Polyurethane-Based Electronic Packaging: The Characterization of Natural Aging over a Decade
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.3. Characterization
3. Results and Discussion
3.1. Appearance Inspection and Electrical Properties Test of Electronic Components
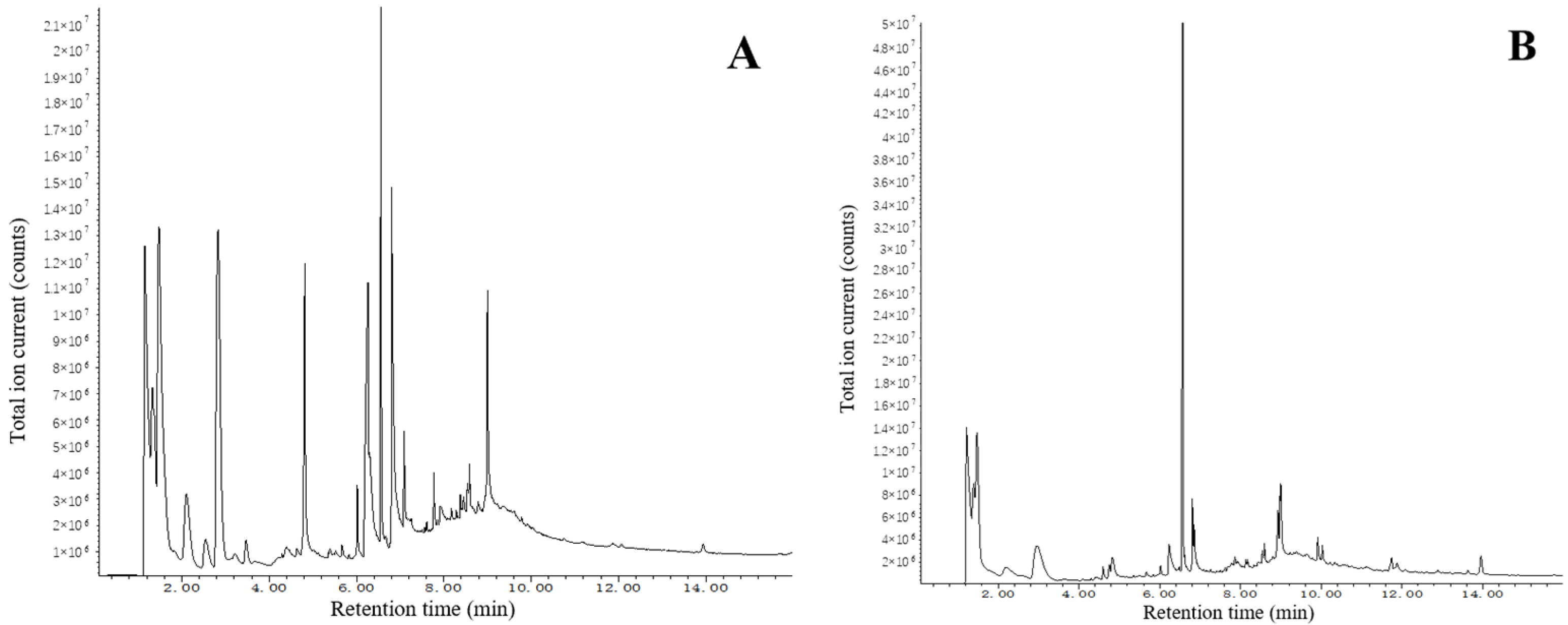
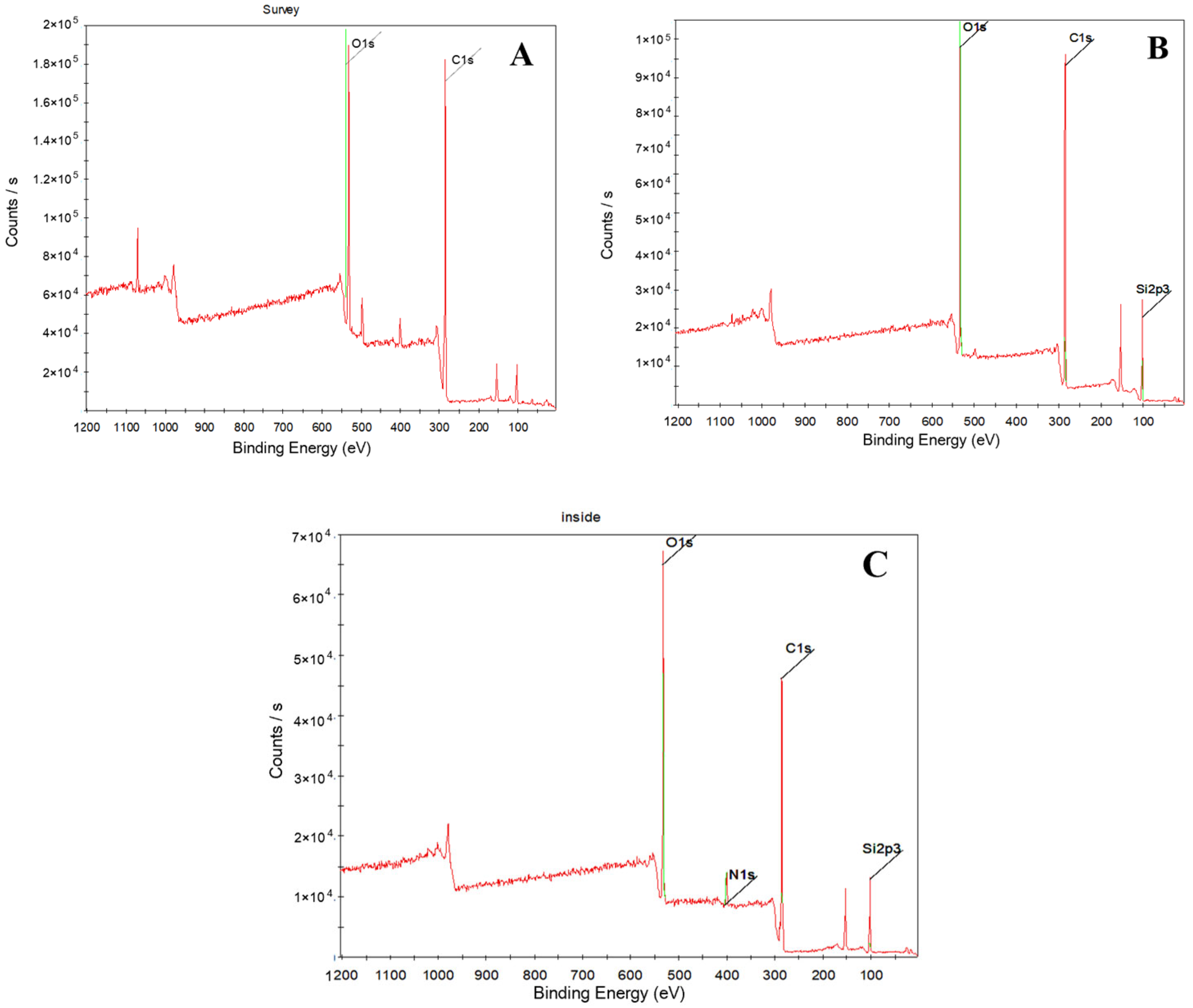
3.2. Elemental Content Analysis
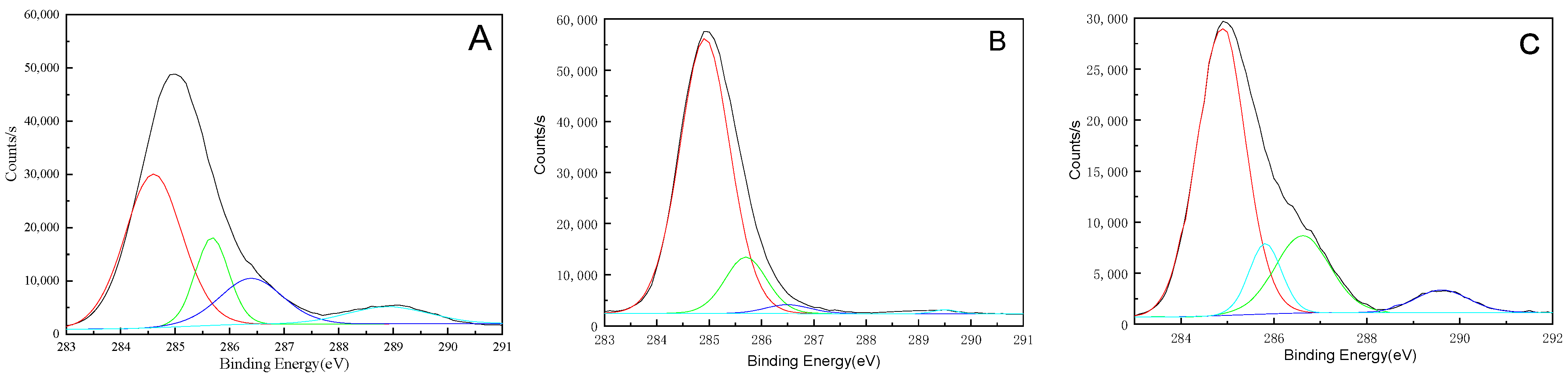
3.3. Chemical Functional Groups Analysis
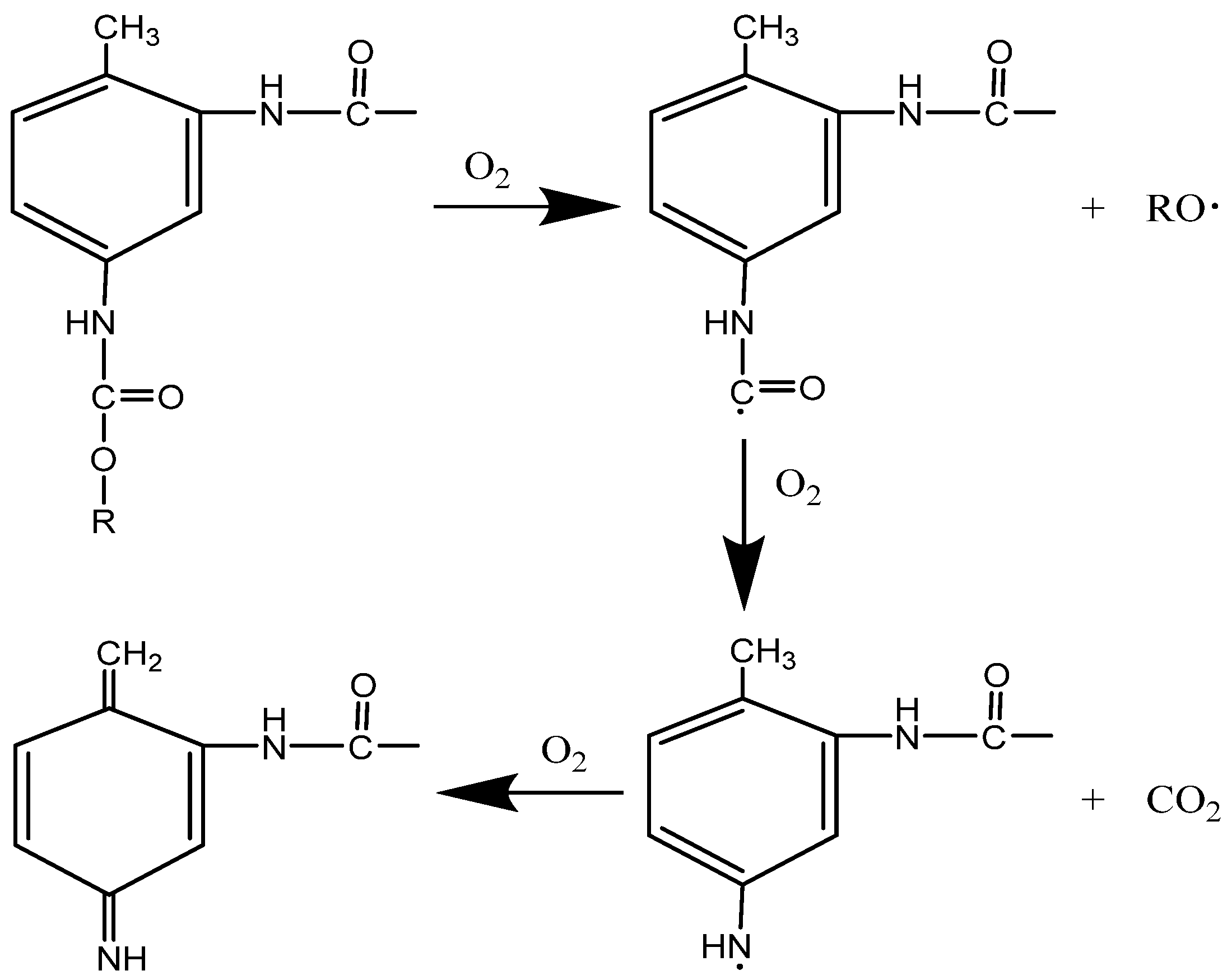
3.4. Aging Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Luo, S.; Zhuo, Q.; Liang, Y.; Li, Y.; Dong, H.; Qin, L.; Li, Y. A comprehensive study on the degradation behavior and mechanism of expanded thermoplastic polyurethane. Polymers 2025, 17, 1033. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-GC/MS and HS SPME-GC/MS. J. Chromatogr. A 2011, 1218, 4498–4508. [Google Scholar] [CrossRef]
- Hu, J.; Huang, M.; Zhou, X.; Luo, R.; Li, L.; Li, X. Research Status of Lignin-Based Polyurethane and Its Application in Flexible Electronics. Polymers 2024, 16, 2340. [Google Scholar] [CrossRef]
- Ji, F.L.; Hu, J.L. Comparison of Shape Memory Polyurethanes and Polyurethane-Ureas Having Crystalline Reversible Phase. High Perform. Polym. 2011, 23, 314–325. [Google Scholar] [CrossRef]
