Optimization and Experimental Analysis of Electroless Nickel Plating on the Diamond Surface
Abstract
1. Introduction
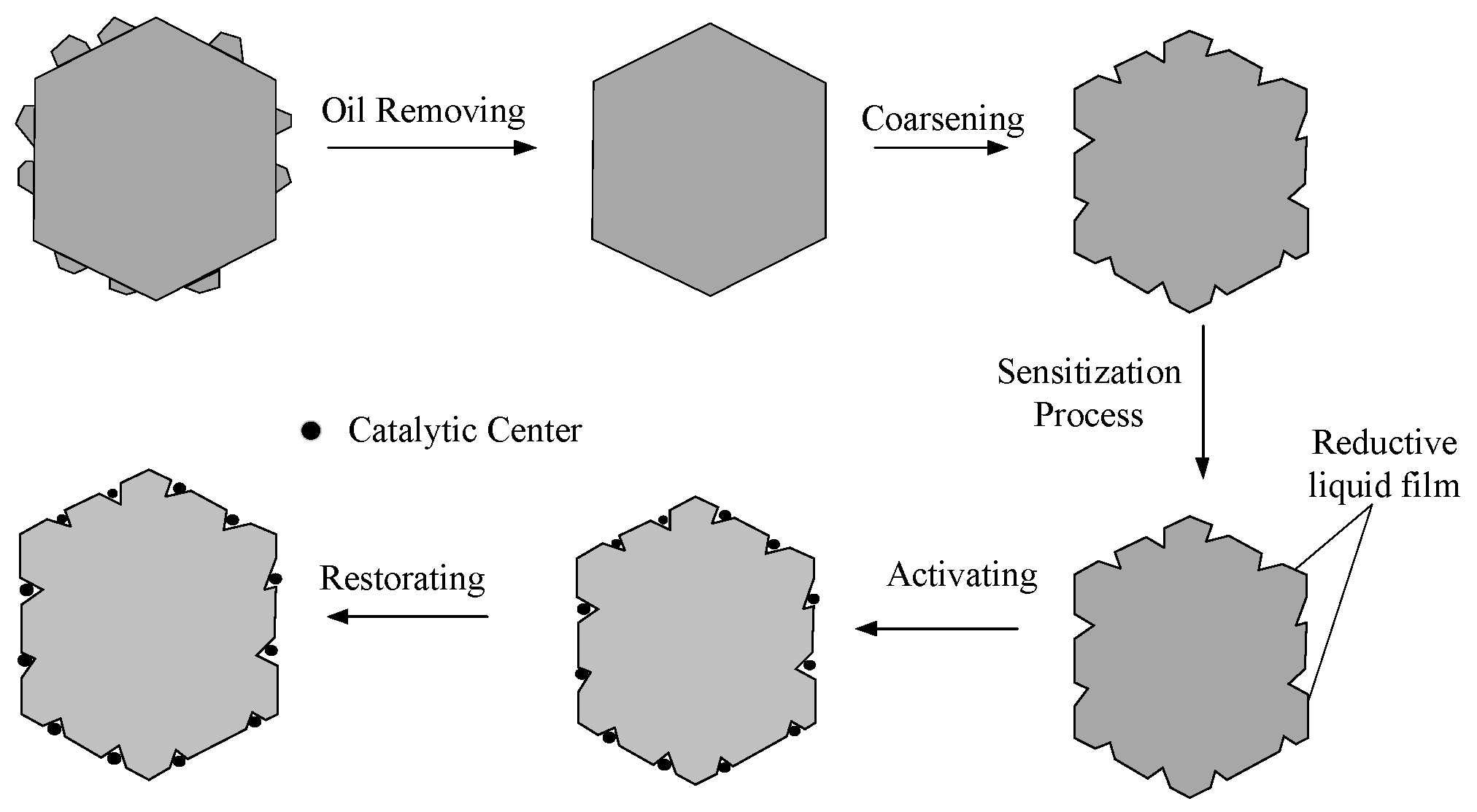
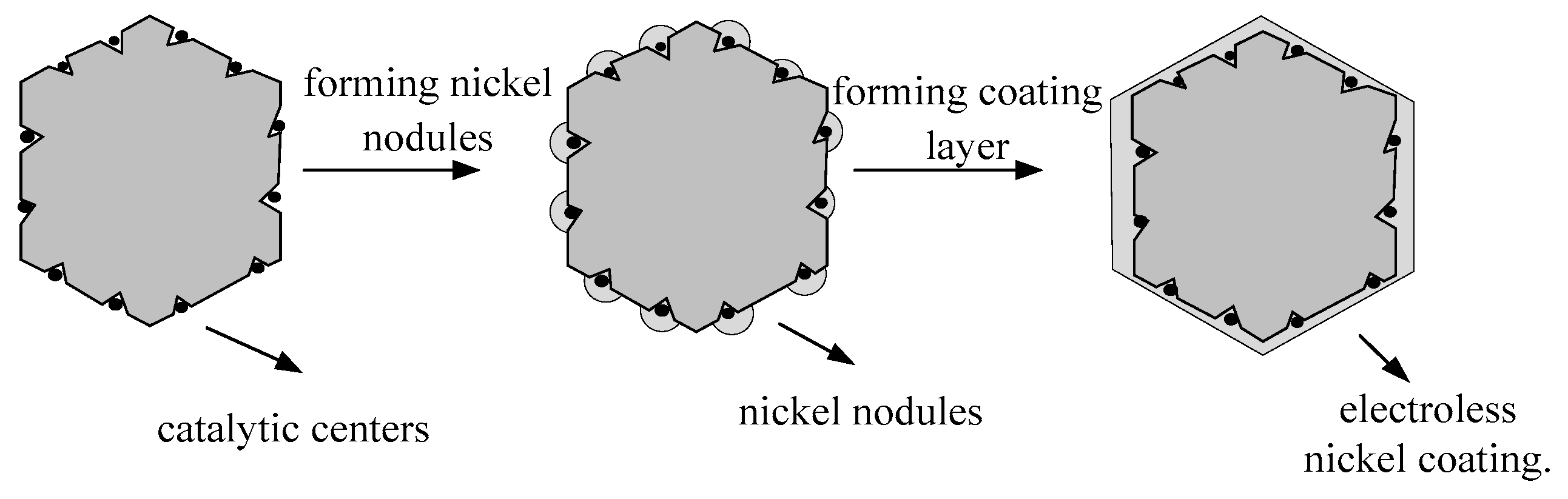
2. Methods and Principles
3. Selection of Activating Agents
4. Experimental Design
4.1. Composition of Electroless Nickel Plating Solution
- (1)
- Weigh an appropriate amount of citric acid and dissolve it in 50 mL of ultrapure water. Continue heating and stirring until the solution becomes transparent. Subsequently, add an appropriate amount of sodium citrate, heat and stir until dissolved to obtain a transparent solution, designated as solution a.
