An Updated Review of BiCuSeO-Based Thermoelectric Materials
Abstract
1. Introduction
2. The Basic Properties of BiCuSeO
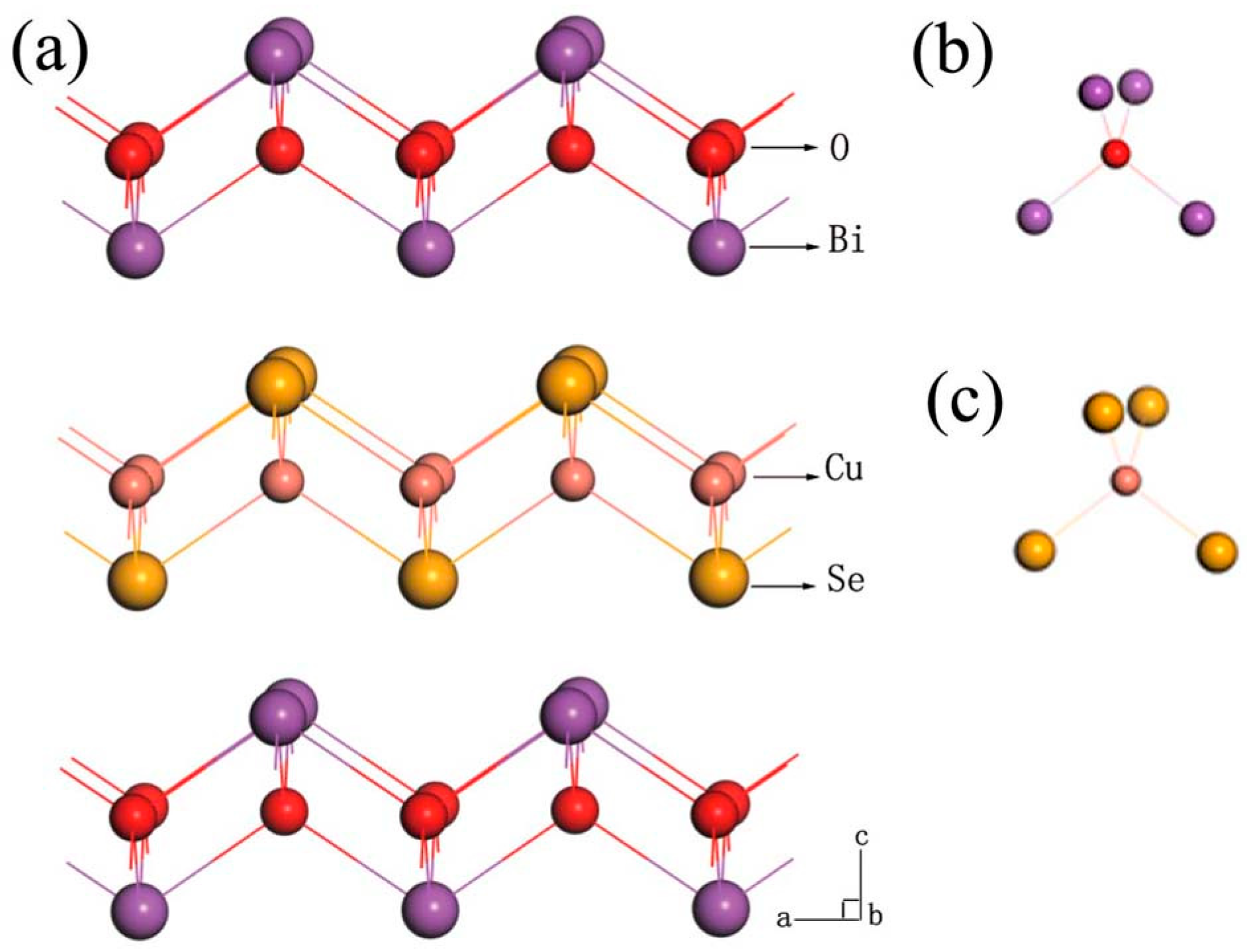
2.1. The Special Natural Super-Lattice Structure of BiCuSeO
2.2. The Vickers Hardness of BiCuSeO
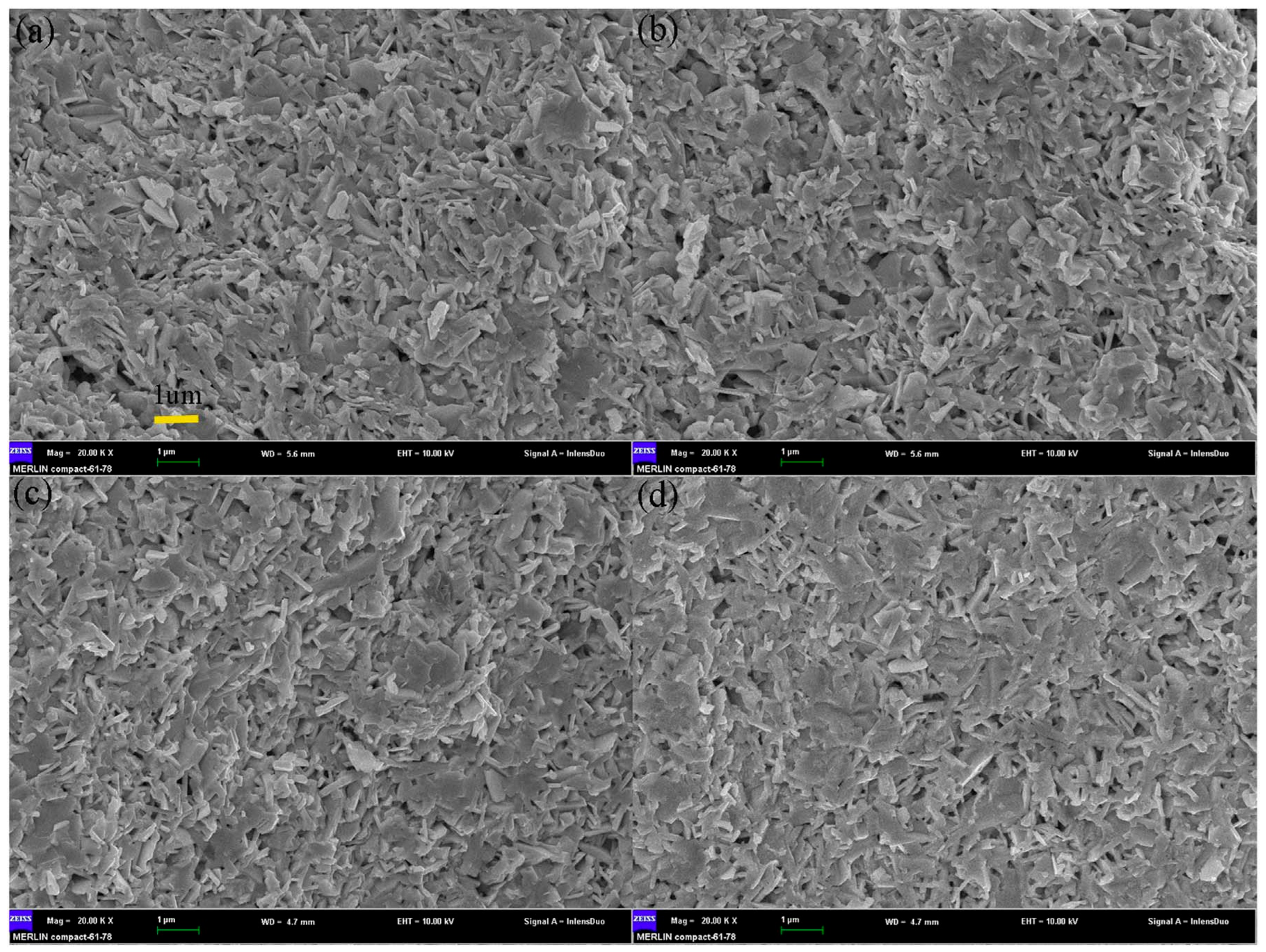
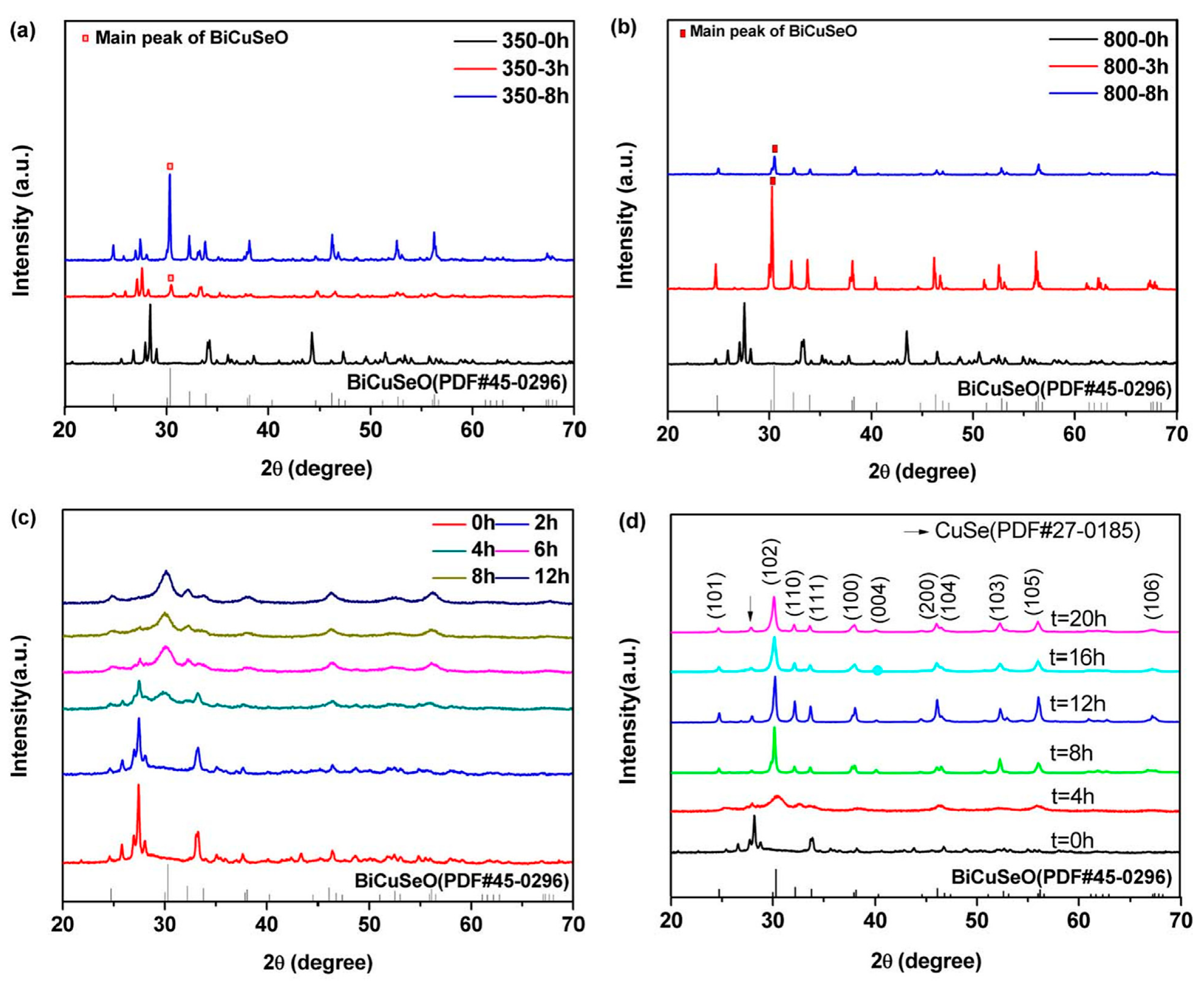
3. The Preparation Methods of BiCuSeO
4. The Composition Adjustments of BiCuSeO
4.1. Low-Valence Element Doping for BiCuSeO
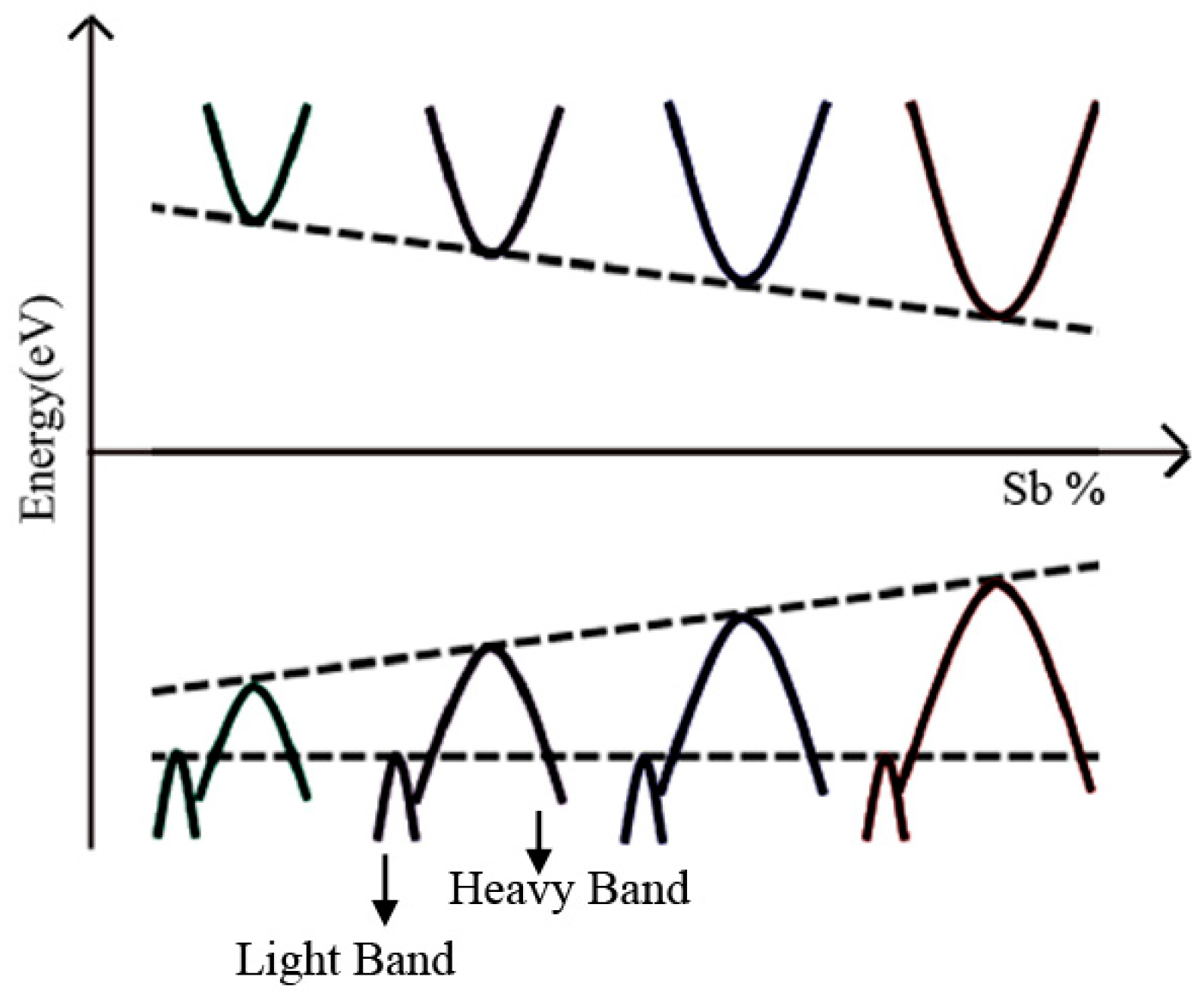
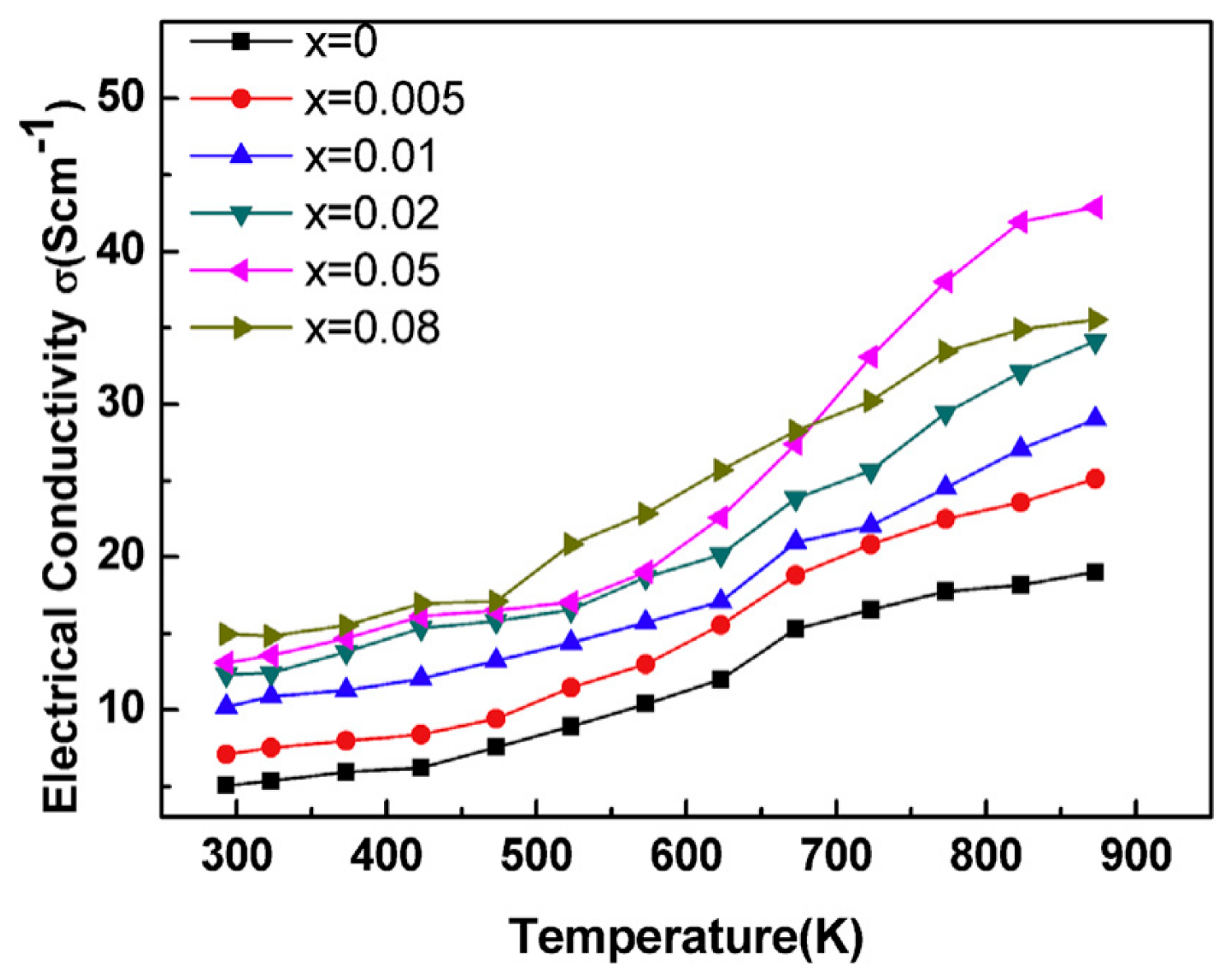
4.2. Equivalent Valence Element Doping for BiCuSeO
4.3. Chemical Defect Engineering of BiCuSeO
4.4. Dual Doping/Vacancies for BiCuSeO
4.5. The Research Status of N-Type BiCuSeO
4.6. Structural Adjustment for BiCuSeO
5. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Majumdar, A. Thermoelectric devices: Helping chips to keep their cool. Nat. Nanotechnol. 2009, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Macklin, W.J.; Moseley, P.T. On the use of oxides for thermoelectric refrigeration. Mater. Sci. Eng. B 1990, 7, 111–117. [Google Scholar] [CrossRef]
- Funahashi, R. Waste Heat Recovery Using Thermoelectric Oxide Materials. Sci. Adv. Mater. 2011, 3, 682–686. [Google Scholar] [CrossRef]
- Funahashi, R.; Urata, S.; Mihara, T. Power Generation Using Oxide Thermoelectric Modules. Adv. Sci. Technol. 2006, 46, 158–167. [Google Scholar]
- Gu, Y.; Shi, X.L.; Pan, L.; Liu, W.D.; Sun, Q.; Tang, X.; Kou, L.Z.; Liu, Q.F.; Wang, Y.F.; Chen, Z.G. Rational electronic and structural designs advance BiCuSeO thermoelectrics. Adv. Funct. Mater. 2021, 31, 2101289. [Google Scholar] [CrossRef]
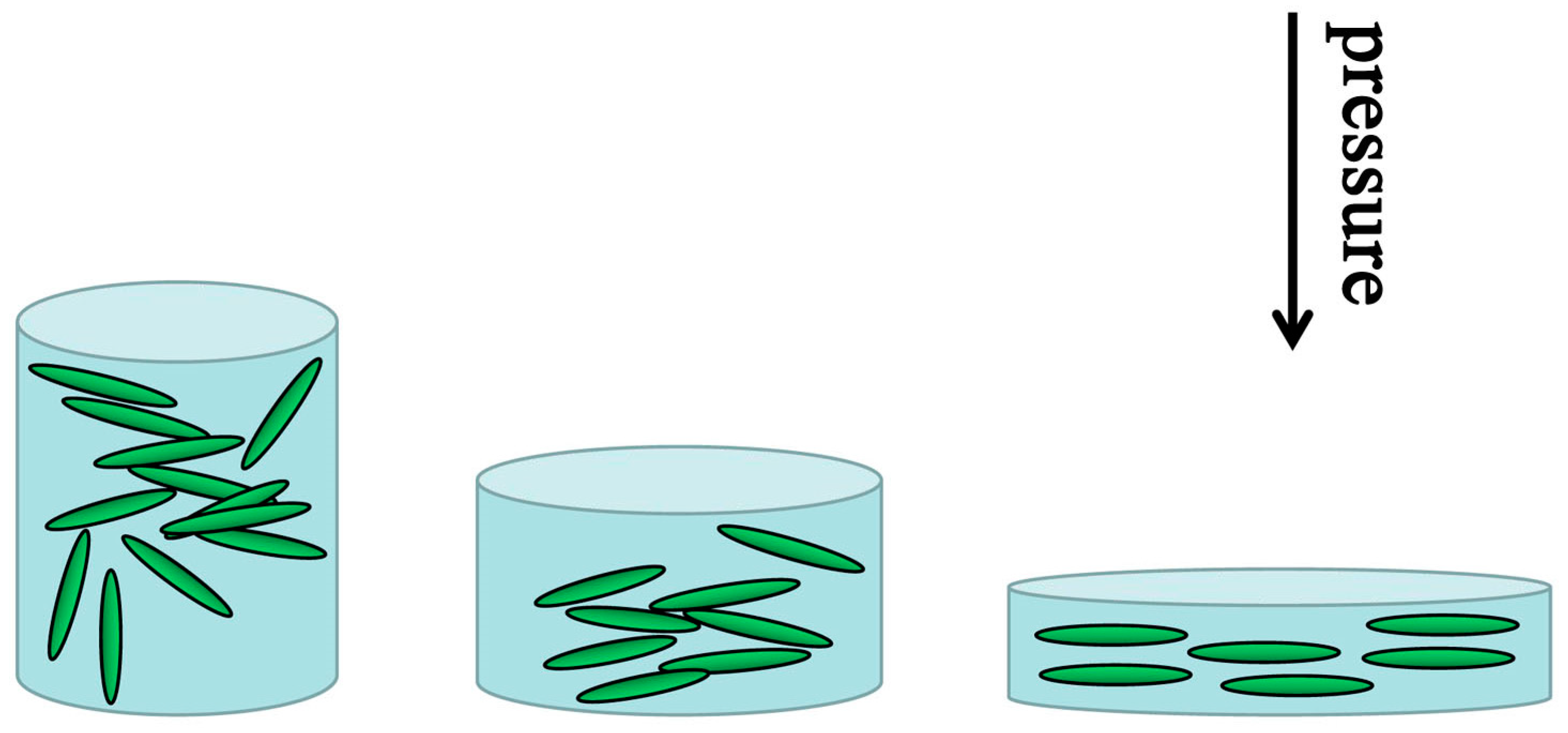
- Yin, Z.; Zhang, H.; Wang, Y.; Wu, Y.; Wang, X.; Fang, X.; Yu, Y.; Guo, X. Ultrahigh-pressure structural modification in BiCuSeO ceramics: Dense dislocations and exceptional thermoelectric performance. Adv. Energy Mater. 2025, 15, 2403174. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Ge, G.; Yang, J. Enhanced Power Factor and Ultralow Lattice Thermal Conductivity Induced High Thermoelectric Performance of BiCuTeO/BiCuSeO Superlattice. Materials 2023, 16, 4318. [Google Scholar] [CrossRef]
