Synthesis of Chitosan Nanoparticles via Microfluidic Approach: The Role of Temperature in Tailoring Aggregation for Enhanced Uniformity
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Characterization Methods
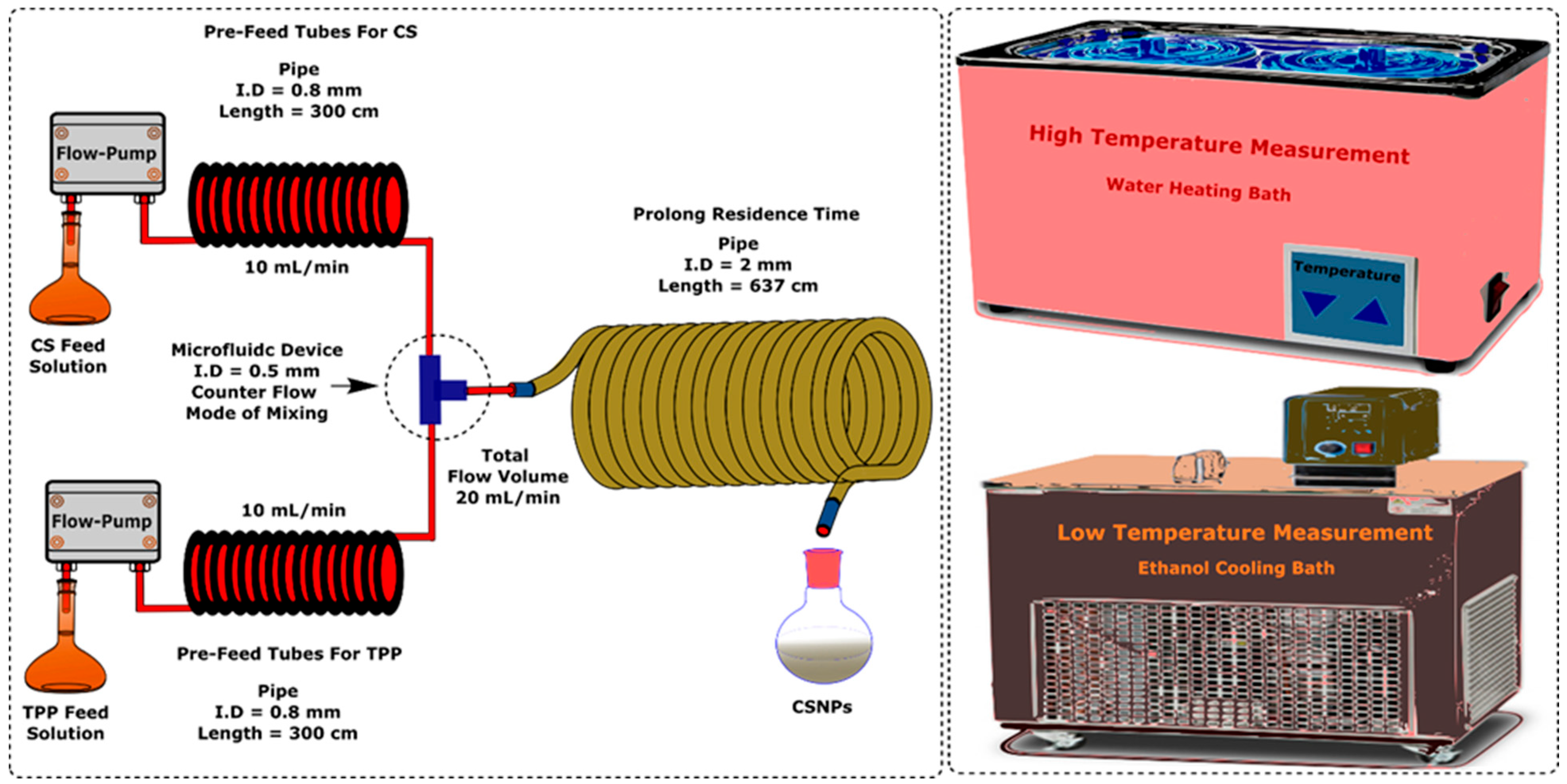
2.3. Equipment and Procedures
3. Results and Discussion
3.1. Tailoring the Fabrication of CSNPs: Insights from Temperature Variation
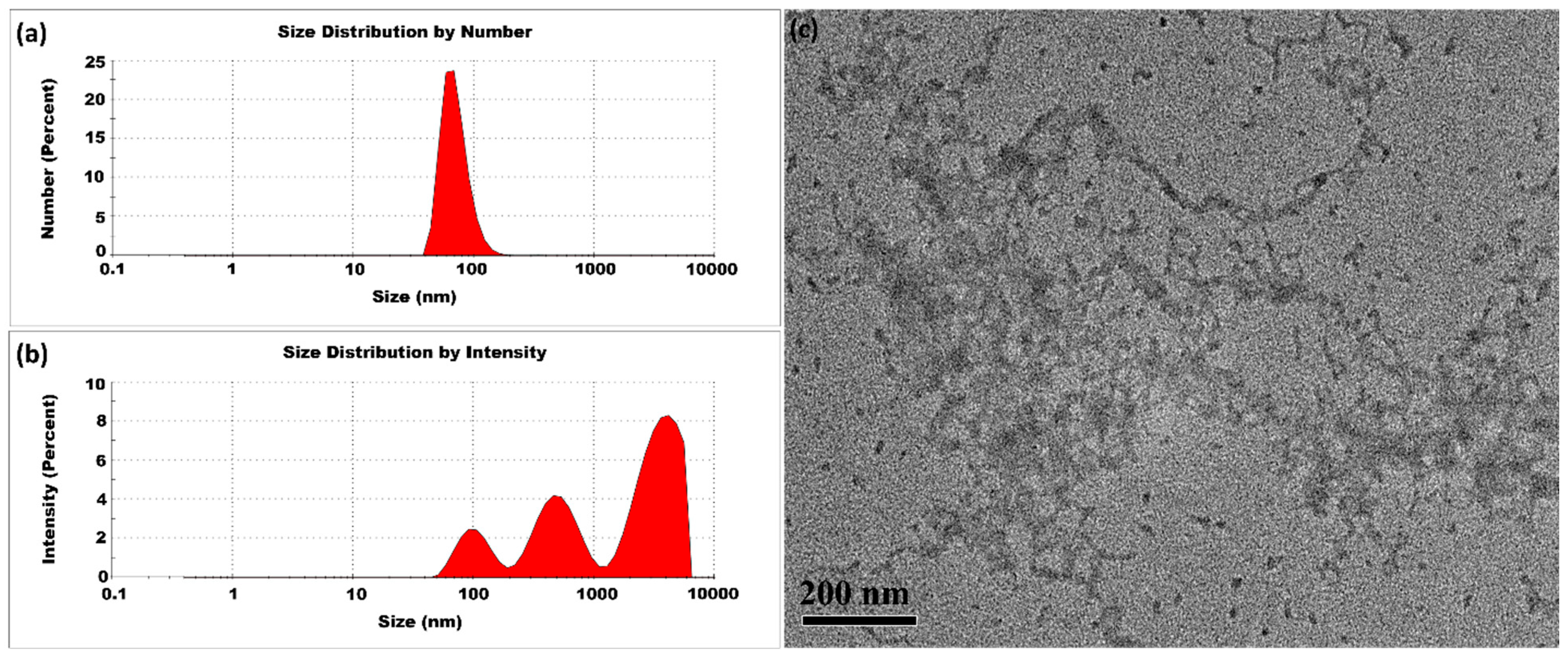
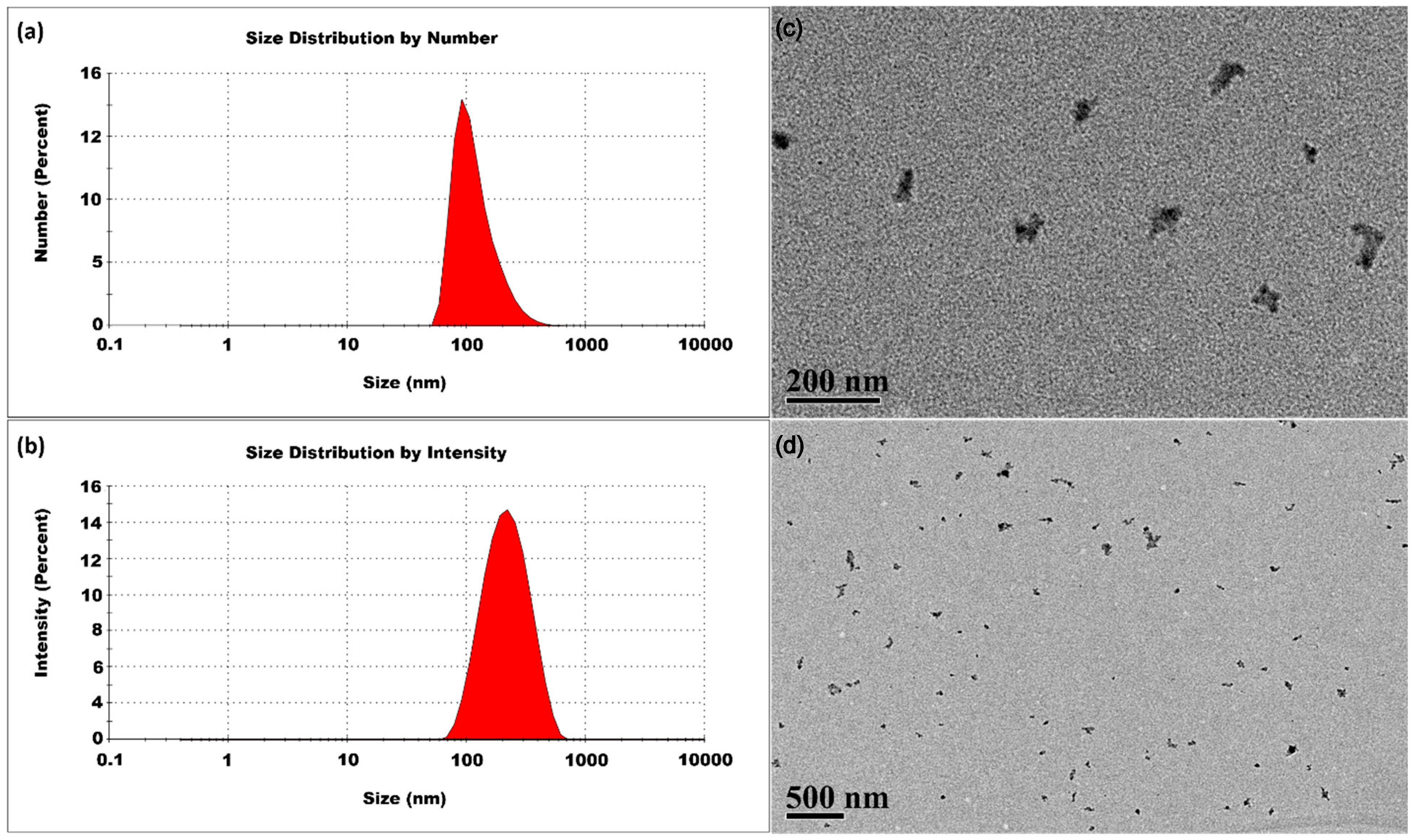
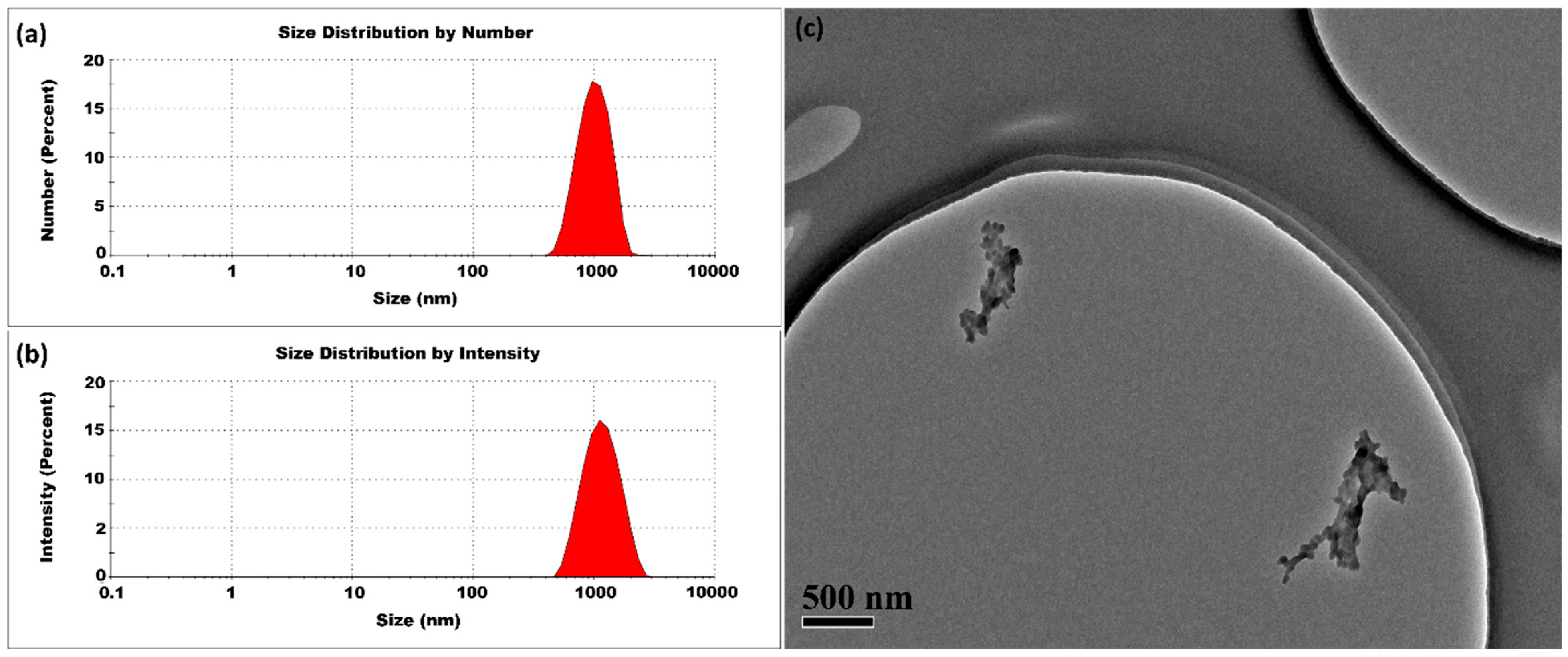
3.1.1. Room Temperature
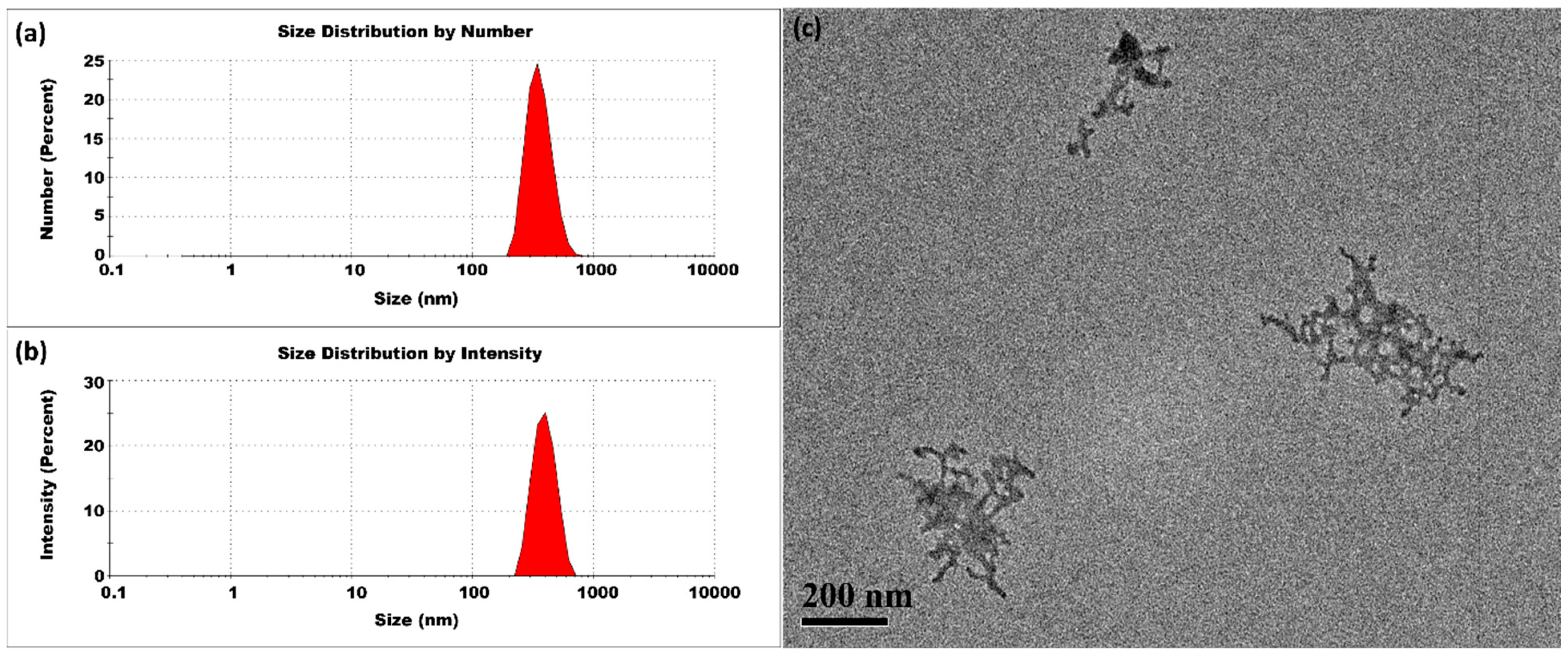
3.1.2. Lower Temperatures
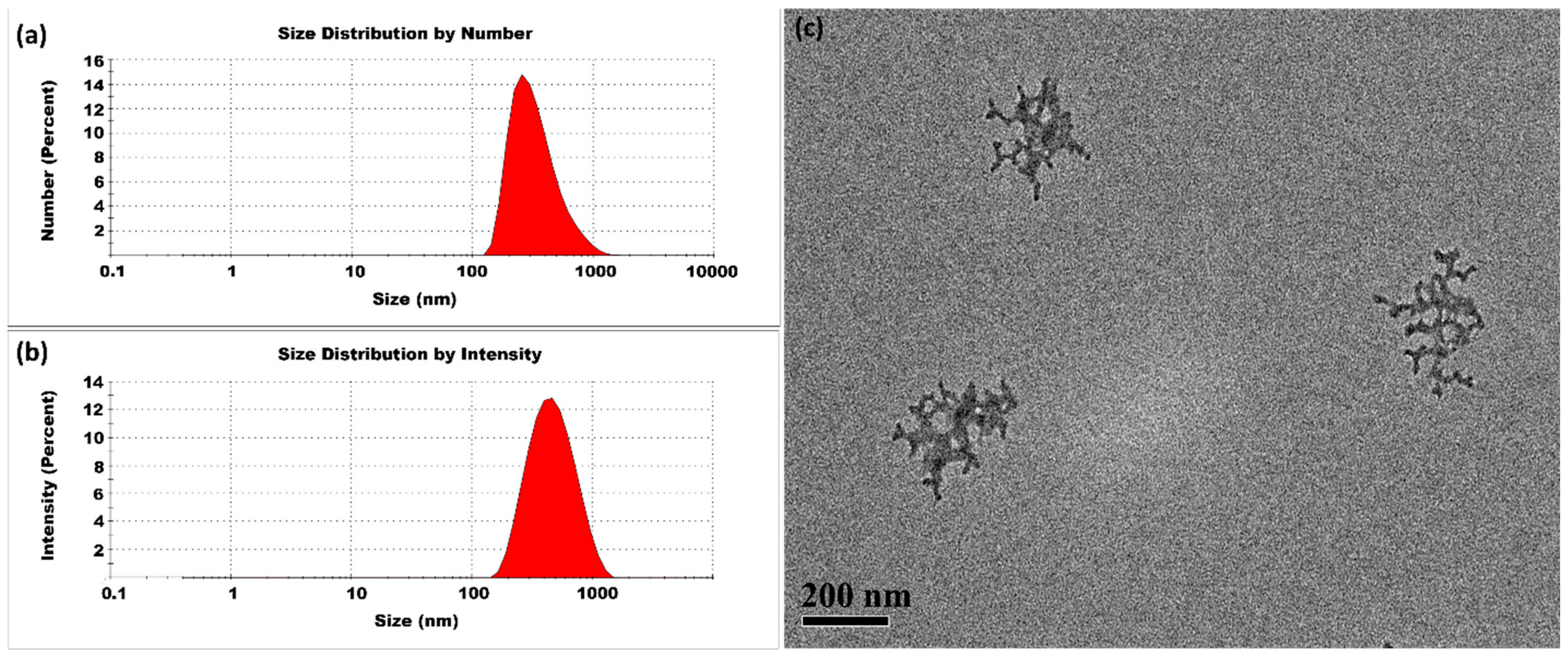
3.1.3. High Temperatures
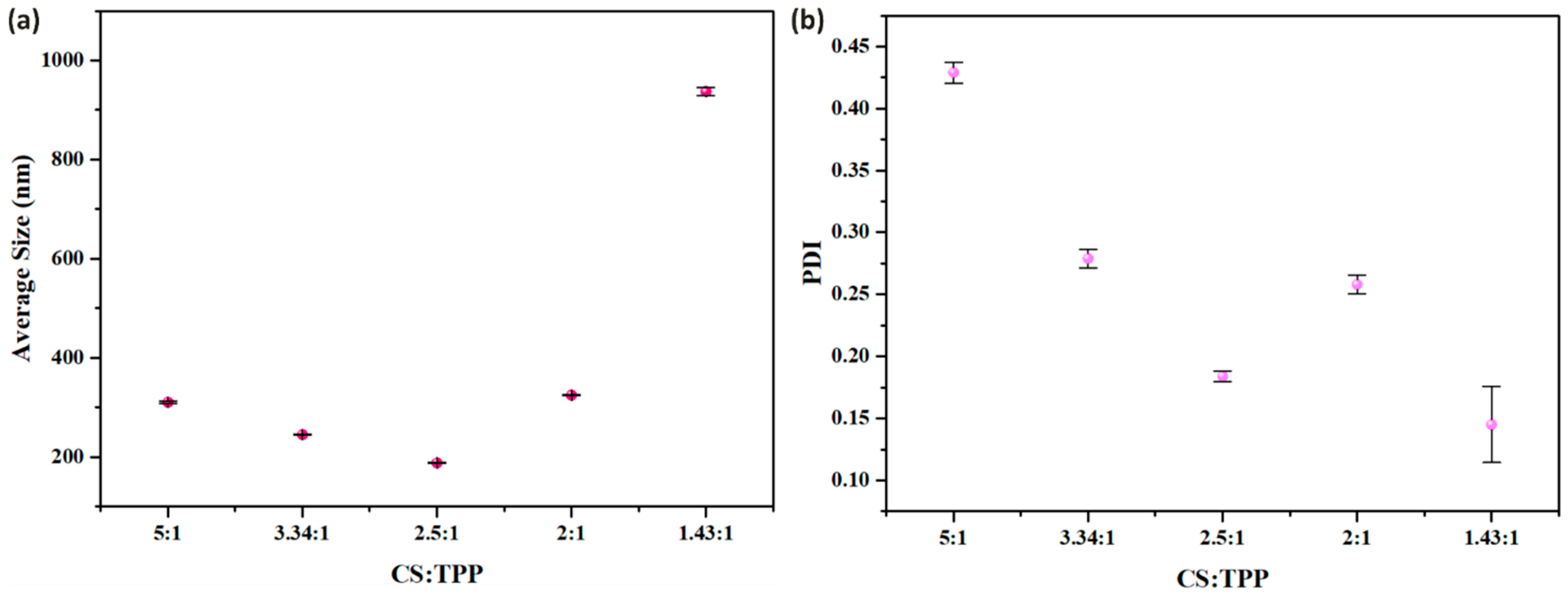
3.2. Impact of TPP Concentration on the Synthesis of CSNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saravanakumar, K.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Venkatachalam, K.; Hu, X.; Wang, M.-H. pH-sensitive release of fungal metabolites from chitosan nanoparticles for effective cytotoxicity in prostate cancer (PC3) cells. Process Biochem. 2021, 102, 165–172. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E. Progresses in targeted drug delivery systems using chitosan nanoparticles in cancer therapy: A mini-review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, H.Y.; Sun, I.C.; Kwon, I.C.; Kim, K. Tumor-Targeting Glycol Chitosan Nanoparticles for Cancer Heterogeneity. Adv. Mater. 2020, 32, e2002197. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 602–623. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Morales, A.; Erdocia, X.; Iturrondobeitia, M.; Labidi, J.; Lizundia, E. Chitosan–chitin nanocrystal films from lobster and spider crab: Properties and environmental sustainability. ACS Sustain. Chem. Eng. 2024, 12, 10363–10375. [Google Scholar] [CrossRef]
