Wearable Medical Devices: Application Status and Prospects
Abstract
1. Introduction
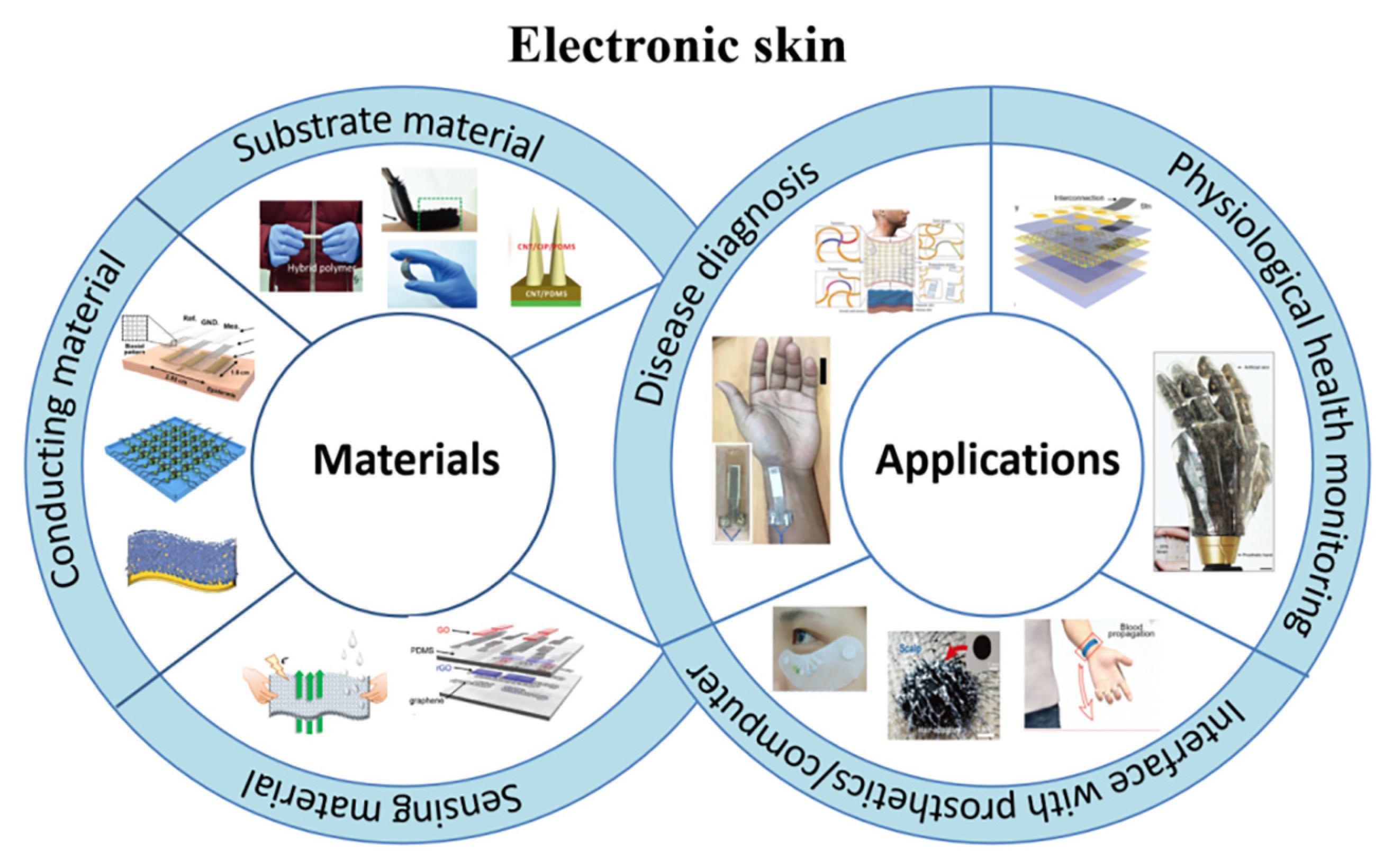
2. Materials
2.1. Substrate Material
