Microfluidic Vortex Enhancement for on-Chip Sample Preparation
Abstract
:1. Introduction
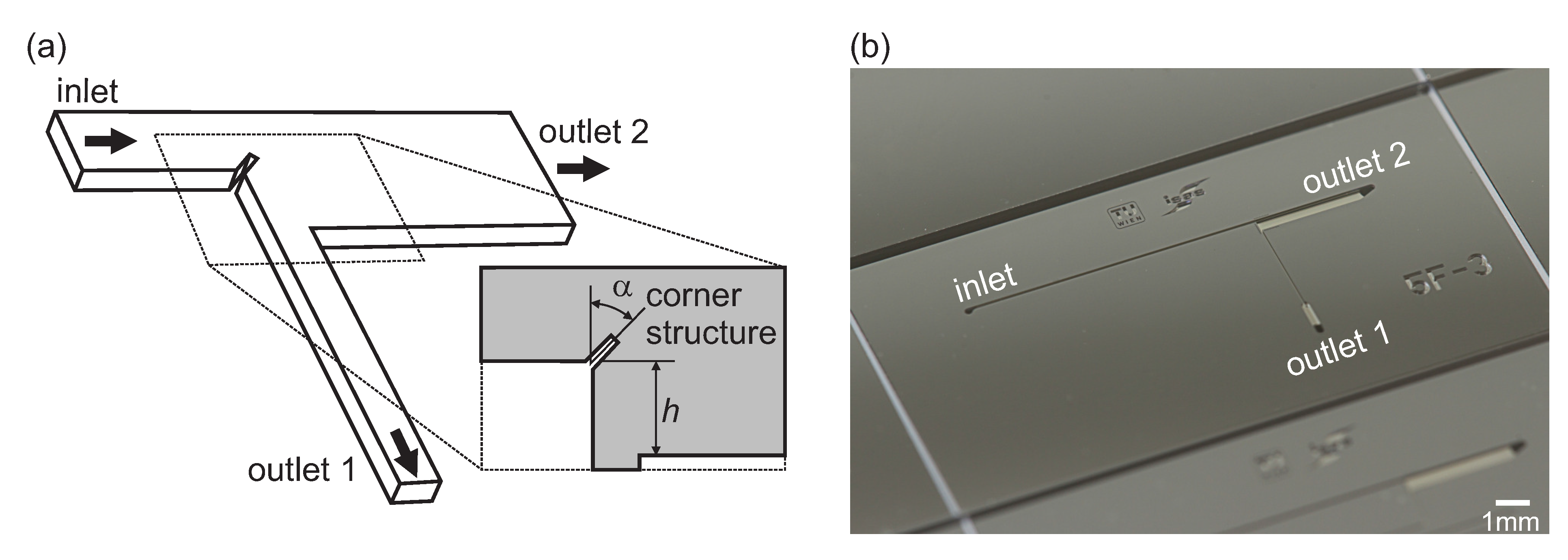
2. Materials and Methods
2.1. Numerical Models
2.2. Device Fabrication
2.3. Particle Suspension and Blood Preparation
2.4. Experimental Setup
2.5. Procedures for Image Analysis
3. Measurements and Results
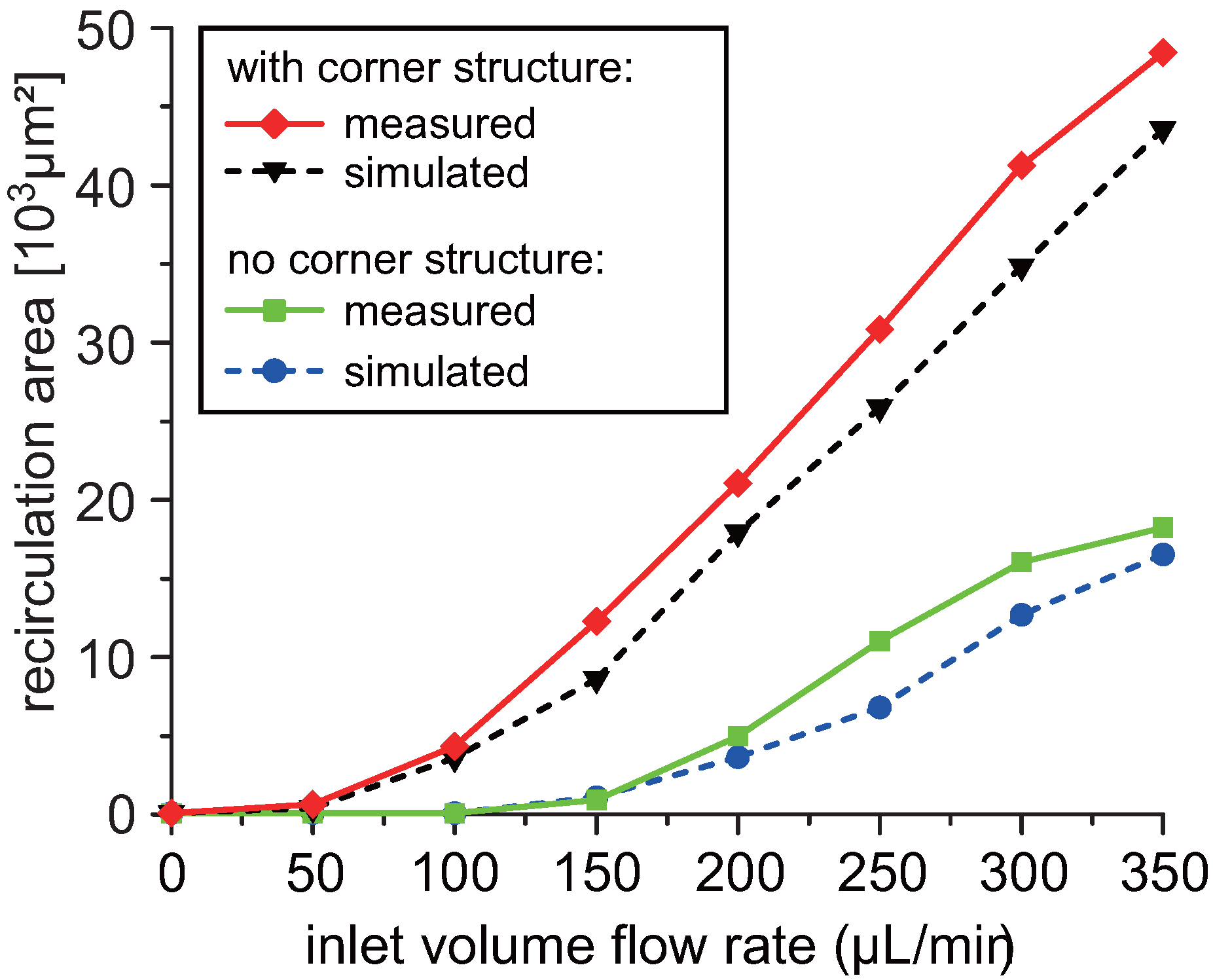
3.1. Design Optimization
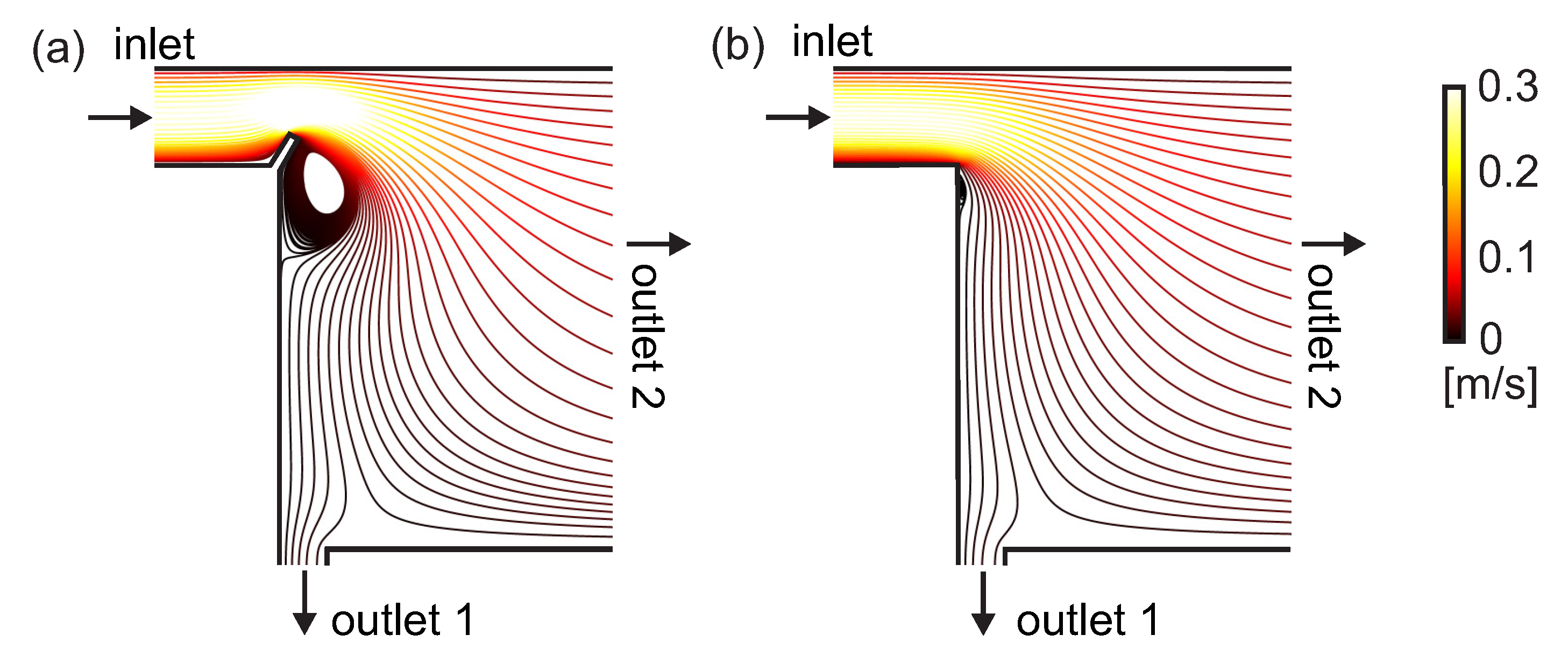
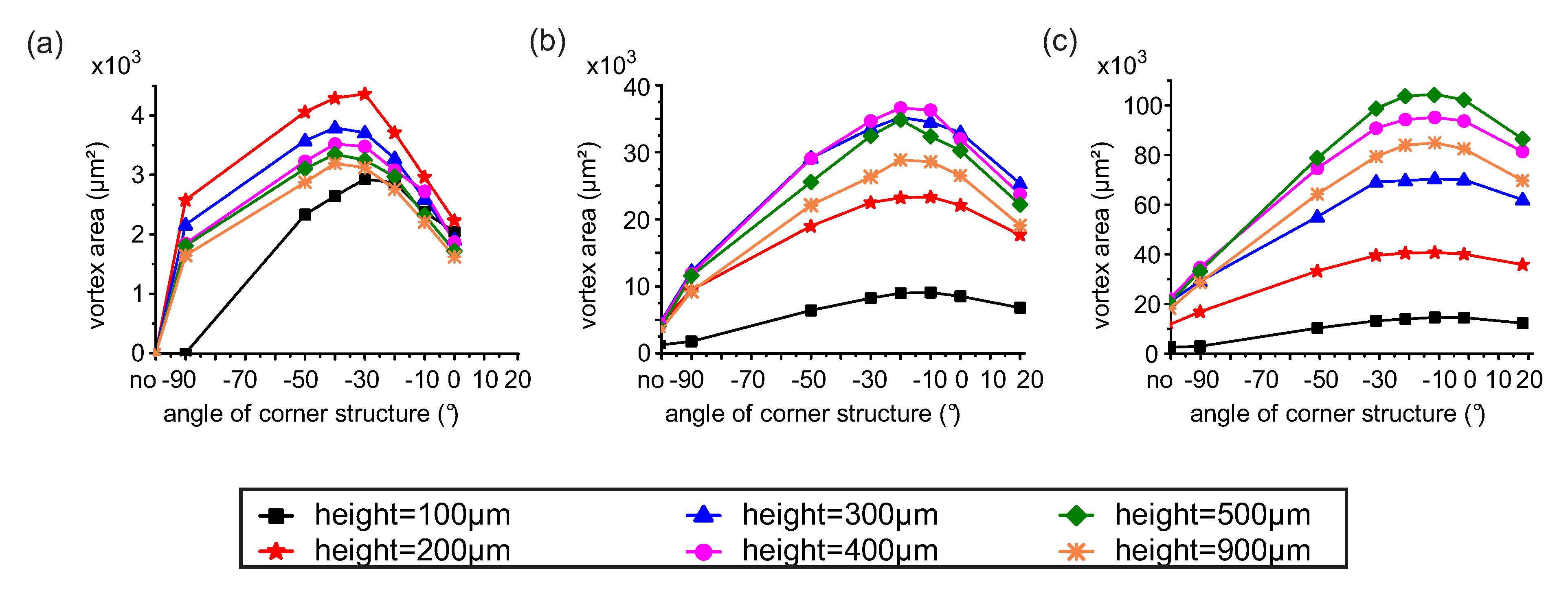
3.2. Streamline Visualization
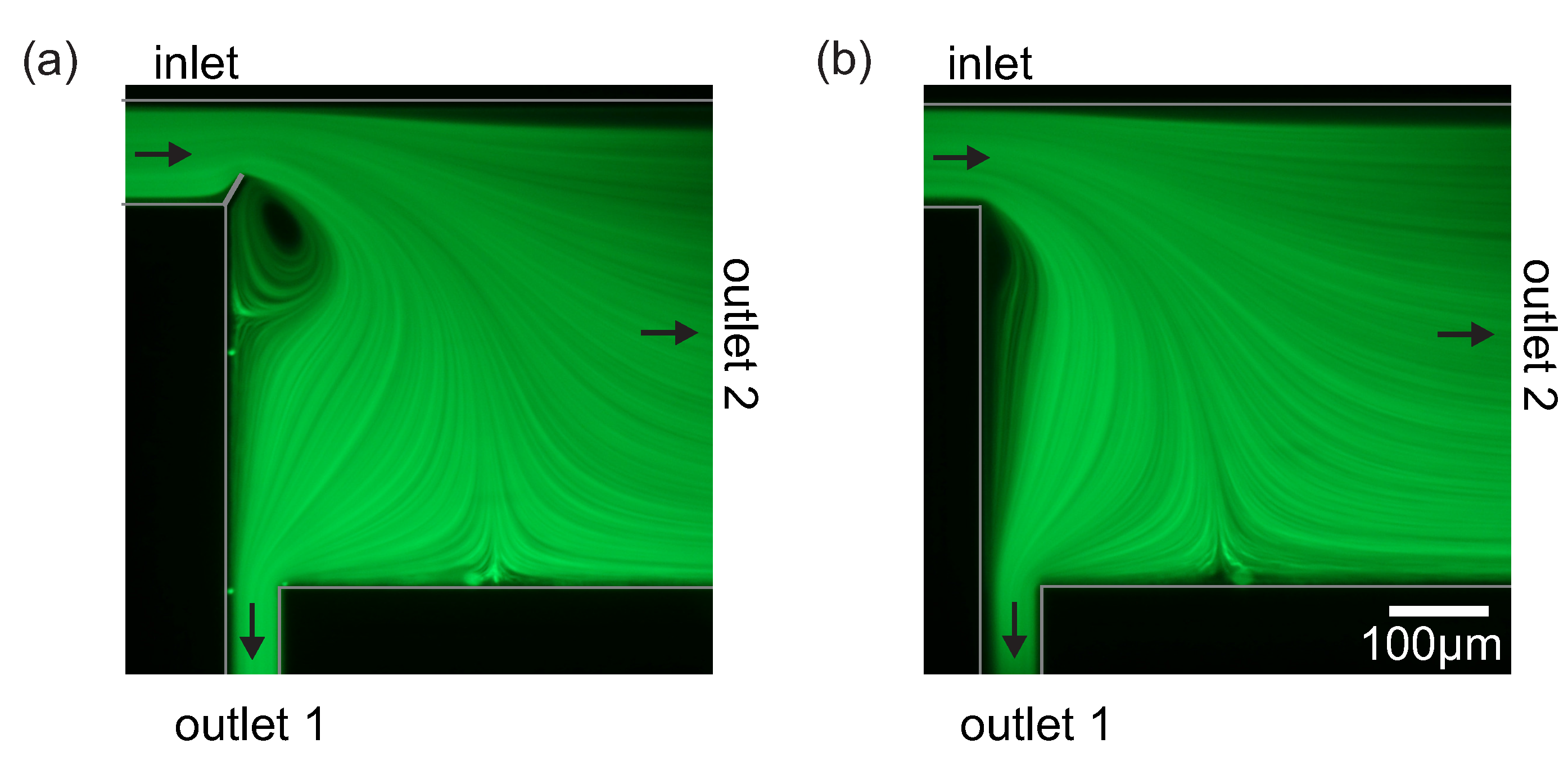
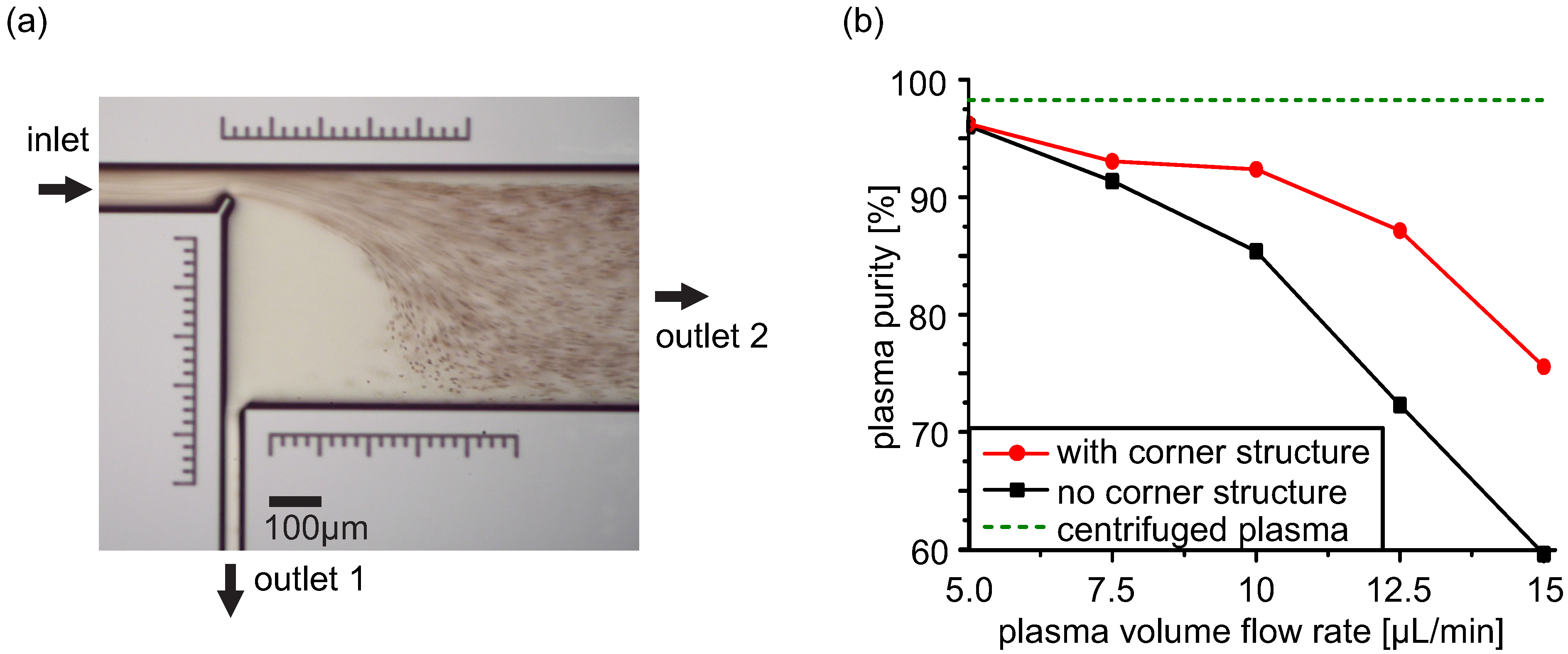
3.3. Human Blood Plasma Separation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mach, A.J.; Adeyiga, O.B.; di Carlo, D. Microfluidic sample preparation for diagnostic cytopathology. Lab Chip 2013, 13, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.T.; Mickleburgh, T.G.; Stewart-James, S.A.; Sellens, K.A.; Pressnall, M. Micro total analysis systems: Fundamental advances and biological applications. Anal. Chem. 2014, 86, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, E. Microfluidic chips for clinical and forensic analysis. Electrophoresis 2002, 23, 677–712. