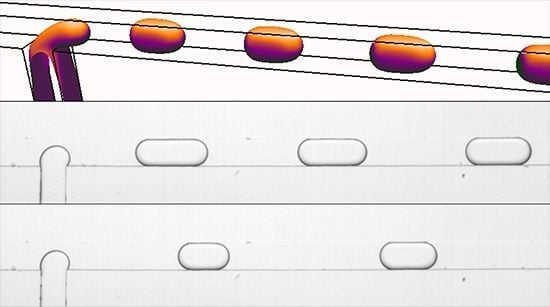
Generation of Oil Droplets in a Non-Newtonian Liquid Using a Microfluidic T-Junction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication
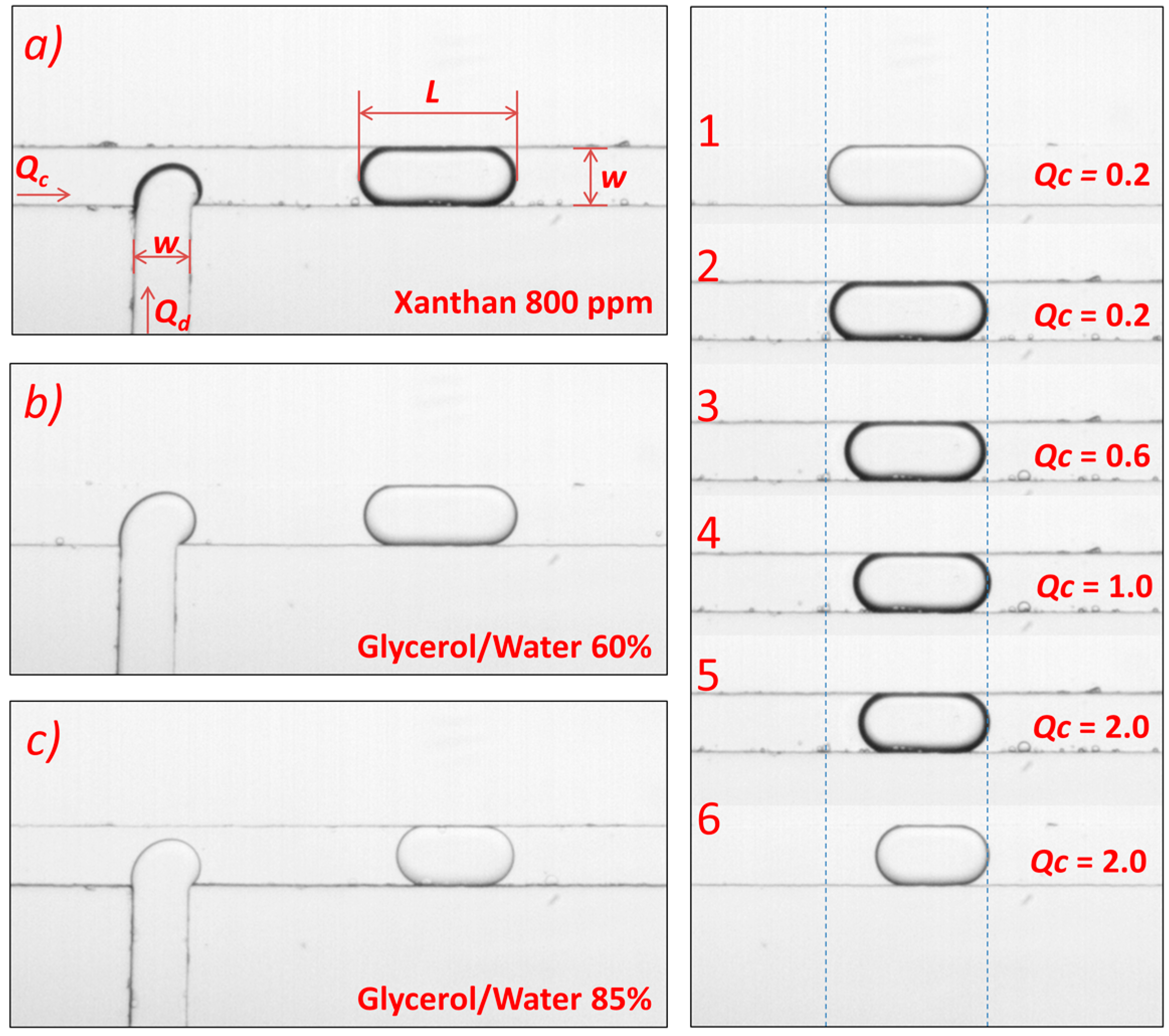
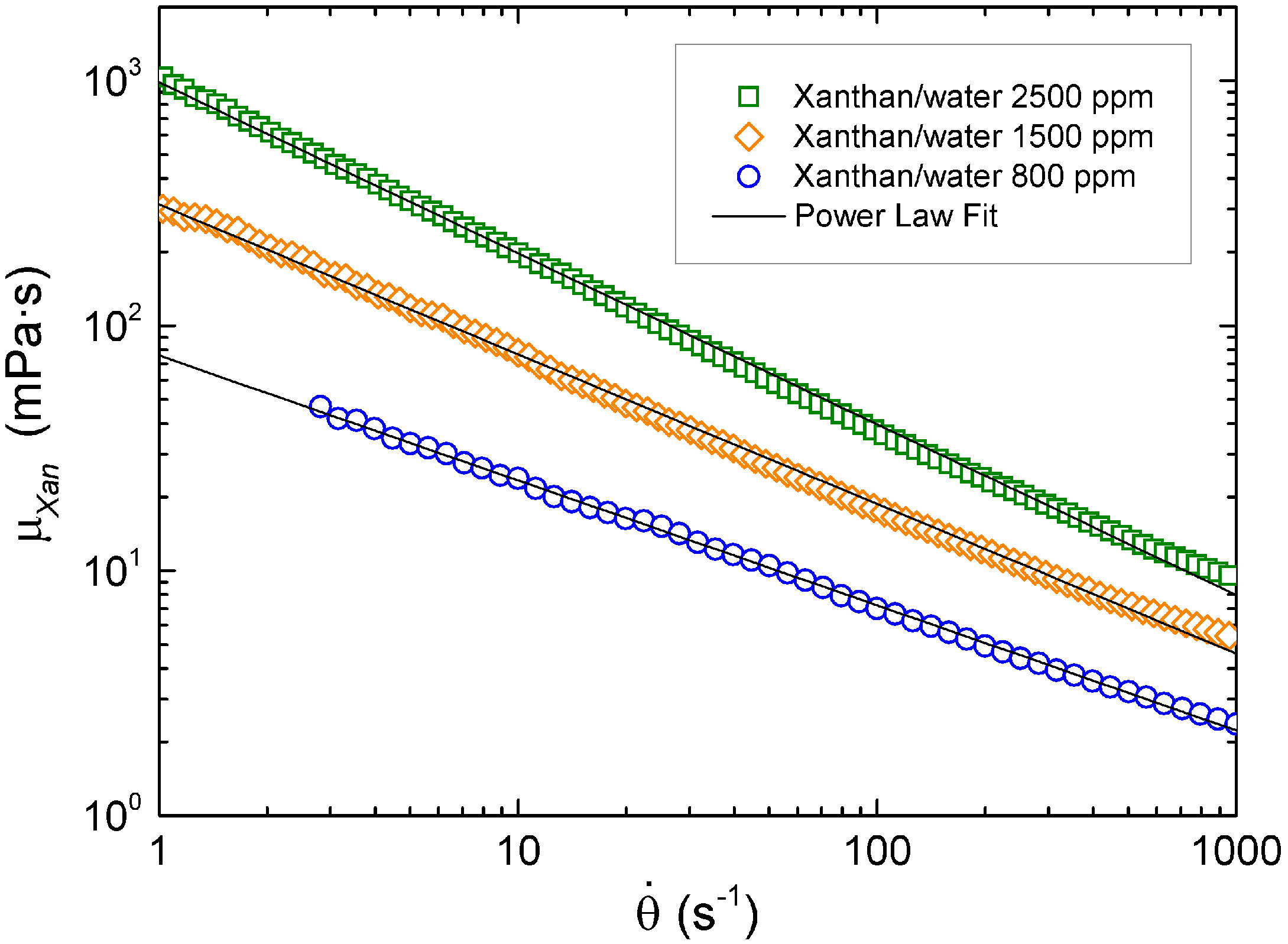
2.2. Liquids
| Liquid | ρ (g/cm3) | γ (mN/m) | μ (mPa·s) |
|---|---|---|---|
| Linseed oil | 0.93 | 22.3 | |
| Water | 0.997 | 72 | 0.89 |
| 60% (w/w) Glycerol/Water | 1.15 | 67.3 | 9 |
| 85% (w/w) Glycerol/Water | 1.22 | 64.4 | 79 |
| 800 ppm (w/w) Xanthan/Water | 0.997 | 72 | 76 |
| 1500 ppm (w/w) Xanthan/Water | 0.997 | 72 | 312 |
| 2500 ppm (w/w) Xanthan/Water | 0.997 | 72 | 985 |
| Liquid | K (mPa·sn) | n |
|---|---|---|
| 800 ppm (w/w) Xanthan/Water | 75.5 ± 0.6 | 0.491 ± 0.002 |
| 1500 ppm (w/w) Xanthan/Water | 312.5 ± 1.6 | 0.389 ± 0.001 |
| 2500 ppm (w/w) Xanthan/Water | 985 ± 6 | 0.302 ± 0.001 |
2.3. Imaging and Analysis
3. Results and Discussion
3.1. Newtonian Droplets in a Newtonian Continuous Phase
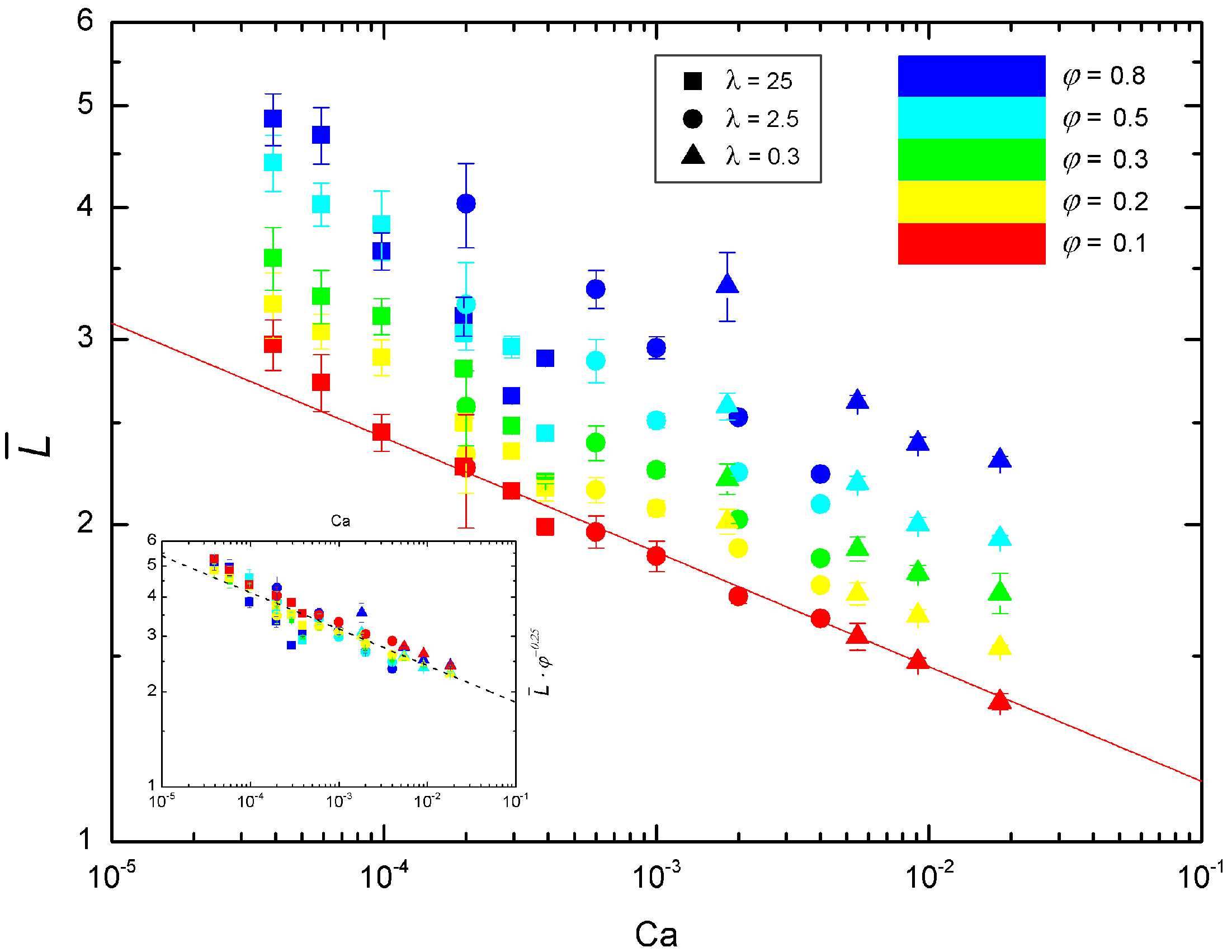
3.2. Newtonian Droplets in a Non-Newtonian Continuous Phase
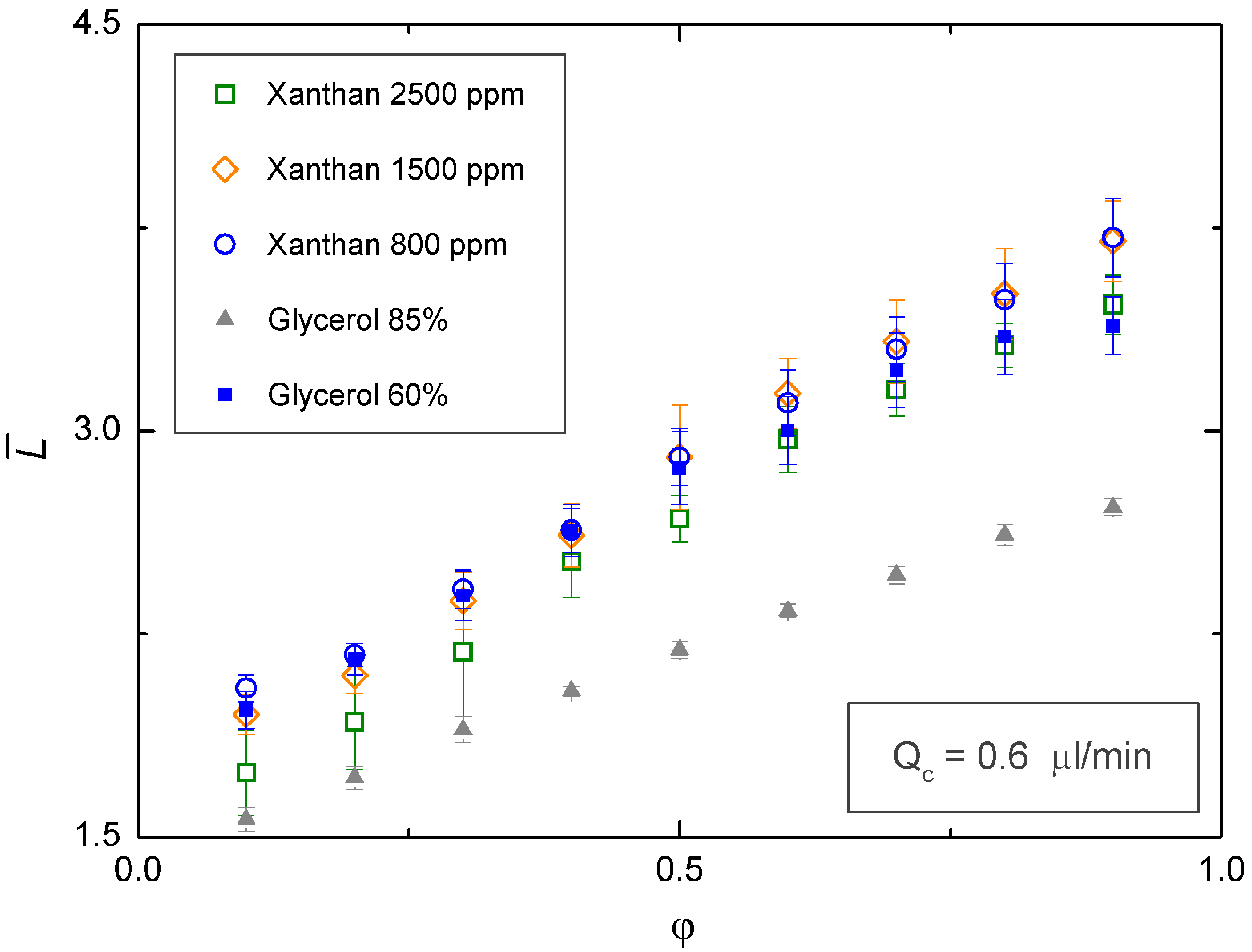
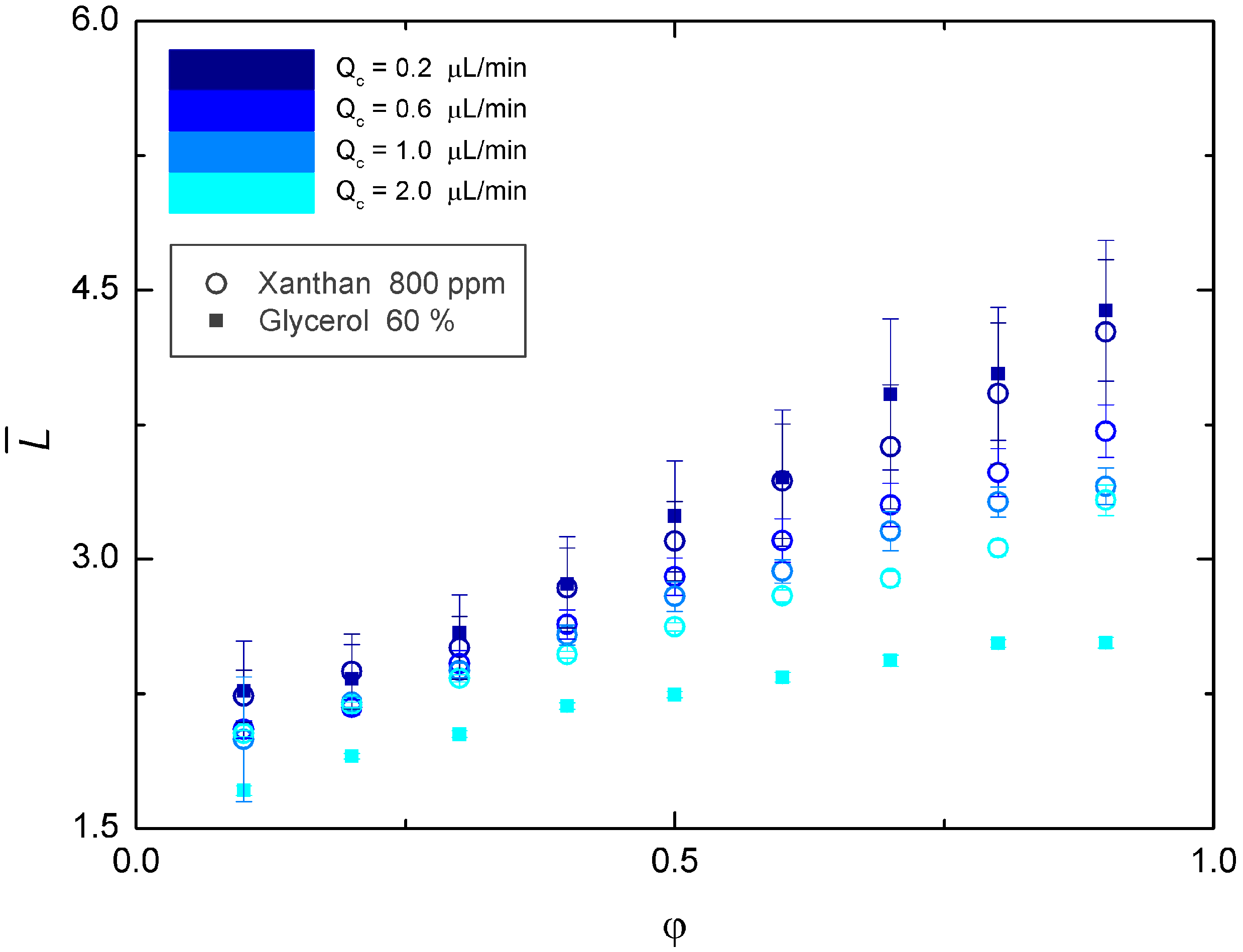
