Investigation into Ultrasonic Oscillation-Assisted Nickel Electroplating onto a Diamond Surface
Abstract
1. Introduction
2. Nickel Electroplating Experiment
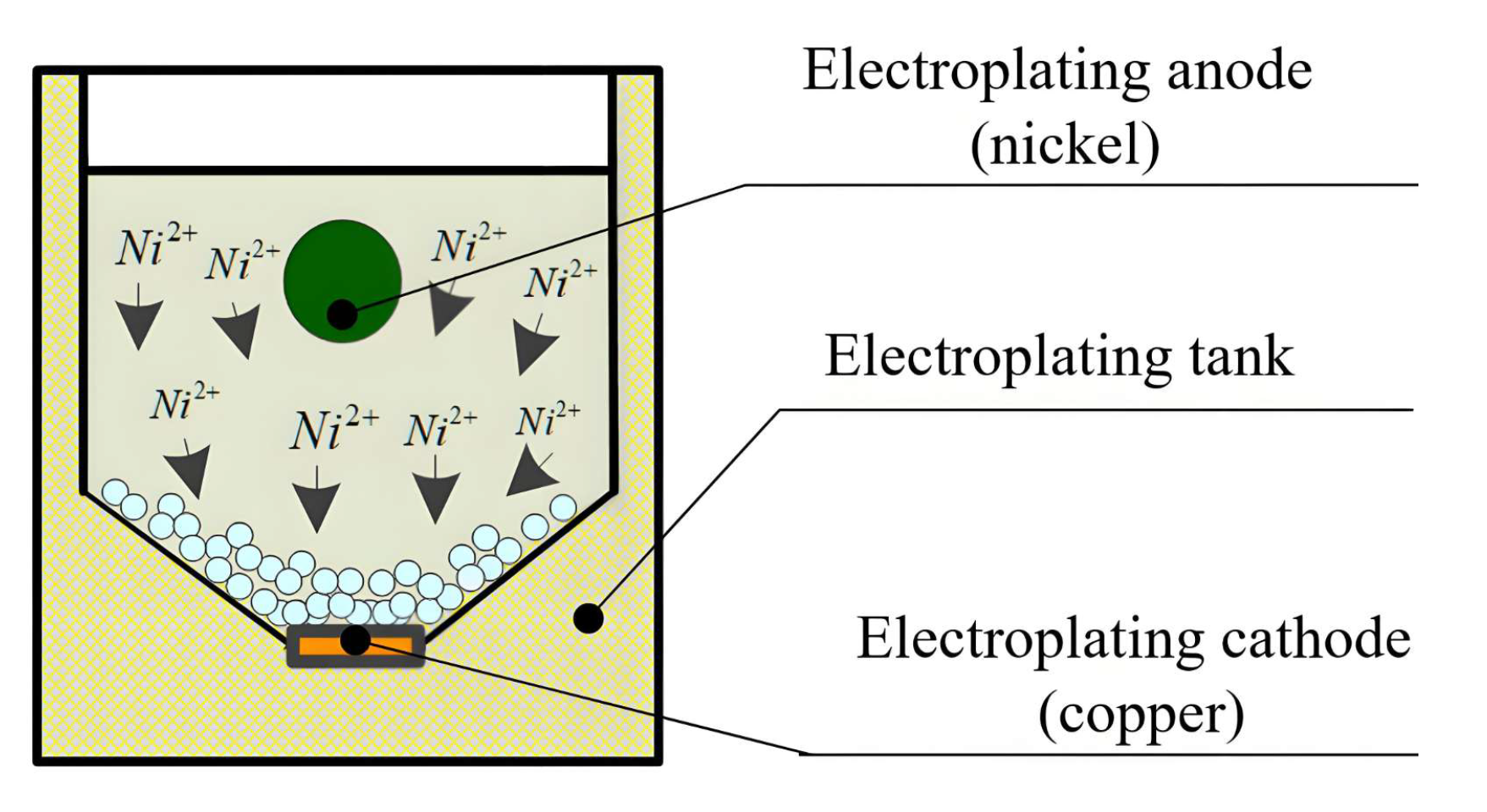
2.1. Mechanism of Ultrasonic Oscillation-Assisted Nickel Electroplating onto the Diamond Surface
2.2. Device of Ultrasonic Oscillation-Assisted Nickel Electroplating on Diamond Surface
2.3. Experiment Scheme
- (1)
- Inject water into the ultrasonic oscillator, and then place the electroplating container on the stainless-steel frame inside the ultrasonic oscillator.
- (2)
- Pour the prepared electroplating solution (the components are shown in Table 2) into the electroplating tank, with the liquid level ensuring immersion of the electroplating anode.
- (3)
- Place a certain quantity of diamond particles that have undergone electroless nickel plating into the electroplating solution. Under the action of gravity, diamond particles accumulate and completely cover the electroplating cathode. Turn on the ultrasonic oscillator and oscillate for 10 min to evenly disperse and fully wet the diamond particles in the electroplating solution.
