Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems
Abstract
1. Introduction
2. AR Bacteria in the Environment
2.1. Occurrence of AR Bacteria in Livestock Waste
2.2. Occurrence of AR Bacteria in Agricultural Waste
2.3. Occurrence of AR Bacteria in Aquaculture Waste
3. Occurrence of AR Bacteria in Retail Foods
4. Prevention and Monitoring of AR Bacteria
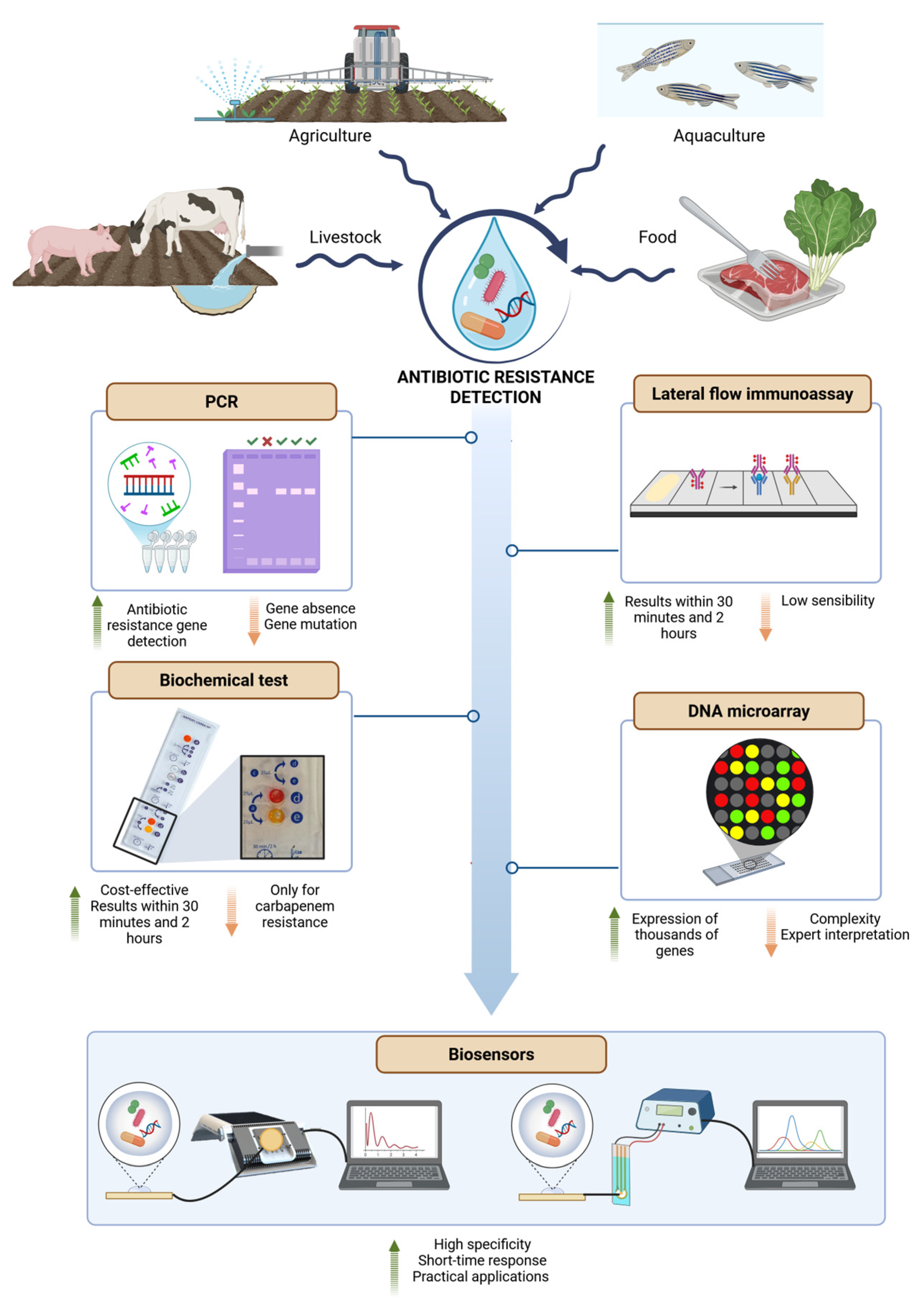
5. Rapid Detection of AR—Contribution of Biosensors
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; pp. 1–256. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Johnson, S.C.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 6736, 1–27. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of Nations. Rev. Antimicrob. Resist. 2014, 11, 1–18. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; pp. 1–52. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of Americas. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Cohen, D.A.; Marchaim, D. Appropriate antimicrobial therapy in the era of multidrug-resistant human pathogens. Clin. Microbiol. Infect. 2015, 21, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Salazar, A.; Verónica Martínez-Vázquez, A.; Aguilera-Arreola, G.; de Jesus de Luna-Santillana, E.; Antonia Cruz-Hernández, M.; Marcial Escobedo-Bonilla, C.; Lara-Ramírez, E.; Sánchez-Sánchez, M.; Guerrero, A.; Rivera, G.; et al. Prevalence of ESKAPE bacteria in surface water and wastewater sources: Multidrug resistance and molecular characterization, an updated review. Water 2023, 15, 3220. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2022, 20, 1–197. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Mwebaza, N.; Odda, J.; Kyegombe, D.B.; Ntale, M. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health 2014, 6, 410–425. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Kotsiri, Z.; Vantarakis, A.; Rizzotto, F.; Kavanaugh, D.; Ramarao, N.; Vidic, J. Sensitive detection of E. coli in artificial seawater by aptamer-coated magnetic beads and direct PCR. Appl. Sci. 2019, 9, 5392. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez‐Ordóñez, A.; Bolton, D.; Bover‐Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. EFSA Role played by the environment in the emergence and spread of Antimicrobial Resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Roccaro, P. Treatment processes for municipal wastewater reclamation: The challenges of emerging contaminants and direct potable reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 46–54. [Google Scholar] [CrossRef]
- Nadimpalli, M.; Delarocque-Astagneau, E.; Love, D.C.; Price, L.B.; Huynh, B.-T.; Collard, J.-M.; Lay, K.S.; Borand, L.; Ndir, A.; Walsh, T.R.; et al. Combating global antibiotic resistance: Emerging One Health concerns in Lower- and Middle-Income Countries. Clin. Infect. Dis. 2018, 66, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; Mcnally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage Rene. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Haskell, K.J.; Schriever, S.R.; Fonoimoana, K.D.; Haws, B.; Hair, B.B.; Wienclaw, T.M.; Holmstead, J.G.; Barboza, A.B.; Berges, E.T.; Heaton, M.J.; et al. Antibiotic resistance is lower in Staphylococcus aureus isolated from antibiotic-free raw meat as compared to conventional raw meat. PLoS ONE 2018, 13, e0206712. [Google Scholar] [CrossRef]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, E.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of livestock- associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef] [PubMed]
