A Highly Sensitive Giant Magnetoresistive (GMR) Biosensor Based on the Magnetic Flux Concentrator Effect
Abstract
1. Introduction
2. Simulation and Analysis
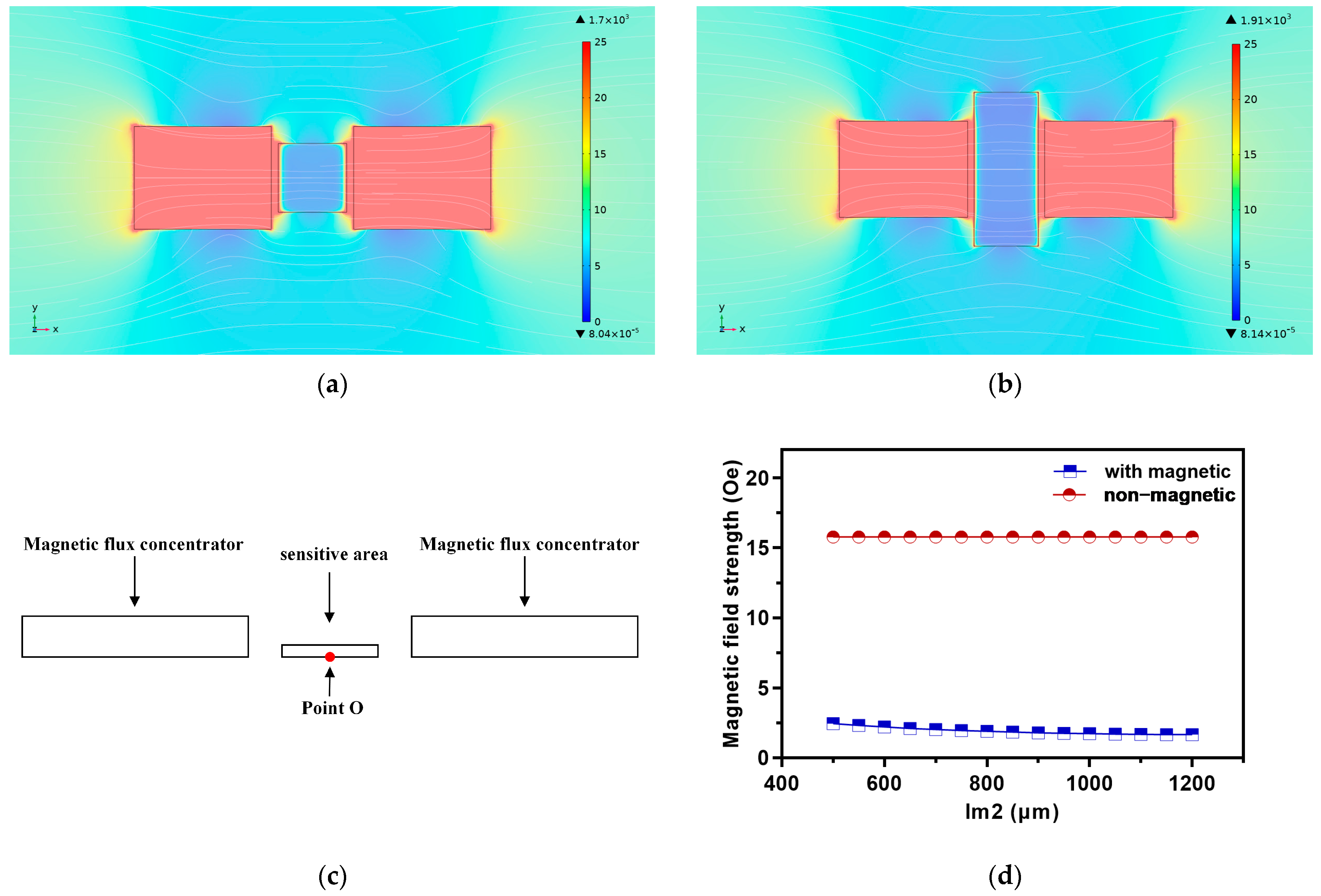
2.1. Principle and Model of Magnetic Flux Concentrator
2.2. Simulation Results and Analysis of MFC
2.2.1. Sensitive Area Width Lm2
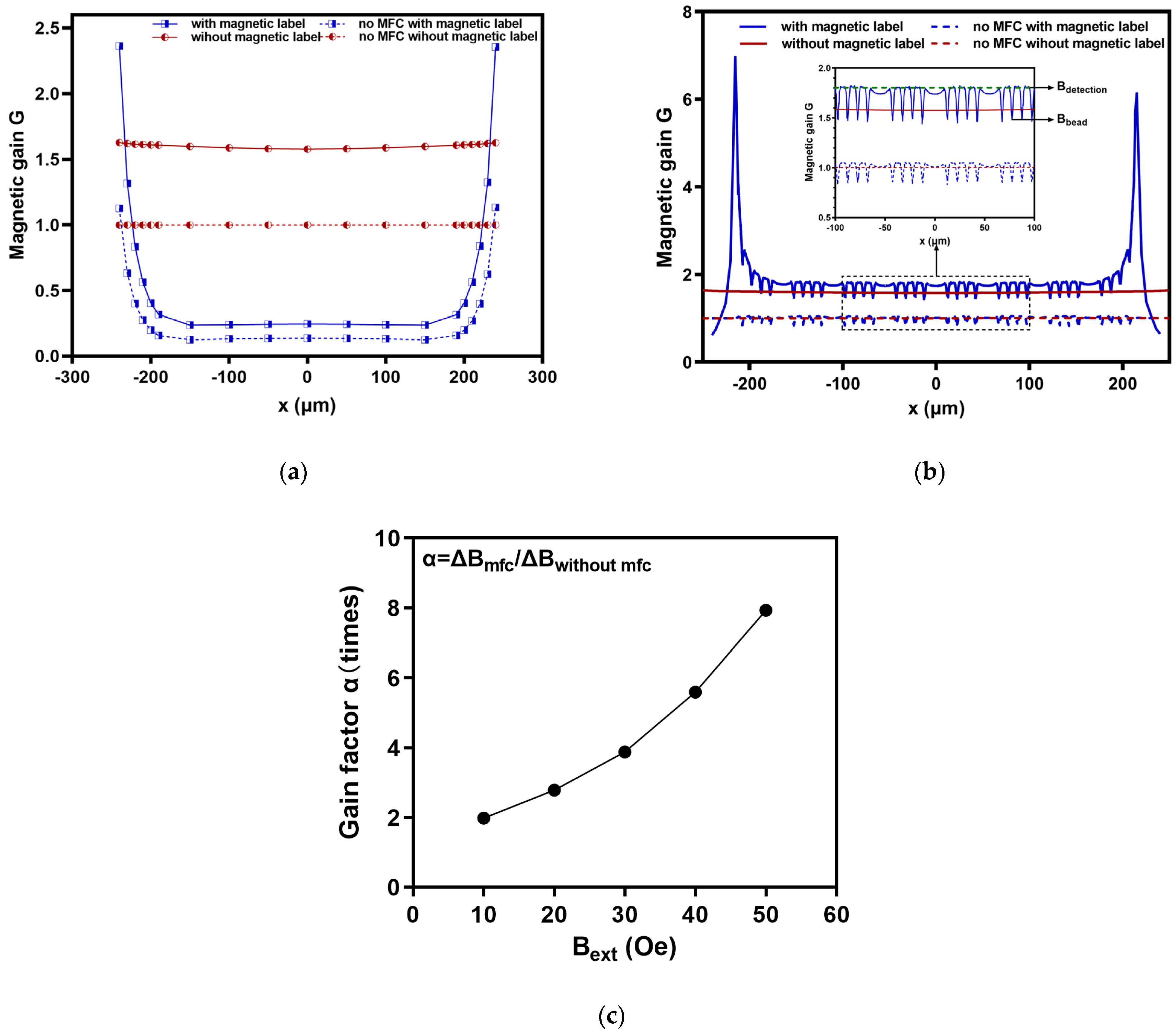
2.2.2. Air Gap Width wg
2.2.3. Magnetic Labels
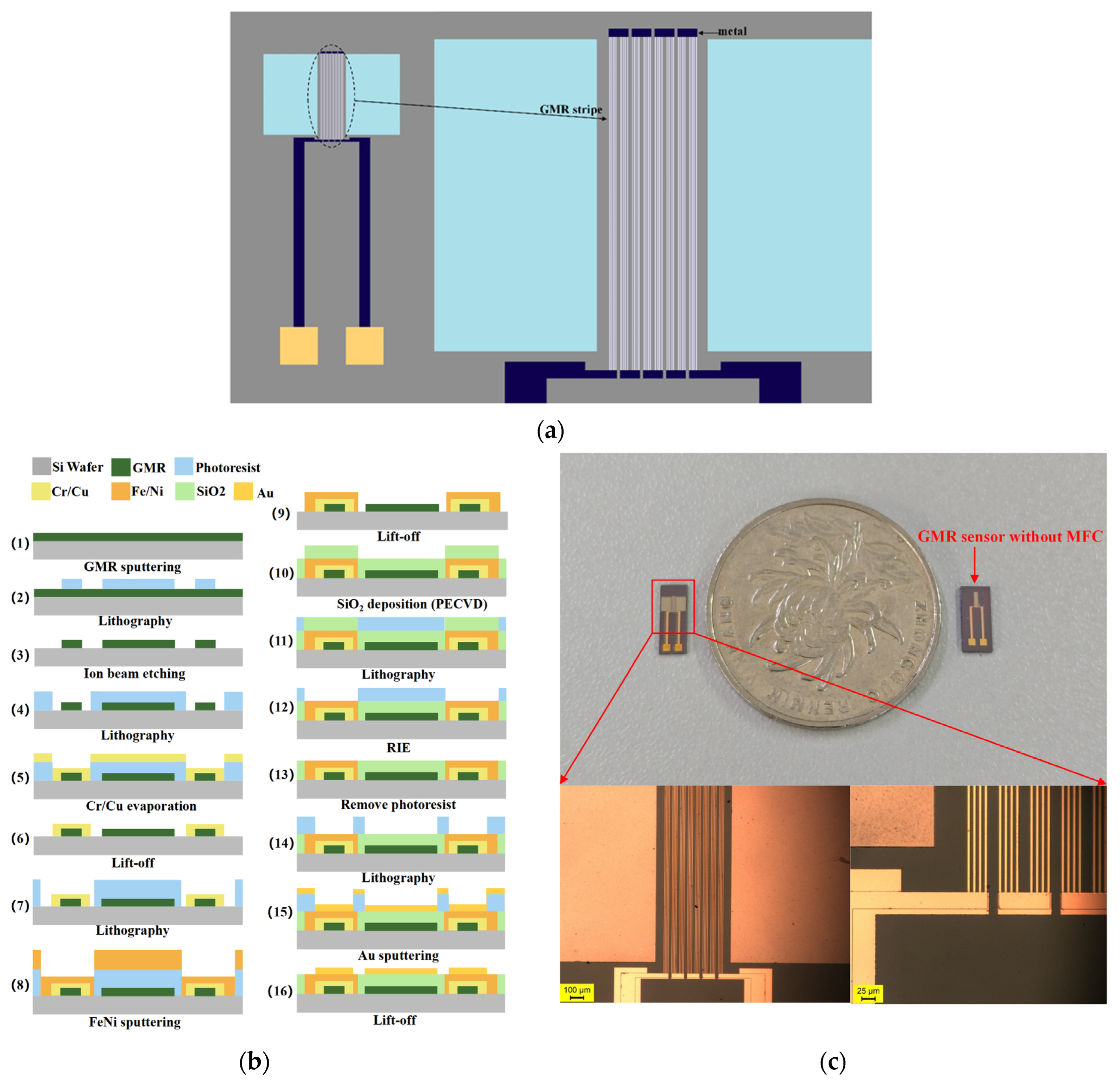
3. Experimental Details
3.1. Design and Fabrication of GMR Magnetic Sensor
3.2. Preparation of Biological Samples
3.2.1. Biological and Chemical Materials
3.2.2. Preparation of Bacterial Samples
3.2.3. Surface Functionalization Modification
4. Results and Discussion
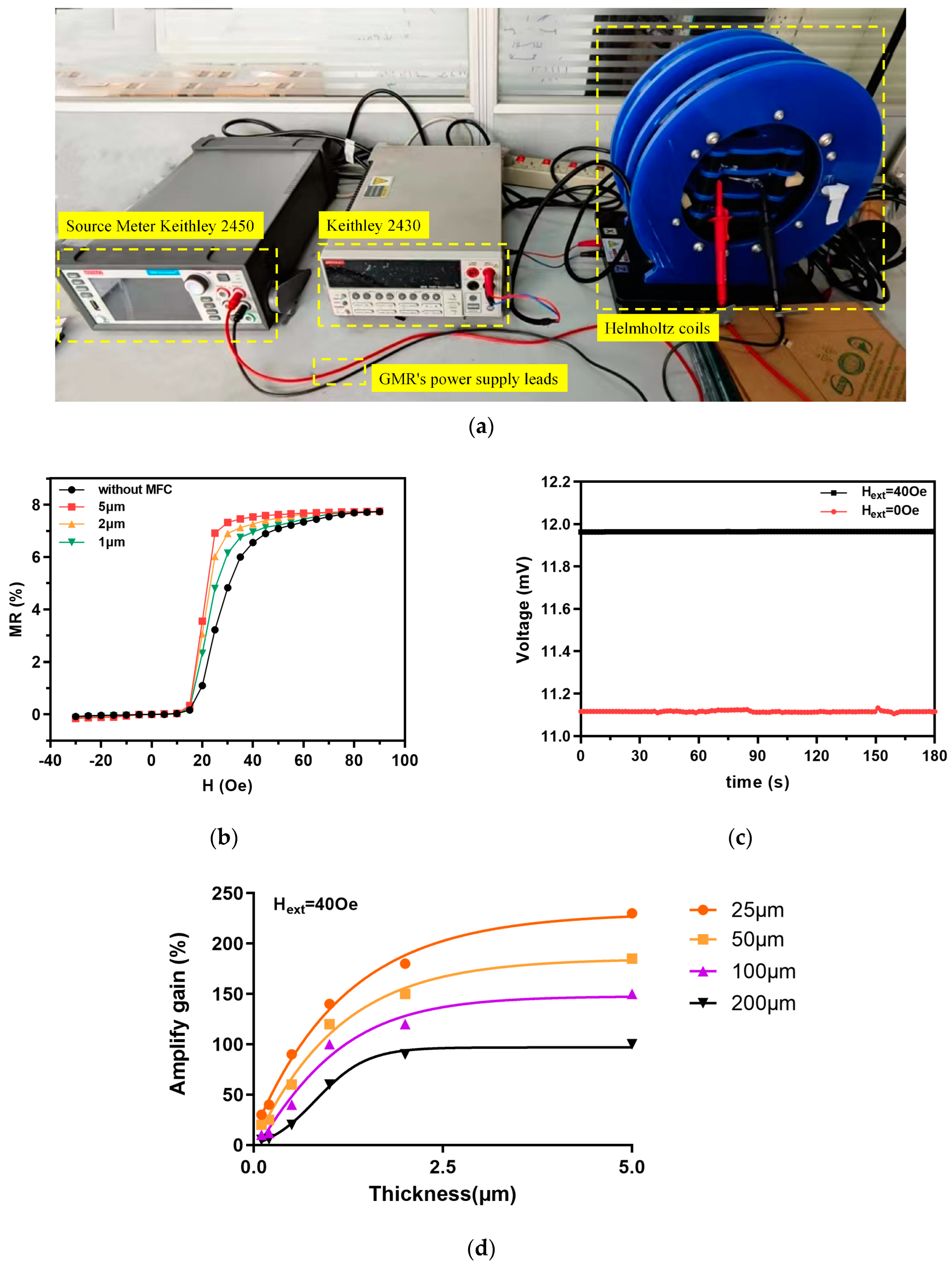
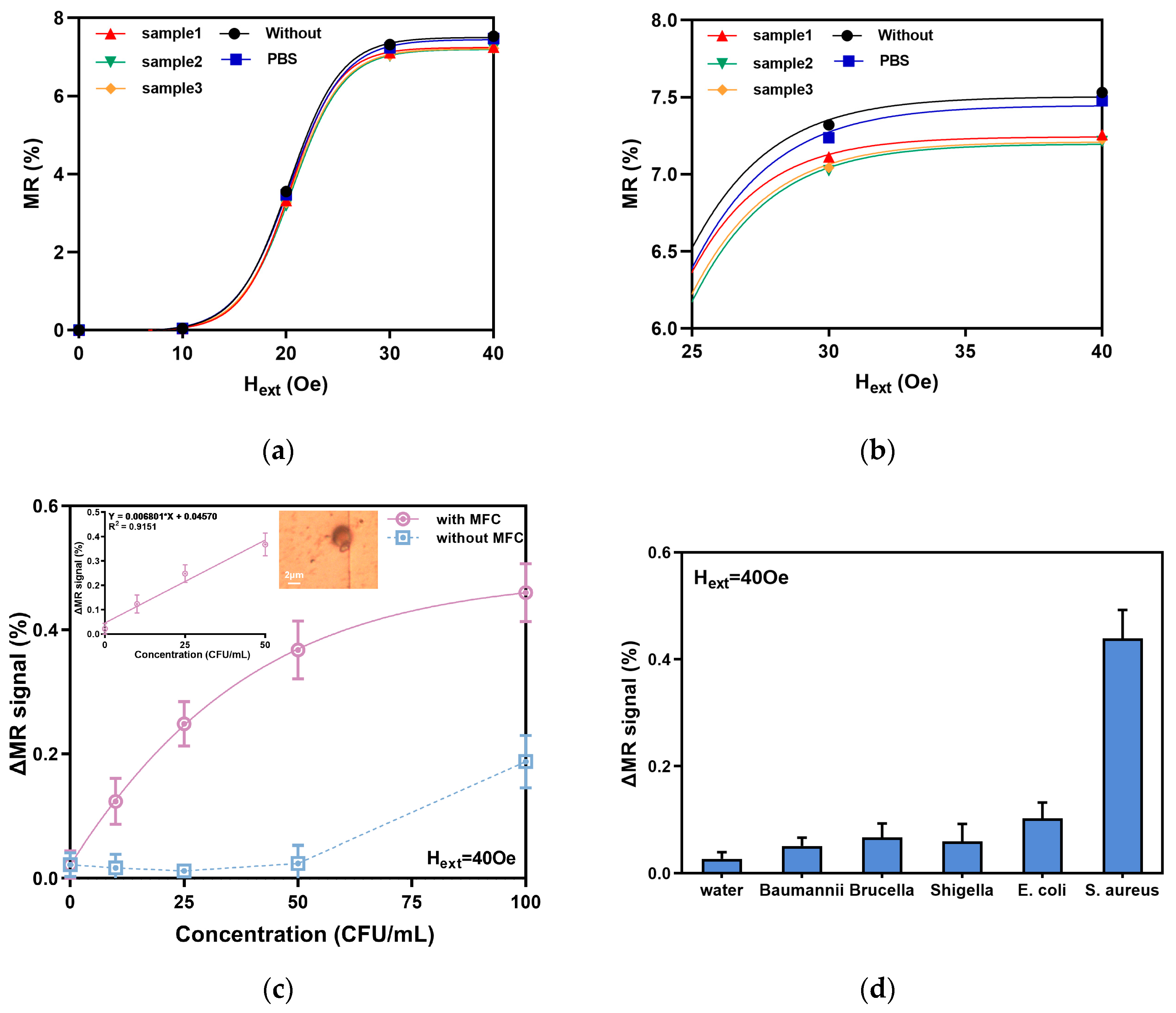
4.1. Measurement of GMR Basic Performance
4.2. Magnetic Biological Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Li, X.; Fan, Y.; Li, J.; Lu, K.; Wen, H.; Ren, J. Recent advances of fluorescent sensors for bacteria detection-A review. Talanta 2023, 254, 124133. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Trends, challenges, and advances in optical sensing for pathogenic bacteria detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.R.; Pu, H.Y. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, G. Advances in giant magnetoresistance biosensors with magnetic nanoparticle tags: Review and outlook. IEEE Trans. Magn. 2008, 44, 1687–1702. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.H.; Chen, Q.S. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Yin, M.; Jing, C.; Li, H.; Deng, Q.; Wang, S. Surface chemistry modified upconversion nanoparticles as fluorescent sensor array for discrimination of foodborne pathogenic bacteria. J. Nanobiotechnol. 2020, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Dursun, A.D.; Borsa, B.A.; Bayramoglu, G.; Arica, M.Y.; Ozalp, V.C. Surface plasmon resonance aptasensor for Brucella detection in milk. Talanta 2022, 239, 123074. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Ozalp, V.C.; Oztekin, M.; Arica, M.Y. Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 2019, 200, 263–271. [Google Scholar] [CrossRef]
- Hianik, T.; Spagnolo, S.; Thompson, M. Trends in Development of Aptamer-Based Biosensor Technology for Detection of Bacteria. Adv. Biochem. Eng./Biotechnol. 2024, 187, 339–380. [Google Scholar] [CrossRef]
- Gao, S.P.; Wei, Z.K.; Zheng, X.Y.; Zhu, J.; Wang, T.X.; Huang, X.W.; Shen, T.T.; Zhang, D.; Guo, Z.M.; Zou, X.B. Advancements in magnetic nanomaterial-assisted sensitive detection of foodborne bacteria: Dual-recognition strategies, functionalities, and multiplexing applications. Food Chem. 2025, 478, 19. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Venkatesan, J.; AlAnezi, A.A.; Walczyk, D.; Farooqi, A.; Malina, D.; Hosseini, S.H.; Tyliszczak, B. Magnetic nanomaterials and sensors for biological detection. Nanomed.-Nanotechnol. Biol. Med. 2016, 12, 2459–2473. [Google Scholar] [CrossRef] [PubMed]
- Issadore, D.; Park, Y.I.; Shao, H.; Min, C.; Lee, K.; Liong, M.; Weissleder, R.; Lee, H. Magnetic sensing technology for molecular analyses. Lab A Chip 2014, 14, 2385–2397. [Google Scholar] [CrossRef]
- Schotter, J.; Shoshi, A.; Brueckl, H. Development of a magnetic lab-on-a-chip for point-of-care sepsis diagnosis. J. Magn. Magn. Mater. 2009, 321, 1671–1675. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Pühler, A.; Brinkmann, D.; Schepper, W.; Brückl, H.; Reiss, G. A biochip based on magnetoresistive sensors. IEEE Trans. Magn. 2002, 38, 3365–3367. [Google Scholar] [CrossRef]
- Bellamkonda, R.; John, T.; Mathew, B.; DeCoster, M.; Hegab, H.; Davis, D. Fabrication and testing of a CoNiCu/Cu CPP-GMR nanowire-based microfluidic biosensor. J. Micromech. Microeng. 2010, 20, 6. [Google Scholar] [CrossRef]
