Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels
Abstract
:1. Introduction
Background
2. Materials and Methods
2.1. Materials
2.2. Cell Preparation and Culture
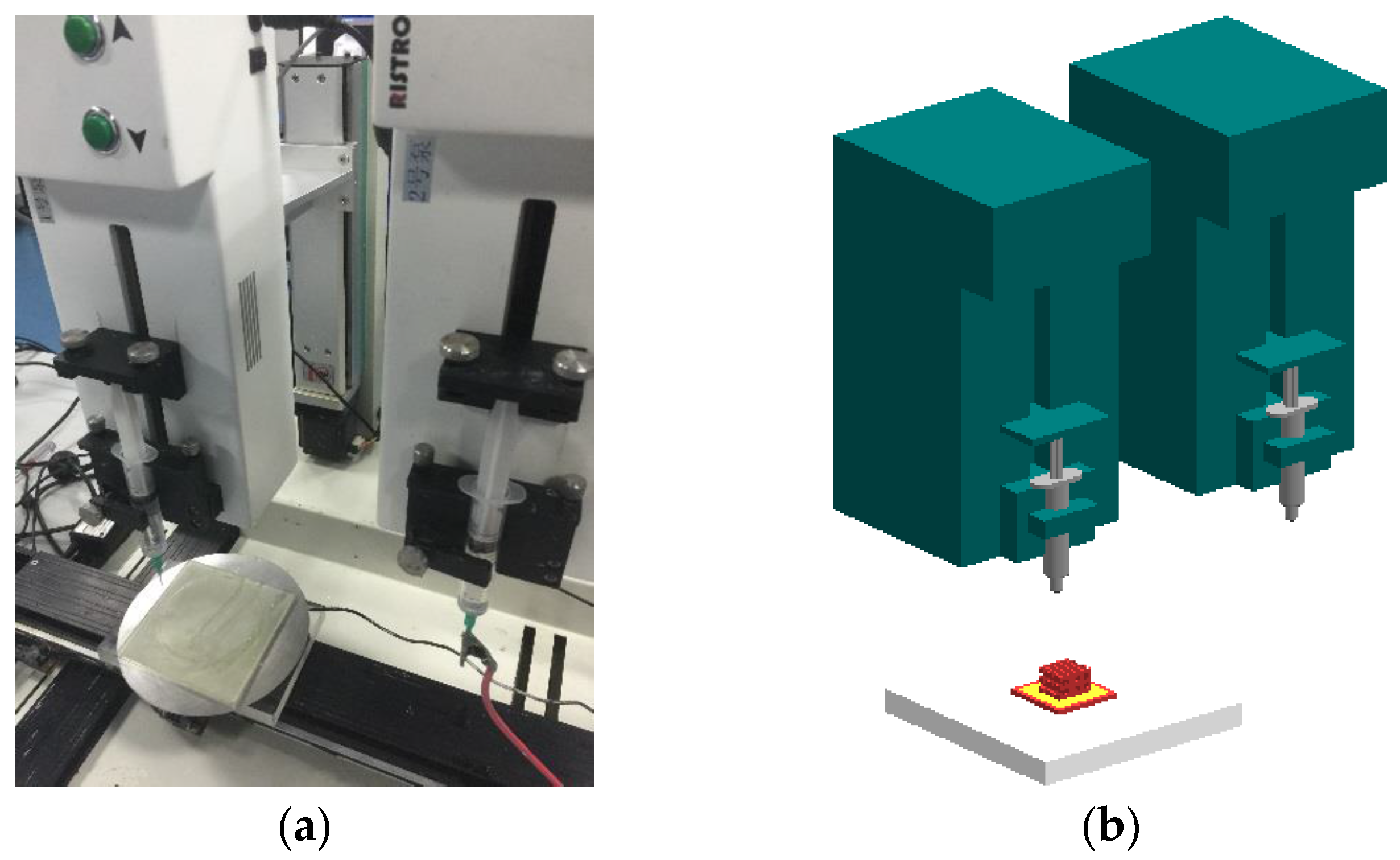
2.3. Bioprinting Setup
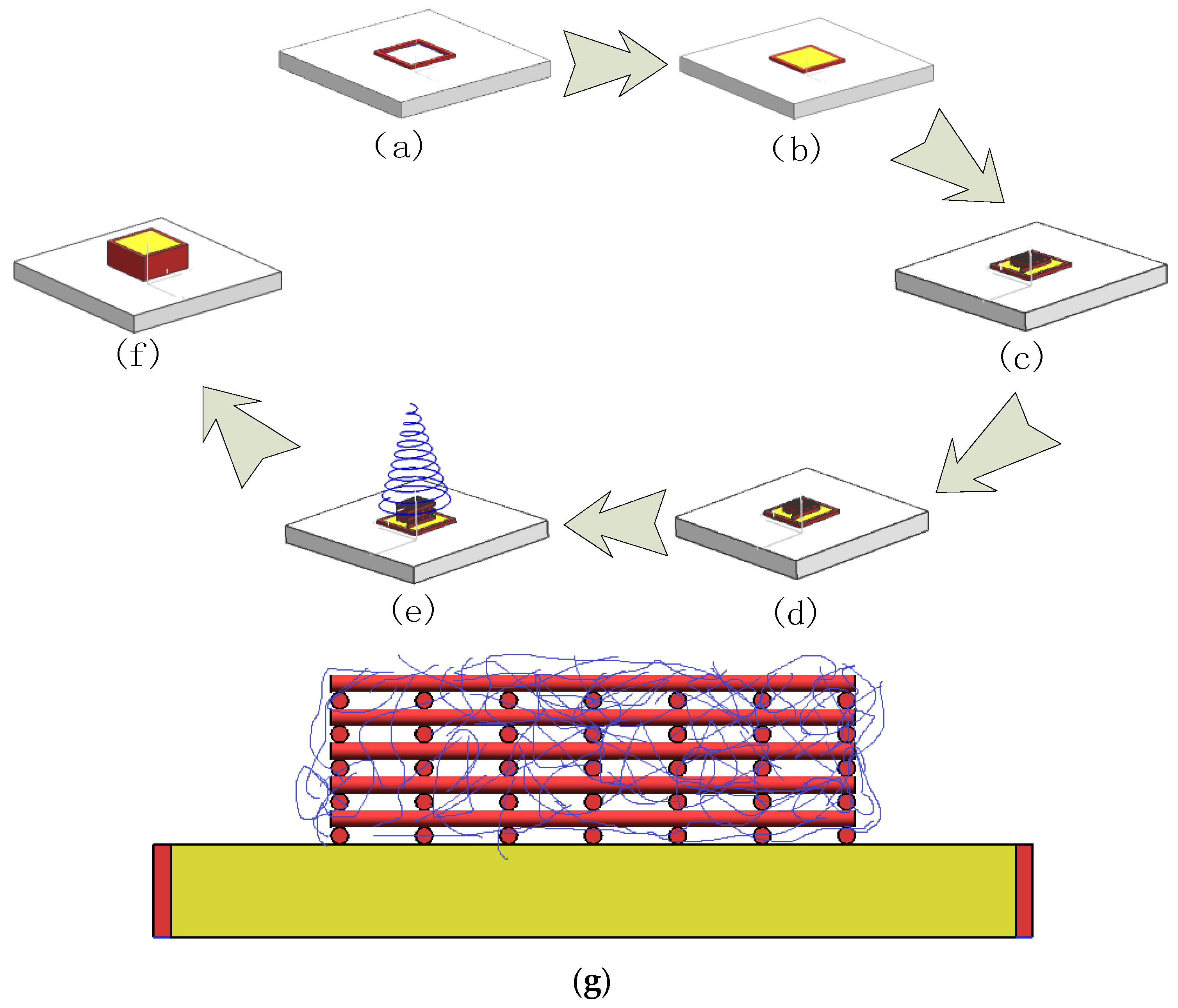
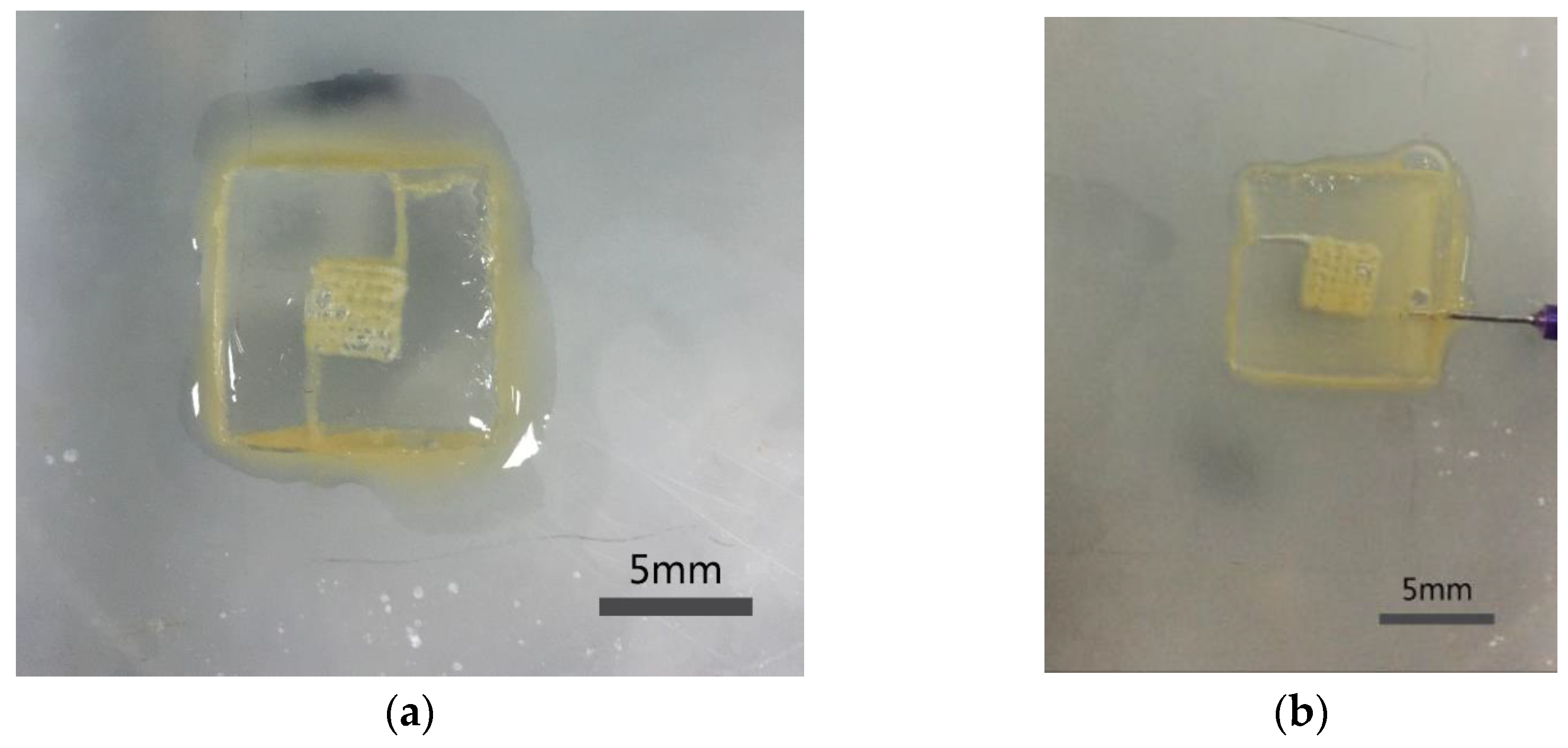
2.4. Construction of the Structure with Multiscale Channels
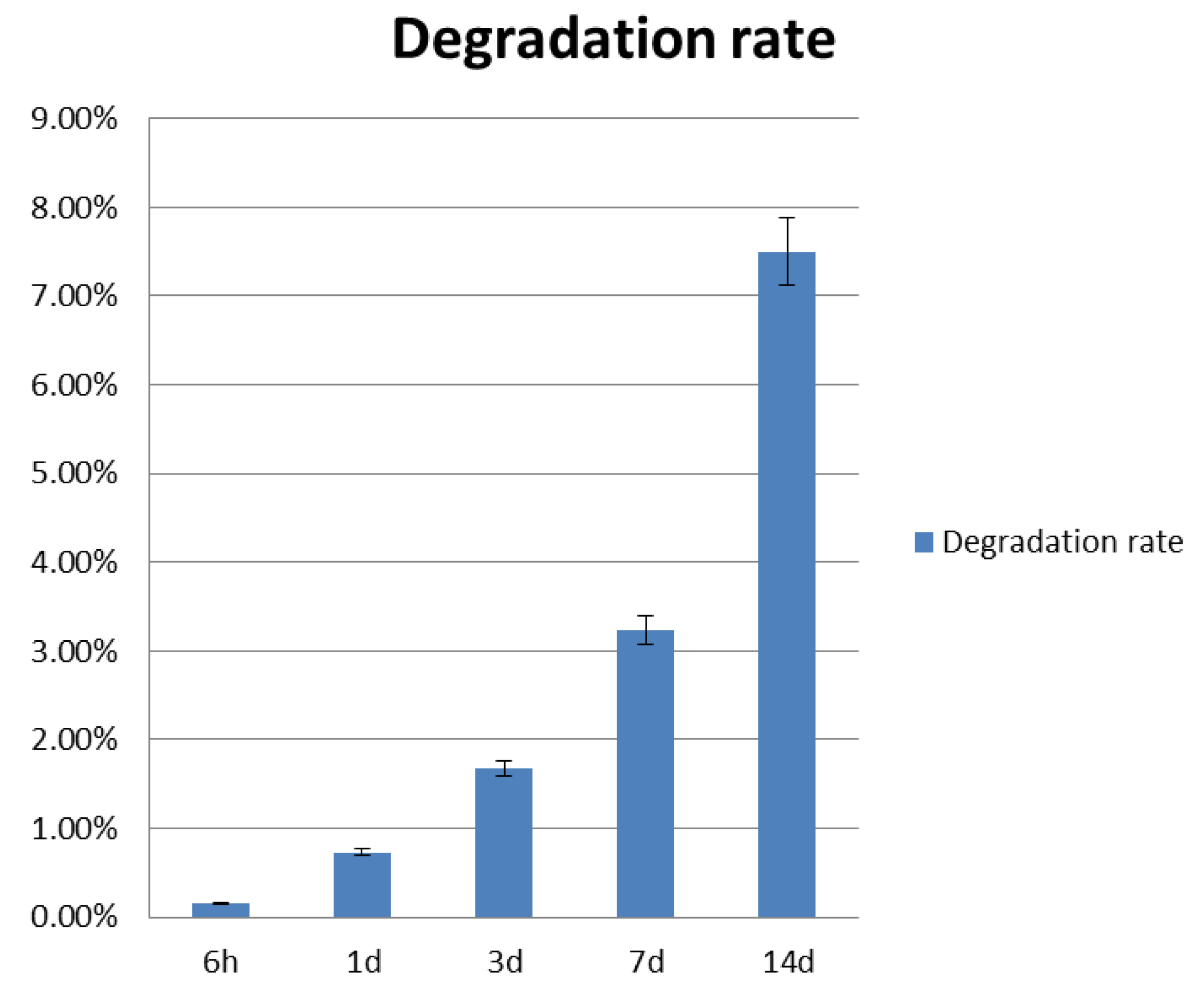
2.5. Construct Degradation
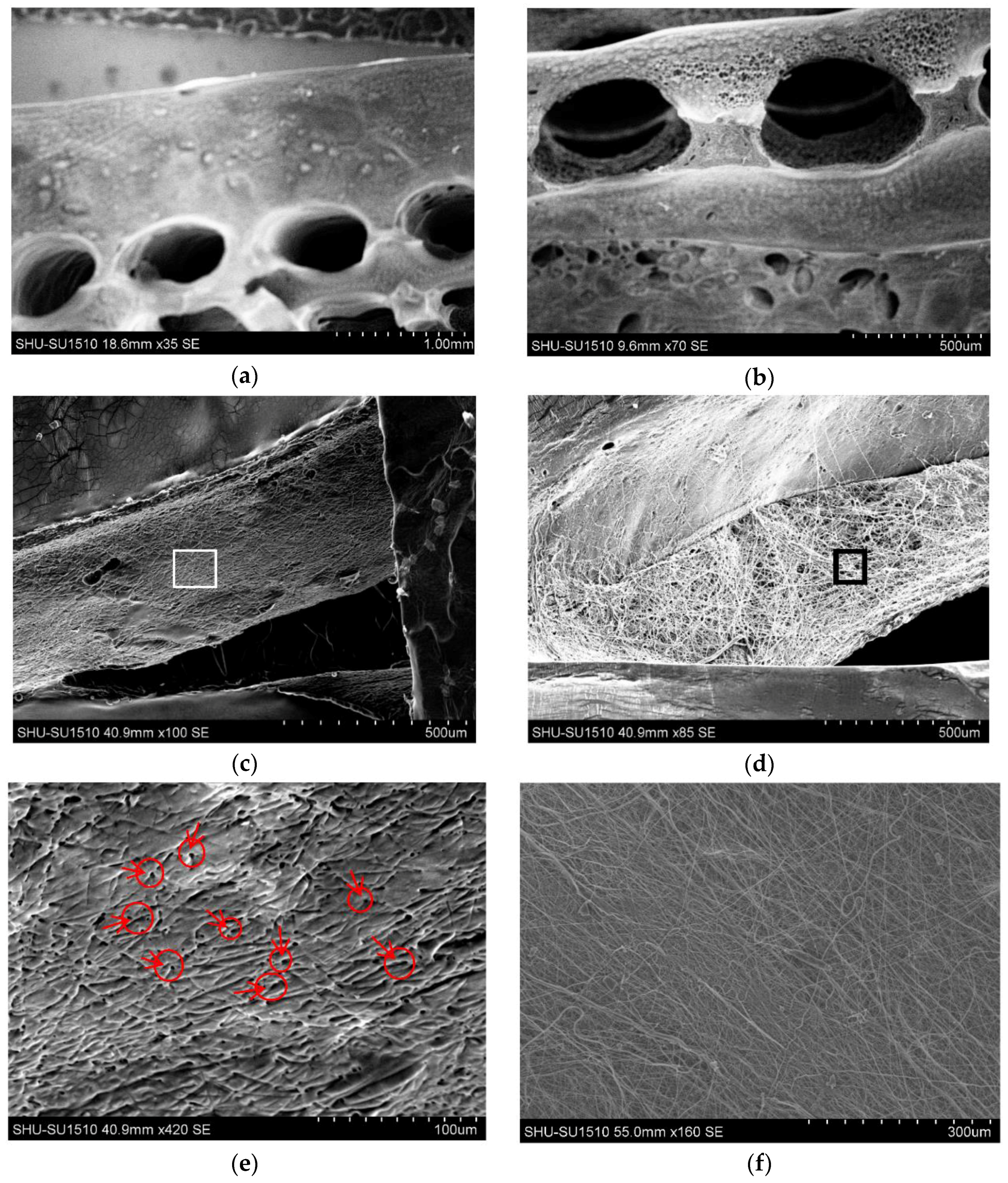
2.6. Morphology
2.7. Viability Analysis
2.8. Endothelialization Analysis
3. Results
3.1. Construct Degradation Rate
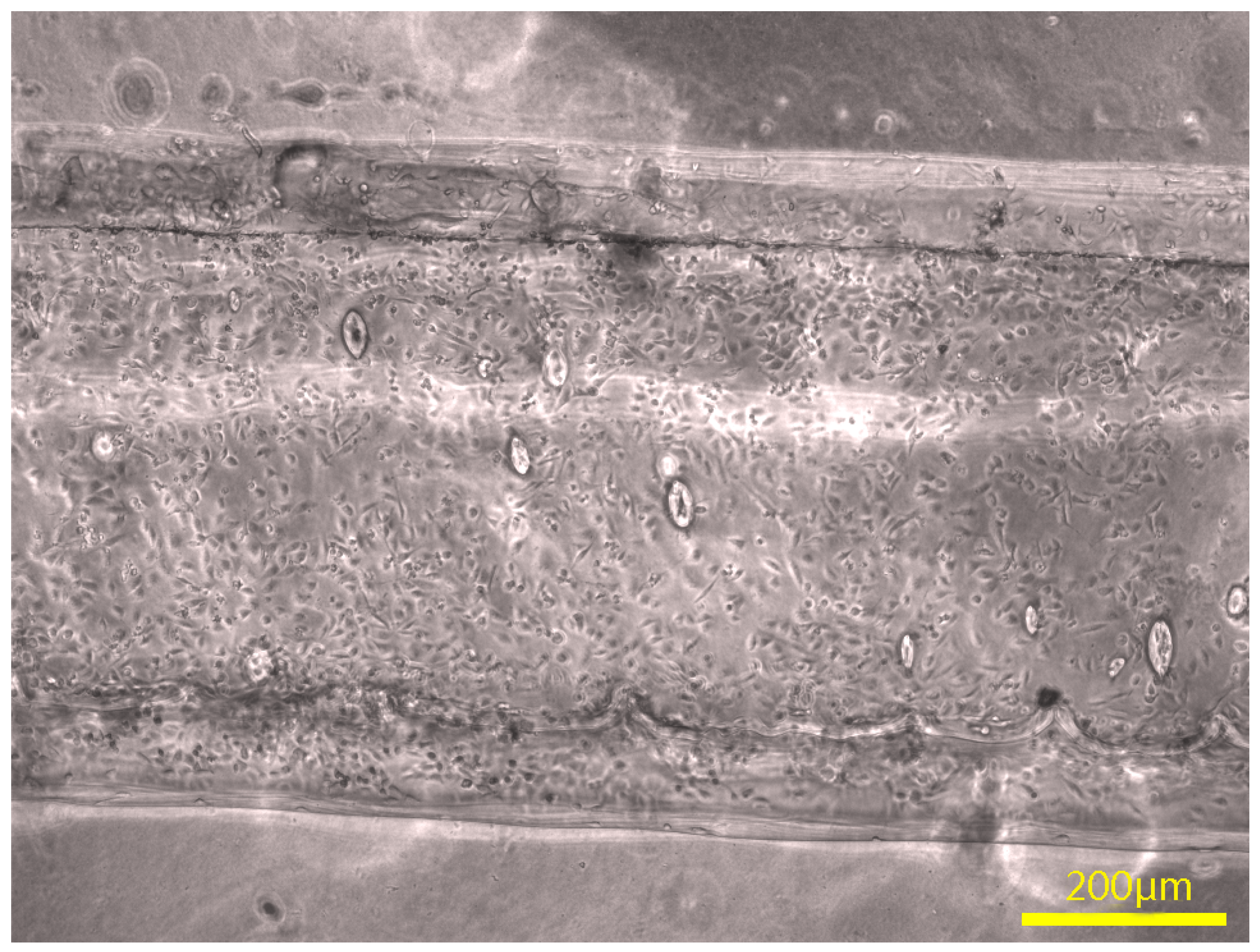
