Application of Vertical Electrodes in Microfluidic Channels for Impedance Analysis
Abstract
:1. Introduction
2. Device Fabrication
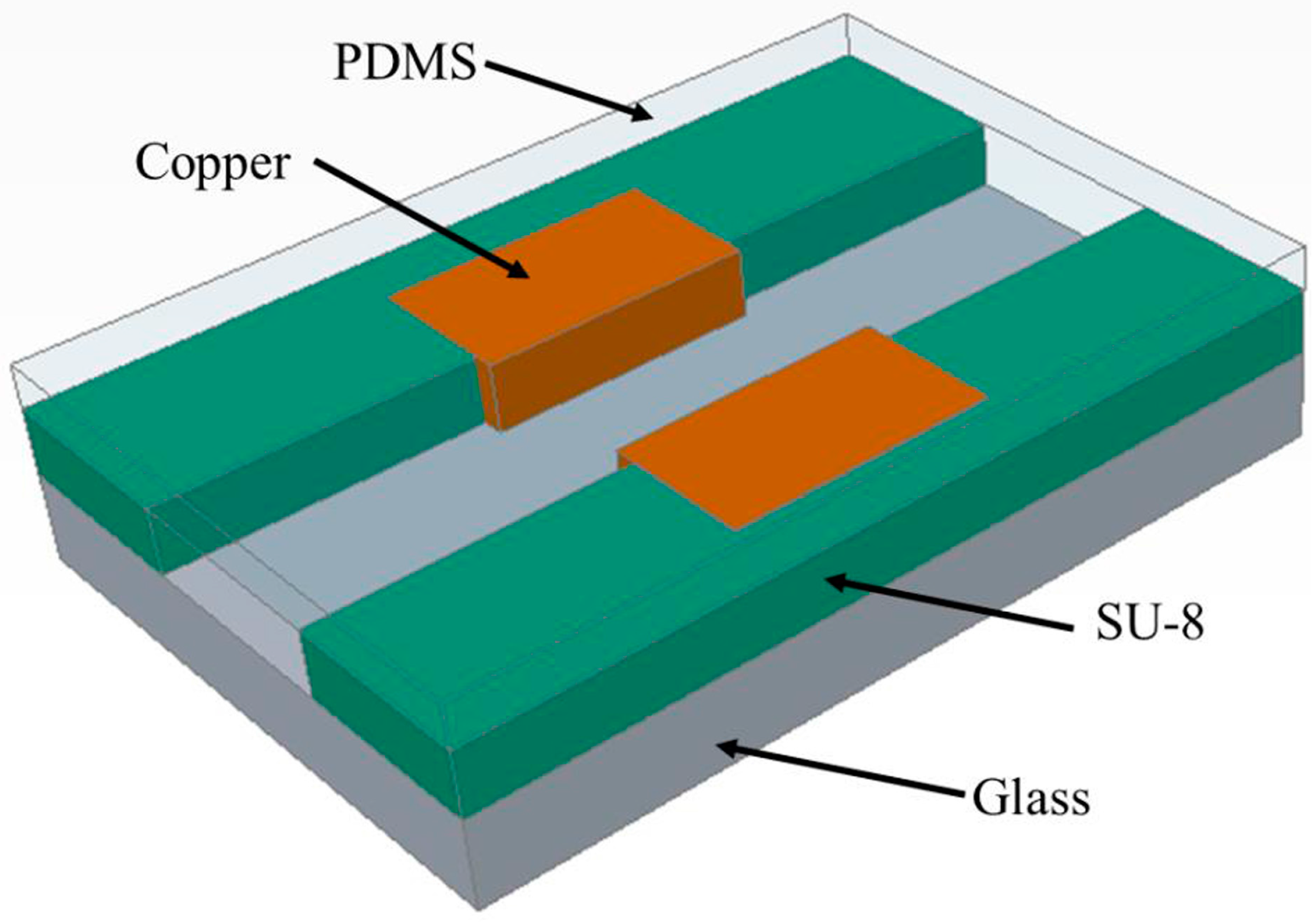
2.1. Design and Layout
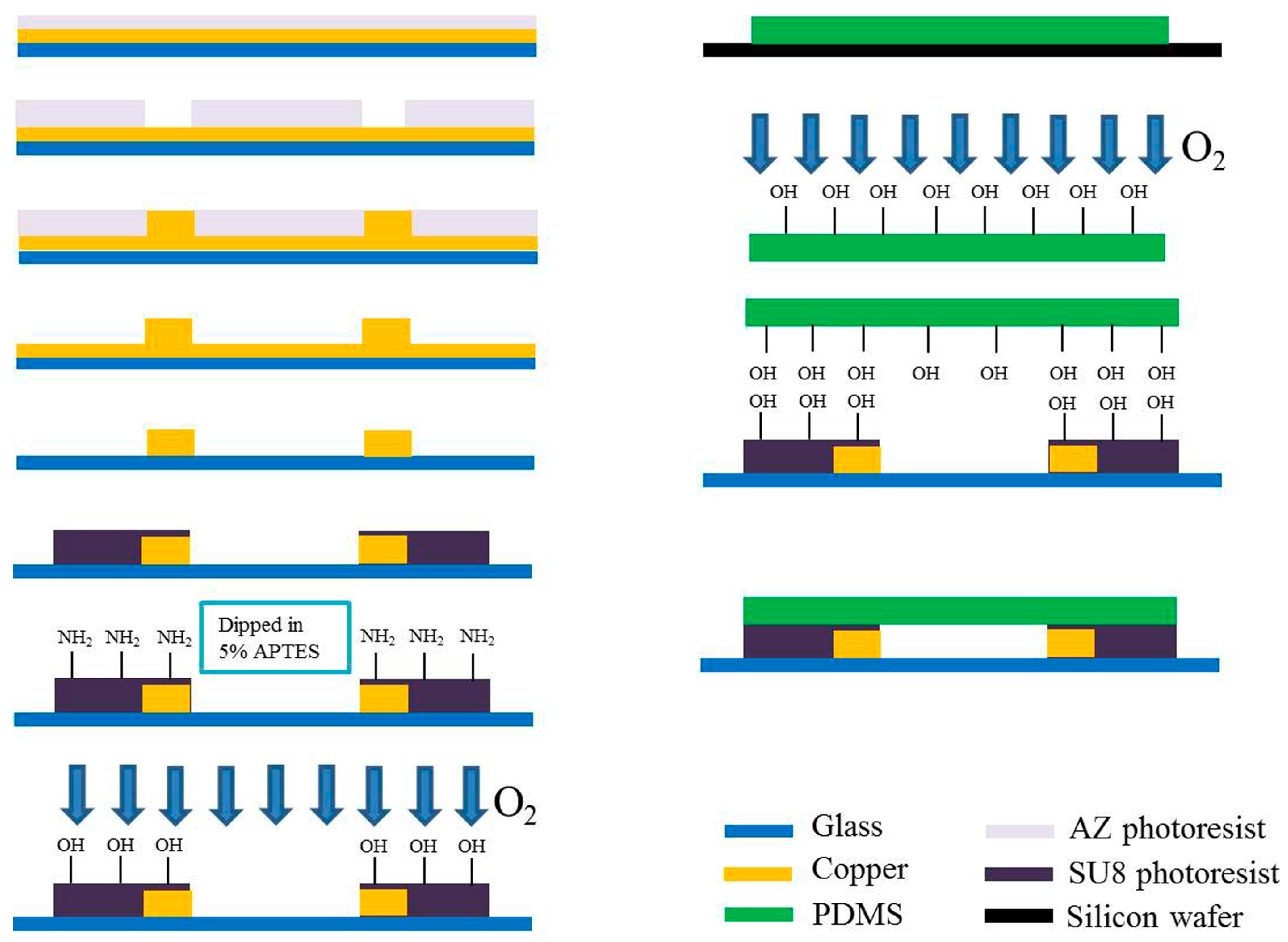
2.2. Fabrication of Impedance Sensor
2.3. Bonding of SU-8 with PDMS
3. Experiment
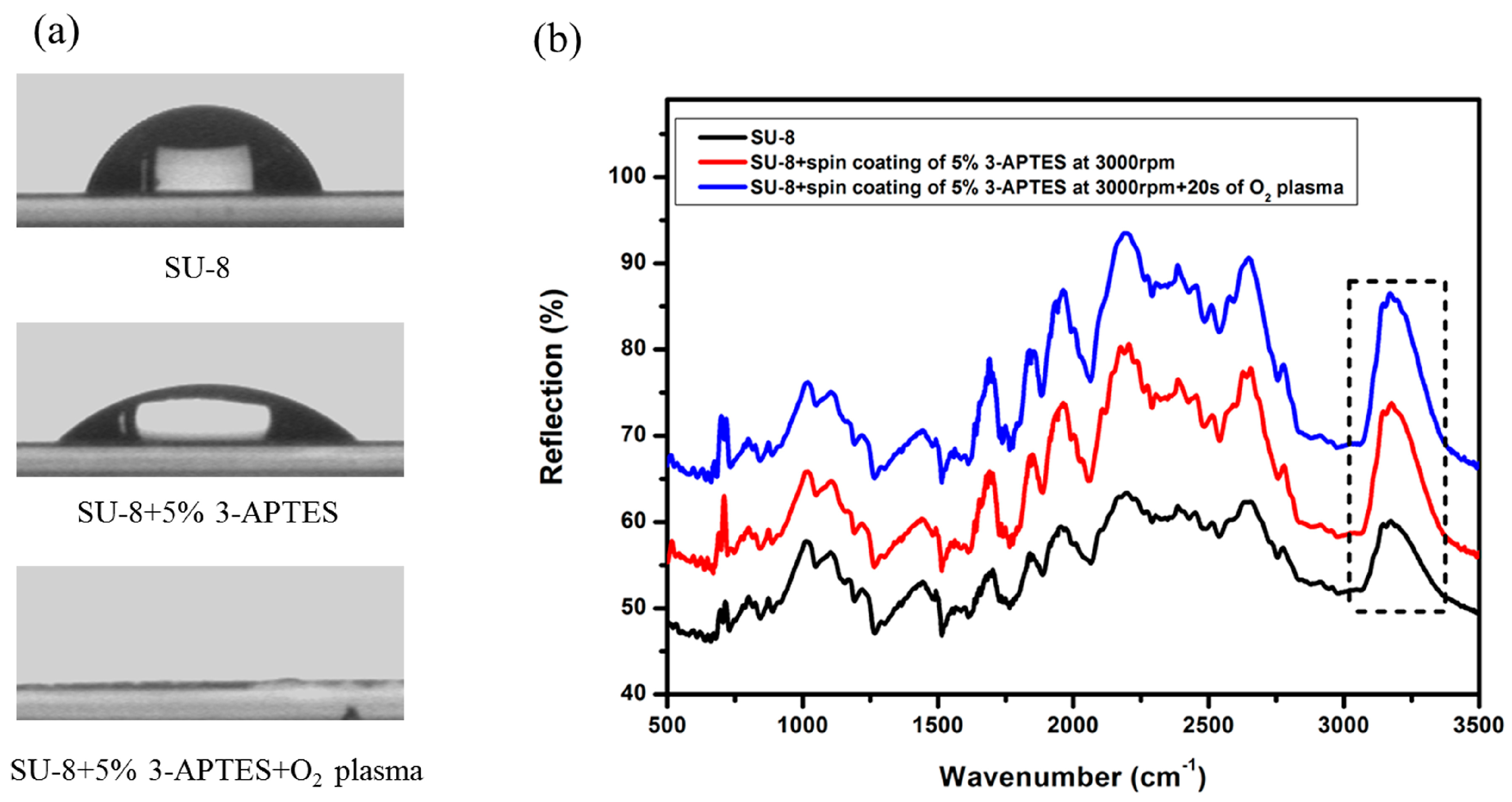
3.1. Bonding Test
3.2. Preparation for Impedance Measurement
4. Results and Discussion
4.1. Contact Angle and FTIR Spectra Characterization of Modified SU-8 Layer
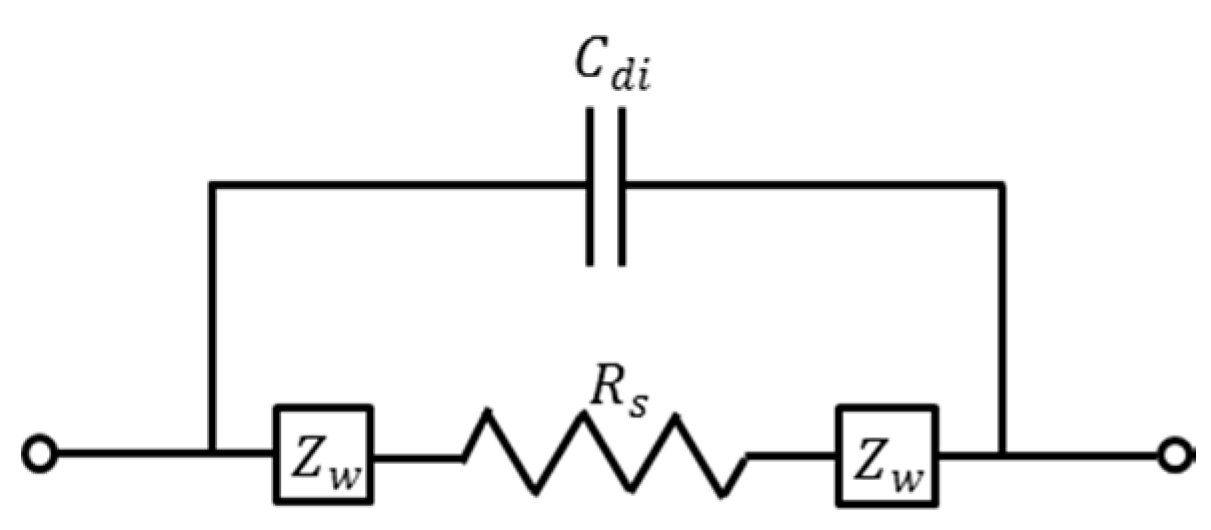
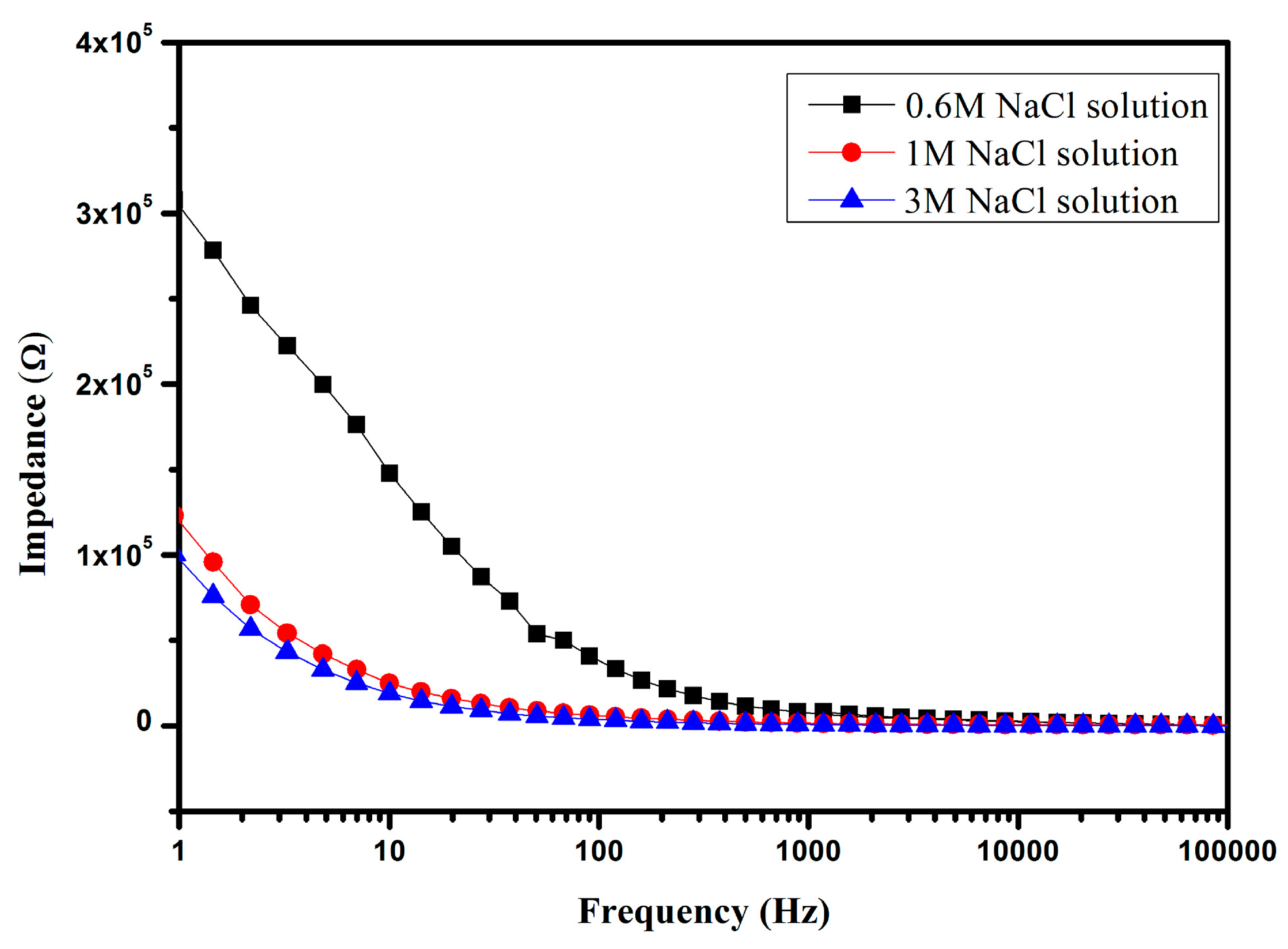
4.2. Impedance Detection of NaCl Solution
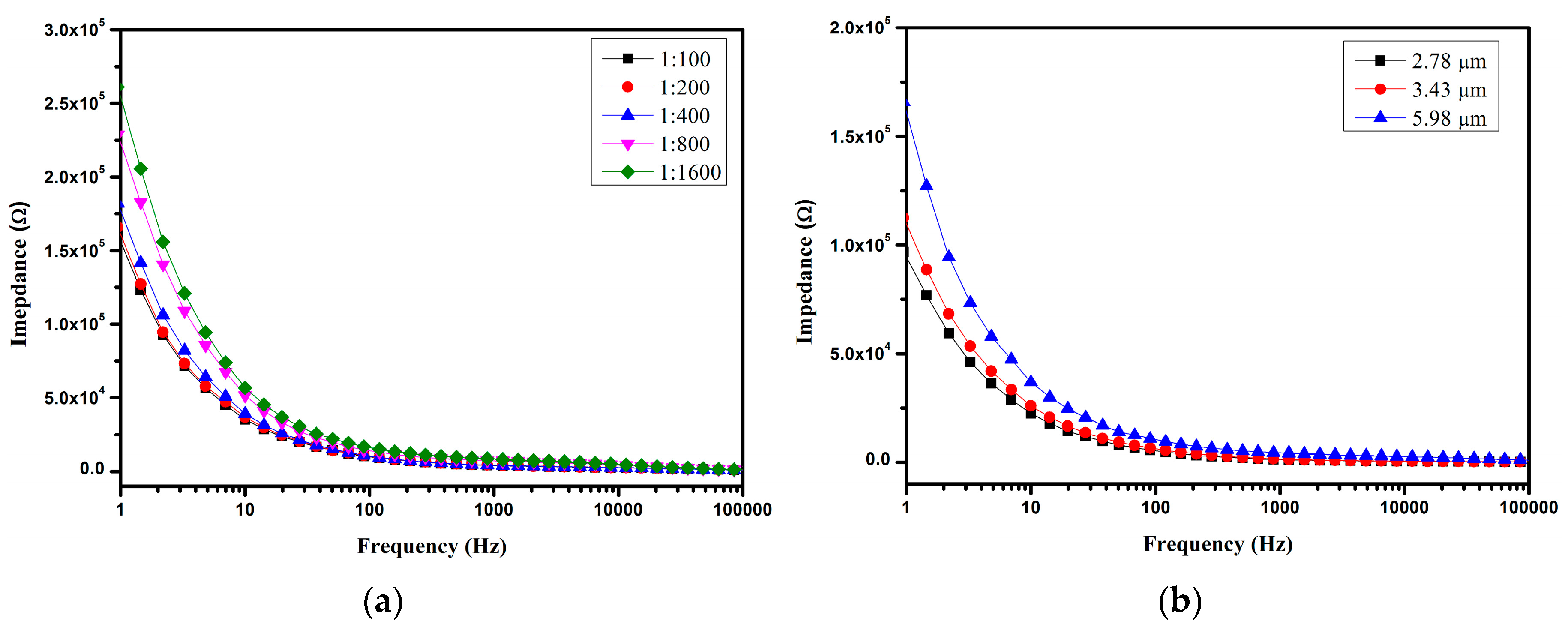
4.3. Impedance Detection of Microspheres
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elvira, K.S.; Casadevall i Solvas, X.; Wootton, R.C.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Keng, P.Y.; Ding, H.; Sadeghi, S.; Shah, G.J.; Dooraghi, A.; Phelps, M.E.; Satyamurthy, N.; Chatziioannou, A.F.; Kim, C.; et al. Micro-chemical synthesis of molecular probes on an electronic microfluidic device. Proc. Natl. Acad. Sci. USA 2012, 109, 690–695. [Google Scholar]