- Wu, K.; Tonini, D.; Liang, S.; Saha, R.; Chugh, V.K.; Wang, J.P. Giant Magnetoresistance Biosensors in Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 9945–9969. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Mujika, M.; Arana, S. GMR sensors: Magnetoresistive behaviour optimization for biological detection by means of superparamagnetic nanoparticles. Biosens. Bioelectron. 2011, 26, 3705–3709. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, L.; Geng, J.W.; Shi, X.Z.; Qian, H. A micro-array bio detection system based on a GMR sensor with 50-ppm sensitivity. Sci. China-Inf. Sci. 2017, 60, 9. [Google Scholar] [CrossRef]
- Rife, J.C.; Miller, M.M.; Sheehan, P.E.; Tamanaha, C.R.; Tondra, M.; Whitman, L.J. Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuators A-Phys. 2003, 107, 209–218. [Google Scholar] [CrossRef]
- Gaster, R.S.; Xu, L.; Han, S.J.; Wilson, R.J.; Hall, D.A.; Osterfeld, S.J.; Yu, H.; Wang, S.X. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 2011, 6, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sutham, P.; Wu, K.; Mallikarjunan, K.; Wang, J.P. Giant Magnetoresistance Biosensors for Food Safety Applications. Sensors 2022, 22, 5663. [Google Scholar] [CrossRef]
- Mak, A.C.; Osterfeld, S.J.; Yu, H.; Wang, S.X.; Davis, R.W.; Jejelowo, O.A.; Pourmand, N. Sensitive giant magnetoresistive-based immunoassay for multiplex mycotoxin detection. Biosens. Bioelectron. 2010, 25, 1635–1639. [Google Scholar] [CrossRef]
