.; Li, H.; Wang, T.; Zhuang, L.; Hu, N.; Wang, P. Cardiomyocyte-Based Biosensor Based on Impedance Sensor Technology and CCD Imaging Analysis for Pharmaceutical Assessment. Sens. Lett. 2015, 13, 40–47. [Google Scholar] [CrossRef]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, T.; Wang, P.; Hu, N. High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip. Micromachines 2016, 7, 122. https://doi.org/10.3390/mi7070122
Zhang X, Wang T, Wang P, Hu N. High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip. Micromachines. 2016; 7(7):122. https://doi.org/10.3390/mi7070122
Chicago/Turabian StyleZhang, Xi, Tianxing Wang, Ping Wang, and Ning Hu. 2016. "High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip" Micromachines 7, no. 7: 122. https://doi.org/10.3390/mi7070122
APA StyleZhang, X., Wang, T., Wang, P., & Hu, N. (2016). High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip. Micromachines, 7(7), 122. https://doi.org/10.3390/mi7070122