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- deMello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; Solvas, X.C.I.; Wootton, R.C.R.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Cramer, C.; Fischer, P.; Windhab, E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004, 59, 3045–3058. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, B.; Shen, A.Q.; Sureshkumar, R. Dynamics of viscoelastic fluid filaments in microfluidic devices. Phys. Fluid. 2007, 19, 073103. [Google Scholar] [CrossRef]
- Arratia, P.E.; Gollub, J.P.; Durian, D.J. Polymeric filament thinning and breakup in microchannels. Phys. Rev. E 2008, 77, 036309. [Google Scholar] [CrossRef] [PubMed]
- Arratia, P.E.; Cramer, L.A.; Gollub, J.P.; Durian, D.J. The effects of polymer molecular weight on filament thinning and drop breakup in microchannels. New J. Phys. 2009, 11, 115006. [Google Scholar] [CrossRef]
- Derzsi, L.; Kasprzyk, M.; Plog, J.P.; Garstecki, P. Flow focusing with viscoelastic liquids. Phys. Fluid. 2013, 25, 092001. [Google Scholar] [CrossRef]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Husny, J.; Cooper-White, J.J. The effect of elasticity on drop creation in T-shaped microchannels. J. Non Newton. Fluid Mech. 2006, 137, 121–136. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Passive breakup of viscoelastic droplets and filament self-thinning at a microfluidic T-junction. J. Rheol. 2009, 53, 663–683. [Google Scholar] [CrossRef]
- Gu, Z.; Liow, J.-L.; Zhu, G. Investigation on the droplet formation time with xanthan gum solutions at a T-junction. In Proceedings of the ASME 2012 Fluids Engineering Division Summer Meeting, Rio Grande, PR, USA, 8–12 July 2012; Volume 2, pp. 299–307.
- Li, X.B.; Li, F.C.; Kinoshita, H.; Oishi, M.; Oshima, M. Dynamics of viscoelastic fluid droplet under very low interfacial tension in a serpentine T-junction microchannel. Microfluid. Nanofluid. 2015, 18, 1007–1021. [Google Scholar] [CrossRef]
- Gupta, A.; Sbragaglia, M. Deformation and breakup of viscoelastic droplets in confined shear flow. Phys. Rev. E 2014, 90, 023305. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, P.; Macosko, C. Rheology of xanthan gum. J. Rheol. 1978, 22, 493–505. [Google Scholar] [CrossRef]
- Zirnsak, M.A.; Boger, D.V.; Tirtaatmadja, V. Steady shear and dynamic rheological properties of xanthan gum solutions in viscous solvents. J. Rheol. 1999, 43, 627–650. [Google Scholar] [CrossRef]