- Haulisah, N.A.; Hassan, L.; Bejo, S.K.; Jajere, S.M.; Ahmad, N.I. High levels of antibiotic resistance in isolates from diseased livestock. Front. Vet. Sci. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Elmi, S.A.; Simons, D.; Elton, L.; Haider, N.; Hamid, M.M.A.; Shuaib, Y.A.; Khan, M.A.; Othman, I.; Kock, R.; Osman, A.Y. Identification of risk factors associated with resistant Escherichia coli isolates from poultry farms in the East Coast of Peninsular Malaysia: A cross sectional study. Antibiotics 2021, 10, 117. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Chou, M.-Y.; Shih, Y.-J.; Huang, T.-Y.; Yang, P.-Y.; Chiu, Y.-C.; Chen, J.-S.; Hsu, B.-M. Distribution and genotyping of aquatic Acinetobacter baumannii strains isolated from the Puzi River and its tributaries near areas of livestock farming. Water 2018, 10, 1374. [Google Scholar] [CrossRef]
- ECDC; EFSA; EMA. Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. EFSA J. 2021, 19, 1–166. [Google Scholar] [CrossRef]
- Tamminen, M.; Karkman, A.; Lõhmus, A.; Muziasari, W.I.; Takasu, H.; Wada, S.; Suzuki, S.; Virta, M. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 2011, 45, 386–391. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, B.; Wang, J.; Feng, C.; Gao, M.; Wang, L. Prevalence of veterinary antibiotics and antibiotic- resistant Escherichia coli in the surface water of a livestock production region in Northern China. PLoS ONE 2014, 9, e111026. [Google Scholar] [CrossRef] [PubMed]
- Keelara, S.; Scott, H.M.; Morrow, W.M.; Gebreyes, W.A.; Correa, M.; Nayak, R.; Stefanova, R.; Thakur, S. Longitudinal study of distributions of similar antimicrobial-resistant Salmonella serovars in pigs and their environment in two distinct swine production systems. Appl. Environ. Microbiol. 2013, 79, 5167–5178. [Google Scholar] [CrossRef]
- Casanova, L.M.; Hill, V.R.; Sobsey, M.D. Antibiotic-resistant Salmonella in swine wastes and farm surface waters. Lett. Appl. Microbiol. 2019, 71, 117–123. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, X.L.; Li, W.; Lu, X.F.; Liu, B.; Wang, J. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Chen, C.-Y.; Young, C.-W.; Chao, W.-L.; Li, M.-H.; Liu, Y.-H.; Lin, C.-M.; Ying, C. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in Northern Taiwan. J. Hazard. Mater. 2014, 277, 34–43. [Google Scholar] [CrossRef]
- Alam, B.; Nasir Uddin, M.; Mridha, D.; M Taslima Akhter, A.H.; Shaheenur Islam, S.; M Ziaul Haque, A.K.; Lutful Kabir, S.M. Occurrence of Campylobacter spp. in selected small scale commercial broiler farms of Bangladesh related to good farm practices. Microorganisms 2020, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Ter Kuile, B.H.; Kraupner, N.; Brul, S. The risk of low concentrations of antibiotics in agriculture for resistance in human health care. FEMS Microbiol. Lett. 2016, 363, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, Northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Brambilla, G.; Patrizii, M.; De Filippis, S.P.; Bonazzi, G.; Mantovi, P.; Barchi, D.; Migliore, L. Oxytetracycline as environmental contaminant in arable lands. Anal. Chim. Acta 2007, 586, 326–329. [Google Scholar] [CrossRef]
- Kay, P.; Blackwell, P.A.; Boxall, A.B.A. Transport of veterinary antibiotics in overland flow following the application of slurry to arable land. Chemosphere 2005, 59, 951–959. [Google Scholar] [CrossRef]
- Sun, P.; Barmaz, D.; Cabrera, M.L.; Pavlostathis, S.G.; Huang, C.H. Detection and quantification of ionophore antibiotics in runoff, soil and poultry litter. J. Chromatogr. A 2013, 1312, 10–17. [Google Scholar] [CrossRef]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship between biofilm formation and antimicrobial resistance in gram-negative bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef]
- Pornsukarom, S.; Thakur, S. Assessing the impact of manure application in commercial swine farms on the transmission of antimicrobial resistant Salmonella in the environment. PLoS ONE 2016, 11, e0164621. [Google Scholar] [CrossRef]