3.2. Morphology and Channel Size
3.3. Channel Connectivity
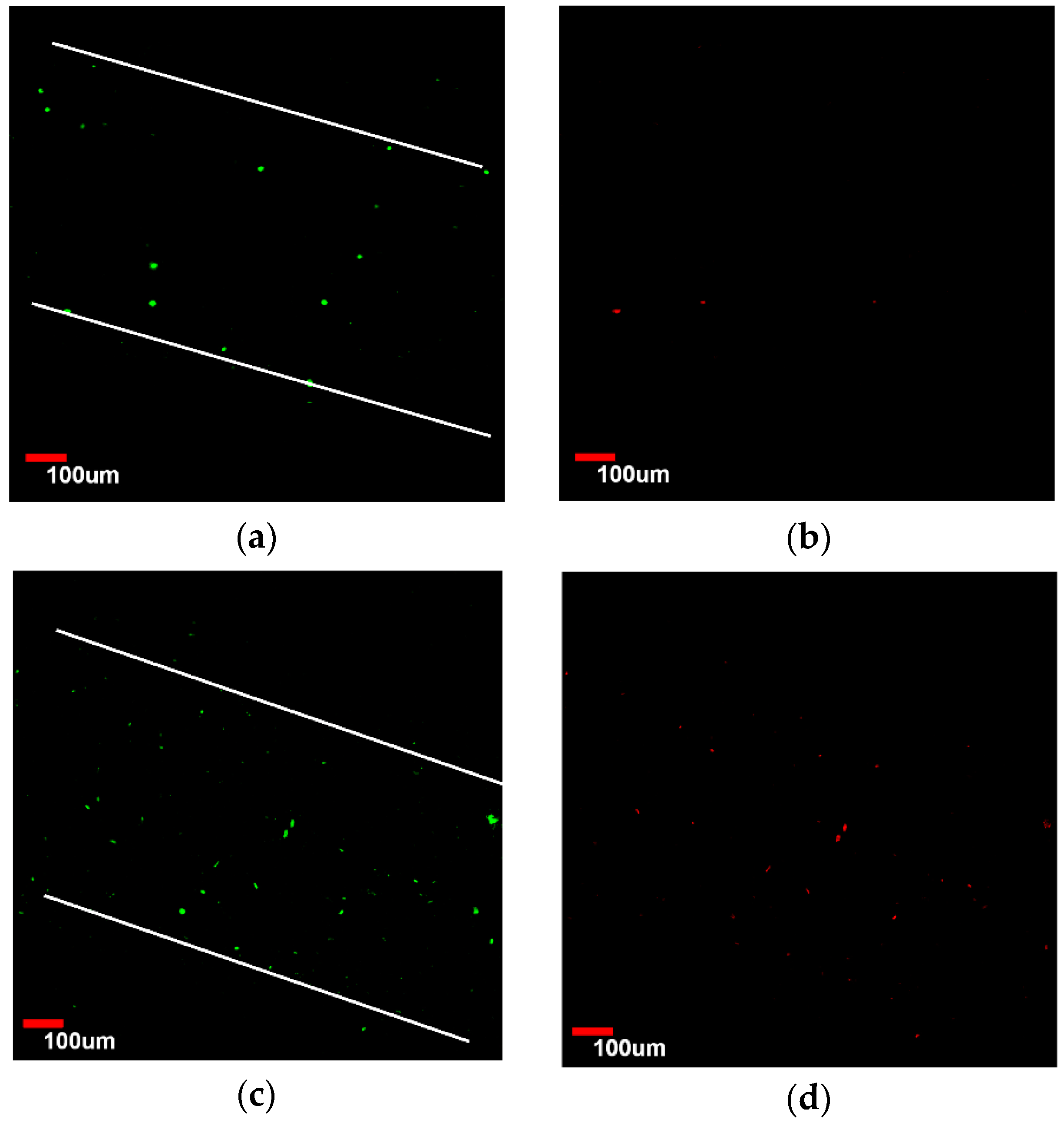
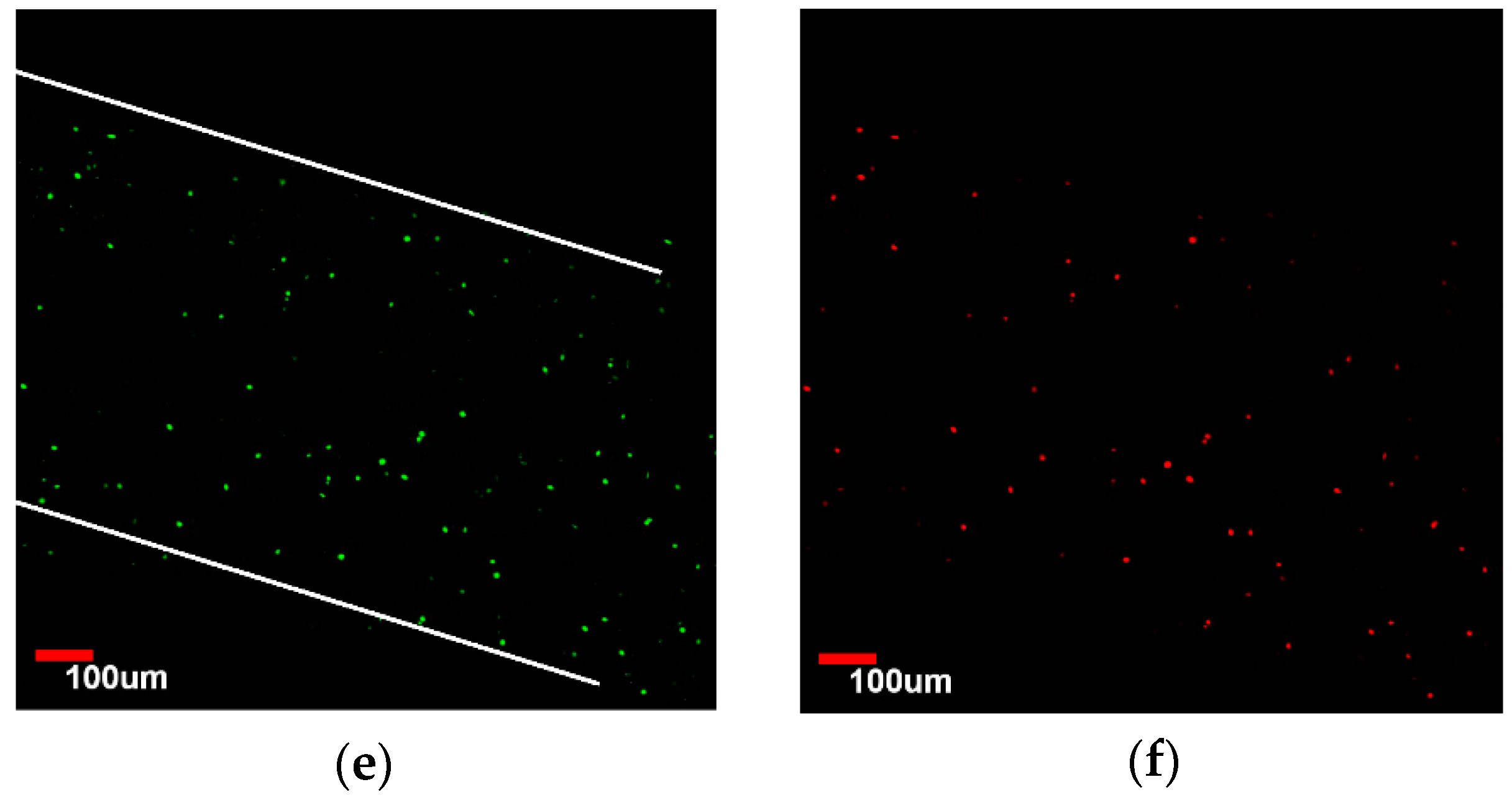
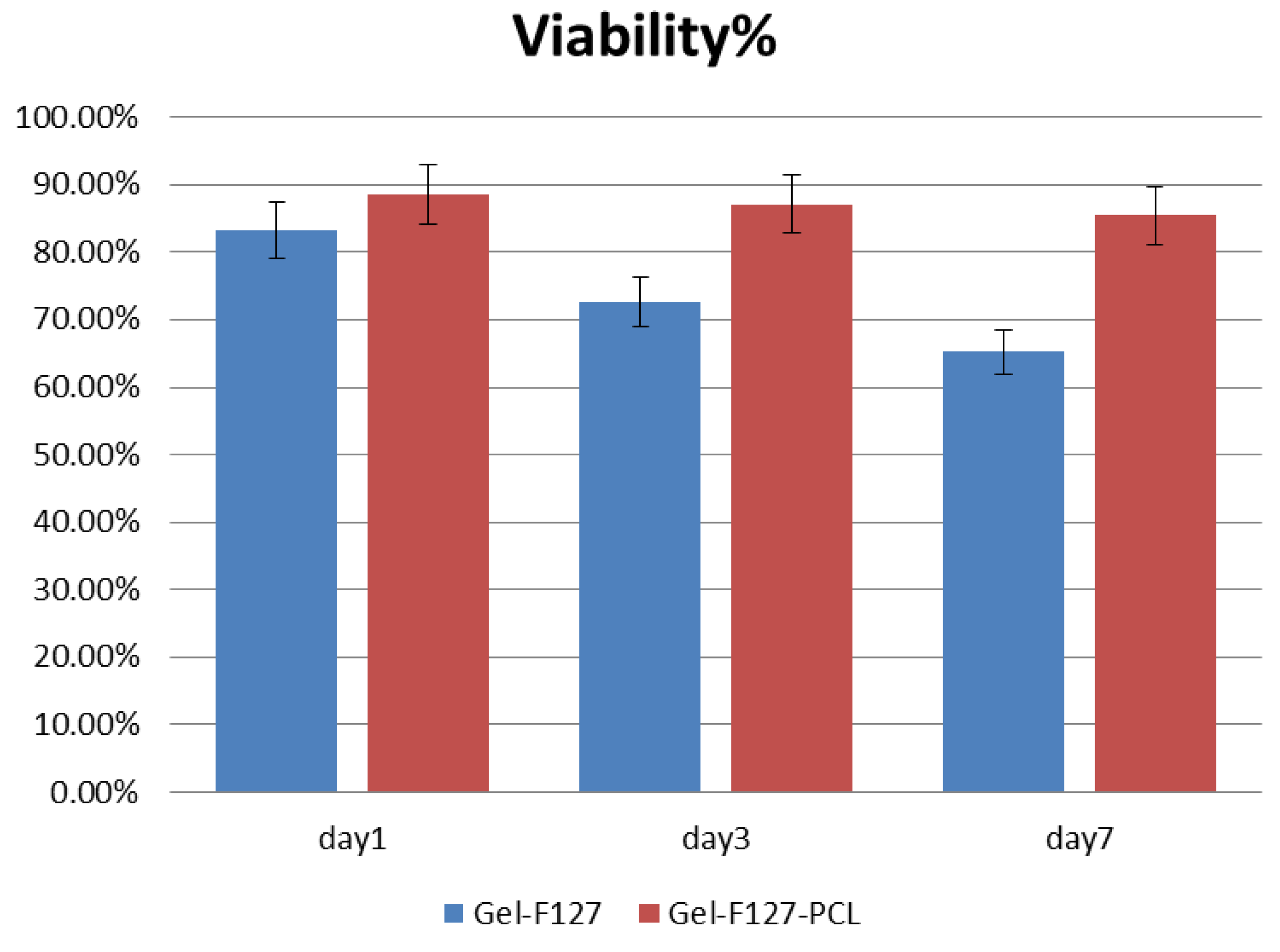
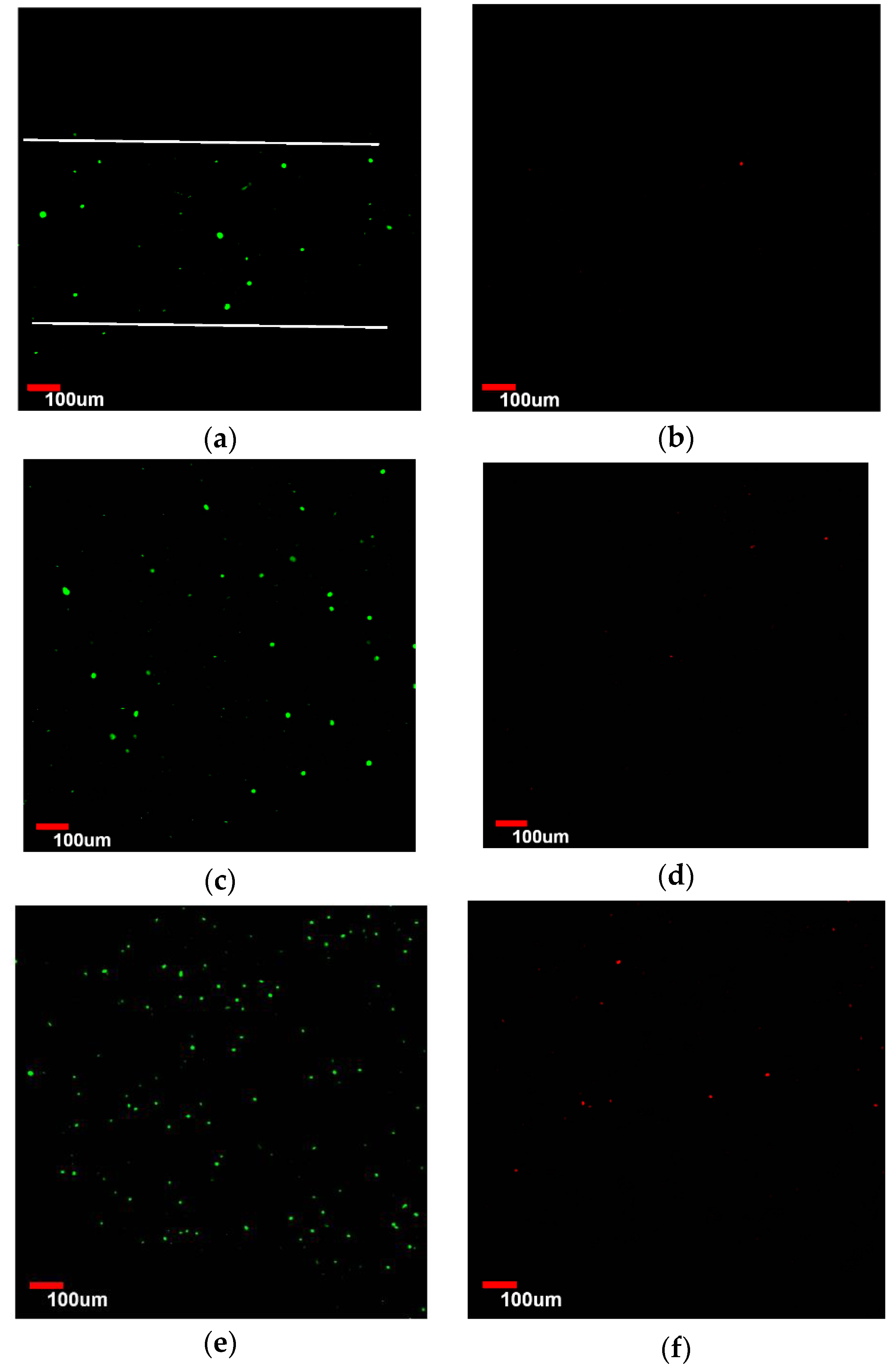
3.4. Viability Analysis of Seeded Human Umbilical Vein Endothelial Cells (HUVEC)
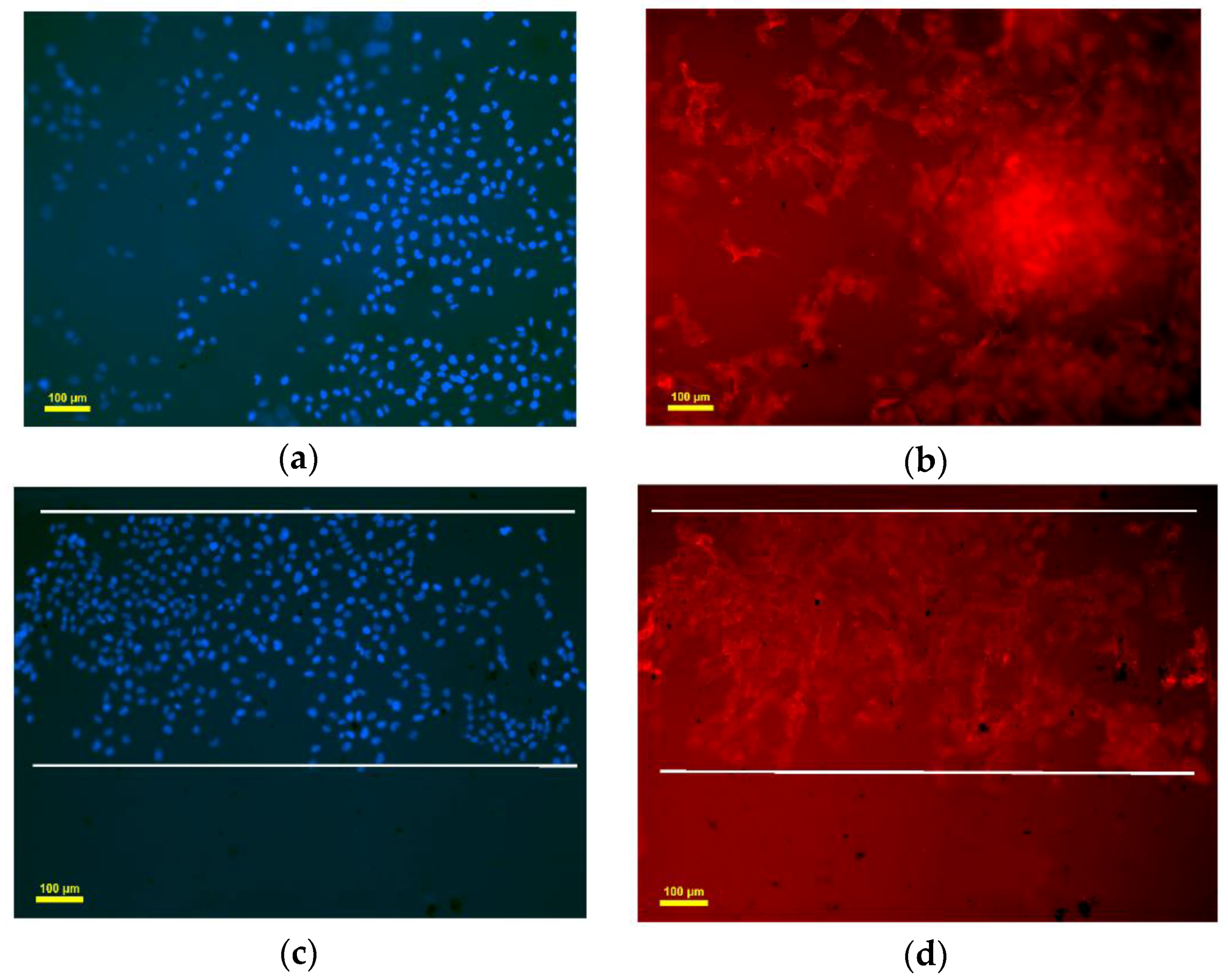
3.5. Endothelialization Analysis of Seeded HUVEC
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puelacher, W.C.; Vacanti, J.P.; Ferraro, N.F.; Schloo, B.; Vacanti, C.A. Femoral shaft reconstruction using tissue-engineered growth of bone. Int. J. Oral Maxillofac. Surg. 1996, 25, 223–228. [Google Scholar] [CrossRef]