- Carre, A.; Eustache, F. Spreading kinetics of shear-thinning fluids in wetting and dewetting modes. Langmuir 2000, 16, 2936–2941. [Google Scholar] [CrossRef]
- Rafai, S.; Bonn, D.; Boudaoud, A. Spreading of non-Newtonian fluids on hydrophilic surfaces. J. Fluid Mech. 2004, 513, 77–85. [Google Scholar] [CrossRef]
- Piccin, E.; Ferraro, D.; Sartori, P.; Chiarello, E.; Pierno, M.; Mistura, G. Generation of water-in-oil and oil-in-water microdroplets in polyester-toner microfluidic devices. Sens. Actuators B Chem. 2014, 196, 525–531. [Google Scholar] [CrossRef]
- Brigo, L.; Carofiglio, T.; Fregonese, C.; Meneguzzi, F.; Mistura, G.; Natali, M.; Tonellato, U. An optical sensor for pH supported onto tentagel resin beads. Sens. Actuators B Chem. 2008, 130, 477–482. [Google Scholar] [CrossRef]
- Silvestrini, S.; Ferraro, D.; Toth, T.; Pierno, M.; Carofiglio, T.; Mistura, G.; Maggini, M. Tailoring the wetting properties of thiolene microfluidic materials. Lab Chip 2012, 12, 4041–4043. [Google Scholar] [CrossRef] [PubMed]
- Glawdel, T.; Ren, C.L. Droplet formation in microfluidic T-junction generators operating in the transitional regime. III. Dynamic surfactant effects. Phys. Rev. E 2012, 86, 026308. [Google Scholar] [CrossRef] [PubMed]
