UV–Vis Detection of Thioacetamide: Balancing the Performances of a Mn(III)-Porphyrin, Gold Colloid, and Their Complex for Selecting the Most Sensitive Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.1.1. Synthesis of 5,10,15,20-Tetrakis(3,4-dimethoxyphenyl)-21H,23H-porphyrin (3,4-diMeOPP)
2.1.2. Obtaining of Mn(III)-5,10,15,20-Tetrakis-(3,4-dimethoxyphenyl)porphyrin Chloride (Mn-3,4-diMeOPP)
2.1.3. Method for Gold Colloid Preparation
2.1.4. Method for Obtaining the Mn-3,4-diMeOPP-AuNP Complex
2.2. Apparatus
2.3. Detection Method for Thioacetamide by UV–Vis Method Using Mn-3,4-diMeOPP as Sensitive Substance
2.4. Optical Detection of Thioacetamide Using Gold Colloid as Sensitive Material
3. Results and Discussion
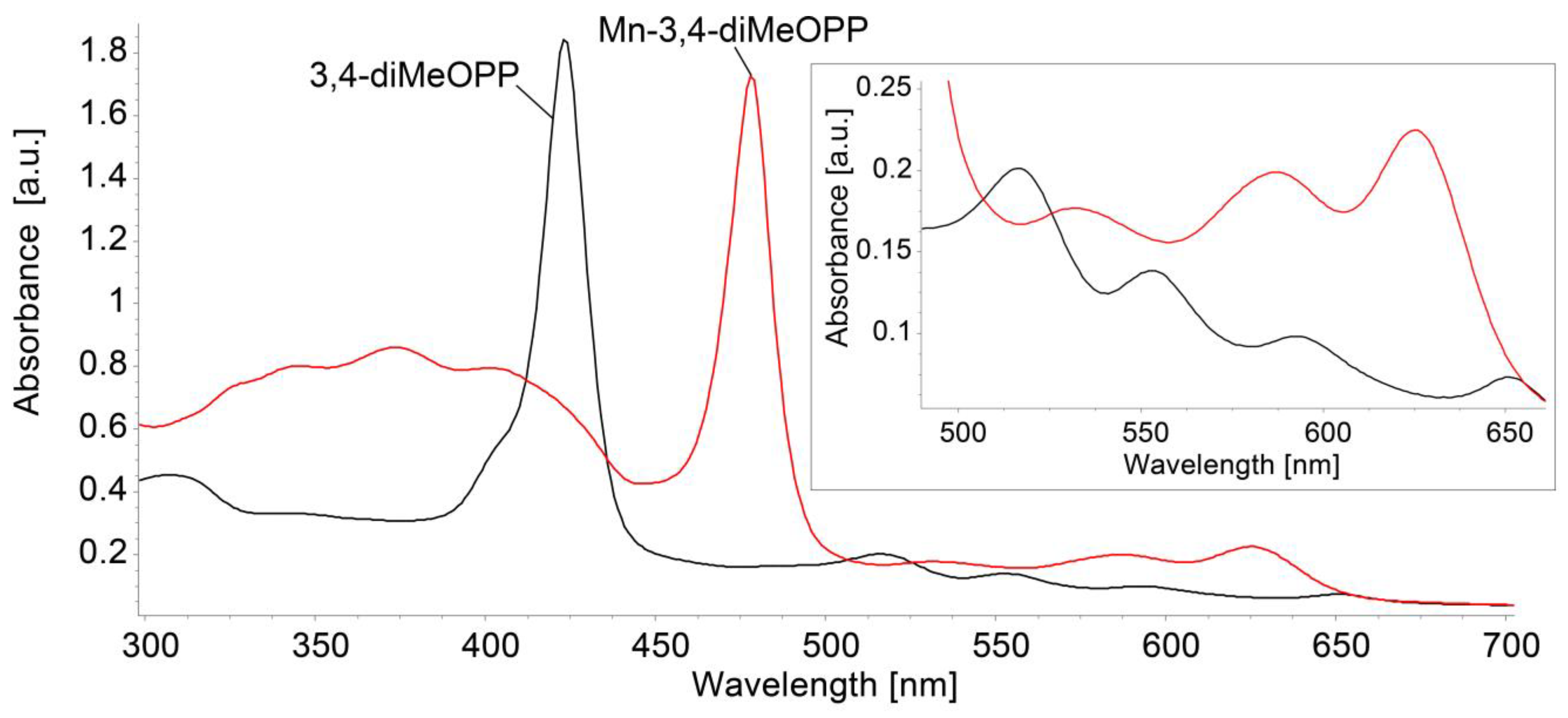
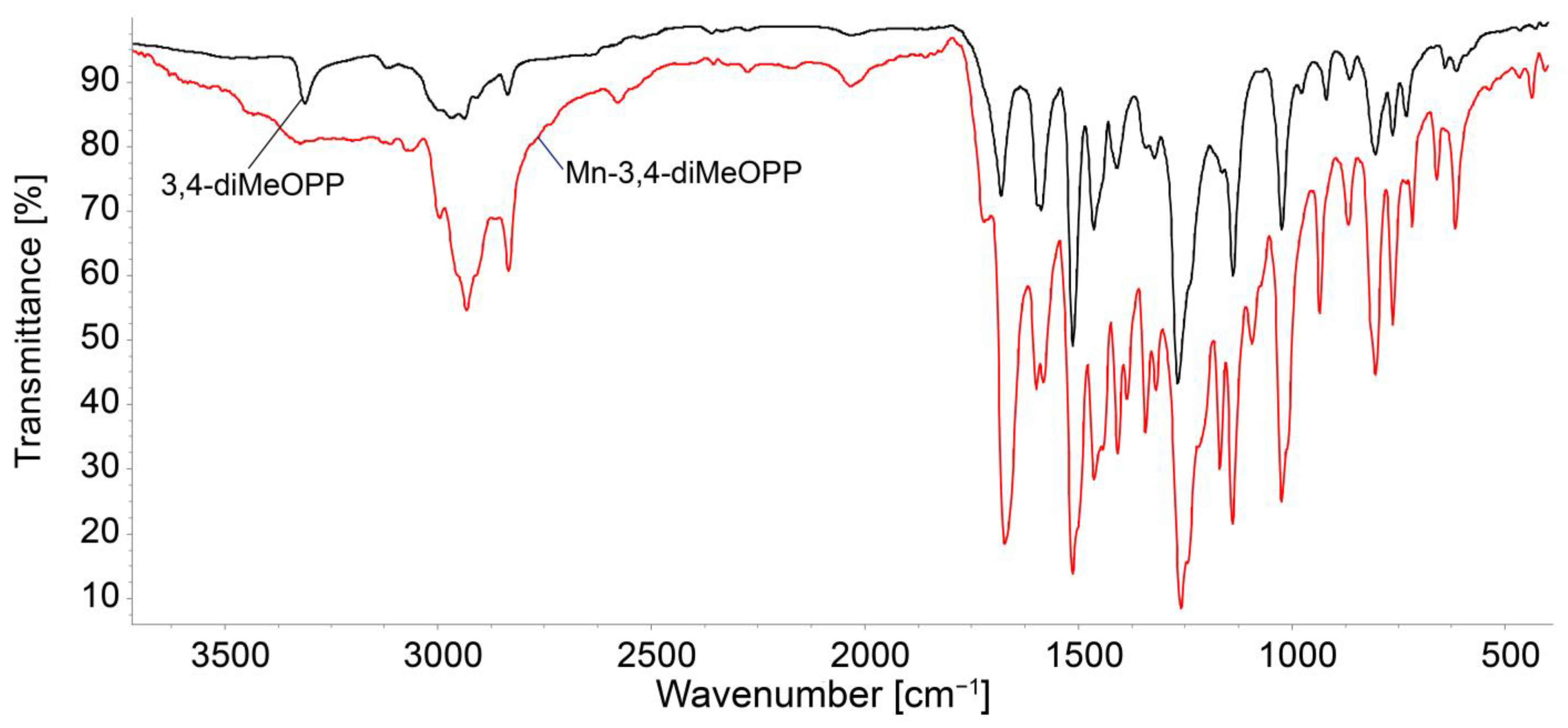
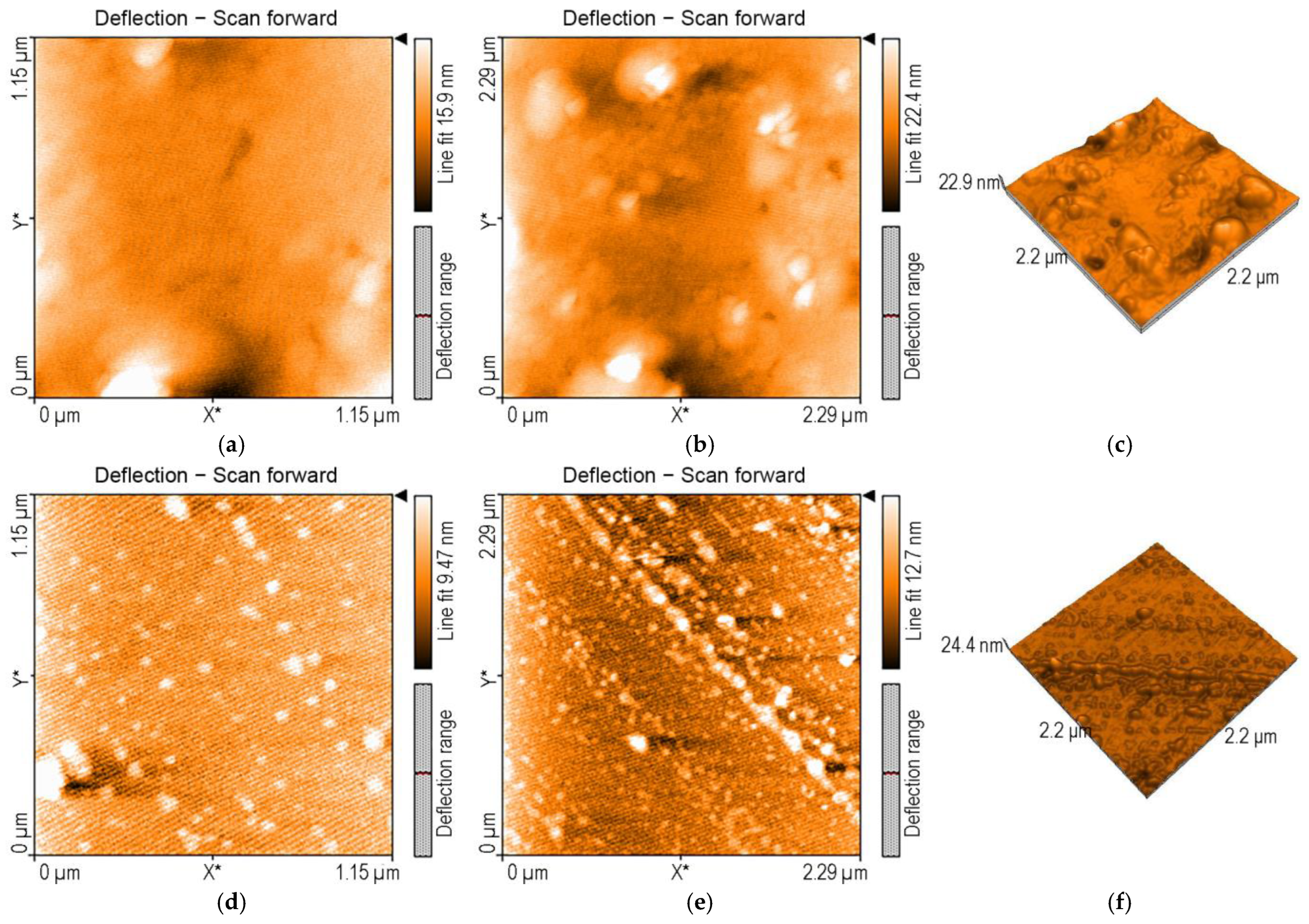
3.1. Comparative Study of Physical–Chemical Characteristics of 3,4-diMeOPP and Mn-3,4-diMeOPP
3.1.1. Comparison of UV–Vis Spectra
3.1.2. FT-IR Analysis
3.1.3. Surface Morphology Studies via AFM Investigations
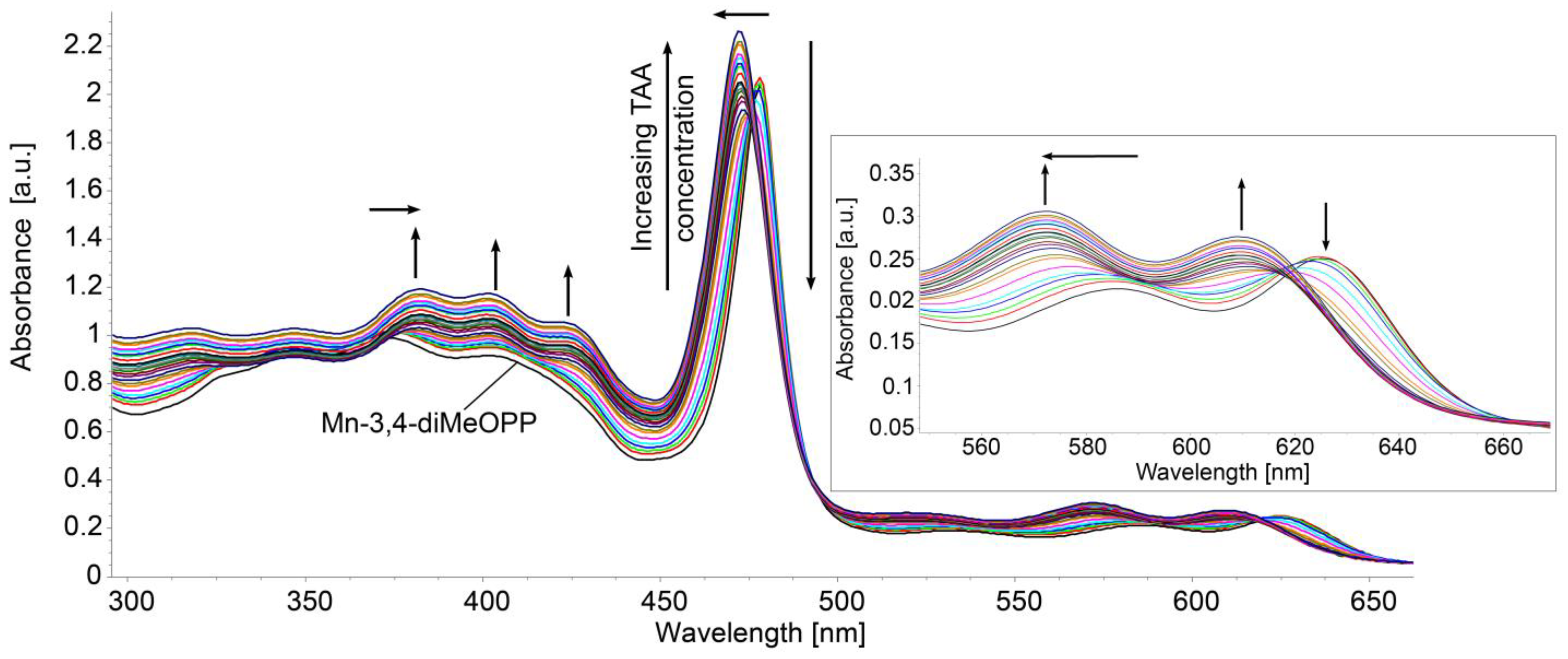
3.2. UV–Vis Method for TAA Detection/Quantification Using Mn-3,4-diMeOPP as Sensitive Material: AFM Morphological Study of Surfaces After TAA Detection
3.3. Detection of TAA Using Gold Colloidal Solution (AuNPs) as Sensitive Material: Study of Morphological Modifications to the Surfaces Before and After Exposure
3.4. Generation of the Mn-3,4-diMeOPP-AuNP Complex
3.5. Detection of TAA Using Mn-3,4-diMeOPP-AuNP Complex as Sensitive Material and AFM Analysis of Morphological Modifications
3.6. TAA Concentration Domain Complementarity for the Three Tested Sensitive Materials
3.7. Impact of Potential Interfering Species on TAA Detection Based on Mn-3,4-diMeOPP
3.8. Proposed Mechanism for the Recognition of TAA by Mn-3,4-diMeOPP
3.9. Comparison of the Efficiency of TAA Detection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, C.M.; Ohkubo, K.; Lammer, A.D.; Kim, D.S.; Kawashima, Y.; Sessler, J.L.; Fukuzumi, S. Photoinduced electron transfer in a supramolecular triad produced by porphyrin anion-induced electron transfer from tetrathiafulvalene calix [4] pyrrole to Li+@ C 60. Chem. Commun. 2015, 51, 9789–9792. [Google Scholar] [CrossRef]
- Lahanas, N.; Kucheryavy, P.; Lalancette, R.A.; Lockard, J.V. Crystallographic identification of a series of manganese porphyrin complexes with nitrogenous bases. Acta Crystallogr. C Struct. Chem. 2019, 75, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nizamidin, P.; Zhang, Y.; Kari, N.; Yimit, A. Detection of Trimethylamine Based on a Manganese Tetraphenylporphyrin Optical Waveguide Sensing Element. Anal. Sci. 2018, 34, 559–565. [Google Scholar] [CrossRef]