- (2)
- Weigh an appropriate amount of NiSO4·6H2O and dissolve it in 50 mL of ultrapure water to obtain solution b. Subsequently, gradually add solution a to solution b, resulting in solution c.
- (3)
- Weigh an appropriate amount of sodium hypophosphite and dissolve it in 50 mL of ultrapure water, stirring continuously to ensure complete dissolution, thereby obtaining solution d. Subsequently, add solution d to solution c and heat while stirring to dissolve, resulting in solution e.
- (4)
- Add an appropriate amount of ammonia solution to solution d, continue heating and stirring until complete dissolution is achieved. Subsequently, introduce an adequate quantity of Sodium Dodecyl Benzene Sulfate (SDBS) and thiourea, and stir for 5 min to obtain the electroless plating solution f.
4.2. Design of the Experimental Scheme
4.3. Analysis of Preliminary Experimental Results
5. Results and Discussion
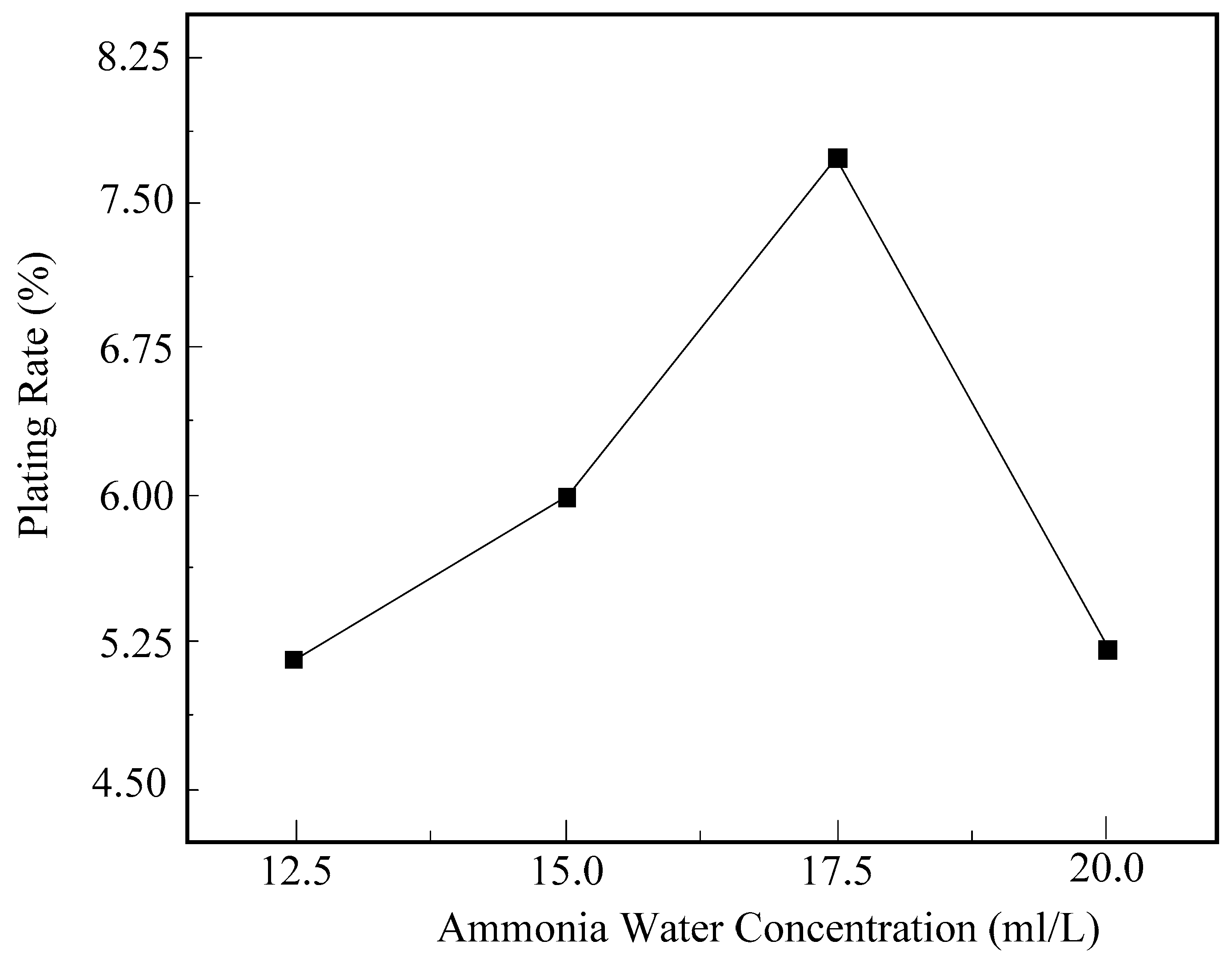
5.1. The Influence of Ammonia Concentration on Electroless Nickel Plating
5.1.1. The Influence of Ammonia Concentration on the Surface Plating Ratio
5.1.2. The Influence of Ammonia Concentration on the Microscopic Morphology of the Coating
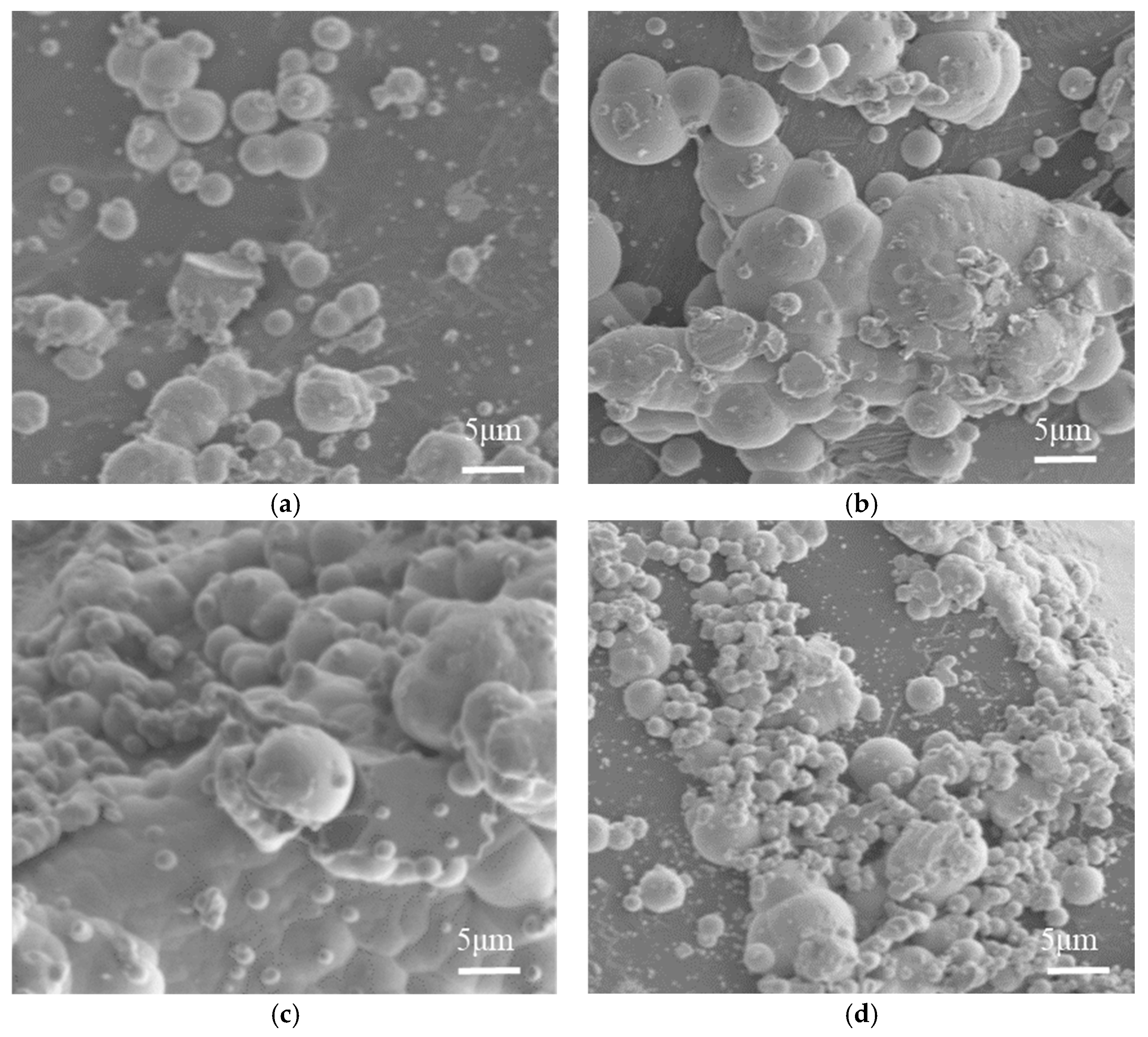
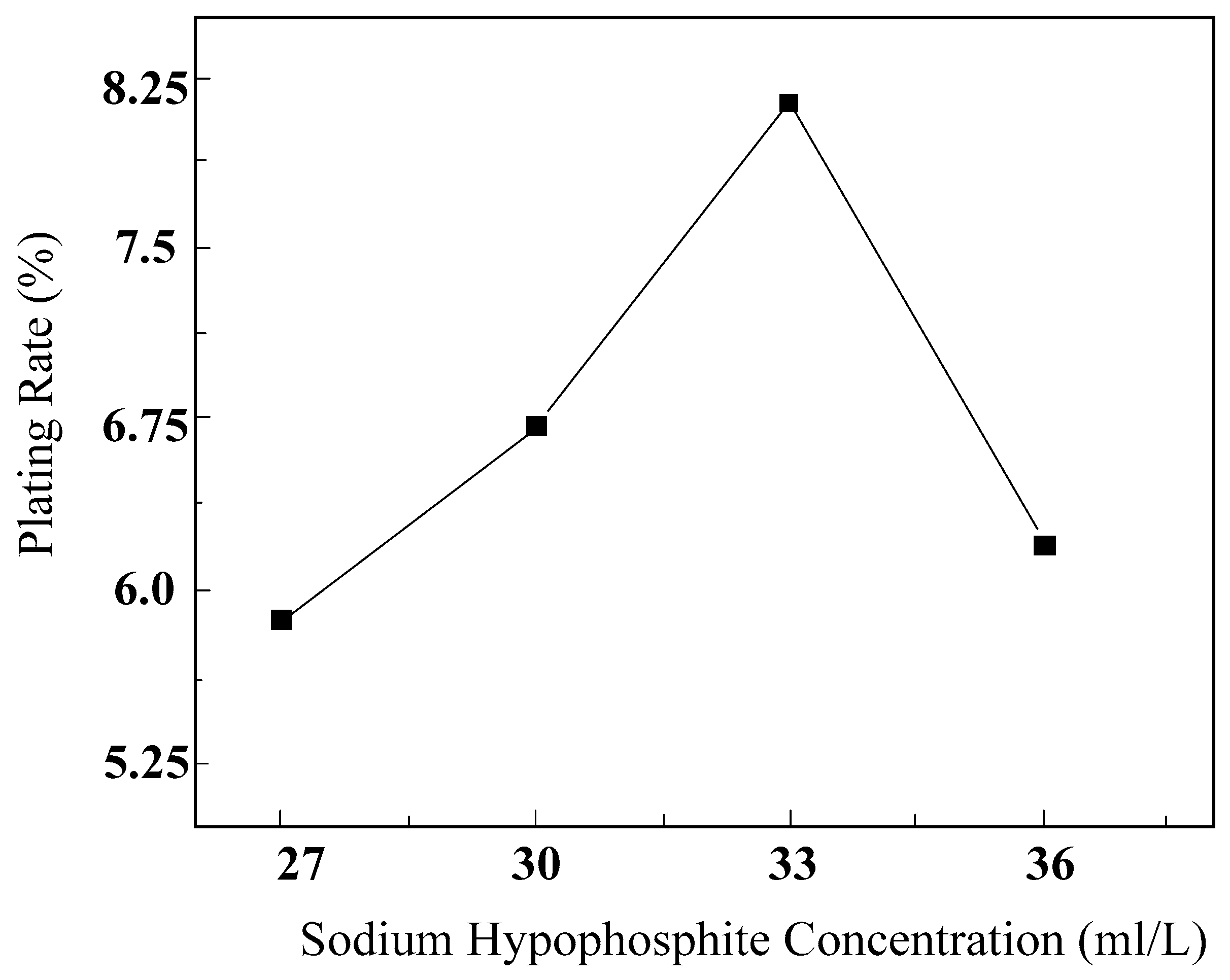
