Effect of Clear Corneal Incisions via Femtosecond Laser Versus Manual Incisions on Corneal Aberrations in Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
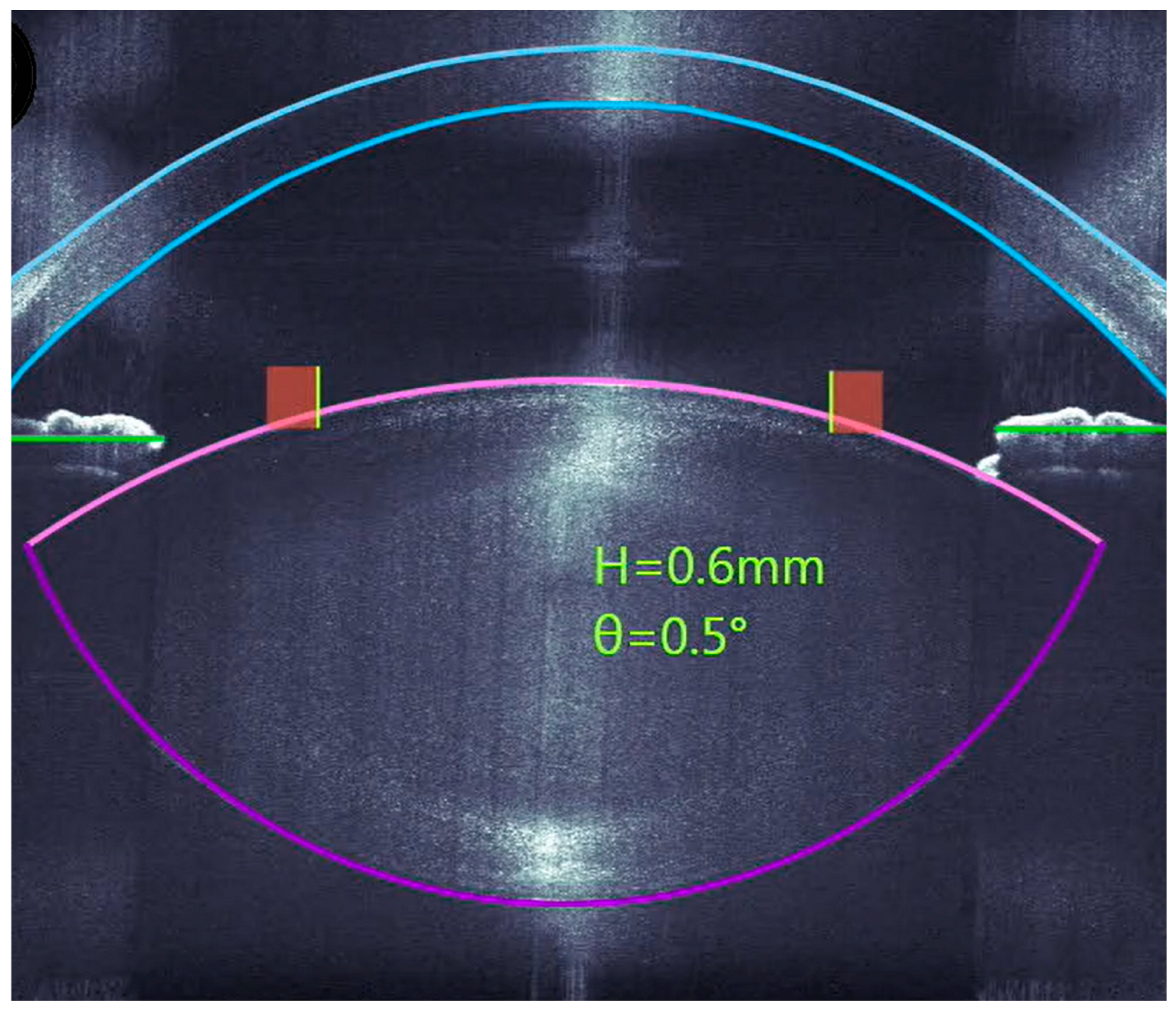
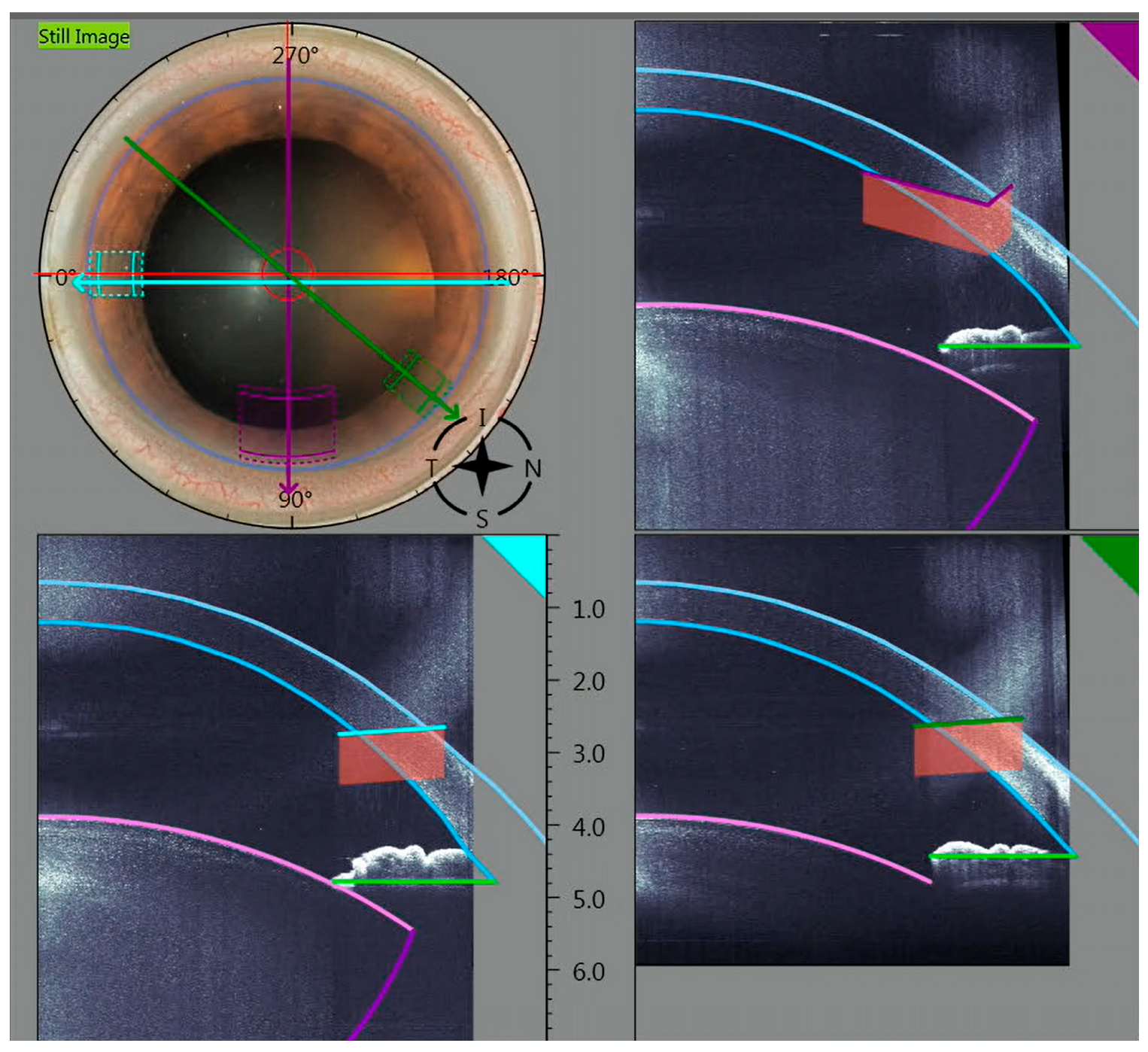
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
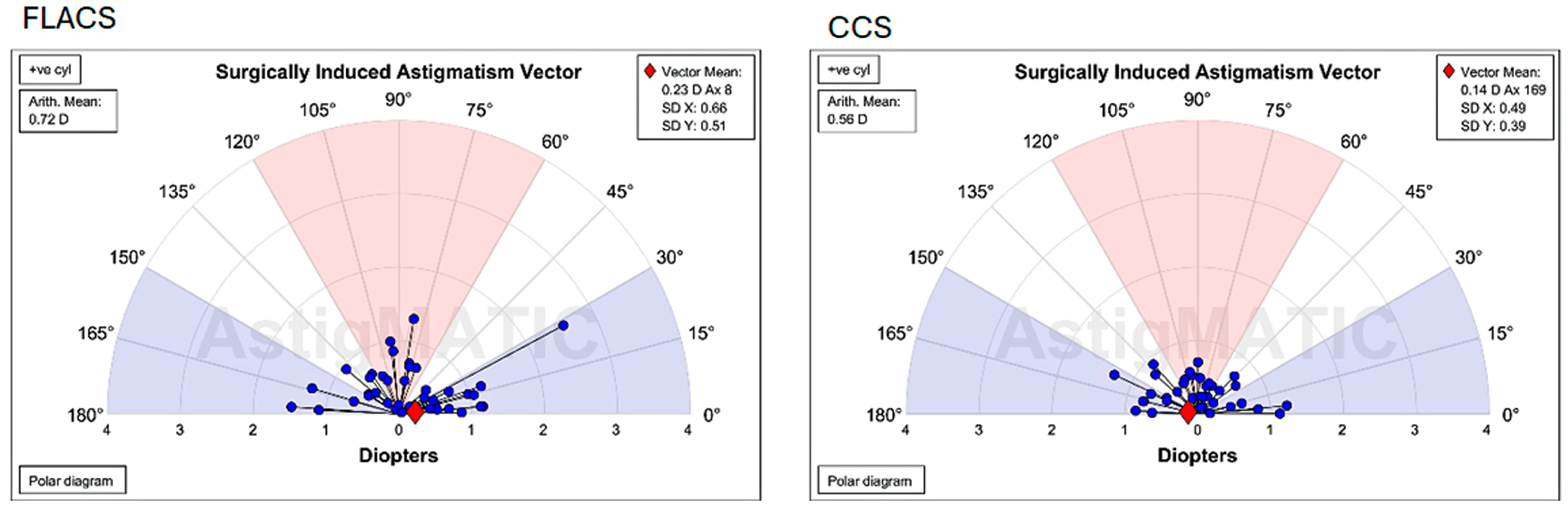
3.1. Surgically Induced Astigmatism After 6 Weeks
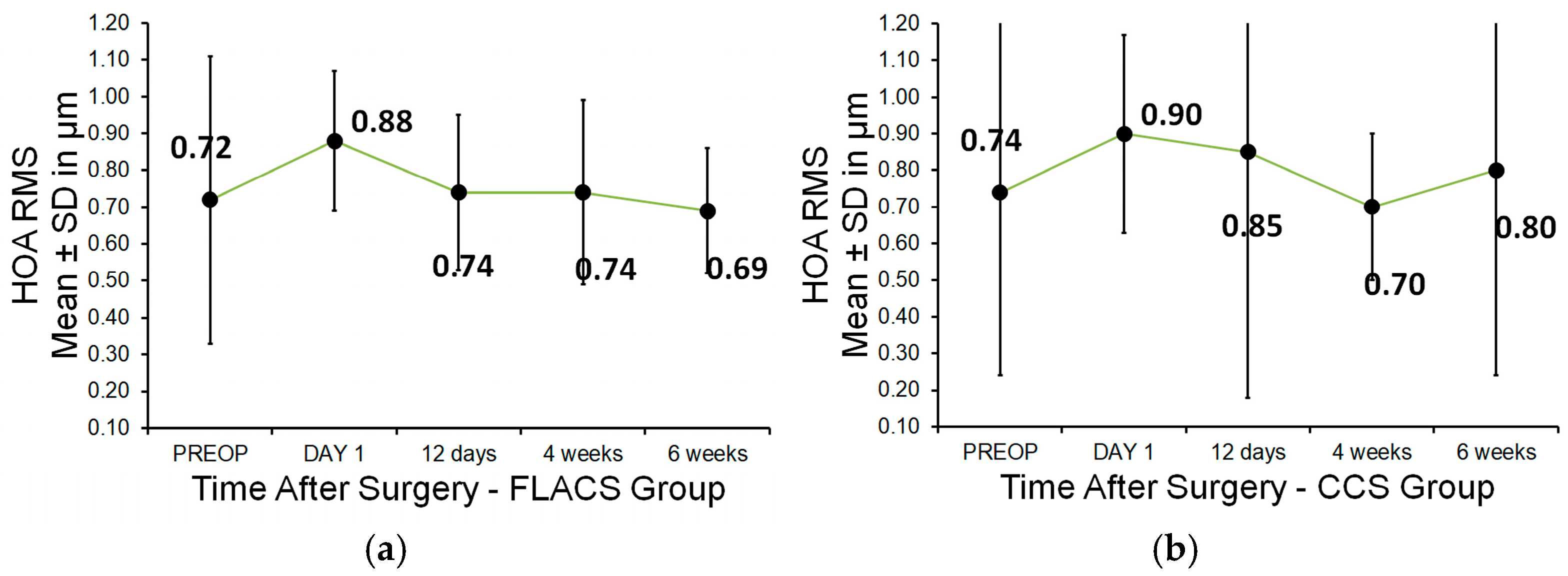
3.2. Higher-Order Aberrations (HOAs)
3.3. Endothelial Cell Density (ECD)
3.4. Central Corneal Thickness (CCT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCIs | clear corneal incisions |
| SIA | induced corneal astigmatism |
| HOAs | higher-order aberrations |
| FLACS | femtosecond laser-assisted cataract surgery |
| CCS | conventional cataract surgery |
| OCT | optical coherence tomography |
| LOCSIII | Lens Opacities Classification System III |
| Yb:YAG | ytterbium-doped yttrium aluminium garnet crystal |
| SESAM | semiconductor saturable absorber mirror |
| BSS | balanced salt solution |
| IFIS | intraoperative floppy iris syndrome |
References
- Nagy, Z.; Takacs, A.; Filkorn, T.; Sarayba, M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J. Refract. Surg. 2009, 25, 1053–1060. [Google Scholar] [CrossRef]
- He, L.; Sheehy, K.; Culbertson, W. Femtosecond laser-assisted cataract surgery. Curr. Opin. Ophthalmol. 2011, 22, 43–52. [Google Scholar] [CrossRef]
- Soong, H.K.; Malta, J.B. Femtosecond lasers in ophthalmology. Am. J. Ophthalmol. 2009, 147, 189–197. [Google Scholar] [CrossRef]
- Pajic, B.; Cvejic, Z.; Pajic-Eggspuehler, B. Cataract Surgery Performed by High Frequency LDV Z8 Femtosecond Laser: Safety, Efficacy, and Its Physical Properties. Sensors 2017, 17, 1429. [Google Scholar] [CrossRef]
- Schroeter, A.; Kropp, M.; Cvejic, Z.; Thumann, G.; Pajic, B. Comparison of femtosecond laser-assisted and ultrasound-assisted cataract surgery with focus on endothelial analysis. Sensors 2021, 21, 996. [Google Scholar] [CrossRef]
