A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators
Abstract
:1. Introduction
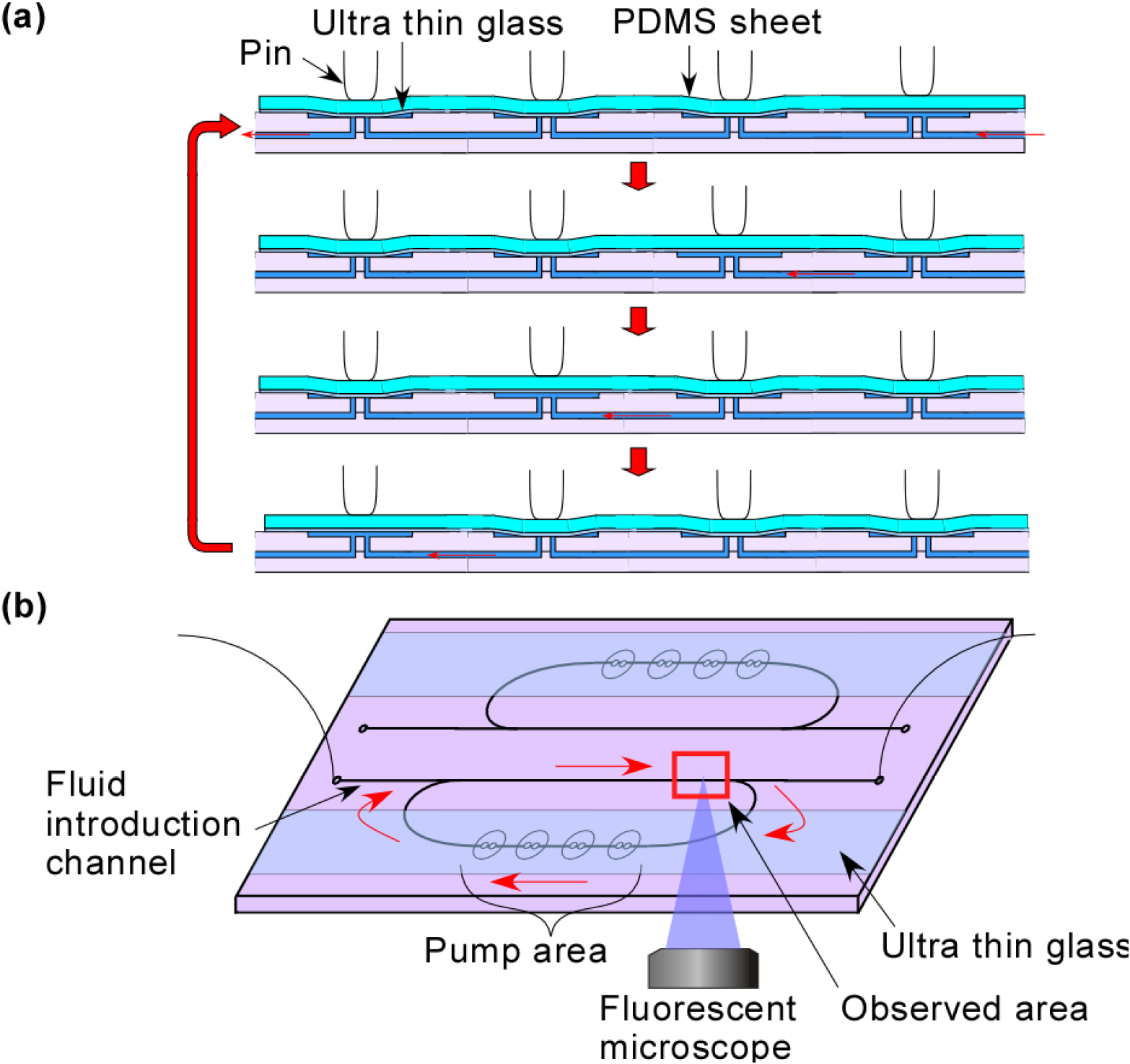
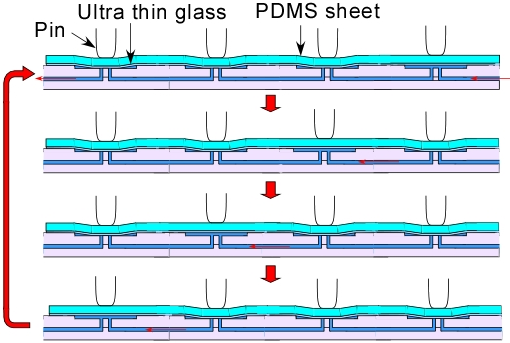
2. Experimental Section
2.1. Materials Preparation
2.2. Fabrication of a Microchip
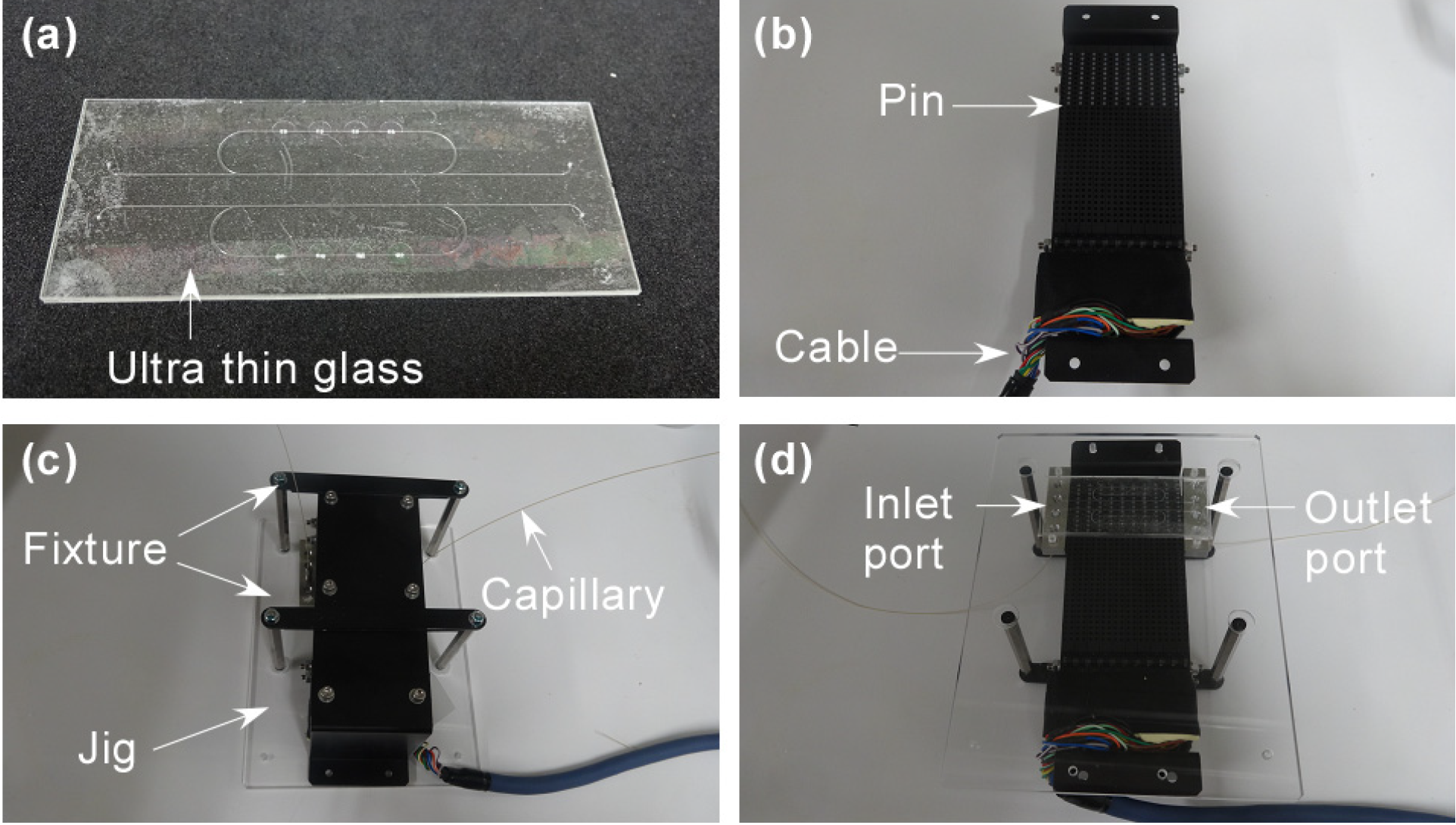
2.3. An Actuator and Software
2.4. Experimental Setting
3. Results and Discussion
3.1. Demonstration of Pumping
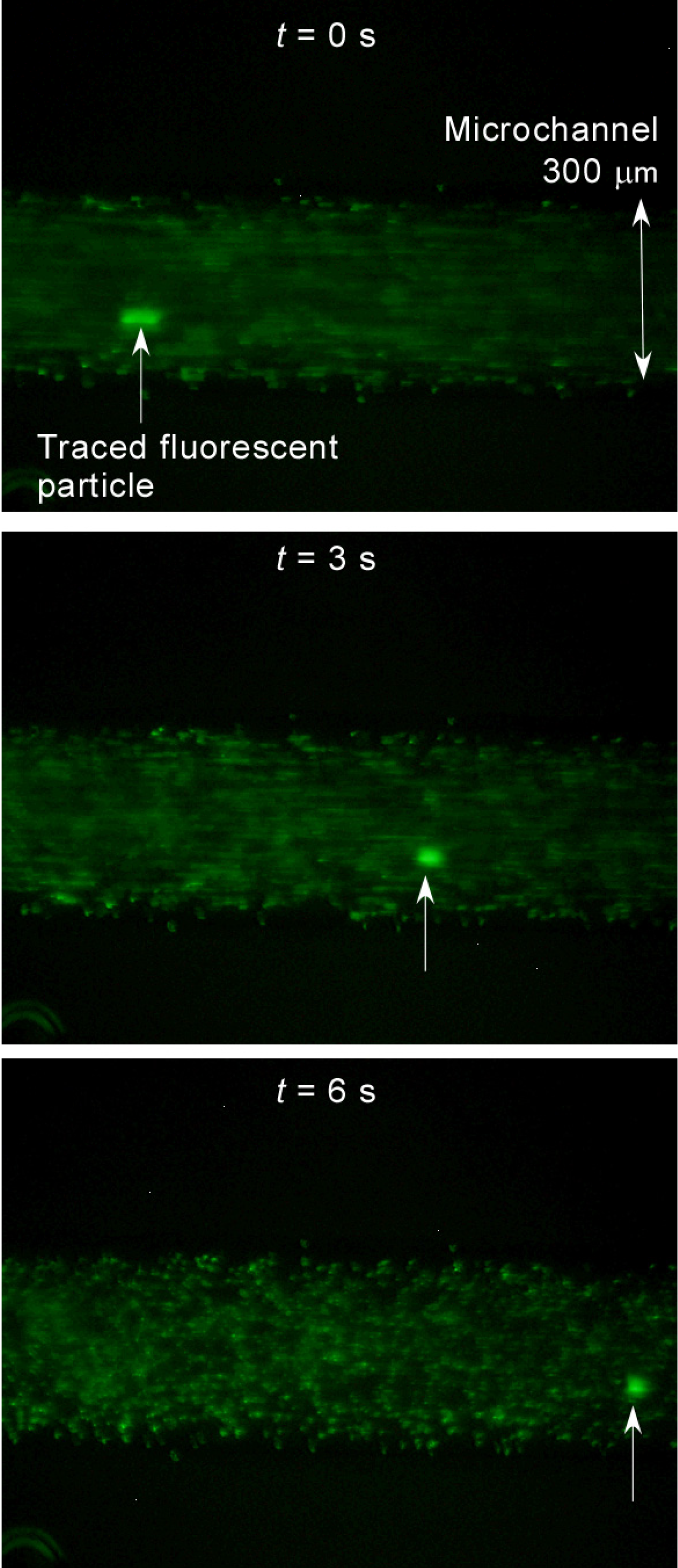
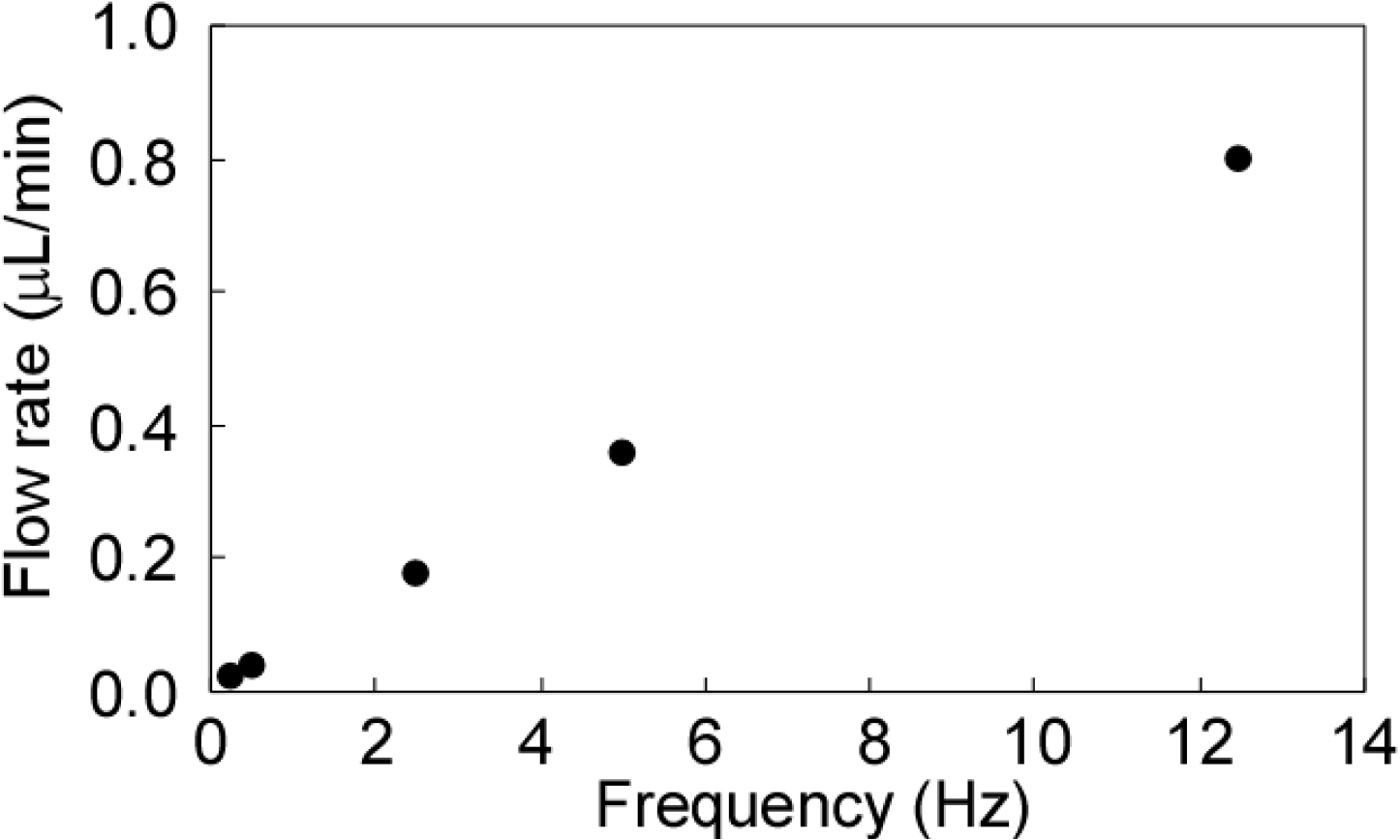
3.2. Dependency on Frequency
3.3. Performance Comparison with Other Micropumps on Chip
| Pump type | Reference | Diaphragm | Sp (mm2) | Qmax (µL/min) | fsp (/min) |
|---|---|---|---|---|---|
| Peristaltic | This study | Glass (Ultra thin) | 1.4 | 0.80 | 0.6 |
| Piezoelectric diaphragm | [6] | Glass | 4100 | 8.0 | 0.0002 |
| [7] | Metal (Brass) | 2500 | 16 | 0.0006 | |
| [8] | Silicon | 98 | 160 | 2 | |
| Capillary | [35] | - | 0.30 | 0.026 | 0.09 |
| Electroosmotic | [36] | - | 0.011 | 0.0054 | 0.5 |
| Bubble | [37] | - | 0.039 | 4.5 | 100 |
| Peristaltic | [43] | Glass | 4000 | 40 | 0.01 |
| [44] | Glass | 1500 | 100 | 0.07 | |
| [39] | Elastomer (PDMS) | 3.5 | 7 | 2 | |
| [15] | Elastomer (PDMS) | 0.11 | 0.6 | 5 | |
| [11] | Elastomer | 0.001 | 1.4 | 100 |