- Yi, C.; Li, C.-W.; Ji, S.; Yang, M. Microfluidics technology for manipulation and analysis of biological cells. Anal. Chim. Acta 2006, 560, 1–23. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.; Hansen, E.H. Miniaturization of environmental chemical assays in flowing systems: The lab-on-a-valve approach vis-à-vis lab-on-a-chip microfluidic devices. Anal. Chim. Acta 2007, 600, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Poenar, D.P.; Carp, M.; Loe, F.C. A microfluidic device for impedance spectroscopy analysis of biological samples. Sens. Actuators B 2007, 123, 168–176. [Google Scholar] [CrossRef]
- Sabuncu, A.C.; Zhuang, J.; Kolb, J.F.; Beskok, A. Microfluidic impedance spectroscopy as a tool for quantitative biology and biotechnology. Biomicrofluidics 2012, 6, 034103. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-Sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Assessment of genotoxicity of catecholics using impedimetric DNA-biosensor. Biosens. Bioelectron. 2014, 53, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Banada, P.P.; Chatni, M.R.; Lim, K.S.; Bhunia, A.K.; Ladisch, M.; Bashir, R. A multifunctional micro-fluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of Listeria monocytogenes. Lab Chip 2006, 6, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, J.; Noutomi, D.; Shutou, M.; Hara, M. Selective detection of specific bacteria using dielectrophoretic impedance measurement method combined with an antigen–antibody reaction. J. Electrost. 2003, 58, 229–246. [Google Scholar] [CrossRef]