- Hao, Z.-W.; Dong, M.-M.; Zhang, R.-Q.; Wang, C.-K.; Fu, X.-X. An ultra-sensitive gas sensor based on a two-dimensional manganese porphyrin monolayer. Phys. Chem. Chem. Phys. 2021, 23, 11852–11862. [Google Scholar] [CrossRef]
- Boakye, A.; Yu, K.; Asinyo, B.K.; Chai, H.; Raza, T.; Xu, T.; Zhang, G.; Qu, L. A portable electrochemical sensor based on manganese porphyrin-functionalized carbon cloth for highly sensitive detection of nitroaromatics and gaseous phenol. Langmuir 2022, 38, 12058–12069. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Patel, S.; Yuan, X.; Mannam, V.; Howard, S.; Batinic-Haberle, I.; Fukuto, J.; Minnion, M.; et al. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants 2019, 8, 639. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; Markel, T.A.; Boone, D.; Stahelin, R.V.; Batinic-Haberle, I.; Straubg, K.D. Effects of Manganese Porphyrins on Cellular Sulfur Metabolism. Molecules 2020, 25, 980. [Google Scholar] [CrossRef] [PubMed]
- Çimen, D.; Bereli, N.; Denizli, A. Advanced plasmonic nanosensors for monitoring of environmental pollutants. Curr. Anal. Chem. 2023, 19, 2–17. [Google Scholar] [CrossRef]
- Boruah, J.S.; Devi, C.; Hazarika, U.; Reddy, P.V.B.; Chowdhury, D.; Barthakur, M.; Kalita, P. Green synthesis of gold nanoparticles using an antiepileptic plant extract: In vitro biological and photo-catalytic activities. RSC Adv. 2021, 11, 28029–28041. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, W.; Huang, P.; Zeng, X.; Mei, L. Smart materials for drug delivery and cancer therapy. View 2021, 2, 20200042. [Google Scholar] [CrossRef]
- Nasser, T.A.; Adel, R.; Badr, A.; Teleb, M.; Bekhit, A.A.; Elkhodairy, K.A.; Abdelhamid, A.S.; Elzoghby, A.O. Combined cancer immunotheranostic nanomedicines: Delivery technologies and therapeutic outcomes. ACS Omega 2023, 8, 4491–4507. [Google Scholar] [CrossRef]
- Madkour, L.H. Applications of gold nanoparticles in medicine and therapy. Pharm. Pharmacol. Int. J. 2018, 6, 157–174. [Google Scholar] [CrossRef]
- Herkert, E.K.; Garcia-Parajo, M.F. Harnessing the Power of Plasmonics for in Vitro and in Vivo Biosensing. ACS Photonics 2025, 12, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Flauraud, V.; Regmi, R.; Winkler, P.M.; Alexander, D.T.L.; Rigneault, H.; Van Hulst, N.F.; García-Parajo, M.F.; Wenger, J.; Brugger, J. In-Plane Plasmonic Antenna Arrays with Surface Nanogaps for Giant Fluorescence Enhancement. Nano Lett. 2017, 17, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Mohr, D.A.; Vidal-Codina, F.; John-Herpin, A.; Jo, M.; Kim, S.; Matson, J.; Caldwell, J.D.; Jeon, H.; Nguyen, N.C.; et al. High-Contrast Infrared Absorption Spectroscopy via Mass-Produced Coaxial Zero-Mode Resonators with Sub-10 Nm Gaps. Nano Lett. 2018, 18, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Kavungal, D.; Magalhães, P.; Kumar, S.T.; Kolla, R.; Lashuel, H.A.; Altug, H. Artificial Intelligence-Coupled Plasmonic Infrared Sensor for Detection of Structural Protein Biomarkers in Neurodegenerative Diseases. Sci. Adv. 2023, 9, eadg9644. [Google Scholar] [CrossRef]
- Lascu, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Hybrid Mn-porphyrin-nanogold nanomaterial applied for the spectrophotometric detection of β-carotene. J. Chem. Hindawi 2018, 2018, 5323561. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and electrochemical mediated detection of ascorbic acid using manganese porphyrin and its gold hybrids. C. R. Chim. 2017, 21, 327–338. [Google Scholar] [CrossRef]
- Fringu, I.; Anghel, D.; Fratilescu, I.; Epuran, C.; Birdeanu, M.; Fagadar-Cosma, E. Nanomaterials Based on 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine Exhibiting Bifunctional Sensitivity for Monitoring Chloramphenicol and Co2+. Biomedicines 2024, 12, 770. [Google Scholar] [CrossRef]
- Lascu, A.; Palade, A.; Birdeanu, M.; Fagadar-Cosma, E. Procaine Detection Using Hybrids of Cobalt-Metalloporphyrin with Gold and Silver Nanoparticles. J. Chem. Soc. Pak. 2019, 41, 43. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Qian, P.; Li, Y.; Ren, W.; Deng, H.; Jiang, L. Synthesis and fungicidal activity of novel benzimidazole derivatives bearing pyrimidine-thioether moiety against Botrytis cinerea. Pest. Manag. Sci. 2021, 77, 5529–5536. [Google Scholar] [CrossRef]
- Kurien, M.; Susamma, A.P.; Kuriakose, A.P. Amidino Thiourea as a Secondary Accelerator in the Sulphur Vulcanization of Natural Rubber Containing Fillers. Prog. Rubber Plast. Recycl. Technol. 2004, 20, 133–161. [Google Scholar] [CrossRef]
- Wei, Y.; Zu, G.; Sun, C.; Yang, X. Flexible, Wash-Resistant Human Mechanical Energy Harvesting and Storage System for Monitoring Human Movement. Langmuir 2023, 39, 4060–4070. [Google Scholar] [CrossRef]
- Behari, K.; Agrawal, U.; Das, R. Gel Free Polymerization of N,N′-Methylenebisacrylamide Initiated by a Peroxodiphosphate-Thioacetamide Redox System. A Kinetic Study. Polym. J. 1993, 25, 1007–1013. [Google Scholar] [CrossRef][Green Version]