- Li, F.; Li, J.F.; Zhao, L.D.; Liu, Y.; Zhang, B.P.; Lin, Y.H.; Nan, C.W.; Zhu, H.M. Polycrystalline BiCuSeO oxide as a potential thermoelectric material. Energy Environ. Sci. 2012, 5, 7188–7195. [Google Scholar] [CrossRef]
- Zhao, L.D.; He, J.; Berardan, D.; Lin, Y.; Li, J.F.; Nan, C.W.; Dragoe, N. BiCuSeO oxyselenides: New promising thermoelectric materials. Energy Environ. Sci. 2014, 7, 2900–2924. [Google Scholar] [CrossRef]
- Barreteau, C.; Pan, L.; Amzallag, E.; Zhao, L.D.; Bérardan, D.; Dragoe, N. Layered oxychalcogenide in the Bi–Cu–O–Se system as good thermoelectric materials. Semicond. Sci. Technol. 2014, 29, 064001. [Google Scholar] [CrossRef]
- Lan, J.L.; Liu, Y.C.; Zhan, B.; Lin, Y.H.; Zhang, B.; Yuan, X.; Zhang, W.; Xu, W.; Nan, C.W. Enhanced thermoelectric properties of Pb-doped BiCuSeO ceramics. Adv. Mater. 2013, 25, 5086–5090. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yu, H.; Pei, Y.; Chen, Y. Resonant doping in BiCuSeO thermoelectrics from first principles. J. Mater. Chem. A 2017, 5, 931–936. [Google Scholar] [CrossRef]
- Feng, D.; Zheng, F.; Wu, D.; Wu, M.; Li, W.; Huang, L.; Zhao, L.D.; He, J. Investigation into the Extremely Low Thermal Conductivity in Ba heavily doped BiCuSeO. Nano Energy 2016, 27, 167–174. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Lan, J.; Xu, W.; Li, F.; Zhang, B.P.; Berardan, D.; Dragoe, N.; Lin, Y.H.; Nan, C.W. Remarkable Enhancement in Thermoelectric Performance of BiCuSeO by Cu Deficiencies. J. Am. Chem. Soc. 2011, 133, 20112–20115. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Hou, Y.; Zhang, C.; Jiang, C.; Hu, J.; Li, Y.; He, Z.; Fan, X.A. Enhanced thermoelectric properties of Sb-doped BiCuSeO due to decreased band gap. J. Alloys Compd. 2017, 712, 386–393. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Pan, Z.; Hu, X.; Liu, P.; Li, Y.; He, Z.; Fan, X.A. Enhancing thermoelectric and mechanical performances in BiCuSeO by increasing bond covalency and nanostructuring. J. Solid State Chem. 2018, 265, 306–313. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.Z.; Zhu, Y.; Liu, Y.; Li, F.; Yu, M.; Liu, D.B.; Xu, W.; Lin, Y.H.; Nan, C.W. Synergistically Optimizing Electrical and Thermal Transport Properties of BiCuSeO via a Dual-Doping Approach. Adv. Energy Mater. 2016, 6, 1502423. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Q.Y.; Zhang, S.D. Carrier mobility and relaxation time in BiCuSeO. Phys. Lett. A 2019, 383, 125990. [Google Scholar] [CrossRef]