- Agostinho Avanci, J.M.; Cardozo, M.V.; Borzi, M.M.; Marin, J.M. Antibiotic resistance and virulence factors among Escherichia coli isolates from avian organic fertilizer. Ciência Rural 2020, 50, 1–10. [Google Scholar] [CrossRef]
- Abakpa, G.O.; Umoh, V.J.; Kamaruzaman, S.; Ibekwe, M. Fingerprints of resistant Escherichia coli O157:H7 from vegetables and environmental samples. J. Sci. Food Agric. 2018, 98, 80–86. [Google Scholar] [CrossRef]
- Hudson, J.A.; Frewer, L.J.; Jones, G.; Brereton, P.A.; Whittingham, M.J.; Stewart, G. The agri-food chain and antimicrobial resistance: A review. Trends Food Sci. Technol. 2017, 69, 131–147. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016, 16, e127–e133. [Google Scholar] [CrossRef]
- Keat Tam, H.; Michael Vui Ling Wong, C.; Ting Yong, S.; Blamey, J.; González, M. Multiple-antibiotic-resistant bacteria from the Maritime Antarctic. Polar Biol. 2015, 38, 1129–1141. [Google Scholar] [CrossRef]
- Sherif, A.H.; Gouda, M.; Darwish, S.; Abdelmohsin, A. Prevalence of antibiotic-resistant bacteria in freshwater fish farms. Aquac. Res. 2021, 52, 2036–2047. [Google Scholar] [CrossRef]
- Su, S.; Li, C.; Yang, J.; Xu, Q.; Qiu, Z.; Xue, B.; Wang, S.; Zhao, C.; Xiao, Z.; Wang, J.; et al. Distribution of antibiotic resistance genes in three different natural water bodies-a lake, river and sea. Int. J. Environ. Res. Public Health 2020, 17, 552. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin‐Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. EFSA Assessment of animal diseases caused by bacteria resistant to antimicrobials: Kept fish species. EFSA J. 2022, 20, 1–23. [Google Scholar] [CrossRef]
- Ng, C.; Chen, H.; Goh, S.G.; Haller, L.; Wu, Z.; Charles, F.R.; Trottet, A.; Gin, K. Microbial water quality and the detection of multidrug resistant E. coli and antibiotic resistance genes in aquaculture sites of Singapore. Mar. Pollut. Bull. 2018, 135, 475–480. [Google Scholar] [CrossRef]
- Shah, S.Q.A.; Cabello, F.C.; L’Abée-Lund, T.M.; Tomova, A.; Godfrey, H.P.; Buschmann, A.H.; Sørum, H. Antimicrobial resistance and antimicrobial resistance genes in marine bacteria from salmon aquaculture and non-aquaculture sites. Environ. Microbiol. 2014, 16, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Marko, V. The resistome of farmed fish feces contributes to the enrichment of antibiotic resistance genes in sediments below Baltic Sea fish farms. Front. Microbiol. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hong, B.; Ba, Y.; Niu, L.; Lou, F.; Zhang, Z.; Liu, H.; Pan, Y.; Zhao, Y. A comprehensive research on antibiotic resistance genes in microbiota of aquatic animals. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Hatosy, S.M.; Martiny, A.C. The ocean as a global reservoir of antibiotic resistance genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef]
- Nanayakkara Sapugahawatte, D.; Li, C.; Zhu, C.; Dharmaratne, P.; Wong, K.T.; Lo, N.; Ip, M. Prevalence and characteristics of Extended-Spectrum-β-Lactamase-producing and Carbapenemase-producing Enterobacteriaceae from freshwater fish and pork in wet markets of Hong Kong. mSphere 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Knorr, D.; Augustin, M.A. Food processing needs, advantages and misconceptions. Trends Food Sci. Technol. 2021, 108, 103–110. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Bhagwat, V.R. Safety of water used in food production. Food Saf. Hum. Health 2019, 9, 219–247. [Google Scholar] [CrossRef]