3.4. Confirmation of Durability against Organic Solvents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kovarik, M.L.; Ornoff, D.M.; Melvin, A.T.; Dobes, N.C.; Wang, Y.; Dickinson, A.J.; Gach, P.C.; Shah, P.K.; Allbritton, N.L. Micro total analysis systems: fundamental advances and applications in the laboratory, clinic, and field. Anal. Chem. 2013, 85, 451–472. [Google Scholar] [CrossRef]
- Kitamori, T.; Tokeshi, M.; Hibara, A.; Sato, K. Thermal lens microscopy and microchip chemistry. Anal. Chem. 2004, 76, 52A–60A. [Google Scholar]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. Biological cells on microchips: New technologies and applications. Biosens. Bioelectron. 2007, 23, 449–458. [Google Scholar] [CrossRef]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35–R64. [Google Scholar] [CrossRef]
- Au, A.K.; Lai, H.; Utela, B.R.; Folch, A. Microvalves and micropumps for BioMEMS. Micromachines 2011, 2, 179–220. [Google Scholar] [CrossRef]
- Van Lintel, H.T.G.; van de Pol, F.C.M.; Bouwstra, S. A piezoelectric micropump based on micromachining of silicon. Sens. Actuators 1988, 15, 153–167. [Google Scholar] [CrossRef]
- Stemme, E.; Stemme, G. A valveless diffuser/nozzle-based fluid pump. Sens. Actuators A 1993, 39, 159–167. [Google Scholar] [CrossRef]
- Zengerle, R.; Ulrich, J.; Kluge, S.; Richter, M.; Richter, A. A bidirectional silicon micropump. Sens. Actuators A 1995, 50, 81–86. [Google Scholar] [CrossRef]
- Loverich, J.J.; Kanno, I.; Kotera, H. Concepts for a new class of all-polymer micropumps. Lab Chip 2006, 6, 1147–1154. [Google Scholar] [CrossRef]