- Hassan, U.A.; Hussein, M.Z.; Alitheen, N.B.; Yahya Ariff, S.A.; Masarudin, M.J. In vitro cellular localization and efficient accumulation of fluorescently tagged biomaterials from monodispersed chitosan nanoparticles for elucidation of controlled release pathways for drug delivery systems. Int. J. Nanomed. 2018, 13, 5075–5095. [Google Scholar] [CrossRef]
- Alamassi, M.N.; Chia, S.L.; Abdullah, C.A.C.; Masarudin, M.J. Increased efficacy of biologics following inhibition of autophagy in A549 lung cancer cells in bimodal treatment of doxorubicin and SAR405-loaded chitosan nanoparticles. OpenNano 2023, 11, 100142. [Google Scholar] [CrossRef]
- Deepa, G.; Sivakumar, K.; Sajeevan, T. Molecular simulation and in vitro evaluation of chitosan nanoparticles as drug delivery systems for the controlled release of anticancer drug cytarabine against solid tumours. 3 Biotech 2018, 8, 493. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Aboelfadl, M.M.S.; Selim, A.M.; Khalil, H.F.; Elkady, G.M. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe (II) and Mn (II) from aqueous phases. Sep. Sci. Technol. 2018, 53, 2870–2881. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Makarova, L.I.; Yusova, A.A. Adsorption removal of anionic dyes from aqueous solutions by chitosan nanoparticles deposited on the fibrous carrier. Chemosphere 2018, 212, 1155–1162. [Google Scholar] [CrossRef]
- Punjabi, K.; Adhikary, R.R.; Patnaik, A.; Bendale, P.; Saxena, S.; Banerjee, R. Lectin-Functionalized Chitosan Nanoparticle-Based Biosensor for Point-of-Care Detection of Bacterial Infections. Bioconjug. Chem. 2022, 33, 1552–1563. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Saharan, V.; Kumaraswamy, R.