- Chang, J.S.; Chen, I.N.; Chan, W.M.; Ng, J.C.; Chan, V.K.; Law, A.K. Initial evaluation of a femtosecond laser system in cataract surgery. J. Cataract. Refract. Surg. 2014, 40, 29–36. [Google Scholar] [CrossRef]
- Masket, S.; Sarayba, M.; Ignacio, T.; Fram, N. Femtosecond laser-assisted cataract incisions: Architectural stability and reproducibility. J. Cataract. Refract. Surg. 2010, 36, 1048–1049. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Ribeiro, F.J.; Pinheiro, J.; Ribeiro, P.; O’Neill, J.G. Comparison of Surgically Induced Astigmatism and Morphologic Features Resulting From Femtosecond Laser and Manual Clear Corneal Incisions for Cataract Surgery. J. Refract. Surg. 2018, 34, 322–329. [Google Scholar] [CrossRef]
- Latz, C.; Asshauer, T.; Rathjen, C.; Mirshahi, A. Femtosecond-Laser Assisted Surgery of the Eye: Overview and Impact of the Low-Energy Concept. Micromachines 2021, 12, 122. [Google Scholar] [CrossRef]
- Riemey, J.; Latz, C.; Mirshahi, A. Intraoperative complications of cataract surgery using a low-energy femtosecond laser: Results from a real-world high-volume setting. PLoS ONE 2022, 7, e0279023. [Google Scholar] [CrossRef]
- Asshauer, T.; Latz, C.; Mirshahi, A.; Rathjen, C. Femtosecond lasers for eye surgery applications: Historical overview and modern low pulse energy concepts. Adv. Opt. Technol. 2021, 10, 393–408. [Google Scholar] [CrossRef]
- Grewal, D.S.; Basti, S. Comparison of morphologic features of clear corneal incisions created with a femtosecond laser or a keratome. J. Cataract. Refract. Surg. 2014, 40, 521–530. [Google Scholar] [CrossRef]
- Uy, H.S.; Shah, S.; Packer, M. Comparison of wound sealability between femtosecond laser–constructed and manual clear corneal incisions in patients undergoing cataract surgery: A pilot study. J. Refract. Surg. 2017, 33, 744–748. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Toto, L.; Mastropasqua, A.; Vecchiarino, L.; Mastropasqua, R.; Pedrotti, E.; Di Nicola, M. Femtosecond laser versus manual clear corneal incision in cataract surgery. J. Refract. Surg. 2014, 30, 27–33. [Google Scholar] [CrossRef]