- Liu, Y.L.; He, J.Y.; Yang, R.J. The Synthesis of Melamine-Based Polyether Polyol and Its Effects on the Flame Retardancy and Physical-Mechanical Property of Rigid Polyurethane Foam. J. Mater. Sci. 2017, 52, 4700–4712. [Google Scholar] [CrossRef]
- Miraftab, R.; Ramezanzadeh, B.; Bahlakeh, G.; Mahdavian, M. An Advanced Approach for Fabricating a Reduced Graphene Oxide-AZO Dye/Polyurethane Composite with Enhanced Ultraviolet (UV) Shielding Properties: Experimental and First-Principles QM Modeling. Chem. Eng. J. 2017, 321, 159–174. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Lysenko, Z.; Brune, D.A.; Wendt, B.L.; Schrock, A.K. Enhancing Polyurethane Properties via Soft Segment Crystallization. Polymer 2005, 46, 10158–10166. [Google Scholar] [CrossRef]
- Feldman, D. Polymer Weathering: Photo-Oxidation. J. Polym. Environ. 2002, 10, 163–173. [Google Scholar] [CrossRef]
- Liu, L.H.; Hu, X.H. Marine Environmental Effect of a Few Encapsulating Materials in Xisha. Equip. Environ. Eng. 2017, 14, 55–64. [Google Scholar]