- Murray, K.; Wu, F.; Shi, J.; Jun Xue, S.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, J.; Fei, P.; Feng, H.; Wang, Y.; Ali, M.A.; Li, S.; Jing, H.; Yang, W. Prevalence, molecular characterization, and antibiotic susceptibility of Bacillus cereus isolated from dairy products in China. J. Dairy Sci. 2020, 103, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 13: Suitability of taxonomic units notified to EFSA until September 2020. EFSA J. 2021, 19, 1–32. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Etayo, L.; González, D.; Vitas, A.I. The aquatic ecosystem, a good environment for the horizontal transfer of antimicrobial resistance and virulence-associated factors among Extended Spectrum β-Lactamases producing. E. coli. Microorganisms 2020, 8, 568. [Google Scholar] [CrossRef]
- Calhau, V.; Mendes, C.; Pena, A.; Mendonça, N.; da Silva, G.J. Virulence and plasmidic resistance determinants of Escherichia coli isolated from municipal and hospital wastewater treatment plants. J. Water Health 2015, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.; Zhang, J.; Qi, W.; Li, Y.; Chen, S.; Zhou, W. Prevalence of antibiotic resistance genes in drinking water and biofilms: The correlation with the microbial community and opportunistic pathogens. Chemosphere 2020, 259, 1–9. [Google Scholar] [CrossRef]
- Maifreni, M.; Frigo, F.; Bartolomeoli, I.; Buiatti, S.; Picon, S.; Marino, M. Bacterial biofilm as a possible source of contamination in the microbrewery environment. Food Control 2015, 50, 809–814. [Google Scholar] [CrossRef]
- Mukherjee, R.; Vidic, J.; Auger, S.; Wen, H.-C.; Pandey, R.P.; Chang, C.-M. Exploring disease management and control through pathogen diagnostics and One Health initiative: A concise review. Antibiotics 2024, 13, 17. [Google Scholar] [CrossRef]
- Gunjan; Vidic, J.; Manzano, M.; Samuel Raj, V.; Pati Pandey, R.; Chang, C.-M. Comparative meta-analysis of antimicrobial resistance from different food sources along with One Health approach in Italy and Thailand. One Health 2023, 16, 1–11. [Google Scholar] [CrossRef]
- Berglund, N.A.; Piggot, T.J.; Jefferies, D.; Sessions, R.B.; Bond, P.J.; Khalid, S. Interaction of the antimicrobial peptide polymyxin b1 with both membranes of E. coli: A molecular dynamics study. PLoS Comput. Biol. 2015, 11, 1–17. [Google Scholar] [CrossRef]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Du, H.; Puri, S.; McCall, A.; Norris, H.L.; Russo, T.; Edgerton, M. Human salivary protein Histatin 5 has potent bactericidal activity against ESKAPE pathogens. Front. Cell Infect. Microbiol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage therapy: Developments and directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef]
- Pallavali, R.R.; Degati, V.L.; Lomada, D.; Reddy, M.C.; Raghava, V.; Durbaka, P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS ONE 2017, 12, e0179245. [Google Scholar] [CrossRef]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef]
- Zanet, V.; Vidic, J.; Auger, S.; Vizzini, P.; Lippe, G.; Iacumin, L.; Comi, G.; Manzano, M. Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 127, 1391–1402. [Google Scholar] [CrossRef]
- Auger, S.; Henry, C.; Péchaux, C.; Lejal, N.; Zanet, V.; Nikolic, M.V.; Manzano, M.; Vidic, J. Exploring the impact of Mg-doped ZnO nanoparticles on a model soil microorganism Bacillus subtilis. Ecotoxicol. Environ. Saf. 2019, 182, 1–10. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Eslamifar, M.; Javan, F. Water disinfection using silver and zinc oxide nanoparticles. J. Nano Res. 2021, 69, 105–121. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Abou-Okada, M.; El-Matbouli, M.; Saleh, M. Silver and zinc oxide nanoparticles for effective aquaculture wastewater treatment. Nanomaterials 2025, 15, 559. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities. Front. Plant Sci. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Wang, C.; Magnuson, J.T.; Zheng, C.; Qiu, W. Incidence of pollution, bioaccumulation, biomagnification, and toxic effects of per- and polyfluoroalkyl substances (PFAS) in aquatic ecosystems: A Review. Aquat. Toxicol. 2025, 286, 1–13. [Google Scholar] [CrossRef]