- Chaves, M.A.P.D.; de Medeiros, A.L.; Vilar, C.M.C.; Magalhães, K.R.P.; Gonçalves, M.R.; Tzelikis, P.F.M.; Hida, W.T.; Carricondo, P.C.; Alves, M.R. Architecture evaluation of the main clear corneal incisions in femtosecond laser-assisted cataract surgery by optical coherence tomography imaging. Clin. Ophthalmol. 2019, 13, 365–372. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Giesen, A.; Hügel, H.; Voss, A.; Witting, K.; Brauch, U.; Opower, H. Scalable concept for diode-pumped high-power solid-state lasers. Appl. Phys. B 1994, 58, 363. [Google Scholar] [CrossRef]
- Keller, U.; Miller, D.A.B.; Boyd, D.G.; Chiu, T.H.; Ferguson, J.F.; Asom, M.T. Solid-state low-loss intracavity saturable absorber for Nd: YLF lasers: An antiresonant semiconductor Fabry–Perot saturable absorber. Opt. Lett. 1992, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Paschotta, R.; Aus der Au, J.; Spuehler, J.; Morier-Genoud, F. Widely turnable pulses durations from a passively mode-locked thin-disk Yb: YAG laser. Opt. Lett. 2001, 26, 379–381. [Google Scholar] [CrossRef]
- Pajic, B.; Vastardis, I.; Pajic-Eggspuehler, B.; Gatzioufas, Z.; Hafezi, F. Femtosecond laser versus mechanical microkeratome-assisted flap creation for LASIK: A prospective, randomized, paired-eye study. Clin. Ophthalmol. 2014, 22, 1883–1889. [Google Scholar]
- Alpins, N.A. A new method of analyzing vectors for changes in astigmatism. J. Cataract. Refract. Surg. 1993, 19, 524–533. [Google Scholar] [CrossRef]
- Alpins, N.A. Vector analysis of astigmatism changes by flattening, steepening, and torque. J. Cataract. Refract. Surg. 1997, 23, 1503–1514. [Google Scholar] [CrossRef]
- Kohnen, T.; Klaproth, O.K.; Bühren, J. Effect of femtosecond laser fragmentation on effective phacoemulsification time in cataract surgery. J. Cataract. Refract. Surg. 2013, 39, 1307–1313. [Google Scholar]