- (4)
- The anode and the cathode of the electroplating tank are, respectively, connected to the positive pole and negative pole of the electroplating power supply.
- (5)
- Heating and oscillating the water area of the ultrasonic oscillator provides a suitable temperature for the electroplating solution, and causes the diamond particles in the electroplating solution to move, thus forming an ultrasonic oscillation electroplating system.
- (6)
- Adjust the electroplating current, the output power of the ultrasonic oscillator, and the diamond particle size to the electroplate nickel on the diamond surface. After electroplating for a certain duration, take out the diamond treated with nickel electroplating and clean, dry, weigh, and test the coating.
| Component | Medicine | Concentration |
|---|---|---|
| Metal salt | Nickel sulfate (NiSO4) | 250 g/L |
| Activator of the anode | Nickel Chloride (NiCl2) | 40 g/L |
| Buffer agent | Boric acid | 20 g/L |
| Wetting agent | Sodium dodecyl sulfate (SDS) | 0.05 g/L |
3. Results and Discussion
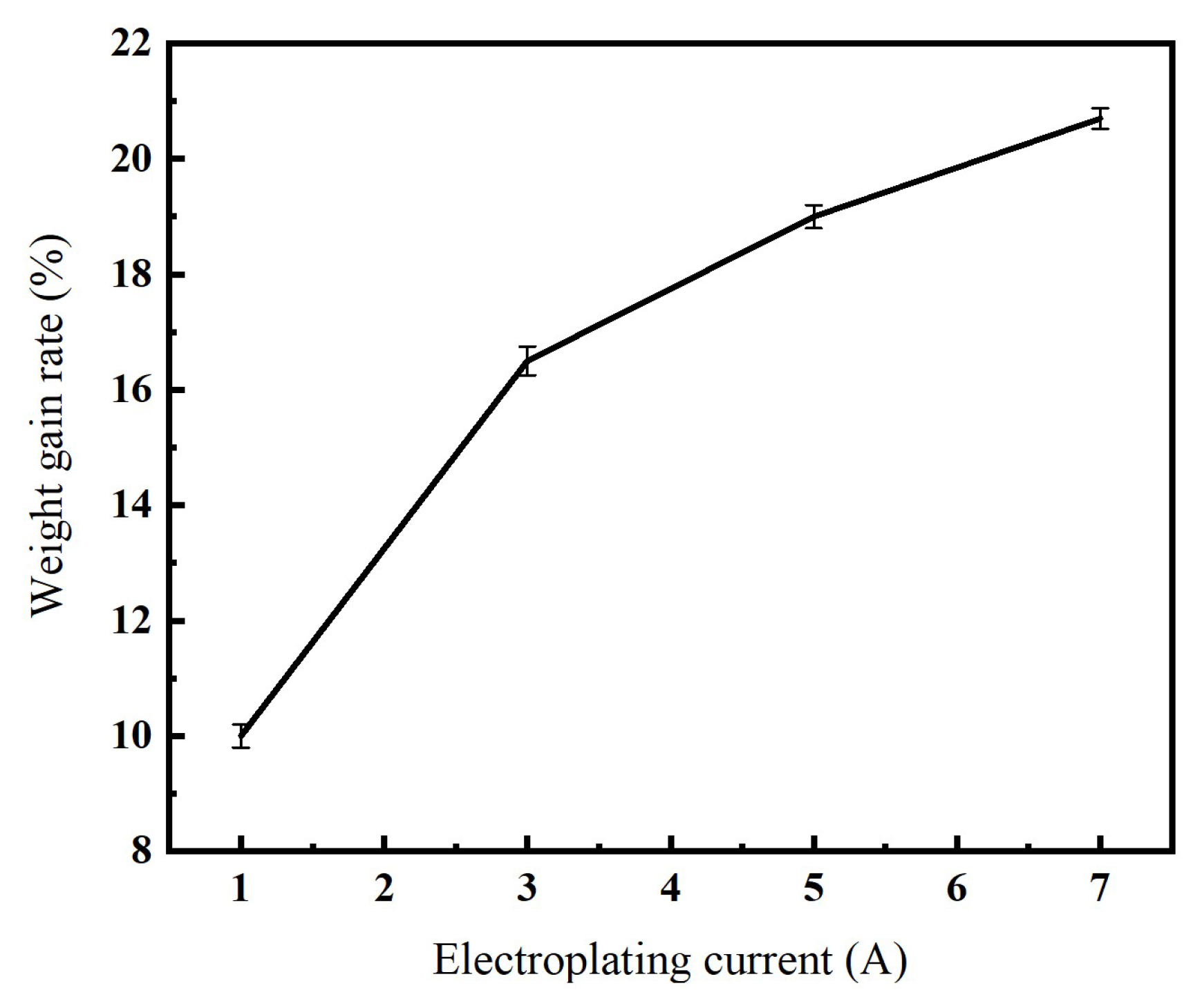
3.1. The Influence of Electroplating Current on Nickel Electroplating onto Diamond Surface
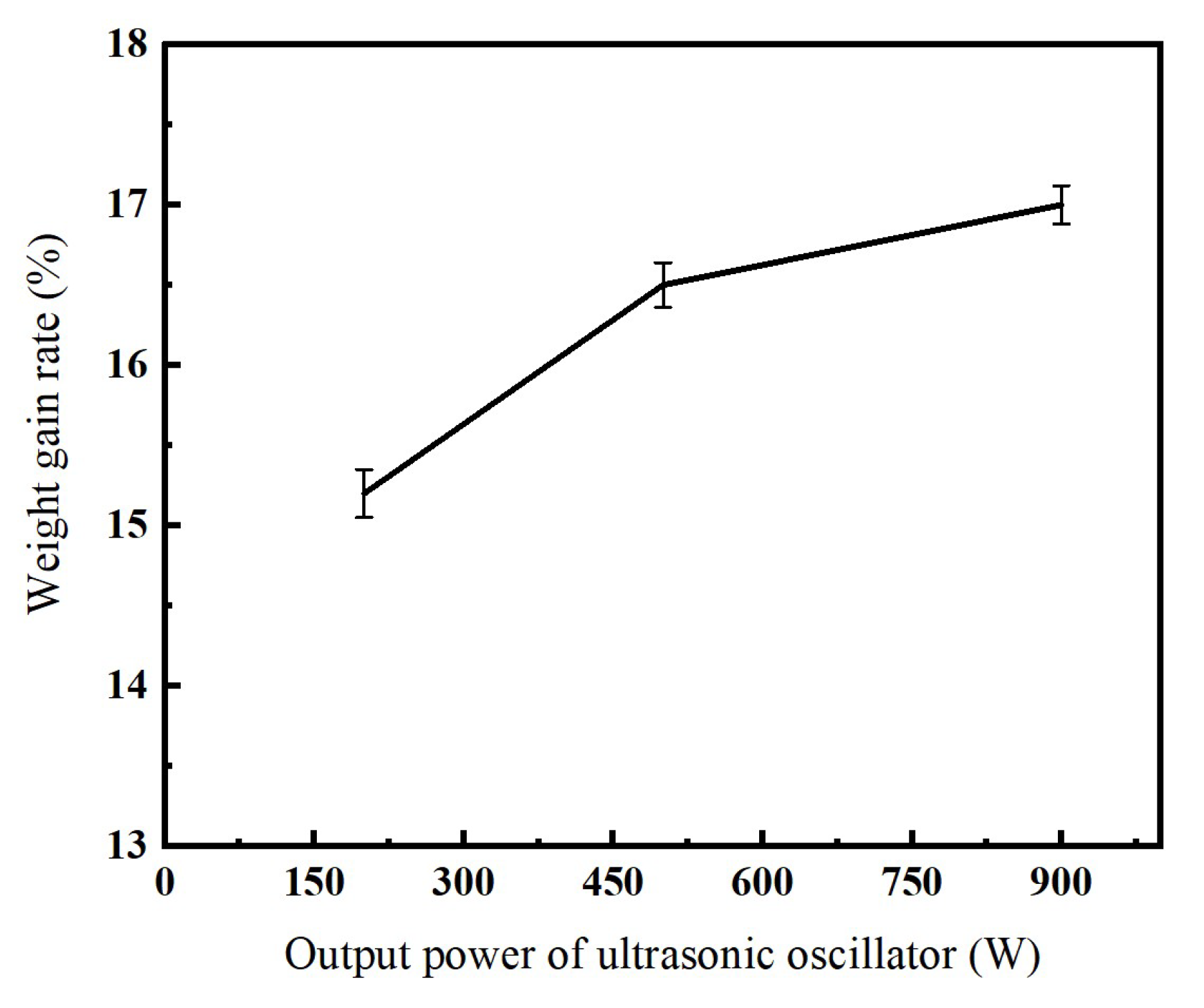
3.2. The Influence of Output Power of an Ultrasonic Oscillator on Nickel Electroplating onto Diamond Surface