- Gharabaghi, M.; Irannajad, M.; Azadmehr, A.R. Selective Sulphide Precipitation of Heavy Metals from Acidic Polymetallic Aqueous Solution by Thioacetamide. Ind. Eng. Chem. Res. 2012, 51, 954–963. [Google Scholar] [CrossRef]
- Han, H.-Y.; Park, S.-M.; Ko, J.-W.; Oh, J.-H.; Kim, S.K.; Kim, T.-W. Integrated transcriptomic analysis of liver and kidney after 28 days of thioacetamide treatment in rats. Toxicol. Res. 2023, 39, 201–211. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J. Unveiling thioacetamide-induced toxicity: Multi-organ damage and omitted bone toxicity. Hum. Exp. Toxicol. 2024, 43, 09603271241241807. [Google Scholar] [CrossRef]
- Mansour, R.S.A.E.; Ezzat, S.F.; Abou-Bakr, D.A. Ameliorative Effect of Propolis Against Thioacetamide Induced Hepatorenal Injury in Adult Male Rats. Kidney Injury Molecule-1 (KIM-1) a Biomarker of Renal Injury. Med. J. Cairo Univ. 2020, 88, 243–258. [Google Scholar] [CrossRef]
- Schyman, P.; Printz, R.L.; Estes, S.K.; O’Brien, T.P.; Shiota, M.; Wallqvist, A. Assessing Chemical-Induced Liver Injury In Vivo From In Vitro Gene Expression Data in the Rat: The Case of Thioacetamide Toxicity. Front. Genet. 2019, 10, 1233. [Google Scholar] [CrossRef]
- Schyman, P.; Printz, R.L.; Estes, S.K.; Boyd, K.L.; Shiota, M.; Wallqvist, A. Identification of the Toxicity Pathways Associated with Thioacetamide-Induced Injuries in Rat Liver and Kidney. Front. Pharmacol. 2018, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.M.M.; Shalaby, R.H.; Mannaa, F.A.; Abdel Wahhab, K.G.; Mohamed, N.R.; Shabana, M.E.; Elwakeel, S.H.B. Hepatoprotective Effects of Chitosan on Thioacetamide Induced Liver Toxicity in Male Albino Rats. Biointerface Res. Appl. Chem. 2021, 11, 14490–14505. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A.; Alamro, A.A.; Ganaie, M.A. Amelioration of thioacetamide-induced liver toxicity in Wistar rats by rutin. Int. J. Immunopathol. Pharmacol. 2017, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, Y.; Li, J.; Cheng, L.; Yao, Y.; Shen, H.; Wang, B.; Ren, J.; Ying, H.; Xu, J. Acute bone damage through liver-bone axis induced by thioacetamide in rats. BMC Pharmacol. Toxicol. 2022, 23, 29. [Google Scholar] [CrossRef]
- Cinghită, D.; Radovan, C.; Dascălu, D. Anodic Voltammetry of Thioacetamide and its Amperometric Determination in Aqueous Media. Sensors 2008, 8, 4560–4581. [Google Scholar] [CrossRef]
- Asghari, A.; Ghaderi, O.; Rajabi, M.; Ameri, M.; Amoozadeh, A. Mechanistic and electrochemical investigation of catechol oxidation in the presence of thioacetamide: Application for voltammetric determination of thioacetamide in aqueous media. Prog. React. Kinet. Mech. 2015, 40, 95–103. [Google Scholar] [CrossRef]