- Xia, F.; Tadigadapa, S.; Zhang, Q.M. Electroactive polymer based microfluidic pump. Sens. Actuators A Phys. 2006, 125, 346–352. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scerer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2007, 288, 113–116. [Google Scholar]
- Quake, S.R.; Scherer, A. From micro- to nanofabrication with soft materials. Science 2000, 290, 1536–1540. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Melin, J.; Quake, S.R. Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 213–231. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef]
- Song, J.W.; Gu, W.; Futai, N.; Warner, K.A.; Nor, J.E.; Takayama, S. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 2005, 77, 3993–3999. [Google Scholar]
- Hisamoto, H.; Shimizu, Y.; Uchiyama, K.; Tokeshi, M.; Kikutani, Y.; Hibara, A.; Kitamori, T. Chemicofunctional membrane for integrated chemical processes on a microchip. Anal. Chem. 2003, 75, 350–354. [Google Scholar] [CrossRef]
- Hiki, S.; Mawatari, K.; Aota, A.; Saito, M.; Kitamori, T. Sensitive gas analysis system on a microchip and application for on-site monitoring of nh3 in a clean room. Anal. Chem. 2011, 83, 5017–5022. [Google Scholar] [CrossRef]
- Jang, K.; Sato, K.; Tanaka, Y.; Xu, Y.; Sato, M.; Nakajima, T.; Mawatari, K.; Konno, T.; Ishihara, K.; Kitamori, T. An efficient surface modification using 2-methacryloyloxyethyl phosphorylcholine to control cell attachment via photochemical reaction in a microchannel. Lab Chip 2010, 10, 1937–1945. [Google Scholar] [CrossRef]