- Tian, L.; George, S.C. Biomaterials to prevascularize engineered tissues. J. Cardiovasc. Transl. Res. 2011, 4, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Au, P.; Tam, J.; Duda, D.G.; Fukumura, D. Engineering vascularized tissue. Nat. Biotechnol. 2005, 23, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Rubin, J.; Deng, Y.; Huang, C.; Demirci, U.; Karp, J.M.; Khademhosseini, A. A cell-laden microfluidic hydrogel. Lab Chip 2007, 7, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Shimizu, T.; Horaguchi, S.; Sekine, H.; Yamato, M.; Umezu, M.; Okano, T. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 2013, 3, 1316. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Z.; Christensen, K.; Huang, Y.; Fu, J.; Markwald, R.R. Freeform Vertical and Horizontal Fabrication of Alginate-Based Vascular-Like Tubular Constructs Using Inkjetting. Manuf. Sci. Eng. 2014, 136, 061020–061028. [Google Scholar] [CrossRef]
- Hammer, J.; Han, L.H.; Tong, X.; Yang, F. A facile method to fabricate hydrogels with microchannel-like porosity for tissue engineering. Tissue Eng. Part C Methods 2014, 20, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Ghosh, R. Fabrication of alginate fibers using a micro-porous membrane based molding technique. Biochem. Eng. J. 2014, 91, 58–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 25004. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S.; Vincent, P.A.; Dai, G.H. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Mironov, V.; Nakamura, M. Bioprinting is Coming of Age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux. Biofabrication 2010, 2, 010201. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Reis, N.; Derby, B. Bioprinting: A beginning. Tissue Eng. 2006, 12, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Ozbolat, I.; Yu, Y. Bioprinting toward Organ Fabrication: Challenges and Future Trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V. Printing Technology to Produce Living Tissue. Expert Opin. Biol. Ther. 2003, 3, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, C.; Venugopal, J.R.; Tham, A.Y.; Ramakrishnam, S.; Kumar, S.D. Biomimetic hybrid nanofibrous substrates for mesenchymal stem cells differentiation into osteogenic cells. Mater. Sci. Eng. C 2014, 49, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Shamaz, B.H.; Anitha, A.; Vijayamohan, M.; Kuttappan, S.; Nair, S.; Nair, M.B. Relevance of fiber integrated gelatin-nanohydroxyapatite composite scaffold for bone tissue regeneration. Nanotechnology 2015, 26, 405101–405116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.; Sina, N.; Zhilian, Y.; Robert, K.; Anita, Q.; Simon, E.; Gordon, G. Bio-Ink Properties and Printability for Extrusion Printing Living Cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Figeys, D.; Pinto, D. Lab-on-a-chip: A revolution in biological and medical sciences. Anal. Chem. 2000, 72, 330A–335A. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 04500223. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, R.; Lu, Y.; Zhan, L.; Liu, Y.; Li, D.; Jin, Z. Fabrication of circular microfluidic network in enzymatically-crosslinked gelatin hydrogel. Mater. Sci. Eng. C 2016, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, Y.; Li, S.; Liu, C.; Hu, Q. Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels. Micromachines 2016, 7, 238. https://doi.org/10.3390/mi7120238
Sun Y, Liu Y, Li S, Liu C, Hu Q. Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels. Micromachines. 2016; 7(12):238. https://doi.org/10.3390/mi7120238
Chicago/Turabian StyleSun, Yuanshao, Yuanyuan Liu, Shuai Li, Change Liu, and Qingxi Hu. 2016. "Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels" Micromachines 7, no. 12: 238. https://doi.org/10.3390/mi7120238
APA StyleSun, Y., Liu, Y., Li, S., Liu, C., & Hu, Q. (2016). Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels. Micromachines, 7(12), 238. https://doi.org/10.3390/mi7120238