- Sui, J.; Li, J.; He, J.; Pei, Y.L.; Berardan, D.; Wu, H.; Dragoe, N.; Cai, W.; Zhao, L.D. Texturation boosts the thermoelectric performance of BiCuSeO oxyselenides. Energy Environ. Sci. 2013, 6, 2916–2920. [Google Scholar] [CrossRef]
- Novitskii, A.P.; Voronin, A.I.; Usenko, A.A.; Gorshenkov, M.V.; Khovaylo, V.V.; Shvanskaya, L.V.; Burkov, A.T.; Vasiliev, A.N. Influence of Sodium Fluoride Doping on Thermoelectric Properties of BiCuSeO. J. Electron. Mater. 2016, 45, 1705–1710. [Google Scholar] [CrossRef]
- Lan, J.L.; Deng, C.; Ma, W.; Ren, G.K.; Lin, Y.H.; Yang, X. Ultra-fast synthesis and high thermoelectric properties of heavy sodium doped BiCuSeO. J. Alloys Compd. 2017, 708, 955–960. [Google Scholar] [CrossRef]
- Ren, G.K.; Lan, J.L.; Butt, S.; Ventura, K.J.; Lin, Y.H.; Nan, C.W. Enhanced thermoelectric properties in Pb-doped BiCuSeO oxyselenides prepared by ultrafast synthesis. RSC Adv. 2015, 5, 69878. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Yan, Y.; Hu, T.; He, J.; Uher, C.; Kanatzidis, M.G.; Tang, X. Manipulating the Combustion Wave during Self-Propagating Synthesis for High Thermoelectric Performance of Layered Oxychalcogenide Bi1–xPbxCuSeO. Chem. Mater. 2016, 28, 4628–4640. [Google Scholar] [CrossRef]
- Achour, A.; Chen, K.; Reece, M.J.; Huang, Z. Enhanced thermoelectric performance of Cs doped BiCuSeO prepared through eco-friendly flux synthesis. J. Alloys Compd. 2017, 735, 861–869. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Yu, M.; Wang, D.; Qin, J.; Xue, X. Effect of high pressure on structure characteristics and electrical transport properties of layered BiCuSeO oxyselenides. J. Alloys Compd. 2020, 812, 152106. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Liang, X. Thermoelectric properties of Bi1-xSnxCuSeO solid solutions. Dalton Trans. 2017, 46, 2510–2515. [Google Scholar] [CrossRef]
- Luu, S.; Vaqueiro, P. Thermoelectric properties of BiOCu1-xMxSe (M = Cd and Zn). Semicond. Sci. Technol. 2014, 29, 064002. [Google Scholar] [CrossRef]