- Tomita, M.; Sotoyama, Y.; Yukawa, S.; Nakamura, T. Comparison of higher-order aberrations and visual outcomes after femtosecond laser–assisted LASIK using two different femtosecond lasers. J. Refract. Surg. 2014, 30, 388–393. [Google Scholar]
- Nagy, Z.Z.; Dunai, A.; Kránitz, K.; Takács, Á.I.; Sándor, G.L.; Hécz, R.; Knorz, M.C. Evaluation of femtosecond laser-assisted and manual clear corneal incisions and their effect on surgically induced astigmatism and higher-order aberrations. J. Refract. Surg. 2014, 30, 522–525. [Google Scholar] [CrossRef]
- Alió, J.L.; Abdou, A.A.; Soria, F.; Javaloy, J.; Fernández-Buenaga, R.; Nagy, Z.Z.; Filkorn, T. Femtosecond laser cataract incision morphology and corneal higher-order aberration analysis. J. Refract. Surg. 2013, 29, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ernest, P.H.; Popovic, M.; Schlenker, M.B.; Klumpp, L.; Ahmed, I.I.K. Higher Order Aberrations in Femtosecond Laser-Assisted Versus Manual Cataract Surgery: A Retrospective Cohort Study. J. Refract. Surg. 2019, 35, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Espaillat, A.; Pérez, O.; Potvin, R. Clinical outcomes using standard phacoemulsification and femtosecond laser-assisted surgery with toric intraocular lenses. Clin. Ophthalmol. 2016, 10, 555–563. [Google Scholar] [CrossRef]
- Diakonis, V.F.; Yesilirmak, N.; Cabot, F.; Kankariya, V.P.; Kounis, G.A.; Warren, D.; Sayed-Ahmed, I.O.; Yoo, S.H.; Donaldson, K. Comparison of surgically induced astigmatism between femtosecond laser and manual clear corneal incisions for cataract surgery. J. Cataract. Refract. Surg. 2015, 41, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.S.; AbouSamra, A.; Helaly, H.A.; Said, A.; Elmassry, A. Comparison between refractive outcomes of femtosecond laser-assisted cataract surgery and standard phacoemulsification. BMC Ophthalmol. 2020, 20, 1. [Google Scholar] [CrossRef]