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Lee, W.; di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Shelby, J.P.; Lim, D.S.W.; Kuo, J.S.; Chiu, D.T. Microfluidic systems: High radial acceleration in microvortices. Nature 2003, 425, 38. [Google Scholar] [CrossRef] [PubMed]
- Shelby, J.P.; Chiu, D.T. Controlled rotation of biological micro- and nano-particles in microvortices. Lab Chip 2004, 4, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.T. Cellular manipulations in microvortices. Anal. Bioanal. Chem. 2007, 387, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mach, A.J.; Kim, J.H.; Arshi, A.; Hur, S.C.; di Carlo, D. Automated cellular sample preparation using a Centrifuge-on-a-Chip. Lab Chip 2011, 11, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Pertaya-Braun, N.; Baier, T.; Hardt, S. Microfluidic centrifuge based on a counterflow configuration. Microfluid. Nanofluid. 2011, 12, 317–324. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.; Bahk, Y.; Yoon, S.; Arakawa, T.; Ko, J.; Shin, B.; Shoji, S.; Go, J. Microfluidic centrifuge of nano-particles using rotating flow in a microchamber. Sens. Actuators B Chem. 2008, 132, 525–530. [Google Scholar] [CrossRef]
- Park, J.S.; Song, S.H.; Jung, H.I. Continuous focusing of microparticles using inertial lift force and vorticity via multi-orifice microfluidic channels. Lab Chip 2009, 9, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Papautsky, I. Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity. Biomicrofluidics 2013, 7, 044119. [Google Scholar] [CrossRef]
- Hsu, C.H.; Di Carlo, D.; Chen, C.; Irimia, D.; Toner, M. Microvortex for focusing, guiding and sorting of particles. Lab Chip 2008, 8, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Frakes, D.H.; Babiker, H.; Chao, S.H.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. Simulation and experimental characterization of microscopically accessible hydrodynamic microvortices. Micromachines 2012, 3, 529–541. [Google Scholar] [CrossRef]
- Zhou, J.; Kasper, S.; Papautsky, I. Enhanced size-dependent trapping of particles using microvortices. Microfluid. Nanofluid. 2013, 15, 611–623. [Google Scholar] [CrossRef]
- Petit, T.; Zhang, L.; Peyer, K.E.; Kratochvil, B.E.; Nelson, B.J. Selective trapping and manipulation of microscale objects using mobile microvortices. Nano Lett. 2012, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Mach, A.J.; di Carlo, D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar] [CrossRef]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Choi, S.; Park, J.K. Rapid