- Jing, L.I.; Sui, J.; Dragoe, N.; Cai, W.; Pei, Y.; Zhao, L.D. Thermoelectric properties of Mg doped p-type BiCuSeO oxyselenides. J. Alloys Compd. 2013, 551, 649–653. [Google Scholar]
- Pei, Y.L.; He, J.; Li, J.F.; Li, F.; Liu, Q.; Pan, W.; Barreteau, C.; Berardan, D.; Dragoe, N.; Zhao, L.D. High thermoelectric performance of oxyselenides: Intrinsically low thermal conductivity of Ca-doped BiCuSeO. NPG Asia Mater. 2013, 5, e47. [Google Scholar] [CrossRef]
- Li, F.; Wei, T.R.; Kang, F.; Li, J.F. Enhanced thermoelectric performance of Ca-doped BiCuSeO in a wide temperature range. J. Mater. Chem. A 2013, 1, 11942–11949. [Google Scholar] [CrossRef]
- Barreteau, C.; Bérardan, D.; Amzallag, E.; Zhao, L.; Dragoe, N. Structural and electronic transport properties in Sr-doped BiCuSeO. Chem. Mater. 2012, 24, 3168–3178. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.H.; Hong, H.Y.; Jung, G.W.; Pi, J.W. Influence of Ba2+ doping on the thermoelectric properties of BiCuSeO fabricated by spark plasma sintering. Ceram. Int. 2019, 45, 9604–9610. [Google Scholar] [CrossRef]
- Li, J.; Sui, J.; Pei, Y.; Berardan, D.; Dragoe, N.; Cai, W.; He, J.; Zhao, L.D. A high thermoelectric figure of merit ZT> 1 in Ba heavily doped BiCuSeO oxyselenides. Energy Environ. Sci. 2012, 5, 8543–8547. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Jiang, Q.; Fu, L. Multi-role of Sodium Doping in BiCuSeO on High Thermoelectric Performance. J. Electron. Mater. 2015, 44, 2849–2855. [Google Scholar] [CrossRef]
- Li, J.; Sui, J.; Pei, Y.; Meng, X.; Berardan, D.; Dragoe, N.; Cai, W.; Zhao, L.D. The roles of Na doping in BiCuSeO oxyselenides as a thermoelectric material. J. Mater. Chem. A 2014, 2, 4903–4906. [Google Scholar] [CrossRef]
- Lee, D.S.; An, T.H.; Jeong, M.; Choi, H.S.; Soo, L.Y.; Seo, W.S.; Park, C.H.; Park, C.; Park, H.H. Density of state effective mass and related charge transport properties in K-doped BiCuOSe. Appl. Phys. Lett. 2013, 103, 232110. [Google Scholar]
- Liu, Y.; Zheng, Y.; Zhan, B.; Chen, K.; Butt, S.; Zhang, B.; Lin, Y.H. Influence of Ag doping on thermoelectric properties of BiCuSeO. J. Eur. Ceram. Soc. 2015, 35, 845–849. [Google Scholar] [CrossRef]
- Farooq, M.U.; Butt, S.; Gao, K.; Pang, X.L.; Sun, X.; Mohmed, F.; Ahmad, A.; Mahmood, A.; Mahmood, N. Improved thermoelectric performance of BiCuSeO by Ag substitution at Cu site. J. Alloys Compd. 2017, 691, 572–577. [Google Scholar] [CrossRef]