- Atanda, S.A.; Shaibu, R.O.; Agunbiade, F.O. Nanoparticles in agriculture: Balancing food security and environmental sustainability. Discov. Agric. 2025, 3, 1–32. [Google Scholar] [CrossRef]
- Suárez-Oubiña, C.; Herbello-Hermelo, P.; Mallo, N.; Vázquez, M.; Cabaleiro, S.; Domínguez-González, R.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Bioaccumulation and human risk assessment of inorganic nanoparticles in aquaculture species. Environ. Sci. Nano 2024, 11, 2937–2947. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Ding, S.; Wang, S. A TaqMan-Based Multiplex Real-Time PCR assay for the rapid detection of tigecycline resistance genes from bacteria, faeces and environmental samples. BMC Microbiol. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P. Antimicrobial resistance in rivers: A review of the genes detected and new challenges. Environ. Toxicol. Chem. 2022, 41, 687–714. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Jousset, A.B.; Emeraud, C.; Oueslati, S.; Dortet, L.; Naas, T. Genetic diversity, biochemical properties, and detection methods of minor carbapenemases in Enterobacterales. Front. Med. 2021, 7, 1–20. [Google Scholar] [CrossRef]
- Buonomini, A.R.; Riva, E.; Di Bonaventura, G.; Gherardi, G. Rapid detection of methicillin-resistant Staphylococcus aureus directly from blood for the diagnosis of bloodstream infections: A mini-review. Diagnostics 2020, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef]
- Volland, H.; Girlich, D.; Laguide, M.; Gonzalez, C.; Paris, V.; Laroche, M.; Oueslati, S.; Dortet, L.; Simon, S.; Naas, T. Improvement of the immunochromatographic NG-Test CArba 5 assay for the detection of IMP variants previously undetected. Antimicrob. Agents Chemother. 2020, 64, e01940-19. [Google Scholar] [CrossRef]
- Dortet, L.; Tandé, D.; de Briel, D.; Bernabeu, S.; Lasserre, C.; Gregorowicz, G.; Jousset, A.B.; Naas, T. MALDI-TOF for the rapid detection of Carbapenemase-producing Enterobacteriaceae: Comparison of the commercialized MBT STAR V R-Carba IVD Kit with Two in-House MALDI-TOF techniques and the RAPIDEC V R CARBA NP. J. Antimicrob. Chemother. 2018, 73, 2352–2359. [Google Scholar] [CrossRef]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-Need DNA testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef]
- Li, R.; Jay, J.A.; Stenstrom, M.K. Fate of antibiotic resistance genes and antibiotic-resistant bacteria in water resource recovery facilities. Water Environ. Res. 2019, 91, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Oniciuc, E.A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; Santos, J.A.; Alvarez-Ordóñez, A. The present and future of Whole Genome Sequencing (WGS) and Whole Metagenome Sequencing (WMS) for surveillance of antimicrobial resistant microorganisms and antimicrobial resistance genes across the food chain. Genes 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Ma, L.; Xia, Y.; Li, B.; Yang, Y.; Li, L.G.; Tiedje, J.M.; Zhang, T. Metagenomic assembly reveals hosts of antibiotic resistance genes and the shared resistome in pig, chicken, and human feces. Environ. Sci. Technol. 2016, 50, 420–427. [Google Scholar] [CrossRef]
- Burnham, C.-A.D.; Leeds, J.; Nordmann, P.; Patel, J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 2017, 15, 697–703. [Google Scholar] [CrossRef]