- Middleton, J.; Burks, B.; Wells, T.; Setters, A.M.; Jasiuk, I.; Predecki, P.; Hoffman, J.; Kumosa, M. The Effect of Ozone and High Temperature on Polymer Degradation in Polymer Core Composite Conductors. Polym. Degrad. Stab. 2013, 98, 2282–2290. [Google Scholar] [CrossRef]
- Ravari, F.; Omrani, A.; Rostami, A.A.; Ehsani, M. Ageing Effects on Electrical, Morphological, and Mechanical Properties of a Low Viscosity Epoxy Nanocomposite. Polym. Degrad. Stab. 2012, 97, 929–935. [Google Scholar] [CrossRef]
- Rabea, A.M.; Mohseni, M.; Mirabedini, S.M.; Tabatabaei, M.H. Surface Analysis and Anti-Graffiti Behavior of a Weathered Polyurethane-Based Coating Embedded with Hydrophobic Silica. Appl. Surf. Sci. 2012, 258, 4391–4396. [Google Scholar] [CrossRef]
- Rossi, S.; Fedel, M.; Petrolli, S.; Deflorian, F. Accelerated Weathering and Chemical Resistance of Polyurethane Powder Coatings. J. Coat. Technol. Res. 2016, 13, 427–437. [Google Scholar] [CrossRef]
- Tuwair, H.; Volz, J.; Elgawady, M.; Mohamed, M.; Chandrashekhara, K.; Birman, V. Behavior of GFRP Bridge Deck Panels Infilled with Polyurethane Foam Under Various Environmental Exposure. Structures 2016, 5, 141–151. [Google Scholar] [CrossRef]
- Yang, X.F.; Vang, C.; Tallman, D.E.; Bierwagen, G.P.; Croll, S.G.; Rohlik, S. Weathering Degradation of a Polyurethane Coating. Polym. Degrad. Stab. 2001, 74, 341–351. [Google Scholar] [CrossRef]