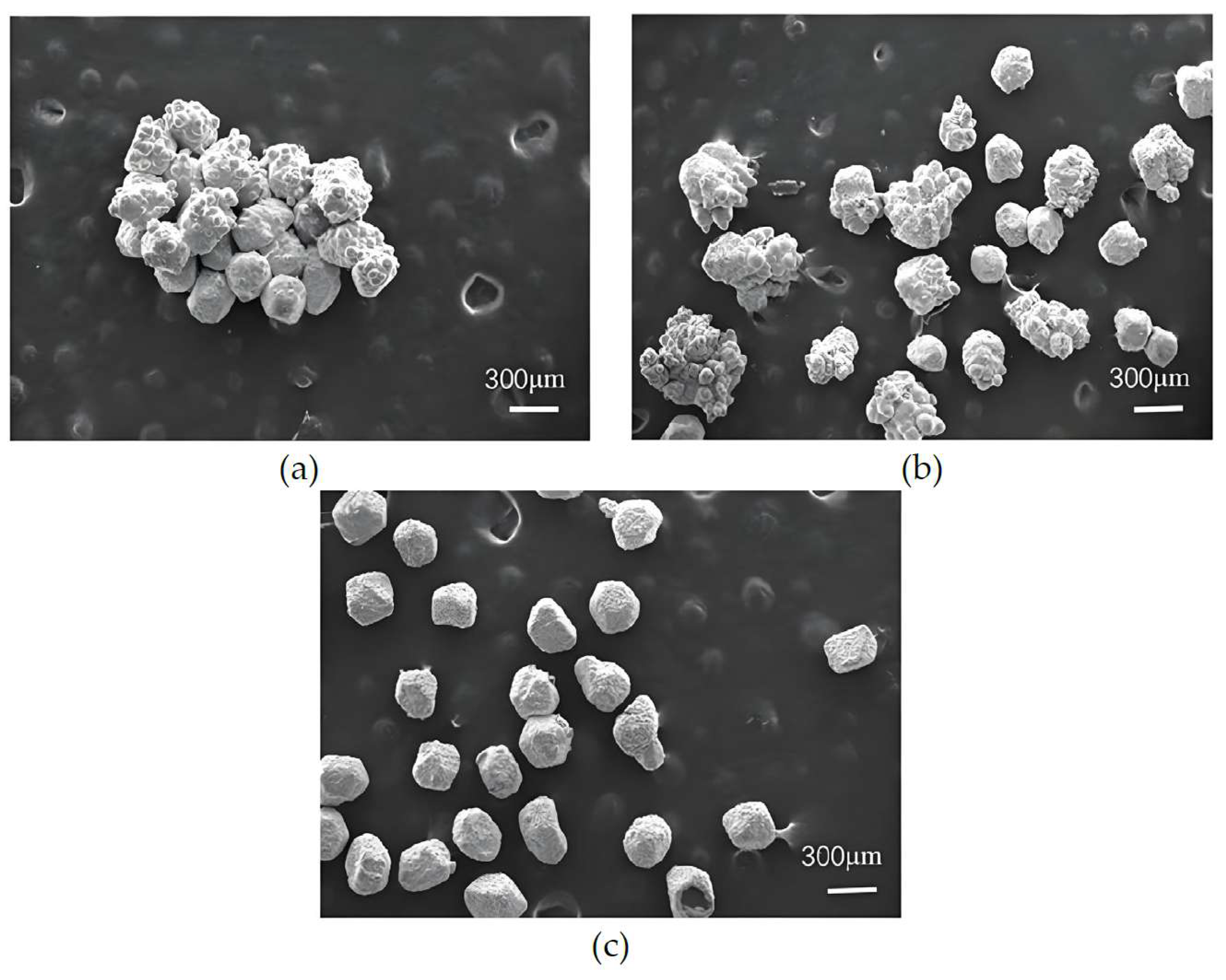
3.3. The Influence of Diamond Particle Size on Nickel Electroplating onto Diamond Surface
4. Conclusions
- (1)
- As the electroplating current increases from 1 A to 7 A, the weight-gain rate on the diamond surface increases correspondingly. However, when the electroplating current reaches 5 A, agglomeration and caking of diamond particles begin to occur.
- (2)
- With the increase in the output power of the ultrasonic oscillator, the plating efficiency of nickel-coated diamonds improves significantly. When the output power is set at 900 W, the highest weight-gain rate is achieved without any signs of particle adhesion and clumping.