- Cossettini, A.; Vidic, J.; Maifreni, M.; Marino, M.; Pinamonti, D.; Manzano, M. Rapid detection of Listeria monocytogenes, Salmonella, Campylobacter spp., and Escherichia coli in food using biosensors. Food Control 2022, 137, 1–10. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 1–15. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Li, Y.; Xie, G. Amplified electrochemical detection of mecA gene in methicillin-resistant Staphylococcus aureus based on target recycling amplification and isothermal strand-displacement polymerization reaction. Sens. Actuators B Chem. 2015, 221, 148–154. [Google Scholar] [CrossRef]
- Dai, G.; Li, Z.; Luo, F.; Lu, Y.; Chu, Z.; Zhang, J.; Zhang, F.; Wang, Q.; He, P. Simultaneous electrochemical determination of nuc and mecA genes for identification of methicillin-resistant Staphylococcus aureus using N-Doped Porous Carbon and DNA-Modified MOF. Mikrochim. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Léguillier, V.; Pinamonti, D.; Chang, C.-M.; Gujan; Mukherjee, R.; Kumar, H.; Cossettini, A.; Manzano, M.; Anba-Mondoloni, J.; Malet-Villemagne, J.; et al. A review and meta-analysis of Staphylococcus aureus prevalence in foods. Microbe 2024, 4, 1–11. [Google Scholar] [CrossRef]
- Butterworth, A.; Pratibha, P.; Marx, A.; Corrigan, D.K. Electrochemical detection of oxacillin resistance using direct-labeling solid-phase isothermal amplification. ACS Sens. 2021, 6, 3773–3780. [Google Scholar] [CrossRef]
- El-Azazy, M. Electrochemical Impedance Spectroscopy (EIS) in food, water, and drug analyses: Recent advances and applications. Electrochem. Impedance Spectrosc. 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Manohar, S.M.; Shah, P.; Nair, A. Flow cytometry: Principles, applications and recent advances. Bioanalysis 2021, 13, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G. Current and future flow cytometry applications contributing to antimicrobial resistance control. Microorganisms 2023, 11, 1300. [Google Scholar] [CrossRef]
- Conteduca, D.; Brunetti, G.; Dell’Olio, F.; Armenise, M.N.; Krauss, T.F.; Ciminelli, C. Monitoring of individual bacteria using electro-photonic traps. Biomed. Opt. Express 2019, 10, 3463–3471. [Google Scholar] [CrossRef]
- Butt, M.A.; Imran Akca, B.; Mateos, X. Integrated photonic biosensors: Enabling next-generation Lab-on-a-Chip platforms. Nanomaterials 2025, 15, 731. [Google Scholar] [CrossRef]
- Calzuola, S.T.; Malet-Villemagne, J.; Pinamonti, D.; Rizzotto, F.; Henry, C.; Péchaux, C.; Blondé, J.B.; Roy, E.; Manzano, M.; Lakisic, G.; et al. Assessing Campylobacter jejuni extracellular vesicle–host interaction using a microfluidic platform with Caco-2 spheroides-on-chip. ACS Biomater. Sci. Eng. 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- di Toma, A.; Brunetti, G.; Chiriacò, M.S.; Ferrara, F.; Ciminelli, C. A novel hybrid platform for live/dead bacteria accurate sorting by on-chip DEP device. Int. J. Mol. Sci. 2023, 24, 7077. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, N.M.; Shrivastava, D.; Bisen, P.S. Contaminant sensors: Nanotechnology-based contaminant sensors. Nanobiosensors 2017, 14, 573–628. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M. Electrochemical biosensors for rapid pathogen detection. Curr. Opin. Electrochem. 2021, 29, 1–6. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. Clinical medicine an overview of healthcare associated infections and their detection methods caused by pathogen bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, D. A novel inductively coupled capacitor wireless sensor system for rapid antibiotic susceptibility testing. J. Biol. Eng. 2023, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naddaf, M. 40 million deaths by 2050: Toll of drug-resistant infections to rise by 70. Nature 2024, 633, 747–748. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance; United Nations Environment Programme: Geneva, Switzerland, 2023; pp. 1–89. [Google Scholar]


| Sources | Country | Antibiotic—Antibiotic Class | Significance in Human Therapeutics * | Reference |