- Suehiro, J.; Hamada, R.; Noutomi, D.; Shutou, M.; Hara, M. Selective detection of viable bacteria using dielectrophoretic impedance measurement method. J. Electrost. 2003, 57, 157–168. [Google Scholar] [CrossRef]
- Costanzo, P.; Liang, E.; Patten, T.E.; Collins, S.D.; Smith, R.L. Biomolecule detection via target mediated nanoparticle aggregation and dielectrophoretic impedance measurement. Lab Chip 2005, 5, 606–610. [Google Scholar] [PubMed]
- Pei, R.J.; Cheng, Z.L.; Wang, E.K.; Yang, X.R. Amplification of antigen–antibody interactions based on biotin labeled protein–streptavidin network complex using impedance spectroscopy. Biosens. Bioelectron. 2001, 16, 355–361. [Google Scholar] [CrossRef]
- Tang, D.P.; Yuan, R.; Chai, Y.Q.; Dai, J.Y.; Zhong, X.; Liu, Y. A novel immunosensor based on immobilization of hepatitis B surface antibody on platinum electrode modified colloidal gold and polyvinyl butyral as matrices via electrochemical impedance spectroscopy. Bioelectrochemistry 2004, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Stegbauer, K.; Wolf, B. Monitoring of cellular behaviour by impedance measurements on interdigitated electrode structures. Biosens. Bioelectron. 1997, 12, 29–41. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Yeh, Y.