5.2. The Influence of Sodium Hypophosphite Concentration on Electroless Nickel Plating
5.2.1. The Influence of Sodium Hypophosphite Concentration on the Plating Efficiency
5.2.2. The Influence of Sodium Hypophosphite Concentration on the Microscopic Morphology of the Coating
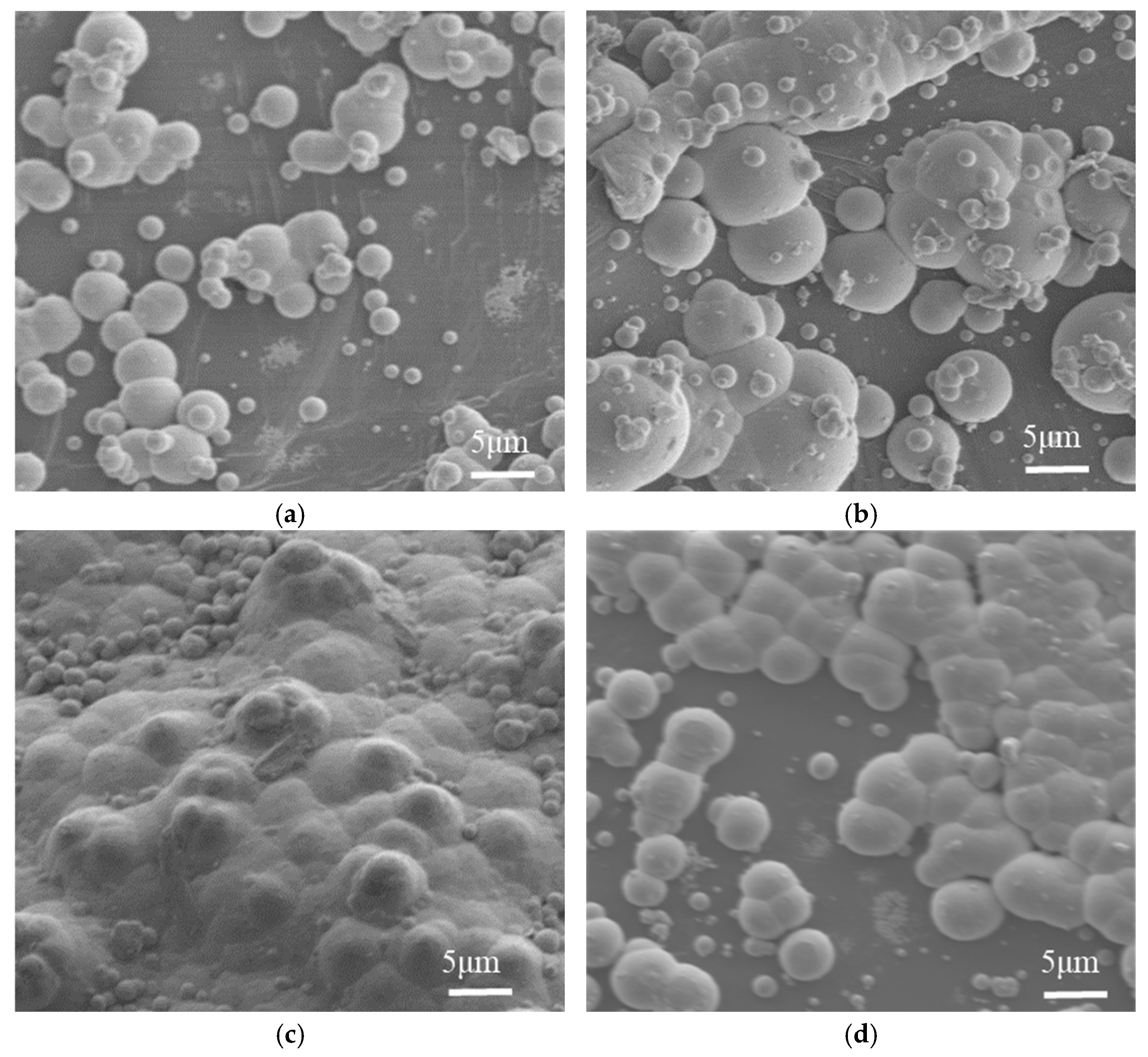
5.3. The Influence of Plating Temperature on Electroless Nickel Plating
5.3.1. The Influence of Plating Temperature on the Surface Plating Ratio
5.3.2. The Influence of Plating Temperature on the Microstructure of the Coating
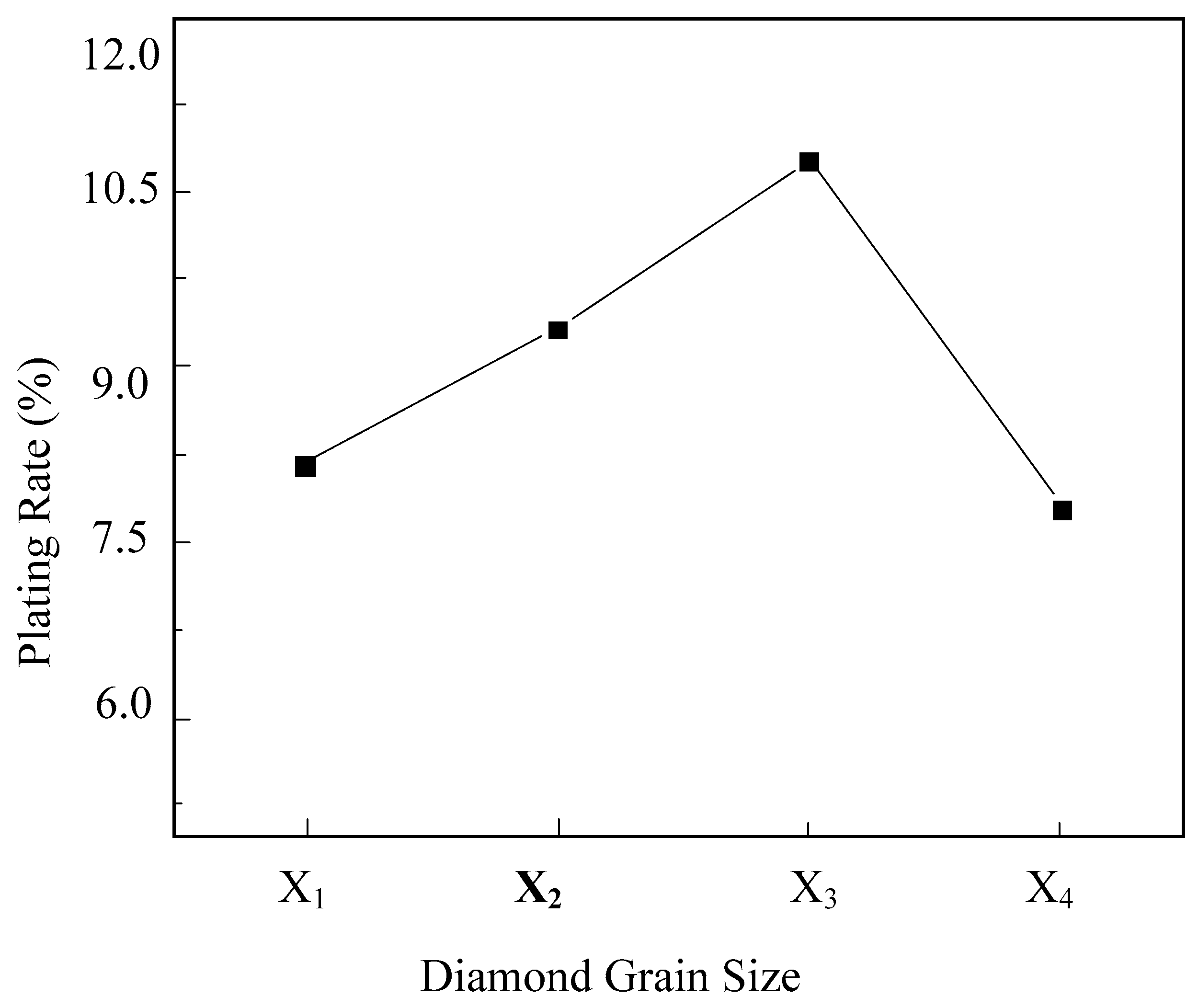
5.4. The Influence of Diamond Grain Size on Electroless Nickel Plating
6. Conclusions
- (1)
- Silver nitrate was employed as the activator for the pretreatment of diamond surfaces prior to electroless nickel plating. The activation effects of two activation solutions, namely a 2 g/L AgNO3 solution and a mixture of a 2 g/L AgNO3 solution and 10 mL/L NH3·H2O, were investigated. The results indicated that the combination of a 2 g/L AgNO3 solution and 10 mL/L NH3·H2O as the activation solution achieved a higher surface plating ratio on electroless nickel-plated diamonds, with a greater number of nickel nodules formed on the plating surface. This demonstrated the feasibility of using silver nitrate as an activator in the pretreatment of diamond surfaces.