2.1.1. High-Molecular Polymer
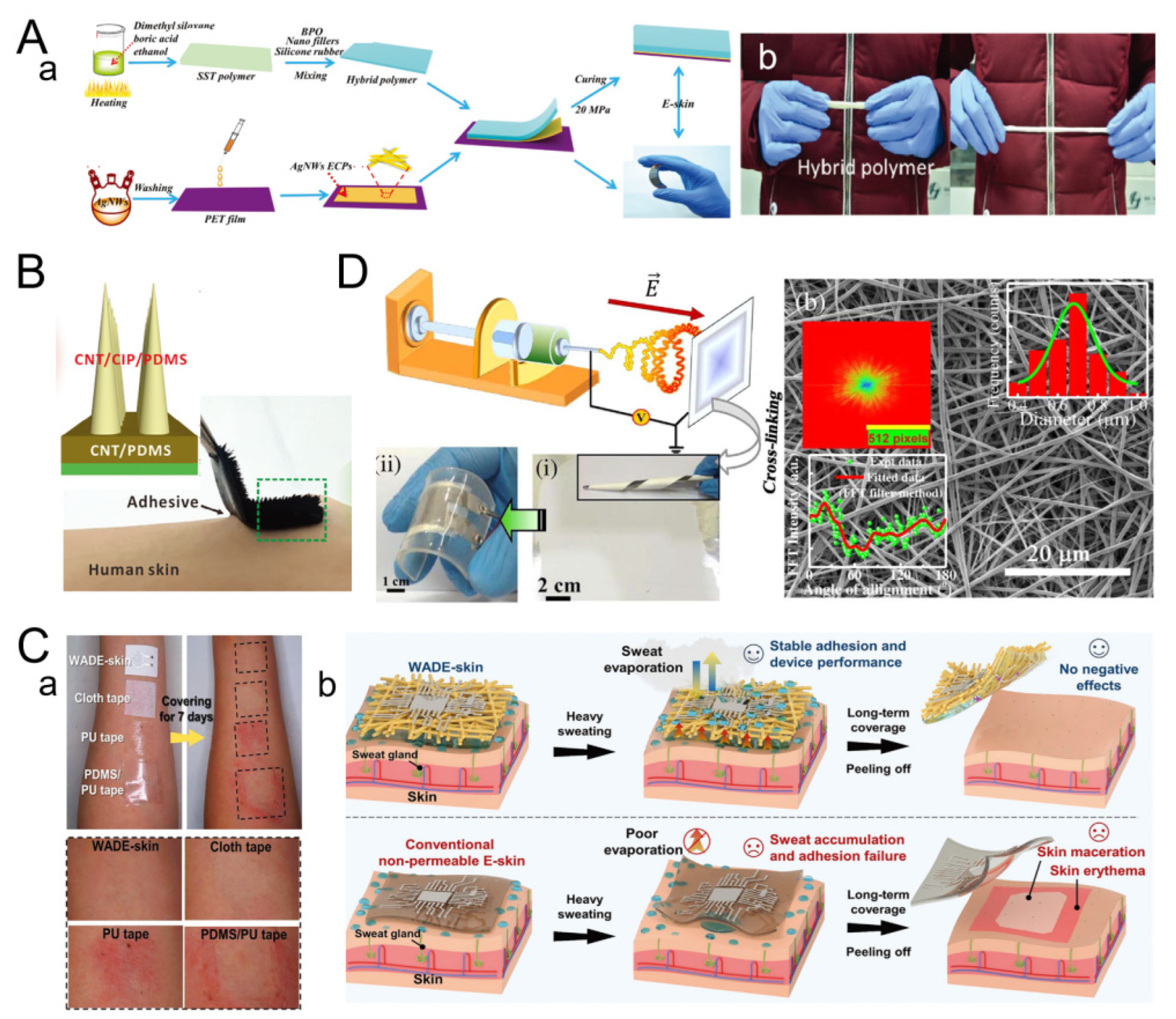
2.1.2. Nanofiber Fabric
2.2. Interconnection Materials
2.2.1. Silver Nanowires (AgNWs)
2.2.2. Carbon Nanomaterials