-T.T.; Zheng, S.-Y. Microfluidic device and system for point-of-care blood coagulation measurement based on electrical impedance sensing. Sens. Actuators B Chem. 2013, 180, 21–27. [Google Scholar] [CrossRef]
- Sabounchi, P.; Morales, A.M.; Ponce, P.; Lee, L.P.; Simmons, B.A.; Davalos, R.V. Sample concentration and impedance detection on a microfluidic polymer chip. Biomed. Microdevices 2008, 10, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Rossier, J.; Reymond, F.; Michel, P.E. Polymer microfluidic chips for electrochemical and biochemical analyses. Electrophoresis 2002, 23, 858–867. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Xiao, G.; Watts, B.R.; Xu, C. Sealing SU-8 microfluidic channels using PDMS. Biomicrofluidics 2011, 5, 046503. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Flanagan, L.; Lee, A.P. Side-wall vertical electrodes for lateral field microfluidic applications. J. Microelectromech Syst. 2007, 16, 454–461. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Tserepi, A.; Pavli, P.; Argitis, P.; Sanopoulou, M.; Misiakos, K. A low temperature surface modification assisted method for bonding plastic substrates. J. Micromech. Microeng. 2009, 19, 015007. [Google Scholar] [CrossRef]
- Talaei, S.; Frey, O.; van der Wal, P.D.; de Rooij, N.F.; Koudelka-Hep, M. Hybrid microfluidic cartridge formed by irreversible bonding of SU-8 and PDMS for multi-layer flow applications. Procedia Chem. 2009, 1, 381–384. [Google Scholar] [CrossRef]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance Characterization and Modeling of Electrodes for Biomedical Applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.H.; Liu, Y.S.; Irimia, D.; Demirci, U.; Yang, L.; Zamir, L.; Rodriguez, W.R.; Toner, M.; Bashir, R. Cell detection and counting through cell lysate impedance spectroscopy in microfluidic devices. Lab Chip 2007, 7, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, J.; Qian, S.Z.; Joo, S.W.; Zheng, X.L. A cell electrofusion microfluidic device integrated with 3D thin-film microelectrode arrays. Biomicrofluidics 2011, 5, 034121. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, J.; Qian, S.Z.; Joo, S.W.; Zheng, X.L. A cell electrofusion microfluidic chip using discrete coplanar vertical sidewall microelectrodes. Electrophoresis 2012, 33, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zheng, X.L.; Yang, J.; Joo, S.W.; Qian, S.Z. A cell electrofusion microfluidic chip with micro-cavity microelectrode array. Microfluid. Nanofluidics 2013, 15, 151–160. [Google Scholar] [CrossRef]