- Yang, X.F.; Tallman, D.E.; Bierwagen, G.P.; Croll, S.G.; Rohlik, S. Blistering and Degradation of Polyurethane Coatings Under Different Accelerated Weathering Tests. Polym. Degrad. Stab. 2002, 77, 103–109. [Google Scholar] [CrossRef]
- Bauer, D.R. Interpreting Weathering Acceleration Factors for Automotive Coatings Using Exposure Models. Polym. Degrad. Stab. 2000, 69, 307–316. [Google Scholar] [CrossRef]
- Han, H.; Yan, H.; Wang, X.; Zhang, K.; Huang, J.; Sun, Y.; Liu, J.; Verlinden, P.J.; Altermatt, P.; Liang, Z.; et al. Analysis of the degradation of encapsulant materials used in photovoltaic modules exposed to different climates in China. Sol. Energy 2019, 194, 177–188. [Google Scholar] [CrossRef]
- ASTM D1729; Standard Practice for Visual Appraisal of Colors and Color Differences of Diffusely-Illuminated Opaque Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Sandten, C.S.; Kreyenschmidt, M.; Albach, R.; Fittschen, U.E.A. The Thermo-Oxidative Degradation of Polyurethane Open-Cell Soft Foam Investigated Through Gas Chromatography and Mass Spectrometry of Volatile Organic Compounds. Polymers 2024, 16, 3342. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Jia, W.; Zhang, F. Analysis of the aging mechanism and life evaluation of elastomers in simulated proton exchange membrane fuel cell environments. e-Polymers 2021, 21, 921–929. [Google Scholar] [CrossRef]
- Blaga, A. Deterioration mechanisms in weathering of plastic materials. In Durability of Building Materials and Components; Sereda, P.J., Litvan, G.G., Eds.; ASTM STP 691; ASTM International: West Conshohocken, PA, USA, 1980; pp. 827–837. [Google Scholar]
- Shaafaey, M.; Bahrololoumi, A.; Mohammadi, H.; Alazhary, S.; Dargazany, R. Investigation of Hygrothermal Aging on Polyurethane-Based (PUB) Adhesive: Substantiating Competition Scenario Between Sub-Aging Thermo-Oxidation and Hydrolytic Phenomena. J. Polym. Res. 2021, 28, 453. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gardette, J.L. Infrared Analysis of the Photochemical Behavior of Segmented Polyurethanes: 1. Aliphatic Poly (Ester-Urethane). Polymer 1997, 38, 4019–4031. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-Change and Yellowing of Polyurethane as a Result of UV Irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- GB/T 6379.2-2004; Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. Standardization Administration of China (SAC): Beijing, China, 2004.