|---|---|---|---|---|
| Swine | North Carolina | Ampicillin—Penicillins | Critically important | [25] |
| Chloramphenicol—Amphenicols | Highly important | |||
| Sulphamethoxazole—Sulphonamides | Highly important | |||
| Sulfisoxazole—Sulphonamides | Highly important | |||
| Streptomycin—Aminoglycosides | Critically important | [24] | ||
| Tetracycline—Tetracyclines | Highly important | |||
| Swine and poultry | China | Ampicillin—Penicillins | Critically important | [23] |
| Chlortetracycline—Tetracyclines | Highly important | |||
| Gentamycin—Aminoglycosides | Critically important | |||
| Sulphamethoxazole/Trimethoprim—Sulphonamides | Highly important | |||
| Tetracycline—Tetracyclines | Highly important | |||
| Livestock (generic) | Taiwan | Sulphamethoxazole/Trimethoprim—Sulphonamides | Highly important | [20] |
| Tetracycline—Tetracyclines | Highly important | |||
| Poultry | Bangladesh | Amoxicillin—Penicillins | Critically important | [28] |
| Azithromycin—Macrolides | Critically important | |||
| Ciprofloxacin—Quinolones | Critically important | |||
| Erythromycin—Macrolides | Critically important | |||
| Gentamycin—Aminoglycosides | Critically important | |||
| Norfloxacin—Quinolones | Critically important | |||
| Streptomycin—Aminoglycosides | Critically important | |||
| Tetracycline—Tetracyclines | Highly important |
| Sources | Country | Antibiotic—Antibiotic Class | Significance in Human Therapeutics * | Reference |
|---|---|---|---|---|
| Manure, soil, vegetables and groundwater | China | Tetracycline—Tetracyclines | Highly important | [30] |
| Sulphonamides | Highly important | |||
| Quinolones | Critically important | |||
| Sediments fertilized by pig slurries | Italy | Oxytetracycline—Tetracyclines | Highly important | [31] |
| Overland flow after putting the slurry to arable land | United Kingdom | Oxytetracycline—Tetracyclines | Highly important | [32] |
| Sulphachloropyridazine—Sulphonamides | Highly important | |||
| Poultry litter-soil-water environment | United States | Monensin, salinomycin and narasin—Ionophores | Currently not used in humans | [33] |
| Swine manure | United States | Streptomycin—Aminoglycosides | Critically important | [35] |
| Sulphisoxazole—Sulphonamides | Highly important | |||
| Tetracycline—Tetracyclines | Highly important | |||
| Avian organic fertilizer | Brazil | Tetracycline—Tetracyclines | Highly important | [36] |
| Gentamycin—Aminoglycosides | Critically important | |||
| Cefotaxime—Cephalosporins | Critically important | |||
| Nitrofurantoin—Nitrofurans | Important | |||
| Trimethoprim/sulfamethoxazole—Sulphonamides | Highly important | |||
| Ampicillin—Penicillins | Critically important | |||
| Irrigation water, manure and soil | Nigeria | Quinolones | Critically important | [37] |
| Sources | Country | Antibiotic—Antibiotic Class | Significance in Human Therapeutics * | Reference |
|---|---|---|---|---|
| Marine environments | United States | Ampicillin—Penicillins | Critically important | [50] |
| Sulfadimethoxine—Sulphonamides | Highly important | |||
| Salmon farm | Chile | Amoxicillin—Penicillins | Critically important | [47] |
| Streptomycin—Aminoglycosides | Critically important | |||
| Tetracycline—Tetracyclines | Highly important | |||
| Trimethoprim/Sulfamethizole—Sulphonamides | Highly important | |||
| Aquatic animals | China | Chloramphenicol—Amphenicols | Highly important | [49] |
| Sulphonamide—Sulphonamides | Highly important | |||
| Tetracycline—Tetracyclines | Highly important | |||
| Fish farms | Finland | Aminoglycoside—Aminoglycosides | Critically important | [48] |
| Chloramphenicol—Amphenicols | Highly important | |||
| Sulfonamide—Sulphonamides | Highly important | |||