- Hemmila, S.; Cauich-Rodriguez, J.V.; Kreutzer, J.; Kallio, P. Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces. Appl. Surf. Sci. 2012, 258, 9864–9875. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Dutil, I.; Farnood, R.R. Preparation and characterization of ethyl cellulose-based core-shell microcapsules containing plant oils. Colloids Surf. A Phys. Eng. Asp. 2012, 394, 74–84. [Google Scholar] [CrossRef]
- Glycerine Producers’ Association. Physical Properties of Glycerine and Its Solutions; Glycerine Producers’ Association: New York, NY, USA, 1963. [Google Scholar]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Hoorfar, M.; Neumann, A.W. Recent progress in Axisymmetric Drop Shape Analysis (ADSA). Adv. Colloid Interf. Sci. 2006, 121, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.; Semprebon, C.; Toth, T.; Locatelli, E.; Pierno, M.; Mistura, G.; Brinkmann, M. Morphological transitions of droplets wetting rectangular domains. Langmuir 2012, 28, 13919–13923. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.F.; Noharuddin, N.N.; Taylor, J.A.; Anna, S.L. Experimental observations of the squeezing-to-dripping transition in T-shaped microfluidic junctions. Phys. Rev. E 2008, 78, 036317. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Li, S.W.; Tan, J.; Luo, G.S. Correlations of droplet formation in T-junction microfluidic devices: from squeezing to dripping. Microfluid. Nanofluid. 2008, 5, 711–717. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R. Effect of geometry on droplet formation in the squeezing regime in a microfluidic T-junction. Microfluid. Nanofluid. 2010, 8, 799–812. [Google Scholar] [CrossRef]
- De Menech, M.; Garstecki, P.; Jousse, F.; Stone, H.A. Transition from squeezing to dripping in a microfluidic T-shaped junction. J. Fluid Mech. 2008, 595, 141–161. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, S.W.; Tan, J.; Wang, Y.J.; Luo, G.S. Preparation of highly monodisperse droplet in a T-junction microfluidic device. AIChE J. 2006, 52, 3005–3010. [Google Scholar] [CrossRef]
- Varagnolo, S.; Mistura, G.; Pierno, M.; Sbragaglia, M. Sliding droplets of xanthan solutions: A joint experimental and numerical study. Eur. Phys. J. E 2015, 38, 126. [Google Scholar]
- Varagnolo, S.; Ferraro, D.; Fantinel, P.; Pierno, M.; Mistura, G.; Amati, G.; Biferale, L.; Sbragaglia, M. Stick-slip sliding of water drops on chemically heterogeneous surfaces. Phys. Rev. Lett. 2013, 111, 066101. [Google Scholar] [CrossRef] [PubMed]