- Pernet-Coudrier, B.; Waeles, M.; Filella, M.; Quentel, F.; Riso, R.D. Simple and simultaneous determination of glutathione, thioacetamide and refractory organic matter in natural waters by DP-CSV. Sci. Total Environ. 2013, 463, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, G.; Ediriweera, M.K.; Kumari, N.; Maria Joseph Raj, N.P.; Cho, S.K.; Kim, S.J. Metal-amino acid nanofibers based triboelectric nanogenerator for self-powered thioacetamide sensor. ACS Appl. Mater. Interfaces 2021, 13, 18887–18896. [Google Scholar] [CrossRef]
- Saha, D.; Negi, D.P. Spectroscopic investigations on the interaction of thioacetamide with ZnO quantum dots and application for its fluorescence sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 516–521. [Google Scholar] [CrossRef]
- Kitte, S.A.; Bushira, F.A.; Li, H.; Jin, Y. Electrochemiluminescence of Ru(bpy) 3 2+/thioacetamide and its application for the sensitive determination of hepatotoxic thioacetamide. Analyst 2021, 146, 5198–5203. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Vlascici, D.; Fagadar-Cosma, G.; Vasile, M.; Bazylak, G. Novel nanomaterials based on 5,10,15,20-tetrakis(3,4-dimethoxyphenyl)-21H,23H-porphyrin entrapped in silica matrices. Mater. Res. Bull. 2009, 44, 2186–2193. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Sil, D.; Bhowmik, S.; Khan, F.S.T.; Rath, S.P. Experimental and Theoretical Investigation of a Series of Novel Dimanganese(III)μ-Hydroxo Bisporphyrins: Magneto–Structural Correlation and Effect of Metal Spin on Porphyrin Core Deformation. Inorg. Chem. 2016, 55, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C.; Parigi, G.; Pierattelli, R. NMR Spectroscopy of Paramagnetic Metalloproteins. ChemBioChem 2005, 6, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Swartjes, A.; White, P.B.; Bruekers, J.P.J.; Elemans, J.A.A.W.; Nolte, R.J.M. Paramagnetic relaxation enhancement NMR as a tool to probe guest binding and exchange in metallohosts. Nat. Commun. 2022, 13, 1846. [Google Scholar] [CrossRef]
- Muthukumar, P.; Abraham John, S. Gold nanoparticles decorated on cobalt porphyrin-modified glassy carbon electrode for the sensitive determination of nitrite ion. J. Colloid. Interf. Sci. 2014, 421, 78–84. [Google Scholar] [CrossRef]
- Yadava, S.; Bharati, S.L. Novel complexes of Mn (III) with macrocylic porphine ligand and ethylenediamine. J. Coord. Chem. 2011, 64, 3950–3959. [Google Scholar] [CrossRef]
- Thandiayyakone, V.; Murugan, A.; Ravikumar, C.R.; Rajkumar, T.; Arasu, P.T.; Yadav, H.S.; Kotteeswaran, P. Studies on redox and axial ligand properties of Meso-Mn (III) porphyrin by cyclic voltammetry and UV–Visible spectrophotometry. Mater. Today Proc. 2021, 47, 933–937. [Google Scholar] [CrossRef]