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, H.; Wang, Y. Nanocomplexes film composed of gallic acid loaded ovalbumin/chitosan nanoparticles and pectin with excellent antibacterial activity: Preparation, characterization and application in coating preservation of salmon fillets. Int. J. Biol. Macromol. 2024, 259, 128934. [Google Scholar] [CrossRef]
- Xu, W.; McClements, D.J.; Zhang, Z.; Zhang, R.; Qiu, C.; Zhao, J.; Jin, Z.; Chen, L. Effect of tannic acid modification on antioxidant activity, antibacterial activity, environmental stability and release characteristics of quercetin loaded zein-carboxymethyl chitosan nanoparticles. Int. J. Biol. Macromol. 2024, 280, 135853. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, S.M.; Crespo, A.M.; Vaz, F.; Filipe, M.; Santos, D.; Jacinto, T.A.; Paiva-Santos, A.C.; Rodrigues, M.; Ribeiro, M.P.; Coutinho, P.; et al. Development and characterization of thermal water gel comprising Helichrysum italicum essential oil-loaded chitosan nanoparticles for skin care. Cosmetics 2023, 10, 8. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and UF-purified annatto and saffron for the preparation of UV protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef]
- Anusha, J.; Raj, C.J.; Cho, B.-B.; Fleming, A.T.; Yu, K.-H.; Kim, B.C. Amperometric glucose biosensor based on glucose oxidase immobilized over chitosan nanoparticles from gladius of Uroteuthis duvauceli. Sens. Actuators B Chem. 2015, 215, 536–543. [Google Scholar] [CrossRef]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel amperometric biosensor based on tyrosinase/chitosan nanoparticles for sensitive and interference-free detection of total catecholamine. Biosensors 2022, 12, 519. [Google Scholar] [CrossRef]