- Masket, S.; Wang, L.; Belani, S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J. Refract. Surg. 2009, 25, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yoshida, M.; Hayashi, H. Corneal shape changes after 2.0-mm or 3.0-mm clear corneal versus scleral tunnel incision cataract surgery. Ophthalmology 2010, 117, 1313–1323. [Google Scholar] [CrossRef]
- Nikose, A.S.; Saha, D.; Laddha, P.M.; Patil, M. Surgically induced astigmatism after phacoemulsification by temporal clear corneal and superior clear corneal approach: A comparison. Clin. Ophthalmol. 2018, 12, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Chen, L.; Yoon, G.Y.; Singer, B.; Yamauchi, Y.; Williams, D.R. Improvement in retinal image quality with dynamic correction of the eye’s aberrations. Opt. Express 2001, 8, 631–643. [Google Scholar] [CrossRef]
- Koch, D.D.; Jenkins, R.B.; Weikert, M.P.; Yeu, E.; Wang, L. Contribution of the posterior cornea to total corneal astigmatism. J. Cataract. Refract. Surg. 2012, 38, 2080–2087. [Google Scholar] [CrossRef]
- Savini, G.; Hoffer, K.J.; Barboni, P.; Carbonelli, M. Influence of posterior corneal astigmatism on total corneal astigmatism in a sample of eyes with a wide range of anterior corneal astigmatism. J. Cataract. Refract. Surg. 2017, 43, 349–354. [Google Scholar]
- Abulafia, A.; Koch, D.D.; Wang, L.; Hill, W.E.; Assia, E.I.; Franchina, M.; Barrett, G.D. New regression formula for toric intraocular lens planning considering posterior corneal astigmatism. J. Cataract. Refract. Surg. 2016, 42, 775–784. [Google Scholar] [CrossRef]
- Thibos, L.N. Retinal image quality for virtual eyes generated by a statistical model of ocular wavefront aberrations. Ophthalmic Physiol. Opt. 2009, 29, 288–291. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Negishi, K.; Ohnuma, K.; Tsubota, K. Correlation between contrast sensitivity and higher-order aberration based on pupil diameter after cataract surgery. Clin. Ophthalmol. 2011, 5, 1701–1707. [Google Scholar] [CrossRef]