- (3)
- An electroplating current of 3 A, an ultrasonic oscillator output power of 900 W, and a diamond particle size of 120/140, nickel-plated diamonds achieve a 20.6% weight-gain rate with uniform, agglomerate-free coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suzuki, T.; Konno, T. Improvement in tool life of electroplated diamond tools by Ni-based carbon nanotube composite coatings. Precis. Eng. 2014, 38, 659–665. [Google Scholar] [CrossRef]
- Tang, H.Q. Wear evolution of metal bond diamond tool in grinding of sapphire. Diam. Relat. Mater. 2023, 137, 110131. [Google Scholar] [CrossRef]
- Zhao, X.J.; Li, J.Y.; Duan, L.C.; Tan, S.C.; Fang, X.H. Effect of Fe-based pre-alloyed powder on the microstructure and holding strength of impregnated diamond bit matrix. Int. J. Refract. Met. Hard Mater. 2019, 79, 115–122. [Google Scholar] [CrossRef]
- Du, Q.B.; Wang, X.X.; Zhang, S.Y.; Long, W.M.; Zhang, L.; Jiu, Y.T.; Yang, C.L.; Zhang, Y.P.; Yang, J. Research status on surface metallization of diamond. Mater. Res. Express 2020, 6, 122005. [Google Scholar] [CrossRef]
- Das, M.K.; Liu, R.; Qin, J.; Zhang, X.; Das, K.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Li, R. Effect of electrodeposition conditions on structure and mechanical properties of Ni-W/diamond composite coatings. Surf. Coat. Technol. 2017, 309, 337–343. [Google Scholar] [CrossRef]
- Kang, Q.P.; He, X.B.; Ren, S.B.; Zhang, L.; Wu, M.; Guo, C.Y.; Cui, W.; Qu, X.H. Preparation of copper–diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Ahn, J.G.; Kim, D.J.; Lee, J.R.; Chung, H.S.; Kim, C.O.; Hai, H.T. Improving the adhesion of electroless-nickel coating layer on diamond powder. Surf. Coat. Technol. 2006, 201, 3793–3796. [Google Scholar] [CrossRef]