| Tetracycline—Tetracyclines | Highly important | |||
| Trimethoprim—Sulphonamides | Highly important | |||
| Aquaculture farms | Singapore | Beta-lactams—Penicillins | Critically important | [46] |
| Farmed freshwater fish | Hong Kong | Beta-lactams—Penicillins | Critically important | [51] |
| Carbapenemase-producers—Carbapenems | Critically important |
| Biosensor Technique | Target Detection | Strengths | Limitations | Refs. |
|---|---|---|---|---|
| Electrochemical biosensors | ARGs | Fast detection (<2 h), cost-effective, highly specific and sensitive, suitable for point-of-care in water environments, requires minimal sample volume | Pre-analysis DNA extraction required; limited to specific target genes | [94,97,98,99] |
| Electrochemical biosensors + isothermal amplification | ARGs | Fast detection (<1 h), combined amplification and target capture directly on electrode surface; avoids need for DNA extraction and ssDNA generation | Not yet optimized; may be inactivated by thermal cycling | [100] |
| Electrochemical Impedance Spectroscopy (EIS) | Bacteria, drugs, pharmaceutical residues | Steady-state, non-destructive technique; label-free; real-time monitoring | Requires large sample volume; sensitivity depends on electrode surface preparation | [101] |
| Quartz Crystal Microbalance (QCM) | ARGs Bacteria Drug effect | Real-time measurement No sample damage | Sensitive to external disturbances | [94] |
| Impedance Flow Cytometry (FCM) | Phenotypic resistance in bacteria | Fast profiling (<2 h) of Gram+/Gram– strains; distinguishes viable, intermediate, and dead cells; no need for culture; can test multiple antibiotics simultaneously | Large sample volume; requires fluorescent labelling for detailed analysis | [102,103] |
| Photonic biosensors | Antibiotic effect on bacteria | Analysis time 1 h, use of photonic crystal cavities to trap cells and monitor metabolic/optical response; low sample volume | Complex fabrication; low portability; high instrumentation cost | [104] |
| Lab-on-Chip with Photonic Integration | Bacterial or cell response | Miniaturized, low-power device; integrates optics and microfluidics for single-chip results | Complexity in design and fluid control; limited scalability | [105] |
| Organ-on-Chip with Impedance Spectroscopy | Cell–drug/ bacteria interaction | Simulates human tissues and physiological conditions; replicates pH, flow, and biochemical environment | Complex design; primarily research-stage | [106] |
| Lab-on-Chip with Dielectrophoresis (DEP) | Cell viability under antibiotic stress | Integrated microfluidic channel with electrodes; distinguishes live/dead cells in ~5 s after 1 h antibiotic exposure; >98% accuracy | Requires precise electrode configuration; fabrication complexity | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinamonti, D.; Vidic, J.; Maifreni, M.; Cossettini, A.; Leguillier, V.; Manzano, M. Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems. Micromachines 2025, 16, 934. https://doi.org/10.3390/mi16080934
Pinamonti D, Vidic J, Maifreni M, Cossettini A, Leguillier V, Manzano M. Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems. Micromachines. 2025; 16(8):934. https://doi.org/10.3390/mi16080934
Chicago/Turabian StylePinamonti, Debora, Jasmina Vidic, Michela Maifreni, Alessia Cossettini, Vincent Leguillier, and Marisa Manzano. 2025. "Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems" Micromachines 16, no. 8: 934. https://doi.org/10.3390/mi16080934
APA StylePinamonti, D., Vidic, J., Maifreni, M., Cossettini, A., Leguillier, V., & Manzano, M. (2025). Water-Mediated Dissemination and Detection of Antibiotic Resistance Across Livestock, Agri-Food, and Aquaculture Systems. Micromachines, 16(8), 934. https://doi.org/10.3390/mi16080934