- Pan, L.; Bérardan, D.; Zhao, L.; Barreteau, C.; Dragoe, N. Influence of Pb doping on the electrical transport properties of BiCuSeO. Appl. Phys. Lett. 2013, 102, 703. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lan, J.L.; Zhan, B.; Ding, J.; Liu, Y.; Lin, Y.H.; Zhang, B.; Nan, C.W. Thermoelectric Properties of Pb-Doped BiCuSeO Ceramics. J. Am. Ceram. Soc. 2013, 96, 2710–2713. [Google Scholar] [CrossRef]
- Ren, G.K.; Wang, S.; Zhou, Z.; Li, X.; Yang, J.; Zhang, W.; Lin, Y.H.; Yang, J.; Nan, C.W. Complex electronic structure and compositing effect in high performance thermoelectric BiCuSeO. Nat. Commun. 2019, 10, 2814. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Butt, S.; Zeng, C.; Liu, Y.; Zhan, B.; Lan, J.; Lin, Y.; Nan, C. Electrical and Thermal Transport Behavior in Zn-Doped BiCuSeO Oxyselenides. J. Electron. Mater. 2015, 44, 1627–1631. [Google Scholar] [CrossRef]
- Farooq, M.U.; Butt, S.; Gao, K.; Zhu, Y.; Sun, X.; Pang, X.; Khan, S.U.; Mohmed, F.; Mahmood, A.; Mahmood, N.; et al. Cd-doping a Facile Approach for Better Thermoelectric Transport Properties of BiCuSeO Oxyselenides. RSC Adv. 2016, 6, 33789–33797. [Google Scholar] [CrossRef]
- Kang, H.; Li, J.; Liu, Y.; Guo, E.; Chen, Z.; Liu, D.; Fan, G.; Zhang, Y.; Jiang, X.; Wang, T. Optimizing the thermoelectric transport properties of BiCuSeO via doping with the rare-earth variable-valence element Yb. J. Mater. Chem. C 2018, 6, 8479–8487. [Google Scholar] [CrossRef]
- Sayan, D.; Suneesh, M.V.; Chen, K.; Satyam, S.; Ramesh, M. Thermoelectric properties of Mn doped BiCuSeO. Mater. Res. Express 2019, 6, 086305. [Google Scholar]
- Barreteau, C.; Berardan, D.; Zhao, L.; Dragoe, N. Influence of Te substitution on the structural and electronic properties of thermoelectric BiCuSeO. J. Mater. Chem. A 2013, 1, 2921–2926. [Google Scholar] [CrossRef]