- Jia, J.H.; Bai, S.X.; Xiong, D.G.; Xiao, J.; Yan, T.N. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Wei, C.L.; Xu, X.; Wei, B.Z.; Chen, P.Q.; Cheng, J.G. Titanium coating on the surface of diamond particles by a novel rapid low-temperature salt bath plating method. Chem. Phys. Lett. 2020, 761, 138091. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, M.L.; Lai, K.L. Corrosion resistance of TiN/TiAlN-coated ADI by cathodic arc deposition. Mater. Sci. Eng. A 2006, 42, 182–190. [Google Scholar] [CrossRef]
- Chang, W.B.; Yong, K.K.; Yoomin, A.; Yong, H.K. Measurement of the mechanical properties of electroplated gold thin films using micromachined beam structures. Sens. Actuators A 2005, 117, 17–27. [Google Scholar]
- Vasudevan, R.; Devanathan, R.; Chidambaram, K. Effect of ultrasonic agitation during electroplating of nickel and copper at room temperature. Metal Finish. 1992, 90, 23–26. [Google Scholar]
- Prasad, P.B.S.N.V.; Vasudevan, R.; Seshadri, S.K.; Ahila, S. The effect of ultrasonic vibration on nickel electrodeposition. Mater. Lett. 1993, 17, 57–359. [Google Scholar] [CrossRef]
- Jensen, J.A.D.; Pocwiardowski, P.; Persson, P.O.A.; Hultman, L.; Møller, P. Acoustic streaming enhanced electrodeposition of nickel. Chem. Phys. Lett. 2003, 368, 732–737. [Google Scholar] [CrossRef]
- Yu, Y.D.; Wei, G.Y.; Guo, H.F.; Lou, J.W.; Ge, H.L. Study on preparation of NiFeP films by pulse electrodeposition. Surf. Eng. 2012, 28, 30–36. [Google Scholar] [CrossRef]
- Yu, Z.M.; Zhu, Y.K.; Niu, Y.S.; Yang, Y.; Wei, J.; Ding, W.J. Preparation and adhesion performance of multilayered Ni coatings deposited by ultrasonic-assisted electroplating. J. Adhes. Sci. Technol. 2013, 27, 136–142. [Google Scholar] [CrossRef]
- Ban, C.L.; Zhu, S.Q.; Ma, J.; Wang, F.; Jia, Z.F.; Wang, J. Structural, electrochemical and mechanical studies of ultrasonic irradiation enhanced electroplating Ni coating. Anti-Corros. Methods Mater. 2018, 65, 333–339. [Google Scholar] [CrossRef]
- Zhao, Z.; Huo, G.Y.; Li, H.F. Solving the bonding problem of the Ni thin coating with the ultrasonic assisted electrochemical potential activation method. Micromachines 2023, 14, 34. [Google Scholar] [CrossRef]