- Giovannetti, R.; Alibabaei, L.; Pucciarelli, F. Spectral and kinetic investigation on oxidation and reduction of water soluble porphyrin–manganese (III) complex. Inorg. Chim. Acta. 2010, 363, 1561–1567. [Google Scholar] [CrossRef]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. In Macro to Nano Spectroscopy; Jamal, U., Ed.; InTech Open: Rijeka, Croatia, 2012; ISBN 987-953-51-0664-7. [Google Scholar] [CrossRef]
- Boucher, L.J. Manganese porphyrin complexes. Coord. Chem. Rev. 1972, 7, 289–329. [Google Scholar] [CrossRef]
- Pop, S.F.; Ion, R.M.; Corobea, M.C.; Raditoiu, V.R. Spectral and thermal investigations of porphyrin and phthalocyanine nanomaterials. J. Optoelectron. Adv. M. 2011, 13, 906–911. [Google Scholar]
- Ibrahim, A.R. Preparation and Characterization of some transition metal complexes of bis Schiff Base Ligand. Int. J. Adv. Res. 2015, 3, 315–324. [Google Scholar]
- Kennedy, B.J.; Murray, K.S. Magnetic properties and zero-field splitting in high-spin manganese (III) complexes. 2. Axially ligated manganese (III) porphyrin complexes. Inorg. Chem. 1985, 24, 1557–1560. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yang, M.; Zhu, M.; Chen, W.; Li, Q.; Sun, D.; Bi, X.; Maletskyi, Z.; Ratnaweera, H. Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length. Processes 2022, 10, 2614. [Google Scholar] [CrossRef]
- Jain, P.S.; Chaudhari, A.J.; Patel, S.A.; Patel, Z.N.; Patel, D.T. Development and validation of the UV-spectrophotometric method for determination of terbinafine hydrochloride in bulk and in formulation. Pharm. Methods 2011, 2, 198–202. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yu, H.; Nie, Y.; Yang, P.; Cheng, H.; Wang, Q.; Qin, X.; Chen, S.; et al. Grafting of organic sulfur-containing functional groups on activated carbon for gold (I) adsorption from thiosulfate solution. Hydrometallurgy 2019, 185, 102–110. [Google Scholar] [CrossRef]
- de la Torre-Miranda, N.; Reilly, L.; Eloy, P.; Poleunis, C.; Hermans, S. Thiol functionalized activated carbon for gold thiosulfate recovery, an analysis of the interactions between gold and sulfur functions. Carbon 2023, 204, 254–267. [Google Scholar] [CrossRef]
- Epuran, C.; Fratilescu, I.; Macsim, A.-M.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors 2022, 10, 133. [Google Scholar] [CrossRef]
- Shelnutt, J.A.; Song, X.-Z.; Ma, J.-G.; Jia, S.-L.; Jentzen, W.; Medforth, C.J. Nonplanar porphyrins and their significance in proteins. Chem. Soc. Rev. 1998, 27, 31–41. [Google Scholar] [CrossRef]