- Berardan, D.; Li, J.; Amzallag, E.; Mitra, S.; Sui, J.; Cai, W. Structure and Transport Properties of the BiCuSeO-BiCuSO Solid Solution. Materials 2015, 8, 1043–1058. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Xu, B.; Lan, J.; Zheng, Y.; Zhan, B.; Zhang, B.; Lin, Y.; Nan, C. Enhanced thermoelectric performance of La-doped BiCuSeO by tuning band structure. Appl. Phys. Lett. 2015, 106, 199–2713. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Hu, X.; Liu, P.; Li, R.; Zhang, Y.; Li, Y.; He, Z.; Fan, X.A. Enhancement of the thermoelectric properties of BiCuSeO via in doping and powder size controlling. J. Electron. Mater. 2020, 49, 611–620. [Google Scholar] [CrossRef]
- Lei, J.; Guan, W.; Zhang, D.; Ma, Z.; Yang, X.; Wang, C.; Wang, Y. Isoelectronic indium doping for thermoelectric enhancements in BiCuSeO. Appl. Surf. Sci. 2019, 473, 985–991. [Google Scholar] [CrossRef]
- Ma, Q.; Deng, J.; Lan, L.; Yang, X.P.; Lin, Y.H. Enhanced Thermoelectric Properties of BiCuSeO Ceramics by Bi Vacancies. Mater. Sci. Forum 2018, 913, 803–810. [Google Scholar] [CrossRef]
- Ishizawa, M.; Yasuzato, Y.; Fujishiro, H.; Naito, T.; Katsui, H.; Goto, T. Oxidation states and thermoelectric properties of BiCuSeO bulks fabricated under Bi or Se deficiencies in the nominal composition. Appl. Phys. Lett. 2018, 123, 245103. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Cao, C.; Fu, J.; Peng, L. Enhanced Thermoelectric Properties in BiCuSeO Oxyselenides via Zn and S Dual-Site Substitution. J. Electron. Mater. 2017, 46, 5909–5915. [Google Scholar] [CrossRef]
- Wen, Q.; Chang, C.; Pan, L.; Li, X.; Yang, T.; Guo, H.; Wang, Z.; Zhang, J.; Xu, F.; Zhang, Z. Enhanced thermoelectric performance of BiCuSeO by increasing Seebeck coefficient through magnetic ion incorporation. J. Mater. Chem. A 2017, 5, 13392–13399. [Google Scholar] [CrossRef]
- Tang, J.; Xu, R.; Zhang, J. Light Element Doping and Introducing Spin Entropy: An Effective Strategy for Enhancement of Thermoelectric Properties in BiCuSeO. ACS Appl. Mater. Interfaces 2019, 11, 15543–15551. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Pan, Z.; Hou, Y.; Zhang, C.; Jiang, C.; Hu, J. Effect of Ba and Pb dual doping on the thermoelectric properties of BiCuSeO ceramics. Mater. Lett. 2018, 217, 189–193. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Pan, Z.; Hu, X.; Liu, P.; He, Z.; Li, Y.; Fan, X.A. Enhanced thermoelectric performance in BiCuSeO oxyselenides via Ba/Te dual-site substitution and 3D modulation doping. J. Solid State Chem. 2018, 266, 297–303. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Pan, Z.; Hu, X.; Liu, P.; Li, Y.; He, Z.; Fan, X.A. Synergistic effects of Bi Deficiencies and Fe-doping on the thermoelectric properties and hardness of BiCuSeO ceramics. J. Ceram. Soc. Jpn. 2018, 126, 699–705. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Fan, S.; Deng, Y.; Zhang, W.; Ye, B. Dual Vacancies: An Effective Strategy Realizing Synergistic Optimization of Thermoelectric Property in BiCuSeO. J. Am. Chem. Soc. 2015, 137, 6587–6593. [Google Scholar] [CrossRef]
- Li, F.; Ruan, M.; Chen, Y.; Wang, W.; Luo, J.; Zheng, Z.; Fan, P. Enhanced thermoelectric properties of polycrystalline BiCuSeO via dual-doping in Bi sites. Inorg. Chem. Front. 2019, 6, 799–807. [Google Scholar] [CrossRef]
- Zhou, Z.; Tan, X.; Ren, G.; Lin, Y.; Nan, C. Thermoelectric Properties of Cl-Doped BiCuSeO Oxyselenides. J. Electron. Mater. 2017, 46, 2593–2598. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Wang, G.; Zhao, L.D. Realizing n-type BiCuSeO through halogens doping. Ceram. Int. 2019, 45, 14953–14957. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, D.; He, J.; Zhao, L.D. Attempting to realize n-type BiCuSeO. J. Solid State Chem. 2018, 258, 510–516. [Google Scholar] [CrossRef]
- Pan, L.; Lang, Y.; Zhao, L.; Berardan, D.; Amzallag, E.; Xu, C.; Gu, Y.; Chen, C.; Zhao, L.D.; Shen, X.; et al. Realization of n-type and enhanced thermoelectric performance of p-type BiCuSeO by controlled iron incorporation. J. Mater. Chem. A 2018, 6, 13340–13349. [Google Scholar] [CrossRef]
- Boona, S.R. Materials science: Nanomagnets boost thermoelectric output. Nature 2017, 549, 169–170. [Google Scholar] [CrossRef]
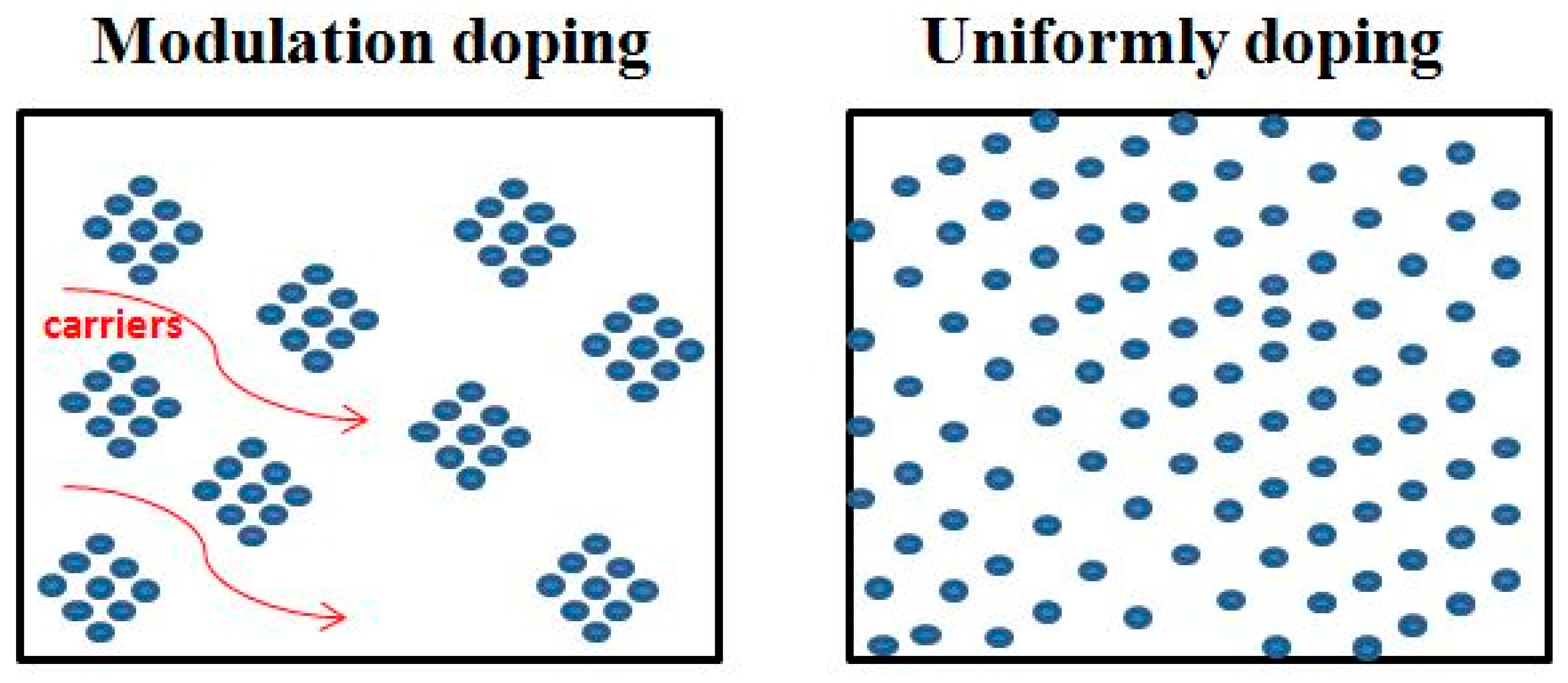
- Pei, Y.L.; Wu, H.; Wu, D.; Zheng, F.; He, J. High thermoelectric performance realized in a BiCuSeO system by improving carrier mobility through 3D modulation doping. J. Am. Chem. Soc. 2014, 136, 13902–13908. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, J.; Li, Y.; Wang, S.; Liu, S.; Zhan, S.; Wang, L.; Zhang, X.; Zhao, L.D. Realizing BiCuSeO-based thermoelectric device for ultrahigh carrier mobility through texturation. Nano Energy 2024, 126, 109649. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Pan, Z.; Hu, X.; Liu, P.; He, Z.; Li, Y.; Fan, X. Effect of synthesis processes on the thermoelectric properties of BiCuSeO oxyselenides. J. Alloys Compd. 2018, 754, 131–138. [Google Scholar] [CrossRef]