multivortex mixing in an alternately formed contraction-expansion array microchannel. Biomed. Microdevices 2010, 12, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, S. Mixing efficiency of a multilamination micromixer with consecutive recirculation zones. Chem. Eng. Sci. 2009, 64, 1223–1231. [Google Scholar] [CrossRef]
- Shih, T.R.; Chung, C.K. A high-efficiency planar micromixer with convection and diffusion mixing over a wide Reynolds number range. Microfluid. Nanofluid. 2007, 5, 175–183. [Google Scholar] [CrossRef]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.M.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012, 12, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Marchalot, J.; Fouillet, Y.; Achard, J.L. Multi-step microfluidic system for blood plasma separation: Architecture and separation efficiency. Microfluid. Nanofluid. 2014, 17, 167–180. [Google Scholar] [CrossRef]
- Sollier, E.; Cubizolles, M.; Fouillet, Y.; Achard, J.L. Fast and continuous plasma extraction from whole human blood based on expanding cell-free layer devices. Biomed. Microdevices 2010, 12, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluid. 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Liou, T.M.; Lin, C.T. Study on microchannel flows with a sudden contraction-expansion at a wide range of Knudsen number using lattice Boltzmann method. Microfluid. Nanofluid. 2013, 16, 315–327. [Google Scholar] [CrossRef]