- Hu, N.; Yang, J.; Yin, Z.Q.; Ai, Y.; Qian, S.; Svir, I.B.; Xia, B.; Yan, J.W.; Hou, W.S.; Zheng, X.L. A high-throughput dielectrophoresis-based cell electrofusion microfluidic device. Electrophoresis 2011, 32, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.M.; Nguyen, N.T. SU-8 as a structural material for labs-on-chips and microelectromechanical systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Perch-Nielsen, I.R.; Poulsen, C.R.; Bang, D.D.; Telleman, P.; Wolff, A. Simulation and experimental validation of a SU-8 based PCR thermocycler chip with integrated heaters and temperature sensor. Sens. Actuators A Phys. 2004, 110, 3–10. [Google Scholar] [CrossRef]
- Chronis, N.; Lee, L.P. Electrothermally activated SU-8 microgripper for single cell manipulation in solution. J. Microelectromech. Syst. 2005, 14, 857–863. [Google Scholar] [CrossRef]
- Ren, Y.F.; Huang, S.-H.; Mosser, S.; Heuschkel, M.O.; Bertsch, A.; Fraering, P.C.; Chen, J.J.; Renaud, P. A Simple and Reliable PDMS and SU-8 Irreversible Bonding Method and Its Application on a Microfluidic-MEA Device for Neuroscience Research. Micromachines 2015, 6, 1923–1934. [Google Scholar] [CrossRef]
- Henry, A.C.; Tutt, T.J.; Galloway, M.; Davidson, Y.Y.; McWhorter, C.S.; Soper, S.A.; McCarley, R.L. Surface modification of poly(methyl methacrylate) used in the fabrication of microanalytical devices. Anal. Chem. 2000, 72, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F.S.; Wenz, G.; Schunk, P.; Schimmel, T. An improved method for the assembly of amino-terminated monolayers on SiO2 and the vapor deposition of gold layers. Langmuir 1999, 15, 4520–4523. [Google Scholar] [CrossRef]
- Gómez-Sjöberg, R.; Morisette, D.T.; Bashir, R. Impedance Microbiology-on-a-Chip: Microfluidic Bioprocessor for Rapid Detection of Bacterial Metabolism. J. Microelectromech. Syst. 2005, 14, 829–838. [Google Scholar] [CrossRef]
- Gómez, R.; Bashir, R.; Bhunia, A.K. Microscale electronic detection of bacterial metabolism. Sens. Actuators B Chem. 2002, 86, 198–208. [Google Scholar] [CrossRef]
- Iliescu, C.; Poenar, D.P.; Selvan, S.T. Frequency dependence on the accuracy of electrical impedance spectroscopy measurements in microfluidic devices. J. Micromech. Microeng. 2010, 20, 022001. [Google Scholar] [CrossRef]
- Jang, L.-S.; Wang, M.H. Microfluidic device for cell capture and impedance measurement. Biomed. Microdevices 2007, 9, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Shilov, V.N.; Delgado, A.V.; Gonzalez-Caballero, F.; Grosse, C. Thin double layer theory of the wide-frequency range dielectric dispersion of suspensions of non-conducting spherical particles including surface conductivity of the stagnant layer. Coll. Surf. A Phys. Eng. Asp. 2001, 192, 253–265. [Google Scholar] [CrossRef]