- Xu, L.; Yu, H.; Akhras, M.S.; Han, S.-J.; Osterfeld, S.; White, R.L.; Pourmand, N.; Wang, S.X. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron. 2008, 24, 99–103. [Google Scholar] [CrossRef]
- Gani, A.W.; Wei, W.; Shi, R.Z.; Ng, E.; Nguyen, M.; Chua, M.S.; So, S.; Wang, S.X. An Automated, Quantitative, and Multiplexed Assay Suitable for Point-of-Care Hepatitis B Virus Diagnostics. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef]
- Sun, X.C.; Lei, C.; Guo, L.; Zhou, Y. Separable detecting of Escherichia coli O157H:H7 by a giant magneto-resistance-based bio-sensing system. Sens. Actuators B-Chem. 2016, 234, 485–492. [Google Scholar] [CrossRef]
- Hall, D.A.; Gaster, R.S.; Lin, T.; Osterfeld, S.J.; Han, S.; Murmann, B.; Wang, S.X. GMR biosensor arrays: A system perspective. Biosens. Bioelectron. 2010, 25, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, Y.; Chen, G.; Liu, J.; Li, J.; Feng, K.; Lv, C.; Wang, W. Influence of size parameters and magnetic field intensity upon the amplification characteristics of magnetic flux concentrators. Aip Adv. 2018, 8, 125222. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, L.; Pong, P.W.T. Magnetic flux concentration at micrometer scale. Microelectron. Eng. 2013, 111, 77–81. [Google Scholar] [CrossRef]
- Mott, N.F. The electrical conductivity of transition metals. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1936, 153, 699–717. [Google Scholar] [CrossRef]