- Shang, F.; Guihen, E.; Glennon, J.D. Recent advances in miniaturisation—The role of microchip electrophoresis in clinical analysis. Electrophoresis 2012, 33, 105–116. [Google Scholar] [CrossRef]
- Yasui, T.; Kaji, N.; Mohamadi, M.R.; Okamoto, Y.; Tokeshi, M.; Horiike, Y.; Baba, Y. Electroosmotic flow in microchannels with nanostructures. ACS Nano 2011, 5, 7775–7780. [Google Scholar] [CrossRef]
- Kaji, N.; Okamoto, Y.; Tokeshi, M.; Baba, Y. Nanopillar, nanoball, and nanofibers for highly efficient analysis of biomolecules. Chem. Soc. Rev. 2010, 39, 948–956. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab Chip 2012, 12, 3576–3589. [Google Scholar] [CrossRef]
- Yıldırım, E.; Sahir Arıkan, M.A.; Külah, H. A normally closed electrostatic parylene microvalve for micro total analysis systems. Sens. Actuators A Phys. 2012, 181, 81–86. [Google Scholar] [CrossRef]
- Rolland, J.P.; van Dam, R.M.; Schorzman, D.A.; Quake, S.R.; DeSimone, J.M. Solvent-resistant photocurable “liquid Teflon” for microfluidic device fabrication. J. Am. Chem. Soc. 2004, 126, 2322–2323. [Google Scholar] [CrossRef]
- Dziuban, J.A.; Mroz, J.; Szczygielska, M.; Malachowski, M.; Gorecka-Drzazga, A.; Walczak, R.; Bula, W.; Zalewski, D.; Nieradko, L.; Lysko, J.; et al. Portable gas chromatograph with integrated components. Sens. Actuators A Phys. 2004, 115, 318–330. [Google Scholar] [CrossRef]