- Sapre, N.; Gumathannavar, R.; Shirolkar, M.; Dalai, S.; Kanojiya, P.; Saroj, S.; Kulkarni, A. Optically tuneable chitosan nanoparticles for biomedical imaging application. Sens. Technol. 2024, 2, 2428590. [Google Scholar] [CrossRef]
- Nesalin, J.A.J.; Smith, A.A. Preparation and evaluation of stavudine loaded chitosan nanoparticles. J. Pharm. Res. 2013, 6, 268–274. [Google Scholar]
- Sawaengsak, C.; Mori, Y.; Yamanishi, K.; Mitrevej, A.; Sinchaipanid, N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech 2014, 15, 317–325. [Google Scholar] [CrossRef]
- Tabynov, K.; Solomadin, M.; Turebekov, N.; Babayeva, M.; Fomin, G.; Yadagiri, G.; Renu, S.; Yerubayev, T.; Petrovsky, N.; Renukaradhya, G.J.; et al. An intranasal vaccine comprising SARS-CoV-2 spike receptor-binding domain protein entrapped in mannose-conjugated chitosan nanoparticle provides protection in hamsters. Sci. Rep. 2023, 13, 12115. [Google Scholar]
- Khan, M.A.; Zafaryab, M.; Mehdi, S.H.; Ahmad, I.; Rizvi, M.M. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int. J. Biol. Macromol. 2016, 93, 242–253. [Google Scholar] [CrossRef]
- Nam, T.; Park, S.; Lee, S.-Y.; Park, K.; Choi, K.; Song, I.C.; Han, M.H.; Leary, J.J.; Yuk, S.A.; Kwon, I.C.; et al. Tumor targeting chitosan nanoparticles for dual-modality optical/MR cancer imaging. Bioconjug. Chem. 2010, 21, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lim, T.M.; Lim, L.-Y. Pharmacological activity of peroral chitosan–insulin nanoparticles in diabetic rats. Int. J. Pharm. 2005, 293, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, F.; Farani, M.R.; Abednejad, A.; Akbari, B.; Mostafavi, E.; Zare, I. Chitosan nanoparticles in tissue engineering and regenerative medicine. In Fundamentals and Biomedical Applications of Chitosan Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2025; pp. 497–526. [Google Scholar]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Dandekar-Jain, P.; Adivarekar, R.V. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 121, 936–946. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.-H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Athavale, R.; Ravindran, S.; Rale, V.; Kulkarni, A.; Deokar, R. Antibiotics functionalization intervened morphological, chemical and electronic modifications in chitosan nanoparticles. Nano-Struct. Nano-Objects 2021, 25, 100657. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic acid-coated chitosan nanoparticles as targeted-carrier of tamoxifen against MCF7 and TMX-resistant MCF7 cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef]
- Song, H.; Su, C.; Cui, W.; Zhu, B.; Liu, L.; Chen, Z.; Zhao, L. Folic acid-chitosan conjugated nanoparticles for improving tumor-targeted drug delivery. BioMed Res. Int. 2013, 2013, 723158. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Holtze, C.; Boehling, R. Batch or flow chemistry?—A current industrial opinion on process selection. Curr. Opin. Chem. Eng. 2022, 36, 100798. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef]
- Yao, F.; Zhu, P.; Chen, J.; Li, S.; Sun, B.; Li, Y.; Zou, M.; Qi, X.; Liang, P.; Chen, Q. Synthesis of nanoparticles via microfluidic devices and integrated applications. Microchim. Acta 2023, 190, 256. [Google Scholar] [CrossRef]
- Alarfaj, A.A. Antibacterial effect of chitosan nanoparticles against food spoilage bacteria. J. Pure Appl. Microbiol. 2019, 13, 1273–1278. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Mohamed, M.I.; Ahmed, H.Y.; Elaasser, M.M.; Kandile, N.G. Fabrication and characterization of unique sustain modified chitosan nanoparticles for biomedical applications. Sci. Rep. 2024, 14, 13869. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.; Pal, A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Jha, A.; Ghormade, V.; Kolge, H.; Paknikar, K.M. Dual effect of chitosan-based nanoparticles on the inhibition of β-amyloid peptide aggregation and disintegration of the preformed fibrils. J. Mater. Chem. B 2019, 7, 3362–3373. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Hussain, Z.; Sahudin, S. Preparation, characterisation and colloidal stability of chitosan-tripolyphosphate nanoparticles: Optimisation of formulation and process parameters. Int. J. Pharm. Pharm. Sci. 2016, 8, 297–308. [Google Scholar]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A simple method for synthesis of chitosan nanoparticles with ionic gelation and homogenization. Molecules 2023, 28, 4328. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J. Preparation and characterization of the fluorescent chitosan nanoparticle probe. Chin. J. Anal. Chem. 2006, 34, 1555–1559. [Google Scholar] [CrossRef]
- Körpe, D.A.; Malekghasemi, S.; Aydın, U.; Duman, M. Fabrication of monodispersive nanoscale alginate–chitosan core–shell particulate systems for controlled release studies. J. Nanopart. Res. 2014, 16, 2754. [Google Scholar] [CrossRef]
- Majedi, F.S.; Hasani-Sadrabadi, M.M.; Emami, S.H.; Taghipoor, M.; Dashtimoghadam, E.; Bertsch, A.; Moaddel, H.; Renaud, P. Microfluidic synthesis of chitosan-based nanoparticles for fuel cell applications. Chem. Commun. 2012, 48, 7744–7746. [Google Scholar] [CrossRef]