- Nejat, A.; Kowsary, F.; Hasanzadeh-Barforoushi, A.; Ebrahimi, S. Unsteady pulsating characteristics of the fluid flow through a sudden expansion microvalve. Microfluid. Nanofluid. 2014, 17, 623–637. [Google Scholar] [CrossRef]
- Balan, C.M.; Broboana, D.; Balan, C. Investigations of vortex formation in microbifurcations. Microfluid. Nanofluid. 2012, 13, 819–833. [Google Scholar] [CrossRef]
- Liu, S.J.; Wei, H.H.; Hwang, S.H.; Chang, H.C. Dynamic particle trapping, release, and sorting by microvortices on a substrate. Phys. Rev. E 2010, 82, 026308. [Google Scholar] [CrossRef]
- Yazdi, S.; Ardekani, A.M. Bacterial aggregation and biofilm formation in a vortical flow. Biomicrofluidics 2012, 6, 044114. [Google Scholar] [CrossRef]
- Shang, X.P.; Cui, X.G.; Huang, X.Y.; Yang, C. Vortex generation in a microfluidic chamber with actuations. Exp. Fluids 2014, 55, 1758. [Google Scholar] [CrossRef]
- Qin, L.; Vermesh, O.; Shi, Q.; Heath, J.R. Self-powered microfluidic chips for multiplexed protein assays from whole blood. Lab Chip 2009, 9, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Arifin, D.R.; Yeo, L.Y.; Friend, J.R. Microfluidic blood plasma separation via bulk electrohydrodynamic flows. Biomicrofluidics 2007, 1, 14103. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Hou, D.; Maheshswari, S.; Chang, H.C. Electrohydrodynamic surface microvortices for mixing and particle trapping. Appl. Phys. Lett. 2006, 88, 233512. [Google Scholar] [CrossRef]
- Fishler, R.; Mulligan, M.K.; Sznitman, J. Mapping low-Reynolds-number microcavity flows using microfluidic screening devices. Microfluid. Nanofluid. 2013, 15, 491–500. [Google Scholar] [CrossRef]
- Tsai, C.H.; Yeh, C.P.; Lin, C.H.; Yang, R.J.; Fu, L.M. Formation of recirculation zones in a sudden expansion microchannel with a rectangular block structure over a wide Reynolds number range. Microfluid. Nanofluid. 2011, 12, 213–220. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, C.H.; Fu, L.M.; Chen, H.C. High-performance microfluidic rectifier based on sudden expansion channel with embedded block structure. Biomicrofluidics 2012, 6, 24108–241089. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Breuer, M.; Durst, F. Backward-facing step flows for various expansion ratios at low and moderate Reynolds numbers. J. Fluids Eng. 2004, 126, 362. [Google Scholar] [CrossRef]