- Abate, A.R.; Lee, D.; Do, T.; Holtze, C.; Weitz, D.A. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip 2008, 8, 516–518. [Google Scholar]
- Kikutani, Y.; Mawatari, K.; Hibara, A.; Kitamori, T. Circulation microchannel for liquid–liquid microextraction. Microchim. Acta 2009, 164, 241–247. [Google Scholar] [CrossRef]
- Fenn, P.M.; Fisk, J.T.; Rhoads, R.L.; Rhoads, R.O. Defect Control in the Making of Sheet Glass by the Fusion Process. U.S. Patent 7,386,999, 17 June 2008. [Google Scholar]
- Fujiwara, K. Ultra thin glass. New Glass 2009, 24, 1033–1036. [Google Scholar]
- Tanaka, Y. Electric actuating valves incorporated into an all glass-based microchip exploiting the flexibility of ultra thin glass. RSC Adv. 2013, 3, 10213–10220. [Google Scholar] [CrossRef]
- Tanaka, Y.; Xi, H.; Sato, K; Mawatari, K.; Renberg, B.; Nilsson, M.; Kitamori, T. Single-molecule DNA patterning and detection by padlock probing and rolling circle amplification in microchannels for analysis of small sample volumes. Anal. Chem. 2011, 82, 3352–3357. [Google Scholar]
- Jang, K.; Xu, Y.; Sato, K.; Tanaka, Y.; Mawatari, K.; Kitamori, T. Micropatterning of biomolecules on a glass substrate in fused silica microchannels by using photolabile linker-based surface activation. Microchim. Acta 2012, 179, 49–55. [Google Scholar] [CrossRef]