- Carrique, F.; Zurita, L.; Delgado, A.V. Dielectric relaxation in polystyrene suspensions. Effect of ionic strength. Coll. Surf. A Phys. Eng. Asp. 1994, 92, 9–21. [Google Scholar] [CrossRef]
- Zhang, C.; Khoshmanesh, K.; Mitchell, A.; Kalantar-Zadeh, K. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Anal. Bioanal. Chem. 2010, 396, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Holmes, D.; Morgan, H. The dielectrophoretic levitation and separation of latex beads in microchips. Electrophoresis 2001, 22, 3893–3901. [Google Scholar] [CrossRef]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yuan, Y.J. Application of Vertical Electrodes in Microfluidic Channels for Impedance Analysis. Micromachines 2016, 7, 96. https://doi.org/10.3390/mi7060096
Li Q, Yuan YJ. Application of Vertical Electrodes in Microfluidic Channels for Impedance Analysis. Micromachines. 2016; 7(6):96. https://doi.org/10.3390/mi7060096
Chicago/Turabian StyleLi, Qiang, and Yong J. Yuan. 2016. "Application of Vertical Electrodes in Microfluidic Channels for Impedance Analysis" Micromachines 7, no. 6: 96. https://doi.org/10.3390/mi7060096
APA StyleLi, Q., & Yuan, Y. J. (2016). Application of Vertical Electrodes in Microfluidic Channels for Impedance Analysis. Micromachines, 7(6), 96. https://doi.org/10.3390/mi7060096