- Haller, A.; Buchegger, W.; Vellekoop, M. Towards an optimized blood plasma separation chip: Finite element analysis of a novel corner structure in a backward-facing step. Procedia Eng. 2011, 25, 439–442. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 16505–1650516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.F.; Aw Yong, K.M.; Fu, J. Microfluidic blood cell sorting: Now and beyond. Small 2014, 10, 1687–703. [Google Scholar] [CrossRef] [PubMed]
- Faivre, M.; Abkarian, M.; Bickraj, K.; Stone, H.A. Geometrical focusing of cells in a microfluidic device: An approach to separate blood plasma. Biorheology 2006, 43, 147–159. [Google Scholar] [PubMed]
- Hou, H.W.; Bhagat, A.A.S.; Lee, W.C.; Huang, S.; Han, J.; Lim, C.T. Microfluidic devices for blood fractionation. Micromachines 2011, 2, 319–343. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Rostaing, H.; Pouteau, P.; Fouillet, Y.; Achard, J.L. Passive microfluidic devices for plasma extraction from whole human blood. Sensors Actuators B Chem. 2009, 141, 617–624. [Google Scholar] [CrossRef]
- Mach, A.J.; di Carlo, D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol. Bioeng. 2010, 107, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Kersaudy-Kerhoas, M.; Kavanagh, D.M.; Dhariwal, R.S.; Campbell, C.J.; Desmulliez, M.P.Y. Validation of a blood plasma separation system by biomarker detection. Lab Chip 2010, 10, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, A.; Spittler, A.; Brandhoff, L.; Zirath, H.; Puchberger-Enengl, D.; Keplinger, F.; Vellekoop, M.J. Microfluidic Vortex Enhancement for on-Chip Sample Preparation. Micromachines 2015, 6, 239-251. https://doi.org/10.3390/mi6020239
Haller A, Spittler A, Brandhoff L, Zirath H, Puchberger-Enengl D, Keplinger F, Vellekoop MJ. Microfluidic Vortex Enhancement for on-Chip Sample Preparation. Micromachines. 2015; 6(2):239-251. https://doi.org/10.3390/mi6020239
Chicago/Turabian StyleHaller, Anna, Andreas Spittler, Lukas Brandhoff, Helene Zirath, Dietmar Puchberger-Enengl, Franz Keplinger, and Michael J. Vellekoop. 2015. "Microfluidic Vortex Enhancement for on-Chip Sample Preparation" Micromachines 6, no. 2: 239-251. https://doi.org/10.3390/mi6020239
APA StyleHaller, A., Spittler, A., Brandhoff, L., Zirath, H., Puchberger-Enengl, D., Keplinger, F., & Vellekoop, M. J. (2015). Microfluidic Vortex Enhancement for on-Chip Sample Preparation. Micromachines, 6(2), 239-251. https://doi.org/10.3390/mi6020239