- Fang, D.; He, F.; Xie, J.; Yu, J.; Hu, Y. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Karroum, H.; Chenakin, S.; Alekseev, S.; Iablokov, V.; Xiang, Y.; Dubois, V.; Kruse, N. Terminal Amines, Nitriles, and Olefins through Catalytic CO Hydrogenation in the Presence of Ammonia. ACS Catal. 2021, 11, 9566–9577. [Google Scholar] [CrossRef]
- Oliveira, M.C.L.; Antunes, R.A.; Costa, I. Effect of the NCO/OH Molar Ratio on the Physical Aging and on the Electrochemical Behavior of Polyurethane-Urea Hybrid Coatings. Int. J. Electrochem. Sci. 2013, 8, 4679–4689. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 202, 43–52. [Google Scholar] [CrossRef]
- Mayer-Trzaskowska, P.; Robakowska, M.; Gierz, Ł.; Pach, J.; Mazur, E. Observation of the Effect of Aging on the Structural Changes of Polyurethane/Polyurea Coatings. Polymers 2024, 16, 23. [Google Scholar] [CrossRef]
- Johannes, C.; Hartung, M.; Heim, H.P. Weathering of a Polyurethane-Based Gel Electrolyte. Polymers 2023, 15, 1448. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Wiśniewski, M.; Kowalska, J. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Schmitz, V.; Lublóy, É.; Gyurkó, Z. Evaluation of the Composition, Thermal and Mechanical Behavior, and Color Changes of Artificially and Naturally Aged Polymers for the Conservation of Stained Glass Windows. Polymers 2023, 15, 2595. [Google Scholar] [CrossRef] [PubMed]


| Detection Site | Information Depth Range | |||
|---|---|---|---|---|
| XPS | ATR-FTIR | Py-GC/MS | ||
| Surface sampling depth | Outermost | Outside to the near surface atomic layer: 5–10 nm | 0.5–3 μm | Outer surface sample |
| Inner sampling depth | Near-component inner | The inner surface; depth: 5–10 nm | 0.5–3 μm | Inner sample |
| Parameter | Leak (Pa·m3/s) | IR (GΩ) | I_l (A) | T_d (s) | I_sb (mA) | V_a (V) | I_max (mA) |
|---|---|---|---|---|---|---|---|
| Requirement | 1.0 × 10−5 | ≥0.045 | ≥0.5 | 0.4~1 s | ≤50 | ≤0.8 | ≤250 |
| Sample 1 (Original) | 8.1 × 10−7 | 99 GΩ | 0.7 | 0.66 | 30.7 | 0.46 | 148.3 |
| Sample 2 (Original) | 8.6 × 10−7 | 99 GΩ | 0.7 | 0.68 | 30 | 0.45 | 146.9 |
| Sample 3 (Original) | 7.3 × 10−7 | 99 GΩ | 0.7 | 0.68 | 29.2 | 0.44 | 146.9 |
| Sample 4 (Original) | 7.5 × 10−7 | 99 GΩ | 0.8 | 0.65 | 30.1 | 0.47 | 148.4 |
| Sample 5 (Original) | 6.9 × 10−7 | 99 GΩ | 0.7 | 0.65 | 30.9 | 0.44 | 147.5 |
| Mean | 7.68 × 10−7 | 99 GΩ | 0.72 | 0.664 | 30.18 | 0.452 | 147.6 |
| Std Dev (S) | 6.01 × 10−8 | 0 | 0.04 | 0.0136 | 0.5980 | 0.0117 | 0.6512 |
| CV | 7.83% | 0.00% | 5.56% | 2.04% | 1.98% | 2.58% | 0.44% |