- Bühren, J.; Martin, T.; Kühne, A.; Kohnen, T. Correlation of aberrometry, contrast sensitivity, and subjective symptoms with quality of vision after LASIK. J. Refract. Surg. 2009, 25, 559–568. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, Y.; Wang, W.; Wang, K.; Liu, X.; Yao, K. Femtosecond laser-assisted cataract surgery versus conventional phacoemulsification: Comparison of internal aberrations and visual quality. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 901–911. [Google Scholar] [CrossRef]
- Lee, J.A.; Song, W.K.; Kim, J.Y.; Kim, M.J.; Tchah, H. Femtosecond laser-assisted cataract surgery versus conventional phacoemulsification: Refractive and aberrometric outcomes with a diffractive multifocal intraocular lens. J. Cataract. Refract. Surg. 2019, 45, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Slezak, F.; Thumann, G.; Kropp, M.; Cvejic, Z.; De Clerck, E.E.B.; Bravetti, G.E.; Guber, I.; Pajic, B. Comparison of Conventional and Femtosecond Laser-Assisted Cataract Surgery Regarding Macula Behavior and Thickness. Medicina 2023, 59, 639. [Google Scholar] [CrossRef]
- Janiszewska-Bil, D.; Czarnota-Nowakowska, B.; Grabarek, B.O.; Dobrowolski, D.; Wylęgała, E.; Lyssek-Boroń, A. Comparison of Vision Correction and Corneal Thickness at 180-Day Follow-Up After Femtosecond Laser-Assisted In-Situ Keratomileusis (FS-LASIK), Photorefractive Keratectomy (PRK), and Small Incision Lenticule Extraction (SMILE): A Study from a Single Center in Poland of 120 Patients with Myopia. Med. Sci. Monit. 2023, 29, e939099. [Google Scholar]
- Janiszewska-Bil, D.; Grabarek, B.O.; Lyssek-Boroń, A.; Kiełbasińska, A.; Kuraszewska, B.; Wylęgała, E.; Krysik, K. Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures. Biomedicines 2024, 12, 2289. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Y.; Li, Y.; Li, Y.; Jiang, X.; Li, X. The impact of corneal higher-order aberrations on dynamic visual acuity post cataract surgery. Front. Neurosci. 2024, 18, 1321423. [Google Scholar] [CrossRef]