- Jang, K.; Xu, Y.; Tanaka, Y.; Sato, K.; Mawatari, K.; Konno, T.; Ishihara, K.; Kitamori, T. Single-cell attachment and culture method using a photochemical reaction in a closed microfluidic system. Biomicrofluidics 2010, 4, 032208. [Google Scholar] [CrossRef]
- Zimmermann, M.; Schmid, H.; Hunziker, P.; Delamarche, E. Capillary pumps for autonomous capillary systems. Lab Chip 2007, 7, 119–125. [Google Scholar] [CrossRef]
- McKnight, T.E.; Culbertson, C.T.; Jacobson, S.C.; Ramsey, J.M. Electroosmotically induced hydraulic pumping with integrated electrodes on microfluidic devices. Anal. Chem. 2001, 73, 4045–4049. [Google Scholar]
- Tsai, J.H.; Lin, L. A thermal-bubble-actuated micronozzle-diffuser pump. J. Microelectromech. Syst. 2002, 11, 665–671. [Google Scholar] [CrossRef]
- Tanaka, Y. Totally Glass-Based Microchips with Valves and Pumps Using Flexibility of Ultra Thin Glass. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences (microTAS), Freiburg, Germany, 27–31 October 2013; pp. 1475–1477.
- Berg, J.M.; Anderson, R.; Anaya, M.; Lahlouh, B.; Holtz, M.; Dallas, T. A two-stage discrete peristaltic micropump. Sens. Actuators A 2003, 104, 6–10. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yanagisawa, Y.; Kitamori, T. Fluid actuation for a bio-micropump powered by previously frozen cardiomyocytes directly seeded on a diagonally stretched thin membrane. Sens. Actuators B Chem. 2011, 156, 494–498. [Google Scholar] [CrossRef]