| Sample 1 (10 years) | 3.8 × 10−6 | 99 | 0.7 | 0.64 | 29.8 | 0.43 | 177.5 |
| Sample 2 (10 years) | 3.9 × 10−6 | 99 | 0.7 | 0.68 | 25.4 | 0.47 | 159.2 |
| Sample 3 (10 years) | 4.7 × 10−6 | 99 | 0.6 | 0.67 | 27.9 | 0.40 | 145.6 |
| Sample 4 (10 years) | 4.2 × 10−6 | 99 | 0.8 | 0.59 | 24.3 | 0.41 | 128.2 |
| Sample 5 (10 years) | 3.7 × 10−6 | 99 | 0.6 | 0.60 | 30.9 | 0.45 | 135.7 |
| Mean | 4.1 × 10−6 | 99 | 0.68 | 0.636 | 27.66 | 0.432 | 149.24 |
| Std Dev (S) | 0.0000004 | 0 | 0.0748 | 0.0361 | 2.5113 | 0.0256 | 17.5334 |
| CV | 9.94% | 0% | 11% | 5.68% | 9.08% | 5.93% | 11.75% |
| Component | Toluene Diisocyanate | Phthalimide | 2-Penten-1-ol |
|---|---|---|---|
| Surface | 0.4% | 27.5% | 0.5% |
| Interior | 1.1% | 20.2% | 0.1% |
| Aging Time | Element | C | N | O | Si |
|---|---|---|---|---|---|
| Binding Energy/eV | 285 | 400.4 | 532 | 102.2 | |
| 0 year | Ontology/% | 51.08 | 3.45 | 26.52 | 18.95 |
| 10 years | Interior/% | 58.87 | 3.51 | 22.33 | 15.29 |
| 10 years | Surface/% | 56.73 | 0 | 20.23 | 23.04 |
| Aging Time | Binding Energy/eV | 284.9 | 285.7 | 286.3 | 289.5 |
|---|---|---|---|---|---|
| Belonging Group | CH2-C6H6 | C6H6-NH | O-CH2(CH2)2CH2-O | HN-COO | |
| 0 year | Ontology/% | 56.87 | 16.31 | 18.07 | 8.75 |
| 10 years | Interior/% | 65.04 | 10.31 | 19.16 | 5.49 |
| 10 years | Surface/% | 81.00 | 12.06 | 5.76 | 1.18 |
| Position | 1046 cm−1 | 1708 cm−1 | 1529 cm−1 | 1222 cm−1 | 2953 cm−1 |
|---|---|---|---|---|---|
| C–OH | C=O | NHCOO | C–O–C | CH2 | |
| Interior | 1028 | 1352 | 591 | 444 | 100 |
| Surface | 1067 | 220 | 335 | 517 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, H.; Zhou, R.; Xie, C.; Ning, H. Polyurethane-Based Electronic Packaging: The Characterization of Natural Aging over a Decade. Micromachines 2025, 16, 1061. https://doi.org/10.3390/mi16091061
Wei X, Li H, Zhou R, Xie C, Ning H. Polyurethane-Based Electronic Packaging: The Characterization of Natural Aging over a Decade. Micromachines. 2025; 16(9):1061. https://doi.org/10.3390/mi16091061
Chicago/Turabian StyleWei, Xiaoqin, Han Li, Rui Zhou, Changcheng Xie, and Honglong Ning. 2025. "Polyurethane-Based Electronic Packaging: The Characterization of Natural Aging over a Decade" Micromachines 16, no. 9: 1061. https://doi.org/10.3390/mi16091061
APA StyleWei, X., Li, H., Zhou, R., Xie, C., & Ning, H. (2025). Polyurethane-Based Electronic Packaging: The Characterization of Natural Aging over a Decade. Micromachines, 16(9), 1061. https://doi.org/10.3390/mi16091061