- Kaplan, W.A.; Suslick, K.S.; Scott, R.A. Core size and flexibility of metallohydroporphyrin macrocycles. Implications for F430 coordination chemistry. J. Am. Chem. Soc. 1991, 113, 9824–9827. [Google Scholar] [CrossRef]
- Kaigorodova, E.Y.; Mamardashvili, G.M.; Mamardashvili, N.Z. Axial Coordination of Pyridine- and Imidazole-Based Drug Molecules to Co(III)-Tetra(4-Carboxyphenyl)porphyrin. Russ. J. Inorg. Chem. 2018, 63, 1192–1198. [Google Scholar] [CrossRef]
- Milanesi, L.; Gomila, R.M.; Frontera, A.; Tomas, S. Binding of a Co(III) Metalloporphyrin to Amines in Water: Influence of the pKa and Aromaticity of the Ligand, and pH-Modulated Allosteric Effect. Inorg. Chem. 2025, 64, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; LeBail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, 420–427. [Google Scholar] [CrossRef]
- Jaguar, version 2025-1. Schrödinger Release. Schrödinger, LLC: New York, NY, USA, 2025.
- Discovery Studio, version 2019, BIOVIA; Dassault Systèmes: San Diego, CA, USA, 2019.
- Dougherty, D.A. The Cation−π Interaction in Chemistry and Biology. Chem. Rev. 2025, 125, 2793–2808. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N. Substituent Effects in Cation/π Interactions and Electrostatic Potentials above the Centers of Substituted Benzenes Are Due Primarily to Through-Space Effects of the Substituents. J. Am. Chem. Soc. 2009, 131, 3126–3127. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J. Quantification of noncovalent interactions—Promises and problems. New J. Chem. 2019, 43, 15498–15512. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 8 May 2025).
- Mandel, J. The validation of measurement through interlaboratory studies. Chemometr. Intell. Lab. Syst. 1991, 11, 109–119. [Google Scholar] [CrossRef]
- Wilrich, P.-T. Critical values of Mandel’s h and k, the Grubbs and the Cochran test statistic. AStA-Adv. Stat. Anal. 2013, 97, 1–10. [Google Scholar] [CrossRef]

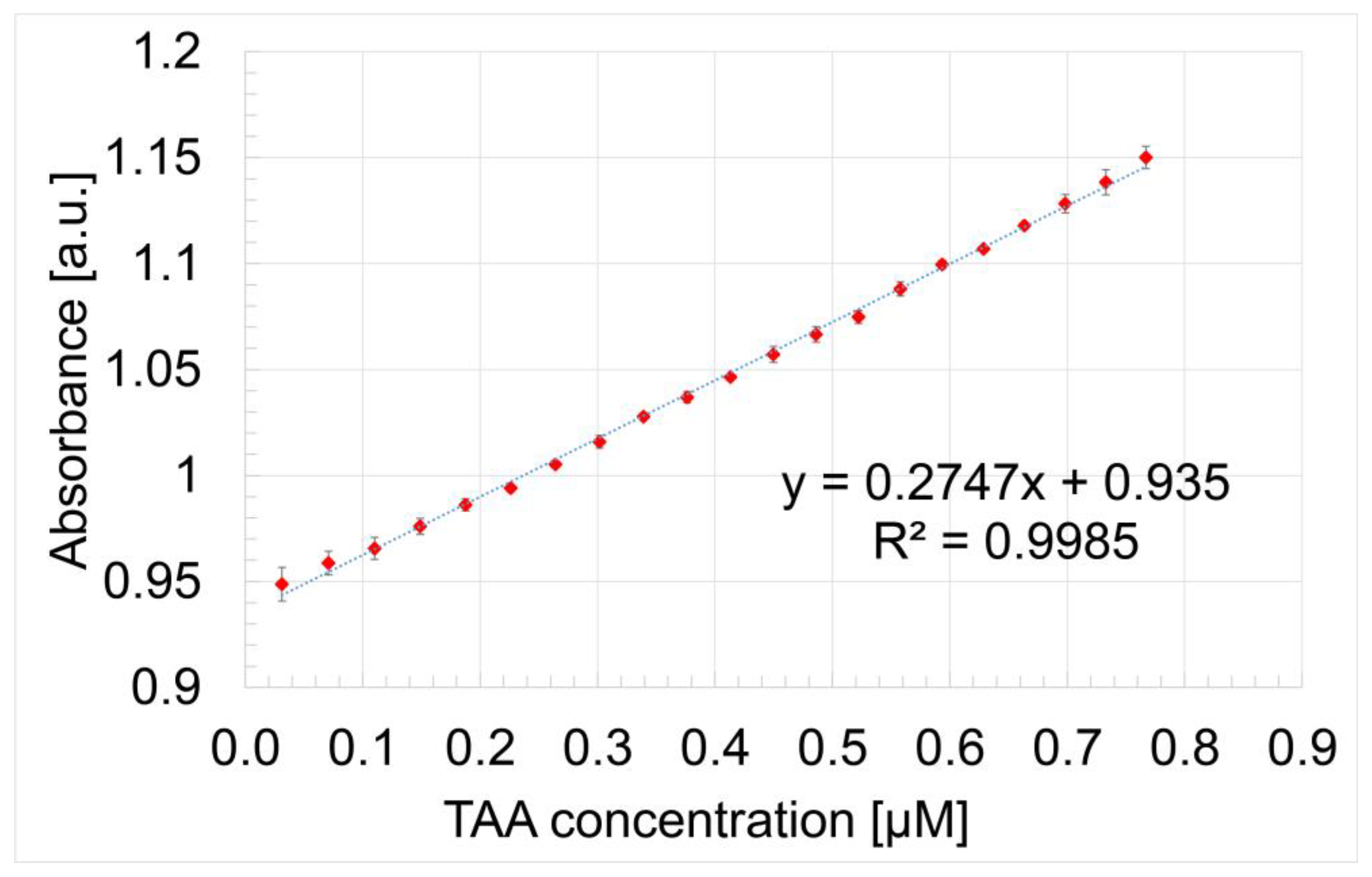
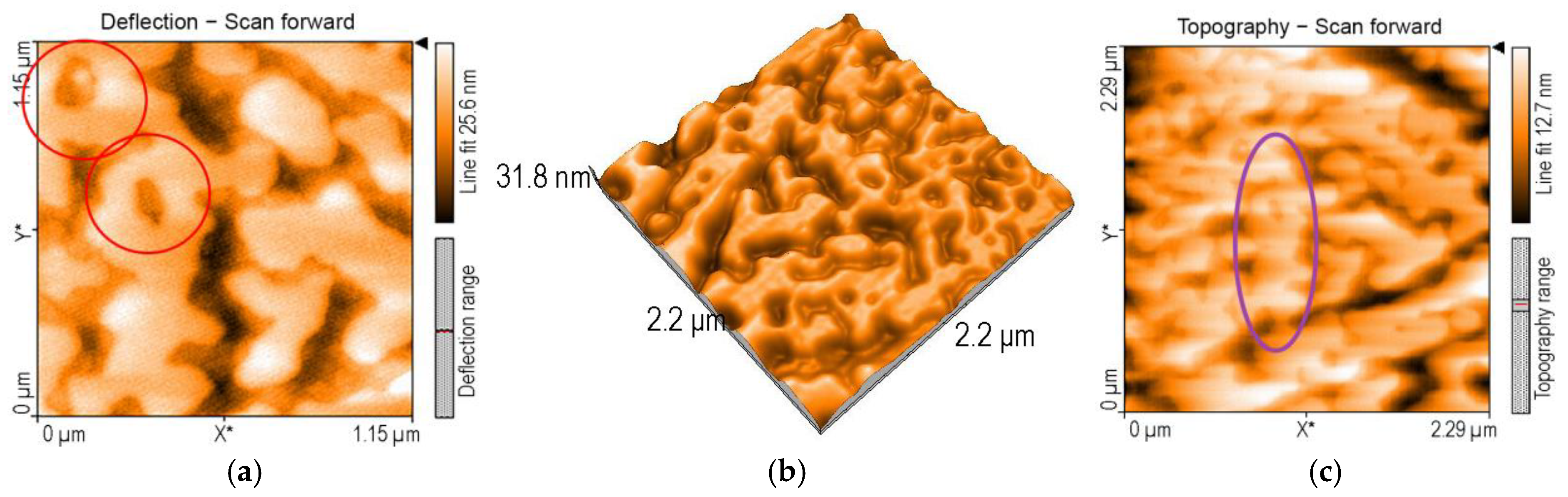
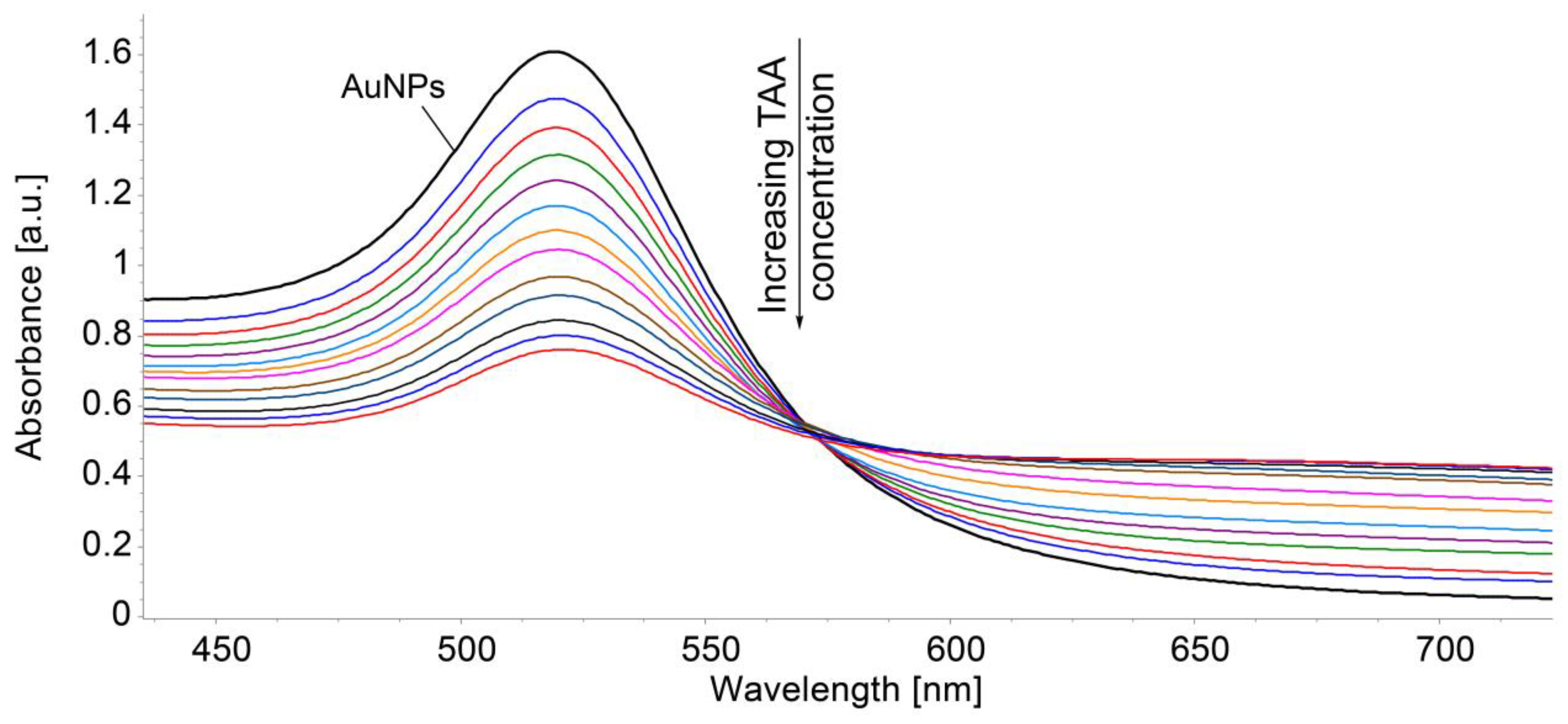

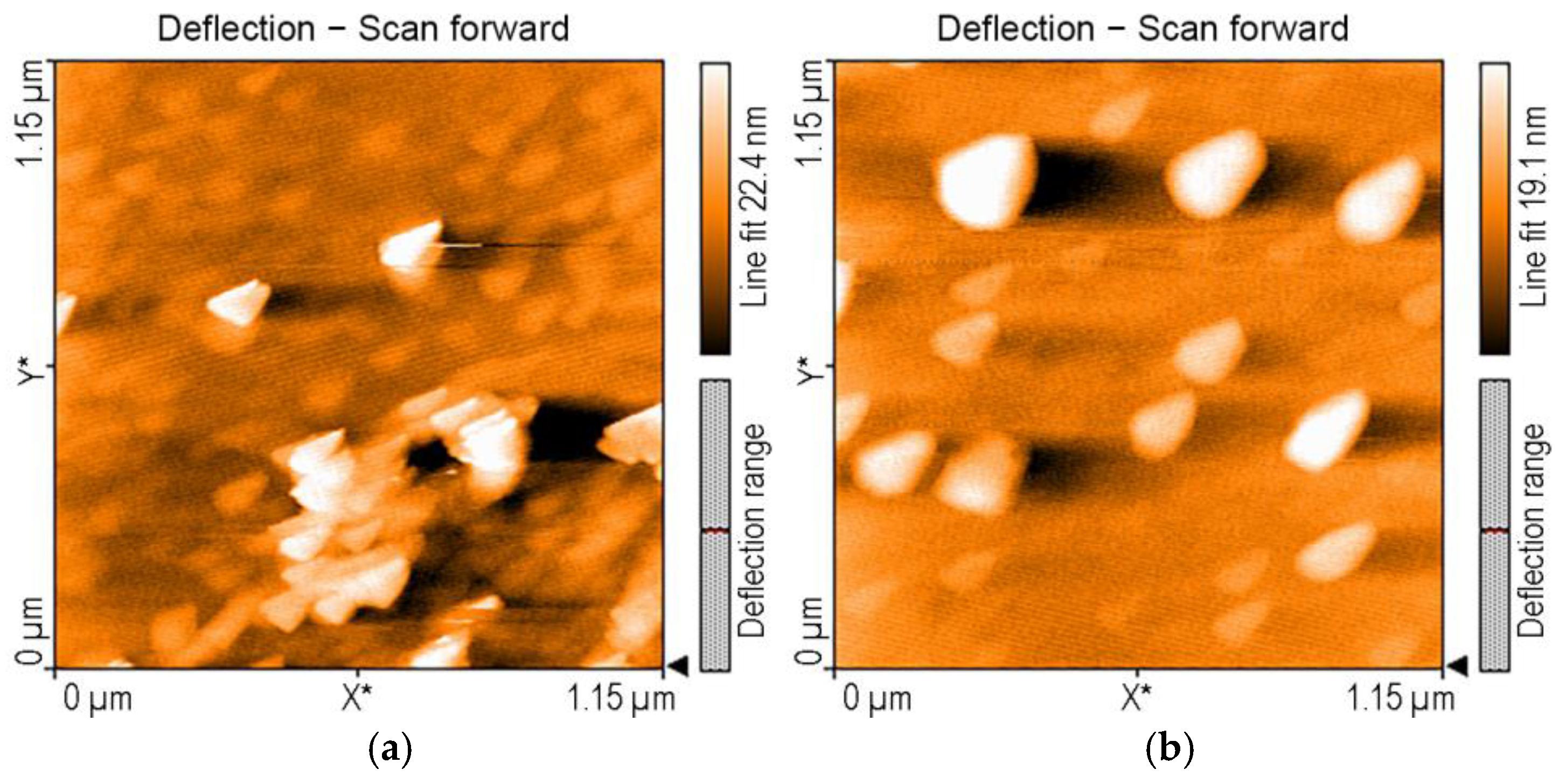
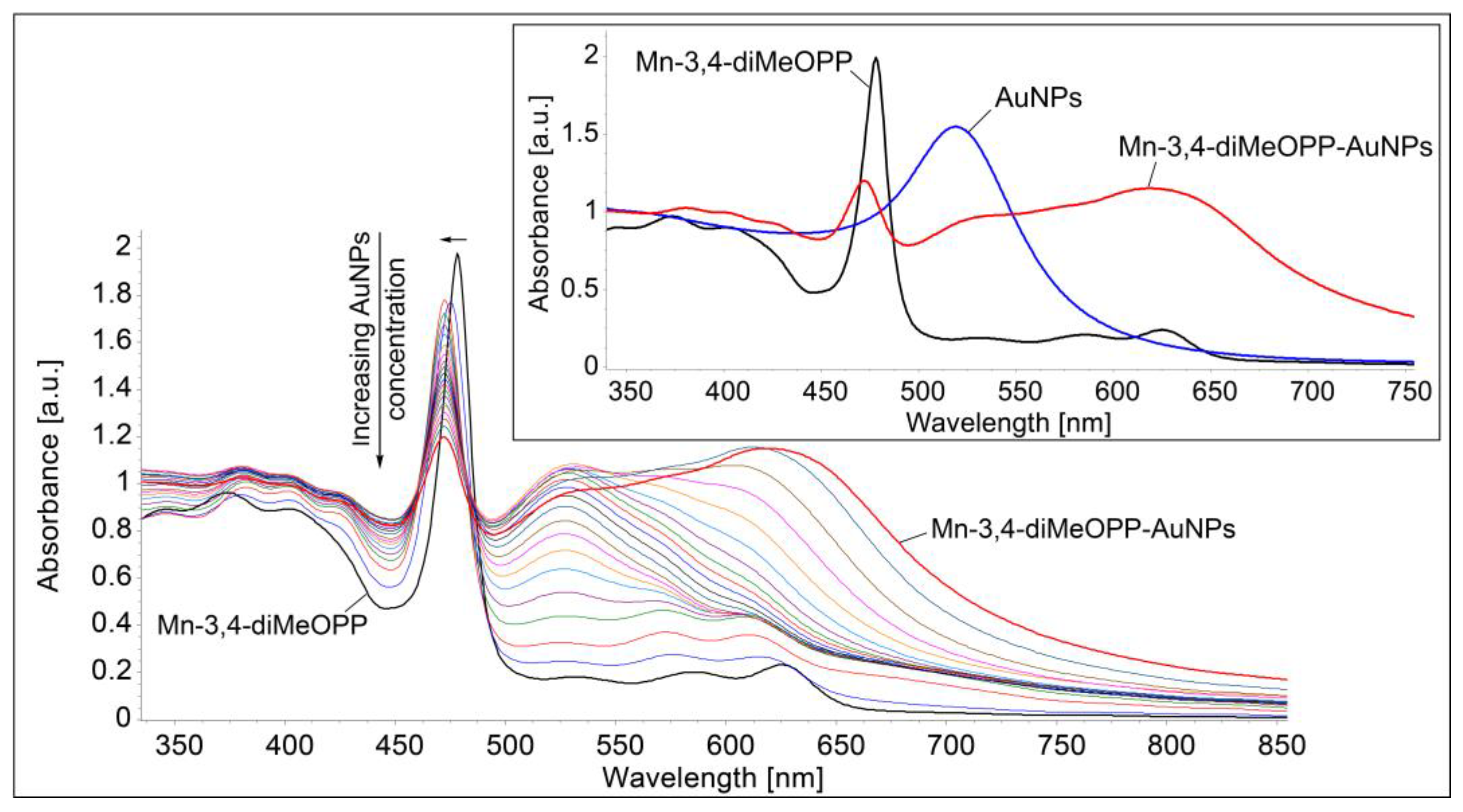
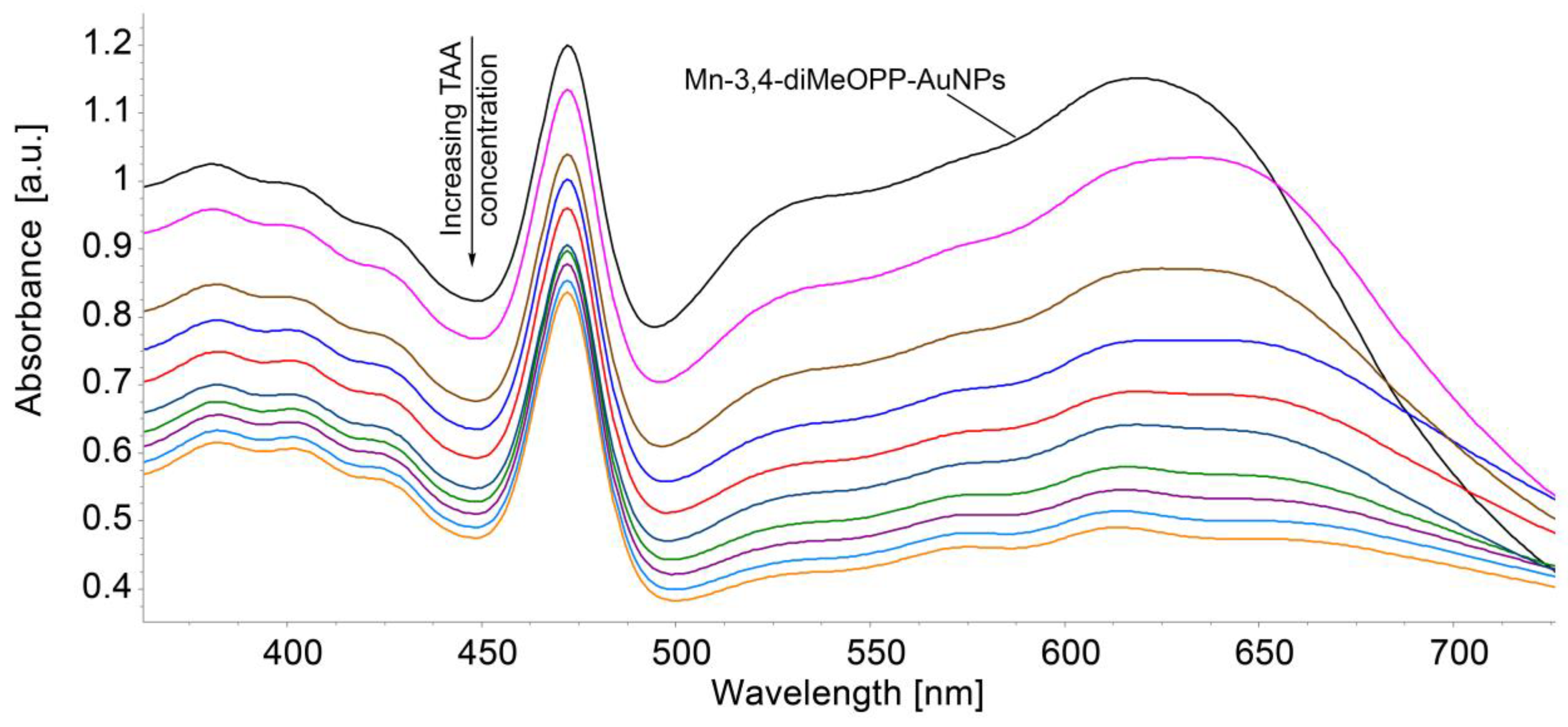
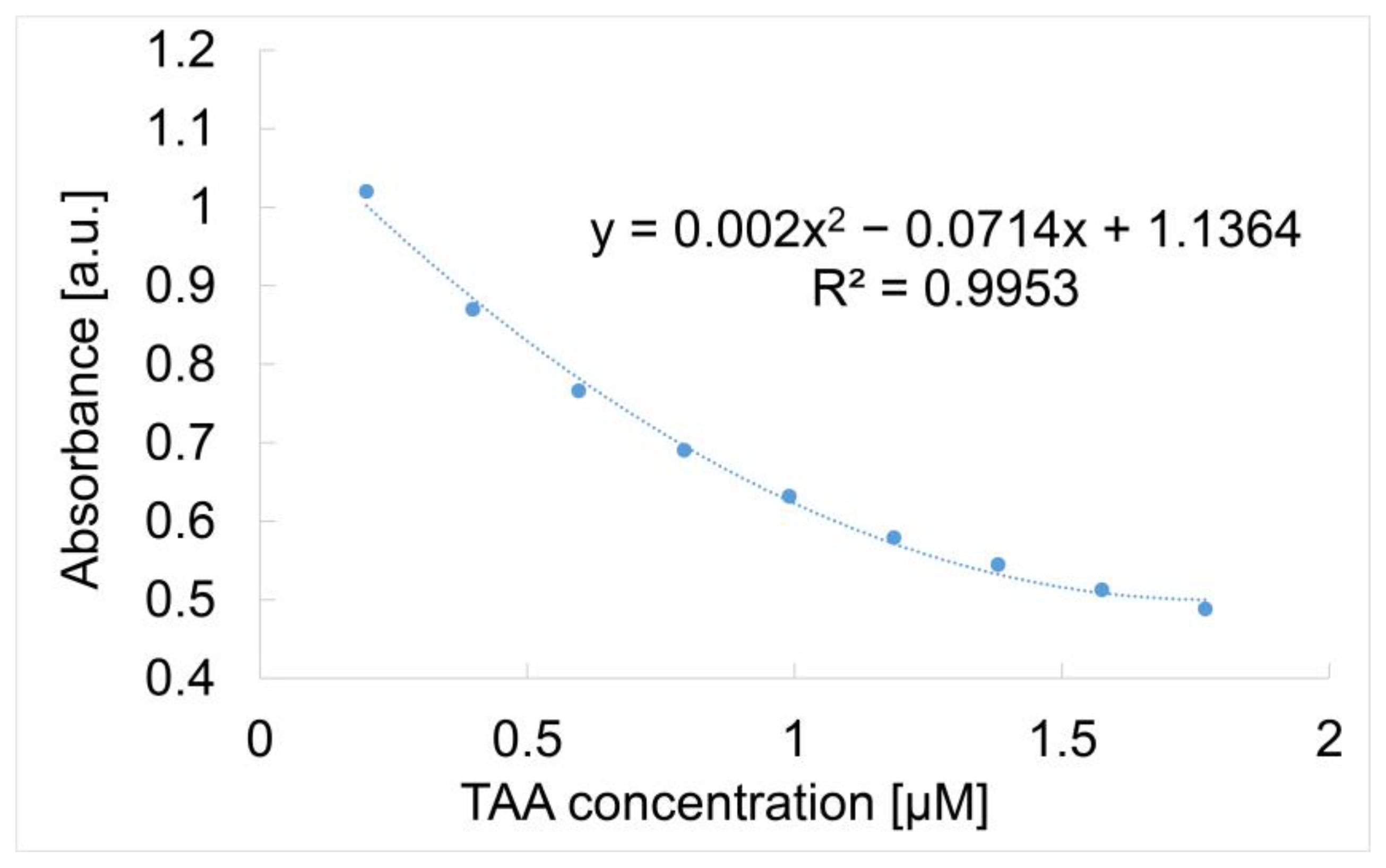
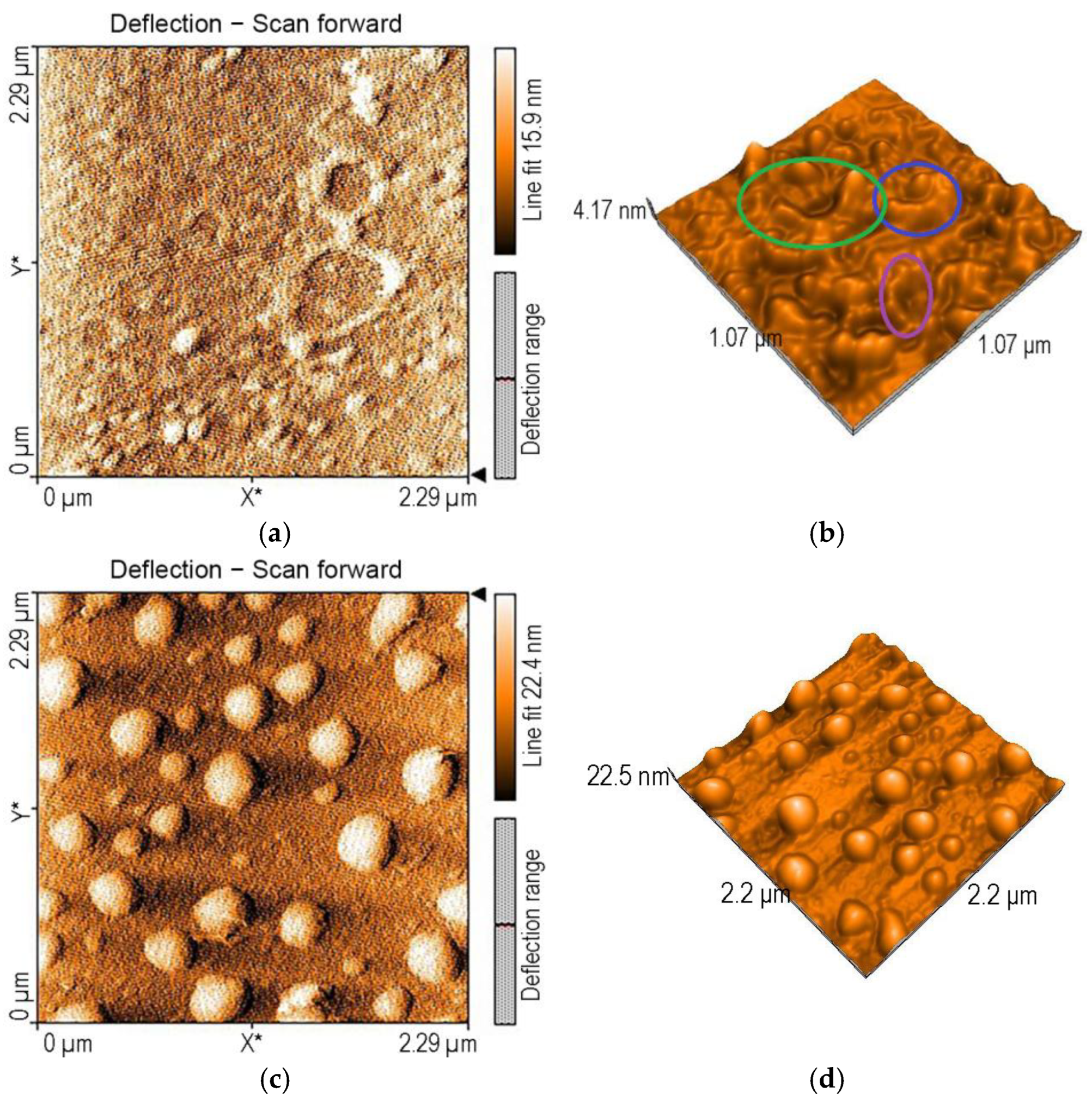
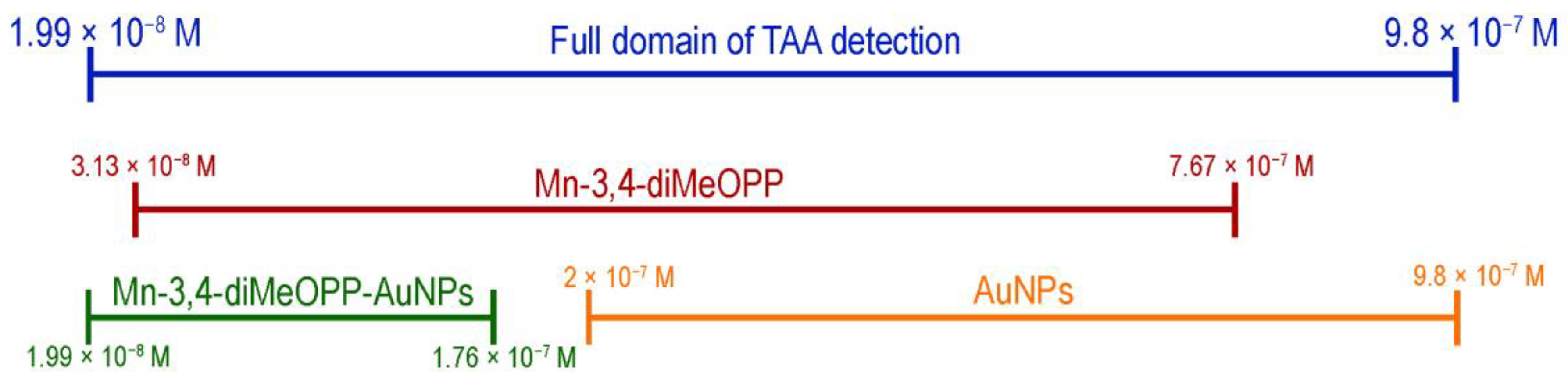
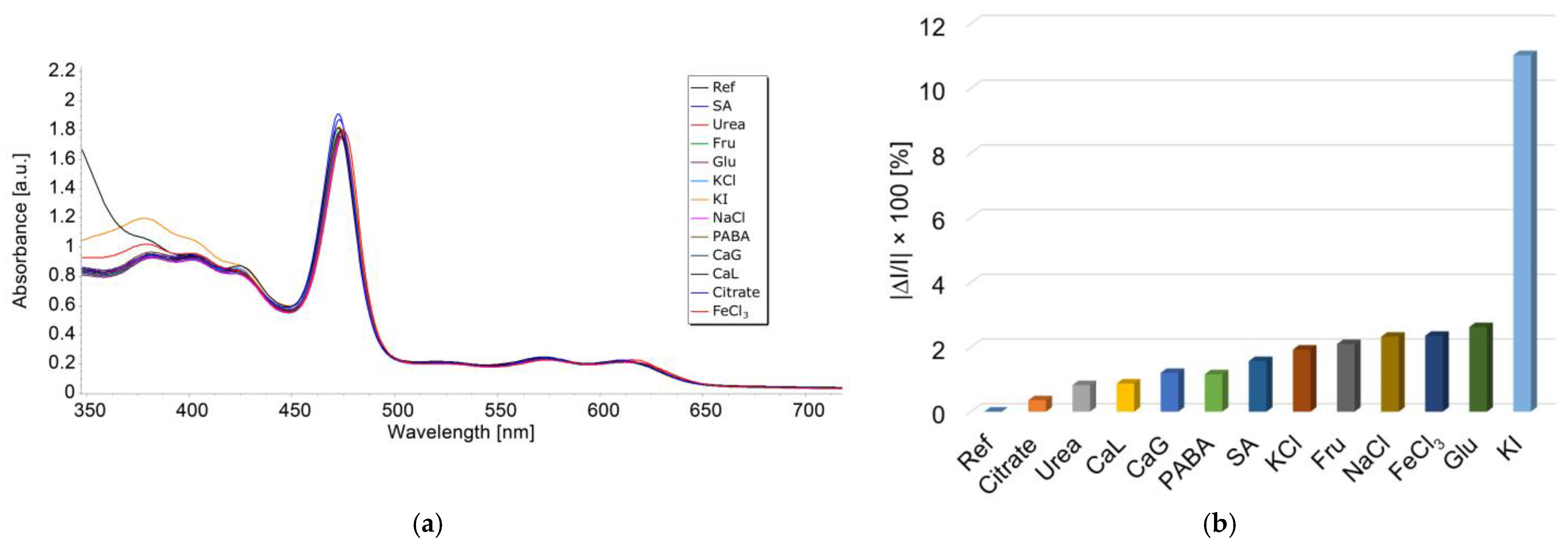
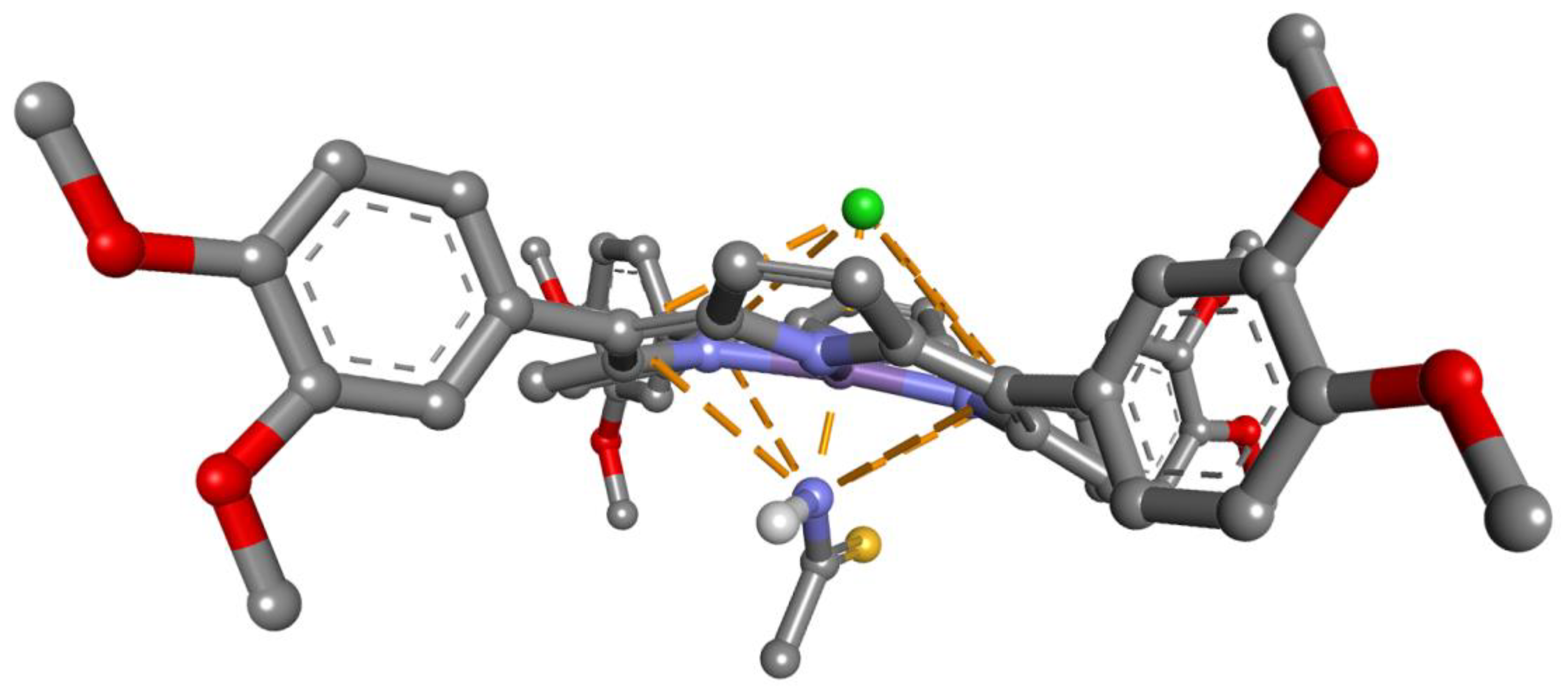
| Sensitive Material/ Detection Method | Concentration Domain [μM] | Detection Limit [μM] | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|