- Oshika, T.; Klyce, S.D.; Applegate, R.A.; Howland, H.C. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am. J. Ophthalmol. 2006, 141, 437–443. [Google Scholar] [CrossRef]
| Total (n = 78) | FLACS (n = 38) | CCS (n = 40) | |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 69.5 ± 1.4 | 69.5 ± 10.2 | 69.4 ± 10.6 |
| Median | 71 | 70.5 | 71.5 |
| (Min/Max) | (43/95) | (47/95) | (43/90) |
| Gender (eyes) | |||
| Male, n (%) | 45 (57.7) | 20 (52.6) | 25 (62.5) |
| Eye | |||
| OS, n (%) | 41 (52.6) | 19 (50) | 22 (55) |
| OD, n (%) | 37 (47.4) | 19 (50) | 18 (45) |
| LOCS III | |||
| Mean ± SD | 2.72 ± 0.74 | 3.13 ± 0.81 | 2.33 ± 0.68 |
| (Min/Max) | (1/5) | (2/5) | (1/4) |
| Grade 1, n | 2 | 0 | 2 |
| Grade 2, n | 34 | 9 | 25 |
| Grade 3, n | 25 | 16 | 9 |
| Grade 4, n | 15 | 12 | 3 |
| Grade 5, n | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onov, V.; Thumann, G.; Kropp, M.; Cvejic, Z.; Slezak, F.; Pajic, B. Effect of Clear Corneal Incisions via Femtosecond Laser Versus Manual Incisions on Corneal Aberrations in Cataract Surgery. Micromachines 2025, 16, 939. https://doi.org/10.3390/mi16080939
Onov V, Thumann G, Kropp M, Cvejic Z, Slezak F, Pajic B. Effect of Clear Corneal Incisions via Femtosecond Laser Versus Manual Incisions on Corneal Aberrations in Cataract Surgery. Micromachines. 2025; 16(8):939. https://doi.org/10.3390/mi16080939
Chicago/Turabian StyleOnov, Vesko, Gabriele Thumann, Martina Kropp, Zeljka Cvejic, Filip Slezak, and Bojan Pajic. 2025. "Effect of Clear Corneal Incisions via Femtosecond Laser Versus Manual Incisions on Corneal Aberrations in Cataract Surgery" Micromachines 16, no. 8: 939. https://doi.org/10.3390/mi16080939
APA StyleOnov, V., Thumann, G., Kropp, M., Cvejic, Z., Slezak, F., & Pajic, B. (2025). Effect of Clear Corneal Incisions via Femtosecond Laser Versus Manual Incisions on Corneal Aberrations in Cataract Surgery. Micromachines, 16(8), 939. https://doi.org/10.3390/mi16080939