- Sbragaglia, M.; Biferale, L.; Amati, G.; Varagnolo, S.; Ferraro, D.; Mistura, G.; Pierno, M. Sliding drops across alternating hydrophobic and hydrophilic stripes. Phys. Rev. E 2014, 89, 012406. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarello, E.; Derzsi, L.; Pierno, M.; Mistura, G.; Piccin, E. Generation of Oil Droplets in a Non-Newtonian Liquid Using a Microfluidic T-Junction. Micromachines 2015, 6, 1825-1835. https://doi.org/10.3390/mi6121458
Chiarello E, Derzsi L, Pierno M, Mistura G, Piccin E. Generation of Oil Droplets in a Non-Newtonian Liquid Using a Microfluidic T-Junction. Micromachines. 2015; 6(12):1825-1835. https://doi.org/10.3390/mi6121458
Chicago/Turabian StyleChiarello, Enrico, Ladislav Derzsi, Matteo Pierno, Giampaolo Mistura, and Evandro Piccin. 2015. "Generation of Oil Droplets in a Non-Newtonian Liquid Using a Microfluidic T-Junction" Micromachines 6, no. 12: 1825-1835. https://doi.org/10.3390/mi6121458
APA StyleChiarello, E., Derzsi, L., Pierno, M., Mistura, G., & Piccin, E. (2015). Generation of Oil Droplets in a Non-Newtonian Liquid Using a Microfluidic T-Junction. Micromachines, 6(12), 1825-1835. https://doi.org/10.3390/mi6121458