| Boron-doped diamond electrode/voltammetry | 5–60 | 0.84 | Good reproducibility and stability/expensive diamond electrode | [35] |
| Catechol/ voltammetry | 5–125 | 2.02 | High sensitivity, no cationic interferences | [36] |
| Differential pulse cathodic stripping voltammetry | − | 0.022 | Low detection limit/toxic mercury is used | [37] |
| Nanofibers composed of copper and aspartate/triboelectric nanogenerator (TENG-sensing) | 1000–100,000 | − | Creation of a triboelectric nanogenerator | [38] |
| ZnO quantum dots/ fluorescence | 0–10 | 0.00214 | Difficult to replicate, narrow concentration domain | [39] |
| Tris(2,2′-bipyridyl)ruthenium(II)/ electrochemiluminescence | 10–1,000,000 | 0.035 | Low detection limit, outstanding recoveries | [40] |
| AuNPs/optical detection | 0.2–0.98 | 0.069 | Detection in low field concentrations/very narrow concentration domain | This work |
| Mn-3,4-diMeOPP-AuNPs/optical detection | 0.019–0.176 | 0.006 | Trace field detection/polynomial dependence between the absorbance and the TAA concentration | |
| Mn-3,4-diMeOPP/optical detection | 0.0313–0.767 | 0.013 | Best performing of all reported sensors; an accurate, sensitive, and selective sensor for trace field detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epuran, C.; Fratilescu, I.; Fringu, I.; Lascu, A.; Halip, L.; Gherban, M.; Fagadar-Cosma, E. UV–Vis Detection of Thioacetamide: Balancing the Performances of a Mn(III)-Porphyrin, Gold Colloid, and Their Complex for Selecting the Most Sensitive Material. Micromachines 2025, 16, 574. https://doi.org/10.3390/mi16050574
Epuran C, Fratilescu I, Fringu I, Lascu A, Halip L, Gherban M, Fagadar-Cosma E. UV–Vis Detection of Thioacetamide: Balancing the Performances of a Mn(III)-Porphyrin, Gold Colloid, and Their Complex for Selecting the Most Sensitive Material. Micromachines. 2025; 16(5):574. https://doi.org/10.3390/mi16050574
Chicago/Turabian StyleEpuran, Camelia, Ion Fratilescu, Ionela Fringu, Anca Lascu, Liliana Halip, Mihaela Gherban, and Eugenia Fagadar-Cosma. 2025. "UV–Vis Detection of Thioacetamide: Balancing the Performances of a Mn(III)-Porphyrin, Gold Colloid, and Their Complex for Selecting the Most Sensitive Material" Micromachines 16, no. 5: 574. https://doi.org/10.3390/mi16050574
APA StyleEpuran, C., Fratilescu, I., Fringu, I., Lascu, A., Halip, L., Gherban, M., & Fagadar-Cosma, E. (2025). UV–Vis Detection of Thioacetamide: Balancing the Performances of a Mn(III)-Porphyrin, Gold Colloid, and Their Complex for Selecting the Most Sensitive Material. Micromachines, 16(5), 574. https://doi.org/10.3390/mi16050574