- Mei, Y.; He, C.; Zeng, W.; Luo, Y.; Liu, C.; Yang, M.; Kuang, Y.; Lin, X.; Huang, Q. Electrochemical Biosensors for Foodborne Pathogens Detection Based on Carbon Nanomaterials: Recent Advances and Challenges. Food Bioprocess Technol. 2022, 15, 498–513. [Google Scholar] [CrossRef]
- Li, Q.; Dou, L.N.; Zhang, Y.J.; Luo, L.; Yang, H.J.; Wen, K.; Yu, X.Z.; Shen, J.Z.; Wang, Z.H. A comprehensive review on the detection of Staphylococcus aureus enterotoxins in food samples. Compr. Rev. Food. Sci. Food Saf. 2024, 23, e13264. [Google Scholar] [CrossRef]
- Mujika, M.; Arana, S.; Castano, E.; Tijero, M.; Vilares, R.; Ruano-Lopez, J.M.; Cruz, A.; Sainz, L.; Berganza, J. Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network. Biosens. Bioelectron. 2009, 24, 1253–1258. [Google Scholar] [CrossRef]
- Guo, P.-L.; Tang, M.; Hong, S.-L.; Yu, X.; Pang, D.-W.; Zhang, Z.-L. Combination of dynamic magnetophoretic separation and stationary magnetic trap for highly sensitive and selective detection of Salmonella typhimurium in complex matrix. Biosens. Bioelectron. 2015, 74, 628–636. [Google Scholar] [CrossRef]
- Schoepp, N.G.; Schlappi, T.S.; Curtis, M.S.; Butkovich, S.S.; Miller, S.; Humphries, R.M.; Ismagilov, R.F. Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci. Transl. Med. 2017, 9, 12. [Google Scholar] [CrossRef]