- Wei, B.; Li, W.; Yang, Y.; Li, J.; Zheng, Y.; Zhang, W.; Zhou, Z.; Lin, C.; Chang, Z.; Jiang, X.; et al. Synergistically optimizing electrical and thermal transport in layered BiCuSeO via biaxial strain modulation. Acta Mater. 2025, 286, 120699. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Lan, J.; Zeng, C.; Zheng, Y.; Zhan, B.; Zhang, B.; Lin, Y. Enhanced thermoelectric performance of BiCuSeO composites with nano inclusion of copper selenides. J. Alloys Compd. 2016, 662, 320–324. [Google Scholar] [CrossRef]
- Farooq, M.U.; Gao, Z.; Butt, S.; Gao, K.; Pang, X.L.; Guangyi, Z.; Shah, H.U.; Jafri, H.M.; Mahmood, A.; Sun, X.; et al. Enhanced Thermoelectric Transport Properties of La0.98Sr0.02CoO3-BiCuSeO Composite. J. Electr. Eng. 2016, 4, 52–57. [Google Scholar]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Kan, C.; Reece, M.; Huang, Z. Tuning of Catalytic Activity by Thermoelectric Materials for Carbon Dioxide Hydrogenation. Adv. Energy Mater. 2017, 8, 1701430. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Feng, B.; Yang, S.; Ruan, R.; Zhang, R.; Xiong, T.; Xu, B.; Zheng, Z.; Zhou, G.; Zhang, Y.; et al. An Updated Review of BiCuSeO-Based Thermoelectric Materials. Micromachines 2025, 16, 703. https://doi.org/10.3390/mi16060703
Zhang H, Feng B, Yang S, Ruan R, Zhang R, Xiong T, Xu B, Zheng Z, Zhou G, Zhang Y, et al. An Updated Review of BiCuSeO-Based Thermoelectric Materials. Micromachines. 2025; 16(6):703. https://doi.org/10.3390/mi16060703
Chicago/Turabian StyleZhang, Haitao, Bo Feng, Suoluosu Yang, Ruolin Ruan, Rong Zhang, Tongqiang Xiong, Biyu Xu, Zhipeng Zheng, Guopeng Zhou, Yang Zhang, and et al. 2025. "An Updated Review of BiCuSeO-Based Thermoelectric Materials" Micromachines 16, no. 6: 703. https://doi.org/10.3390/mi16060703
APA StyleZhang, H., Feng, B., Yang, S., Ruan, R., Zhang, R., Xiong, T., Xu, B., Zheng, Z., Zhou, G., Zhang, Y., Wang, K., Zhong, Y., Fan, Y., & Zuo, X. (2025). An Updated Review of BiCuSeO-Based Thermoelectric Materials. Micromachines, 16(6), 703. https://doi.org/10.3390/mi16060703