- Selvam, M. Nickel plating of synthetic diamond particles. Electroplat. Finish. 2011, 30, 1–4. [Google Scholar]
- Zhang, Y.; Cui, Z.M.; Feng, C.C.; Yang, T.B.; Zhang, Y.H.; Xu, B.C. Nickel plating method and process on diamond surface with rotating electrode. Diam. Abras. Eng. 2021, 41, 44–50. [Google Scholar]
- Feng, C.C.; Cui, Z.M.; Zhang, Y.; Yang, T.B. Electroplating technology of suspended diamond particles surface based on rotating electrode. Diam. Relat. Mater. 2022, 128, 109270. [Google Scholar] [CrossRef]
- Sun, G.; Chen, C.; Peng, F.; Wang, J.H.; Zhang, M.G.; Huang, S.; Niu, Q.L. Electrodeposition of Ni and Fe alloy on diamond grains. Surf. Technol. 2007, 36, 49–51. [Google Scholar]
- Fang, L.L.; Xue, L.S. Effect of current on the property of electroplated diamond. Diam. Abras. Eng. 2015, 35, 38–42+54. [Google Scholar]
- Deng, J.H.; Zhang, J.Q.; Tu, Y.B.; Yang, P.X.; An, M.Z.; Wang, P. Effect of BEO in the electrodeposition process of Ni/diamond composite coatings for preparation of ultra-thin dicing blades: Experiments and theoretical calculations. Ceram. Int. 2018, 44, 16828–16836. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Y.W.; Wang, X.L. Preparing a high-particle-content Ni/diamond composite coating with strong abrasive ability. Surf. Coat. Technol. 2013, 235, 489–494. [Google Scholar] [CrossRef]
- Sun, Y.J.; Tan, Y.T.; Zhao, Y.; Teo, A.Z.; Zhou, Y.J.; Wong, J.K.S. Machine learning approach for process optimization of black nickel electroplating. Int. J. Adv. Manuf. Technol. 2024, 135, 4715–4730. [Google Scholar] [CrossRef]
- Wu, M.; Yao, Z.Q.; Verbeke, M.; Karsmakers, P.; Gorissen, B.; Reynaerts, D. Data-driven models with physical interpretability for real-time cavity profile prediction in electrochemical machining processes. Eng. Appl. Artif. Intel. 2025, 160, 111807. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.X.; Ma, K.; Dai, C.N.; Wang, D.Q.; Wang, J.F.; Pan, F.S. Towards development of anticorrosive CaCO3-coated passive layer on Mg alloy with ultrasound-assisted electrodeposition. Corros. Sci. 2023, 224, 111546. [Google Scholar] [CrossRef]