- Greco, A.; Gabold, B.; Chen, S.; Wang, X.; Xu, Z.; Hartschuh, A.; Chiesa, E.; Genta, I.; Ried, C.L.; Merdan, T. Microfluidic mixing as platform technology for production of chitosan nanoparticles loaded with different macromolecules. Eur. J. Pharm. Biopharm. 2023, 188, 170–181. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Ghorbani-Bidkorbeh, F.; Fallah, N.; Farnam, G.; Mortazavi, S.A.; Shirazi, F.H.; Tehrani, M.H.H.; Hamedi, M.H. Microfluidic platform for synthesis and optimization of chitosan-coated magnetic nanoparticles in cisplatin delivery. Carbohydr. Polym. 2021, 265, 118027. [Google Scholar] [CrossRef]
- Nathanael, K.; Cheng, S.; Kovalchuk, N.M.; Arcucci, R.; Simmons, M.J. Optimization of microfluidic synthesis of silver nanoparticles: A generic approach using machine learning. Chem. Eng. Res. Des. 2023, 193, 65–74. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Chiesa, E.; Bellotti, M.; Caimi, A.; Conti, B.; Dorati, R.; Conti, M.; Genta, I.; Auricchio, F. Development and optimization of microfluidic assisted manufacturing process to produce PLGA nanoparticles. Int. J. Pharm. 2022, 629, 122368. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cito, S.; Zhang, Y.; Wang, C.-F.; Sikanen, T.M.; Santos, H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015, 27, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.-H.; Lee, A.P. Microfluidic devices for the synthesis of nanoparticles and biomaterials. J. Med. Biol. Eng. 2007, 27, 1–6. [Google Scholar]
- Matsunaga, T.; Lee, H.-J.; Nishino, K. An approach for accurate simulation of liquid mixing in a T-shaped micromixer. Lab Chip 2013, 13, 1515–1521. [Google Scholar] [CrossRef]
- Makgwane, P.R.; Ray, S.S. Synthesis of nanomaterials by continuous-flow microfluidics: A review. J. Nanosci. Nanotechnol. 2014, 14, 1338–1363. [Google Scholar] [CrossRef]