- Mostufa, S.; Liang, S.; Chugh, V.K.; Wang, J.-P.; Wu, K. Spintronic devices for biomedical applications. npj Spintron. 2024, 2, 26. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, S.; Chen, L.; Li, M.; Zhang, Y.; Zhou, N. Self-Assembled DNA Nanoflowers Triggered by a DNA Walker for Highly Sensitive Electrochemical Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2021, 13, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Liu, J.; Feng, X.; Xie, G.; Zhang, W.; Xu, H. Rapid enrichment and detection of Staphylococcus aureus in milk using polyethyleneimine functionalized magnetic nanoparticles. Microchem. J. 2022, 178, 107388. [Google Scholar] [CrossRef]
- Li, R.; Yan, J.; Feng, B.; Sun, M.; Ding, C.; Shen, H.; Zhu, J.; Yu, S. Ultrasensitive Detection of Multidrug-Resistant Bacteria Based on Boric Acid-Functionalized Fluorescent MOF@COF. ACS Appl. Mater. Interfaces 2023, 15, 18663–18671. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Describe |
|---|---|
| Lm1 | The length of the magnetic sensitive region |
| Lm2 | Width of magnetic sensitive region |
| lfc | Length of MFC |
| wfc | Width of MFC |
| tfc | Thickness of MFC |
| wg | Width of air gap |
| Reference | Year | Linear Range (CFU/mL) | LOD (CFU/mL) | Methods | Bacteria |
|---|---|---|---|---|---|
| [30] | 2016 | 102–103 | 100.0 | Magnetoresistance | E. coli |