- Guo, J.; Yao, X.L. Preparation of Ni-Co-Fe Alloy Coating on Copper by Ultrasound-assisted Electrodeposition and Its Mechanical Property. Int. J. Electrochem. Sci. 2022, 17, 220740. [Google Scholar] [CrossRef]
- Tudela, I.; Zhang, Y.; Pal, M.; Kerr, I.; Mason, T.J.; Cobley, A.J. Ultrasound-assisted electrodeposition of nickel: Effect of ultrasonic power on the characteristics of thin coatings. Surf. Coat. Technol. 2015, 264, 49–59. [Google Scholar] [CrossRef]


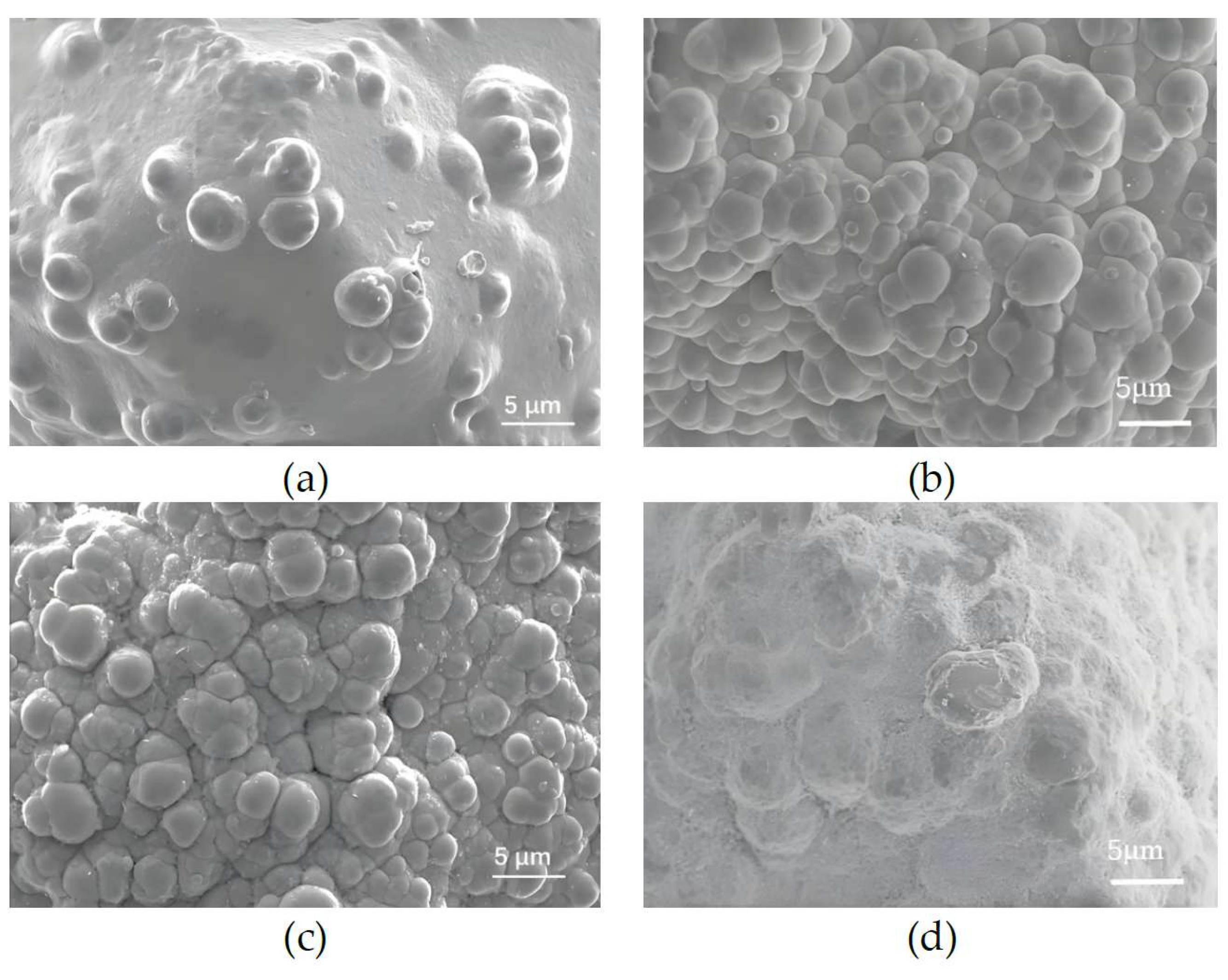

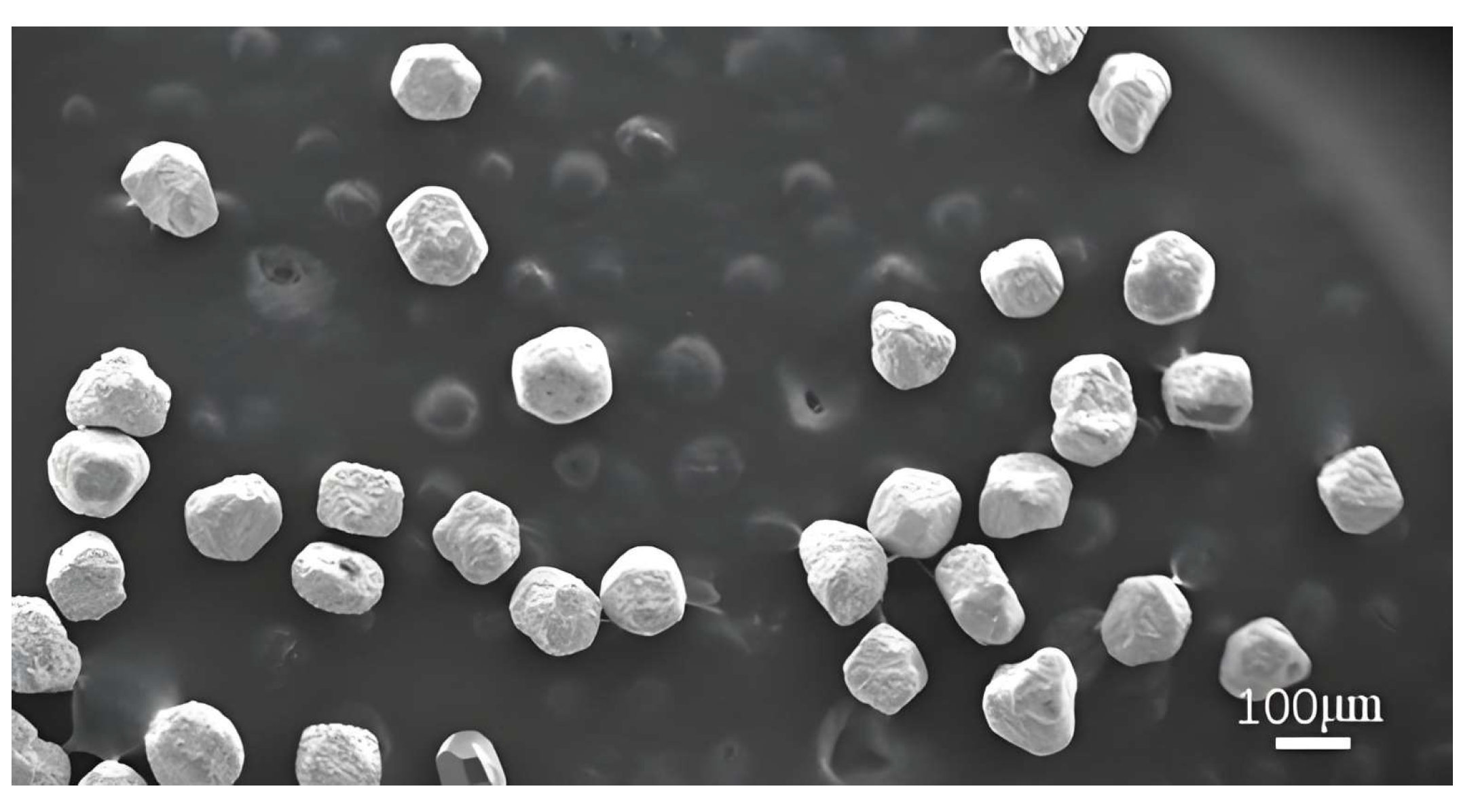
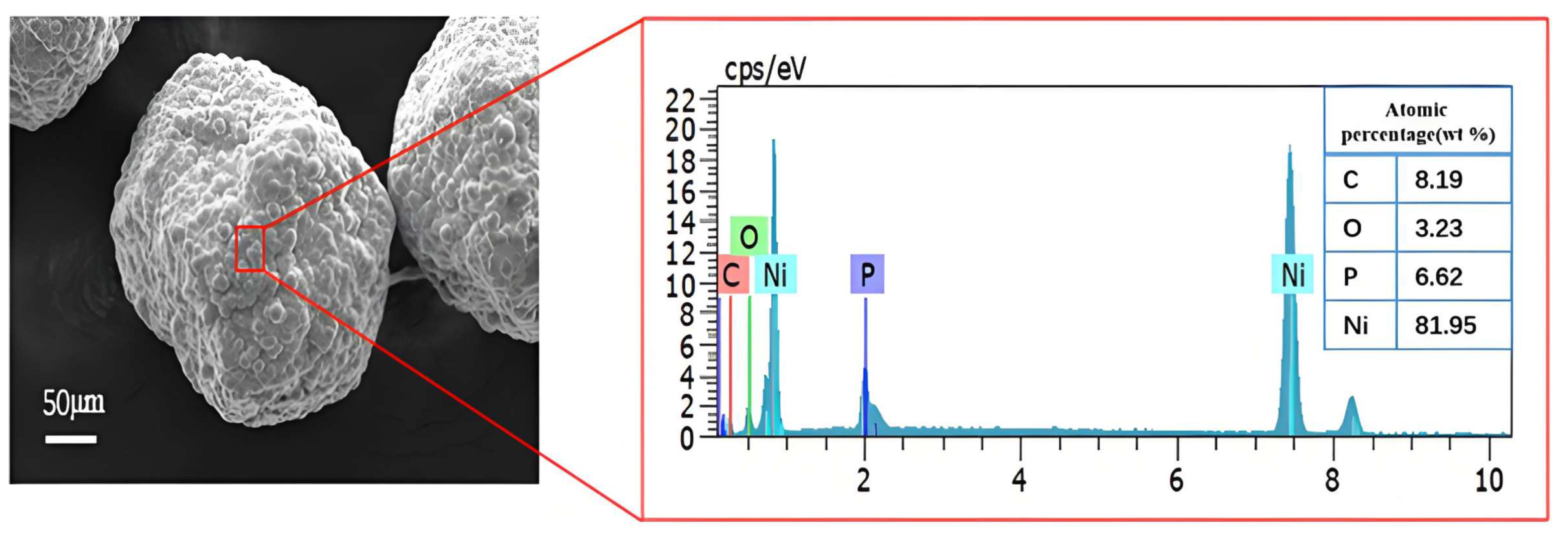
| Parameters | Value |
|---|---|
| Electroplating current (A) | 1, 3, 5, 7 |
| Output power of the ultrasonic oscillator (W) | 200, 500, 900 |
| Diamond particle size | 45/50, 70/80, 120/140, 230/270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Guo, B.; Su, G.; Qi, H.; Li, P.; Zhang, C.; Cheng, K. Investigation into Ultrasonic Oscillation-Assisted Nickel Electroplating onto a Diamond Surface. Micromachines 2025, 16, 962. https://doi.org/10.3390/mi16080962
Fan Q, Guo B, Su G, Qi H, Li P, Zhang C, Cheng K. Investigation into Ultrasonic Oscillation-Assisted Nickel Electroplating onto a Diamond Surface. Micromachines. 2025; 16(8):962. https://doi.org/10.3390/mi16080962
Chicago/Turabian StyleFan, Qingming, Bin Guo, Guokang Su, Hui Qi, Pengfan Li, Chuanyun Zhang, and Kai Cheng. 2025. "Investigation into Ultrasonic Oscillation-Assisted Nickel Electroplating onto a Diamond Surface" Micromachines 16, no. 8: 962. https://doi.org/10.3390/mi16080962
APA StyleFan, Q., Guo, B., Su, G., Qi, H., Li, P., Zhang, C., & Cheng, K. (2025). Investigation into Ultrasonic Oscillation-Assisted Nickel Electroplating onto a Diamond Surface. Micromachines, 16(8), 962. https://doi.org/10.3390/mi16080962