- (2)
- An activation solution consisting of AgNO3 2 g/L and NH3·H2O 10 mL/L was employed for the pretreatment of diamond surfaces, facilitating the investigation of electroless nickel plating on diamonds. The results of orthogonal experiments indicated that the primary and secondary factors affecting the process were in the following order: plating temperature > ammonia concentration > sodium hypophosphite concentration. The findings from single-factor experiments revealed that as the plating temperature, ammonia concentration, and sodium hypophosphite concentration increased, the surface plating ratio of electroless nickel on diamonds, as well as the quantity and size of nickel nodules formed on the diamond surface, exhibited an initial increase followed by a decrease.
- (3)
- Based on the analysis of the experimental results, the optimal process conditions were determined as follows: an ammonia solution concentration of 17.5 mL/L, a hypophosphite concentration of 33 g/L, and a plating temperature of 80 °C. Under these process conditions, the highest deposition rate of electroless nickel on diamonds was achieved, and a continuous and dense coating layer was formed on the diamond surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, Z.; Yang, L.; Zeng, Q.; Lou, H.; Sheng, H.; Wen, J.; Miller, D.; Meng, Y.; Yang, W.; Mao, W.; et al. Synthesis of quenchable amorphous diamond. Nat. Commun. 2017, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Liu, Y.; Lu, X.; Tang, W. Effect of Laser Ablation on Microwave Attenuation Properties of Diamond Films. Materials 2019, 12, 3700. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, J.; Lei, Y.; Wang, X.; Fu, W.; Song, X.; Long, W. Micropatterning of synthetic diamond by metal contact etching with Ti powder. Diam. Relat. Mater. 2022, 129, 109299. [Google Scholar] [CrossRef]
- Kondrin, M.; Lebed, Y.; Brazhkin, V. Intrinsic planar defects in diamond and the upper limit on its melting temperature. Diam. Relat. Mater. 2020, 110, 108114. [Google Scholar] [CrossRef]
- Peng, J.W.; Zhang, F.L.; Wu, Y.X.; Zhou, Y.M.; Tang, H.Q. Wear evolution of metal bond diamond tool in grinding of sapphire. Diam. Relat. Mater. 2023, 137, 110131. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Duan, L.; Tan, S.; Fang, X. Effect of Fe-based pre-alloyed powder on the microstructure and holding strength of impregnated diamond bit matrix. Int. J. Refract. Met. Hard Mater. 2019, 79, 115–122. [Google Scholar] [CrossRef]
- Suzuki, T.; Konno, T. Improvement in tool life of electroplated diamond tools by Ni-based carbon nanotube composite coatings. Precis. Eng. 2014, 38, 659–665. [Google Scholar] [CrossRef]
- Du, Q.; Wang, X.; Zhang, S.; Long, W.; Zhang, L.; Jiu, Y.; Yang, C.; Zhang, Y.; Yang, J. Research status on surface metallization of diamond. Mater. Res. Express 2020, 6, 122005. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, D.; Lee, J.; Chung, H.; Kim, C.; Hai, H. Improving the adhesion of electroless-nickel coating layer on diamond powder. Surf. Coat. Technol. 2006, 201, 3793–3796. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Wei, C.; Xu, X.; Wei, B.; Chen, P.; Cheng, J. Titanium coating on the surface of diamond particles by a novel rapid low-temperature salt bath plating method. Chem. Phys. Lett. 2020, 761, 138091. [Google Scholar] [CrossRef]