- Schmidt, S.; Nguyen, A.T.; Vu, H.Q.; Tran, N.N.; Sareela, M.; Fisk, I.; Hessel, V. Microfluidic Spontaneous Emulsification for Generation of O/W Nanoemulsions—Opportunity for In-Space Manufacturing. Adv. Healthc. Mater. 2023, 12, 2203363. [Google Scholar] [CrossRef]
- Zhang, S.; So, L.L.C.; Faucher, S.; Xi, L. Polymer coating over solid particles with in situ thermal curing. Ind. Eng. Chem. Res. 2016, 55, 5574–5584. [Google Scholar] [CrossRef]
- Lamkin-Kennard, K.A.; Popovic, M.B. Molecular and cellular level—Applications in biotechnology and medicine addressing molecular and cellular level. In Biomechatronics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–233. [Google Scholar]
- An, F.; Jia, H.; Feng, Y. Effect of stress, concentration and temperature on gas diffusion coefficient of coal measured through a direct method and its model application. Fuel 2022, 312, 122991. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Lu, Y. Synthesis of Chitosan Nanoparticles via Microfluidic Approach: The Role of Temperature in Tailoring Aggregation for Enhanced Uniformity. Micromachines 2025, 16, 642. https://doi.org/10.3390/mi16060642
Ahmed M, Lu Y. Synthesis of Chitosan Nanoparticles via Microfluidic Approach: The Role of Temperature in Tailoring Aggregation for Enhanced Uniformity. Micromachines. 2025; 16(6):642. https://doi.org/10.3390/mi16060642
Chicago/Turabian StyleAhmed, Muqarrab, and Yangcheng Lu. 2025. "Synthesis of Chitosan Nanoparticles via Microfluidic Approach: The Role of Temperature in Tailoring Aggregation for Enhanced Uniformity" Micromachines 16, no. 6: 642. https://doi.org/10.3390/mi16060642
APA StyleAhmed, M., & Lu, Y. (2025). Synthesis of Chitosan Nanoparticles via Microfluidic Approach: The Role of Temperature in Tailoring Aggregation for Enhanced Uniformity. Micromachines, 16(6), 642. https://doi.org/10.3390/mi16060642