- Goto, M.; Sato, K.; Murakami, A.; Tokeshi, M.; Kitamori, T. Development of a microchip-based bioassay system using cultured cells. Anal. Chem. 2005, 77, 2125–2131. [Google Scholar] [CrossRef]
- Sato, K.; Tokeshi, M.; Odake, T.; Kimura, H.; Ooi, T.; Nakao, M.; Kitamori, T. Integration of an immunosorbent assay system: analysis of secretory human immunoglobulin A on polystyrene beads in a microchip. Anal. Chem. 2000, 72, 1144–1147. [Google Scholar] [CrossRef]
- Shoji, S.; Nakagawa, S.; Esashi, M. Micropump and sample-injector for integrated chemical analyzing systems. Sens. Actuators A 1990, 21, 189–192. [Google Scholar] [CrossRef]
- Smits, J.G. Piezoelectric micropump with 3 valves working peristaltically. Sens. Actuators A 1990, 21, 203–206. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, Y. A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators. Micromachines 2014, 5, 289-299. https://doi.org/10.3390/mi5020289
Tanaka Y. A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators. Micromachines. 2014; 5(2):289-299. https://doi.org/10.3390/mi5020289
Chicago/Turabian StyleTanaka, Yo. 2014. "A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators" Micromachines 5, no. 2: 289-299. https://doi.org/10.3390/mi5020289
APA StyleTanaka, Y. (2014). A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators. Micromachines, 5(2), 289-299. https://doi.org/10.3390/mi5020289