- Das, M.K.; Liu, R.; Qin, J.; Zhang, X.; Das, K.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Li, R. Effect of electrodeposition conditions on structure and mechanical properties of Ni-W/diamond composite coatings. Surf. Coat. Technol. 2017, 309, 337–343. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Cui, W.; Qu, X. Preparation of copper–diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Tian, D.; Li, D.; Wang, F.; Xiao, N.; Liu, R.; Gao, W.; Li, N.; Li, Q.; Gao, W.; Wu, G. A Pd-free activation method for electroless nickel deposition on copper. Surf. Coat. Technol. 2013, 228, 27–33. [Google Scholar] [CrossRef]
- Luo, L.; Lu, Z.; Huang, X.; Tan, X.; Ding, X.; Cheng, J.; Zhu, L.; Wu, Y. Electroless copper plating on PC engineering plastic with a novel palladium free surface activation process. Surf. Coat. Technol. 2014, 251, 69–73. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, X. Environment-friendly Pd free surface activation technics for ABS surface. Appl. Surf. Sci. 2012, 258, 5328–5331. [Google Scholar] [CrossRef]
- Dong, Y.; He, X.; Ud-din, R.; Guo, C.; Xu, L.; Huang, Y.; Qu, X. Fabrication and thermal stability of Ni-P coated diamond powder using electroless plating. Int. J. Min. Met. Mater. 2011, 18, 479–486. [Google Scholar] [CrossRef]
- Ahn, J.; Jin, K.; Wu, J.; Hai, H.; Lee, J.; Saeng, C. Charateristics of Nickel-Diamond Composite Powders by Eletroless Plating. J. Korean. Powe. Met. 2004, 11, 233–241. [Google Scholar]
- Liu, P.; Zhu, Y. Interaction Between Fine Diamond Particles in Electroless Nickel Solutions. J. Dispersion Sci. Technol. 2015, 36, 1170–1177. [Google Scholar] [CrossRef]
- Salvaga, G.; Cavallotti, P. Characteristics of the Reduction of Nickel Alloys with HypopHoslHite. Plating 1972, 59, 665–671. [Google Scholar]
- Manik, B.; Tapan, K.B.; Prasanta, S. Effect of Coating Bath Parameters on Properties of Electroless Nickel-Boron Alloy Coatings. Int. J. Surf. Eng. Interdiscip. Mater. Sci. (IJSEIMS) 2022, 10, 1–26. [Google Scholar]
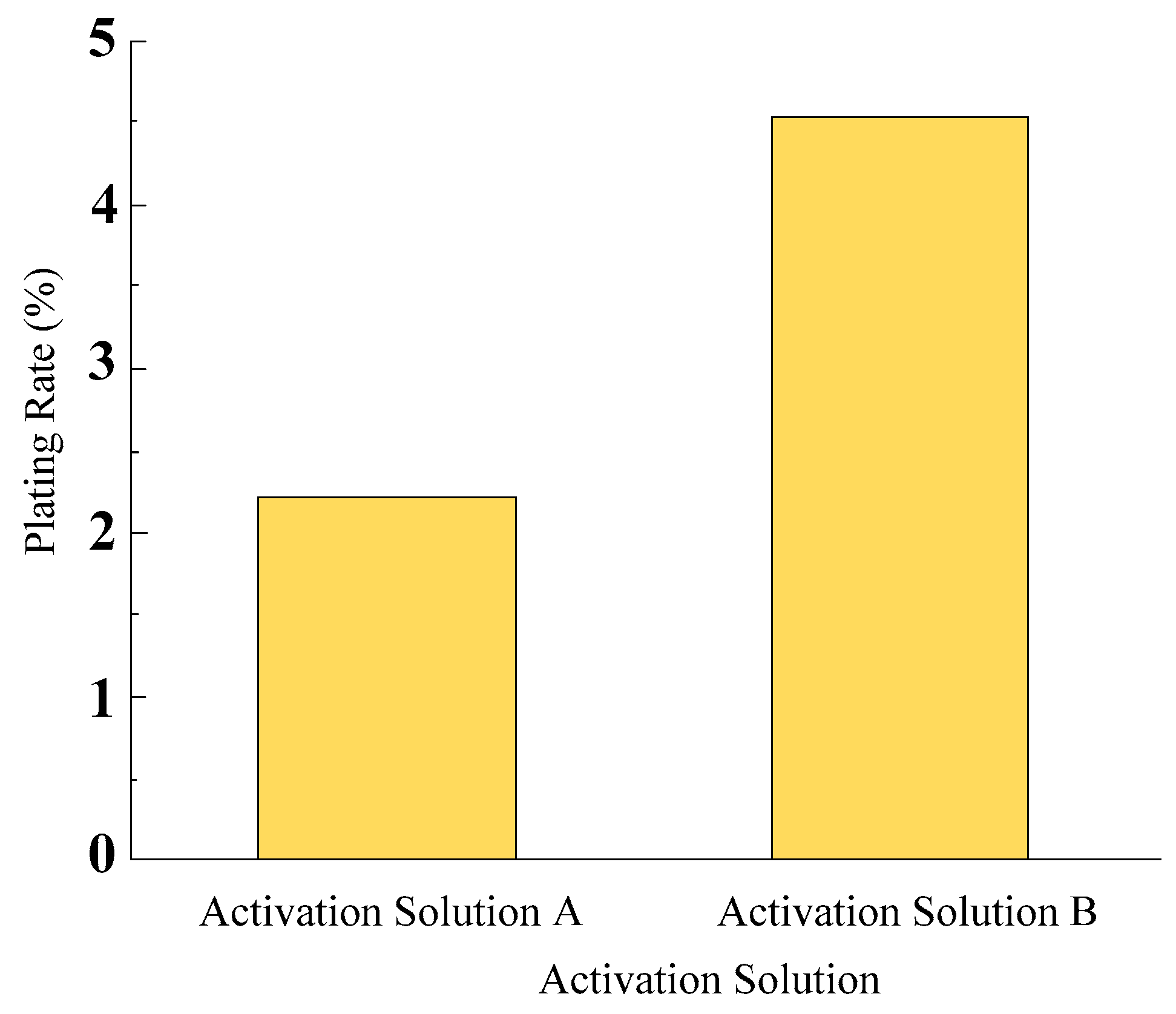



| Activation Solution | Chemical Composition |
|---|---|
| A | AgNO3 (2 g/L) |
| B | AgNO3 (2 g/L) + NH3·H2O (10 mL/L) |
| Components | Medicine | Concentration | Role |
|---|---|---|---|
| Metal salt | Nickel Sulfate | 25 g/L | Provision of metal ions |
| Reducing agent | Sodium Hypophosphite | 33 g/L | Reduction in metal ions |
| Sodium Citrate | 12.5 g/L- | ||
| Complexant | Ammonia solution | 17.5 mL/L | Prevent the precipitation of precipitates from the plating solution |
| Citric Acid | 20 g/L | ||
| Stabilizer | Thiourea | 1.4 mg/L | Stable plating solution |
| Dispersant | Sodium Dodecyl Benzene Sulfonate | 1 g/L | Improving the phenomenon of particles |