| [41] | 2021 | 6.0 × 101–6.0 × 107 | 9.0 | Electrochemical (DPV) | S. aureus |
| [42] | 2022 | 101–107 | 29.0 | Fluorescence | S. aureus |
| [43] | 2023 | 2.0 × 101–108 | 7.0 | Fluorescence | S. aureus |
| This study | 2025 | 101–102 | 10.0 | Magnetoresistance | S. aureus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Li, J.; Zhao, C.; Ren, C.; Tian, T.; Lei, C.; Sun, X. A Highly Sensitive Giant Magnetoresistive (GMR) Biosensor Based on the Magnetic Flux Concentrator Effect. Micromachines 2025, 16, 559. https://doi.org/10.3390/mi16050559
Sun H, Li J, Zhao C, Ren C, Tian T, Lei C, Sun X. A Highly Sensitive Giant Magnetoresistive (GMR) Biosensor Based on the Magnetic Flux Concentrator Effect. Micromachines. 2025; 16(5):559. https://doi.org/10.3390/mi16050559
Chicago/Turabian StyleSun, Hao, Jiao Li, Changhui Zhao, Chunming Ren, Tian Tian, Chong Lei, and Xuecheng Sun. 2025. "A Highly Sensitive Giant Magnetoresistive (GMR) Biosensor Based on the Magnetic Flux Concentrator Effect" Micromachines 16, no. 5: 559. https://doi.org/10.3390/mi16050559
APA StyleSun, H., Li, J., Zhao, C., Ren, C., Tian, T., Lei, C., & Sun, X. (2025). A Highly Sensitive Giant Magnetoresistive (GMR) Biosensor Based on the Magnetic Flux Concentrator Effect. Micromachines, 16(5), 559. https://doi.org/10.3390/mi16050559