| Factors | A | B | C | |
|---|---|---|---|---|
| Ammonia Solution Concentration | Sodium Hypophosphite Concentration | Plating Temperature | ||
| Levels | (mL/L) | (g/L) | (°C) | |
| 1 | 15 | 30 | 75 | |
| 2 | 17.5 | 33 | 80 | |
| 3 | 20 | 36 | 85 | |
| Factors | Ammonia Solution Concentration | Sodium Hypophosphite Concentration | Plating Temperature | Surface Plating Ratio | |
|---|---|---|---|---|---|
| Study Number | (mL/L) | (g/L) | (°C) | (%) | |
| 1 | 15 | 30 | 75 | 4.56 | |
| 2 | 15 | 33 | 80 | 6.72 | |
| 3 | 15 | 36 | 85 | 5.54 | |
| 4 | 17.5 | 30 | 80 | 7.74 | |
| 5 | 17.5 | 33 | 85 | 7.13 | |
| 6 | 17.5 | 36 | 75 | 5.83 | |
| 7 | 20 | 30 | 85 | 6.71 | |
| 8 | 20 | 33 | 75 | 5.75 | |
| 9 | 20 | 36 | 80 | 6.32 | |
| Factors | A | B | C | |
|---|---|---|---|---|
| Levels | ||||
| R1 | 5.61 | 6.33 | 5.38 | |
| R2 | 6.9 | 6.53 | 6.93 | |
| R3 | 6.26 | 5.89 | 6.46 | |
| range | 1.29 | 0.64 | 1.55 | |
| Factors | Ammonia Solution (mL/L) | Nickel Sulfate (g/L) | Sodium Hypophosphite (g/L) | Citric Acid (g/L) | Sodium Citrate (g/L) | SDBS (g/L) | Thiourea (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Number | ||||||||
| 1 | 12.5 | 25 | 33 | 20 | 12.5 | 1 | 1.4 | |
| 2 | 15 | 25 | 33 | 20 | 12.5 | 1 | 1.4 | |
| 3 | 17.5 | 25 | 33 | 20 | 12.5 | 1 | 1.4 | |
| 4 | 20 | 25 | 33 | 20 | 12.5 | 1 | 1.4 | |
| Factors | Nickel Sulfate (g/L) | Sodium Hypophosphite (g/L) | Citric Acid (g/L) | Sodium Citrate (g/L) | Ammonia Solution (mL/L) | SDBS (g/L) | Thiourea (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Number | ||||||||
| 1 | 25 | 27 | 20 | 12.5 | 17.5 | 1 | 1.4 | |
| 2 | 25 | 30 | 20 | 12.5 | 17.5 | 1 | 1.4 | |
| 3 | 25 | 33 | 20 | 12.5 | 17.5 | 1 | 1.4 | |
| 4 | 25 | 36 | 20 | 12.5 | 17.5 | 1 | 1.4 | |
| Chemical Composition | Concentration |
|---|---|
| Nickel Sulfate | 25 g/L |
| Citric Acid | 20 g/L |
| Sodium Citrate | 12.5 g/L |
| Thiourea | 1.4 mg/L |
| SDBS | 1 g/L |
| Ammonia solution | 17.5 mL/L |
| Sodium Hypophosphite | 33 g/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Su, G.; Zhu, C.; Qi, H.; Li, P.; Shen, X.; Zhang, C.; Cheng, K. Optimization and Experimental Analysis of Electroless Nickel Plating on the Diamond Surface. Micromachines 2025, 16, 709. https://doi.org/10.3390/mi16060709
Fan Q, Su G, Zhu C, Qi H, Li P, Shen X, Zhang C, Cheng K. Optimization and Experimental Analysis of Electroless Nickel Plating on the Diamond Surface. Micromachines. 2025; 16(6):709. https://doi.org/10.3390/mi16060709
Chicago/Turabian StyleFan, Qingming, Guokang Su, Congmin Zhu, Hui Qi, Pengfan Li, Xiumei Shen, Chuanyun Zhang, and Kai Cheng. 2025. "Optimization and Experimental Analysis of Electroless Nickel Plating on the Diamond Surface" Micromachines 16, no. 6: 709. https://doi.org/10.3390/mi16060709
APA StyleFan, Q., Su, G., Zhu, C., Qi, H., Li, P., Shen, X., Zhang, C., & Cheng, K. (2025). Optimization and Experimental Analysis of Electroless Nickel Plating on the Diamond Surface. Micromachines, 16(6), 709. https://doi.org/10.3390/mi16060709