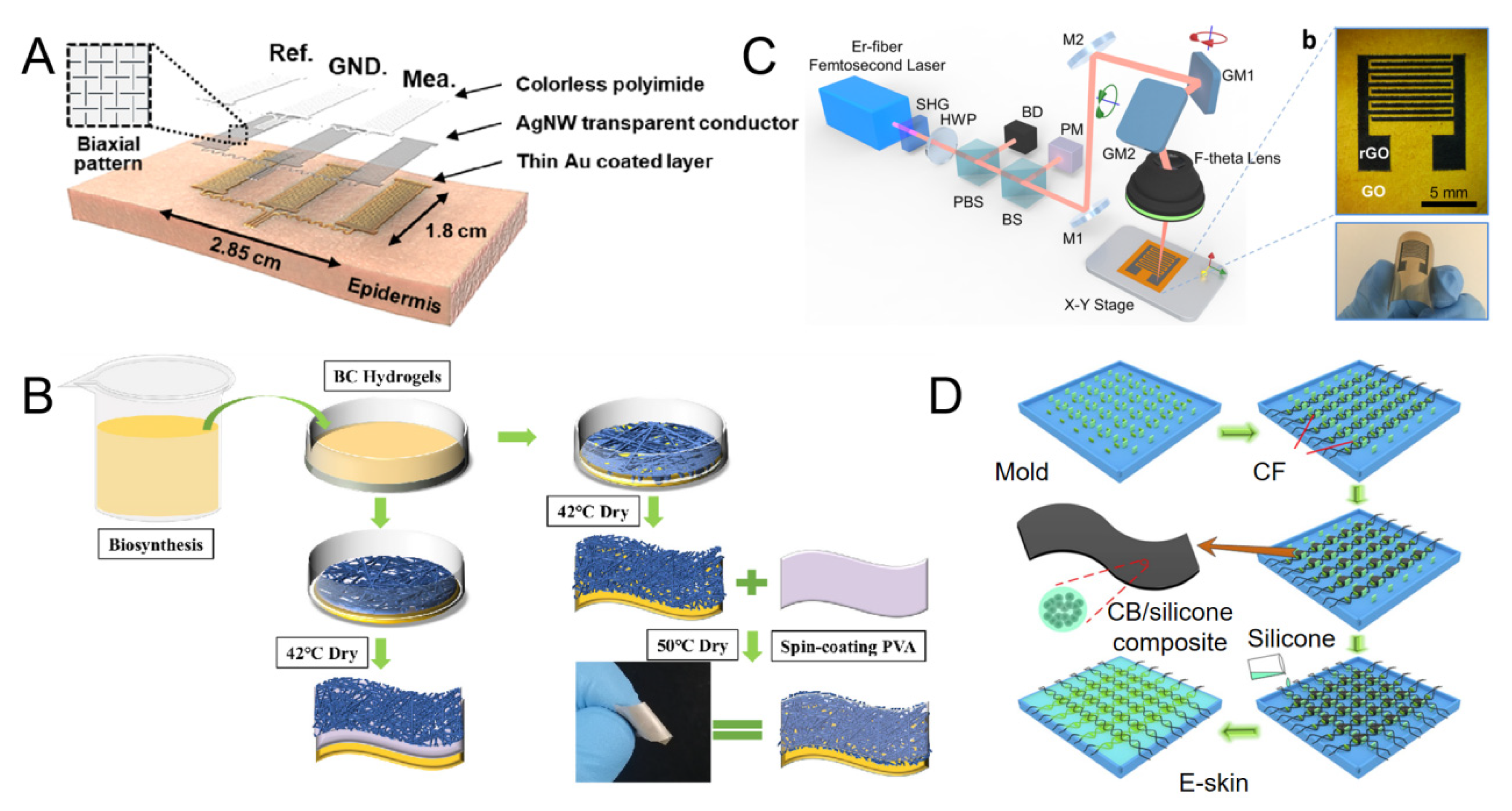
2.2.3. Liquid Metal (LM)
2.2.4. Pure Metal
2.3. Sensing Material
2.3.1. Carbon Materials
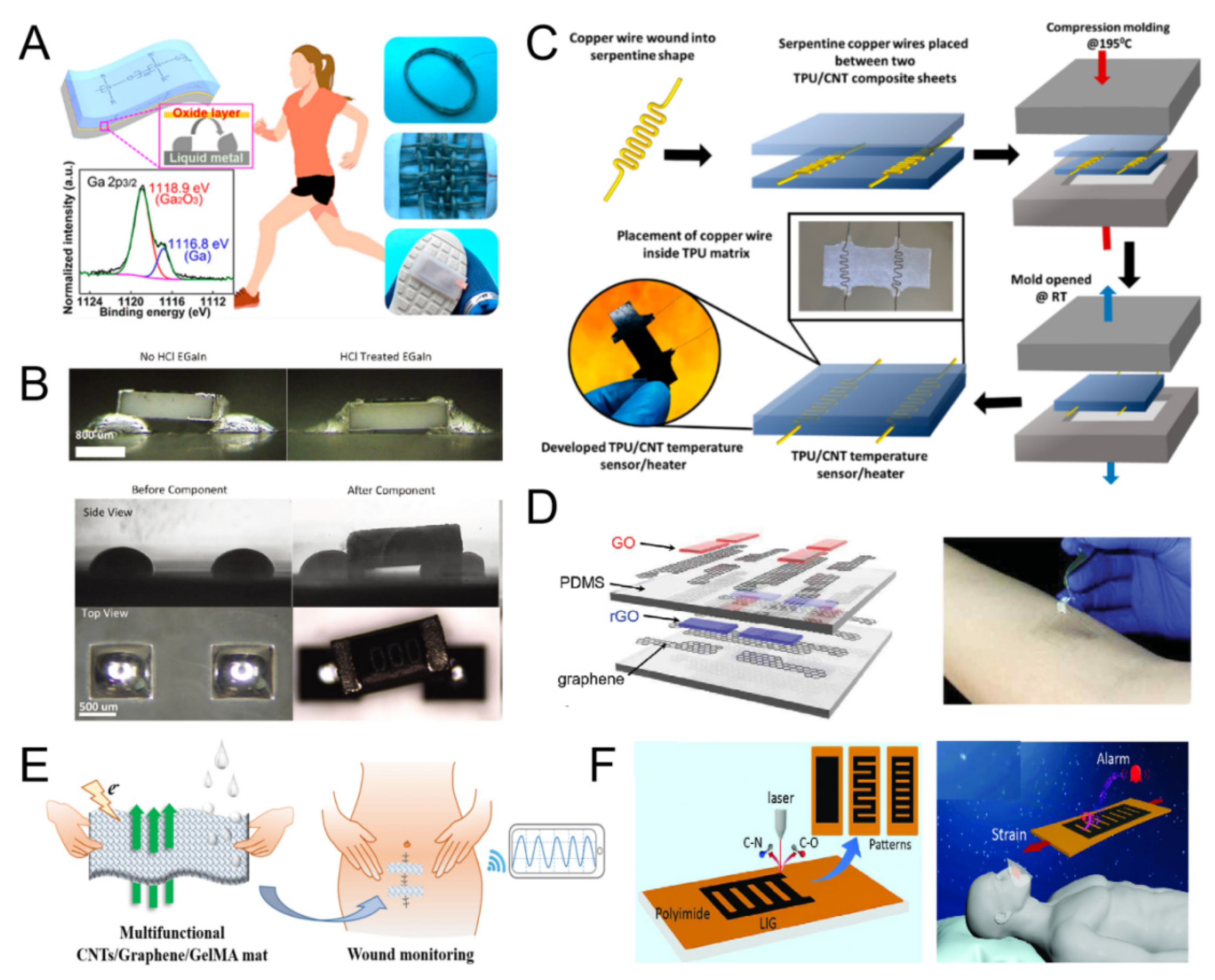
2.3.2. Metal Oxides
3. Sensing Mechanisms
3.1. Piezoelectric Sensing
3.2. Piezoresistive Sensing
3.3. Triboelectric Sensing
3.4. Capacitive Sensing
4. Applications
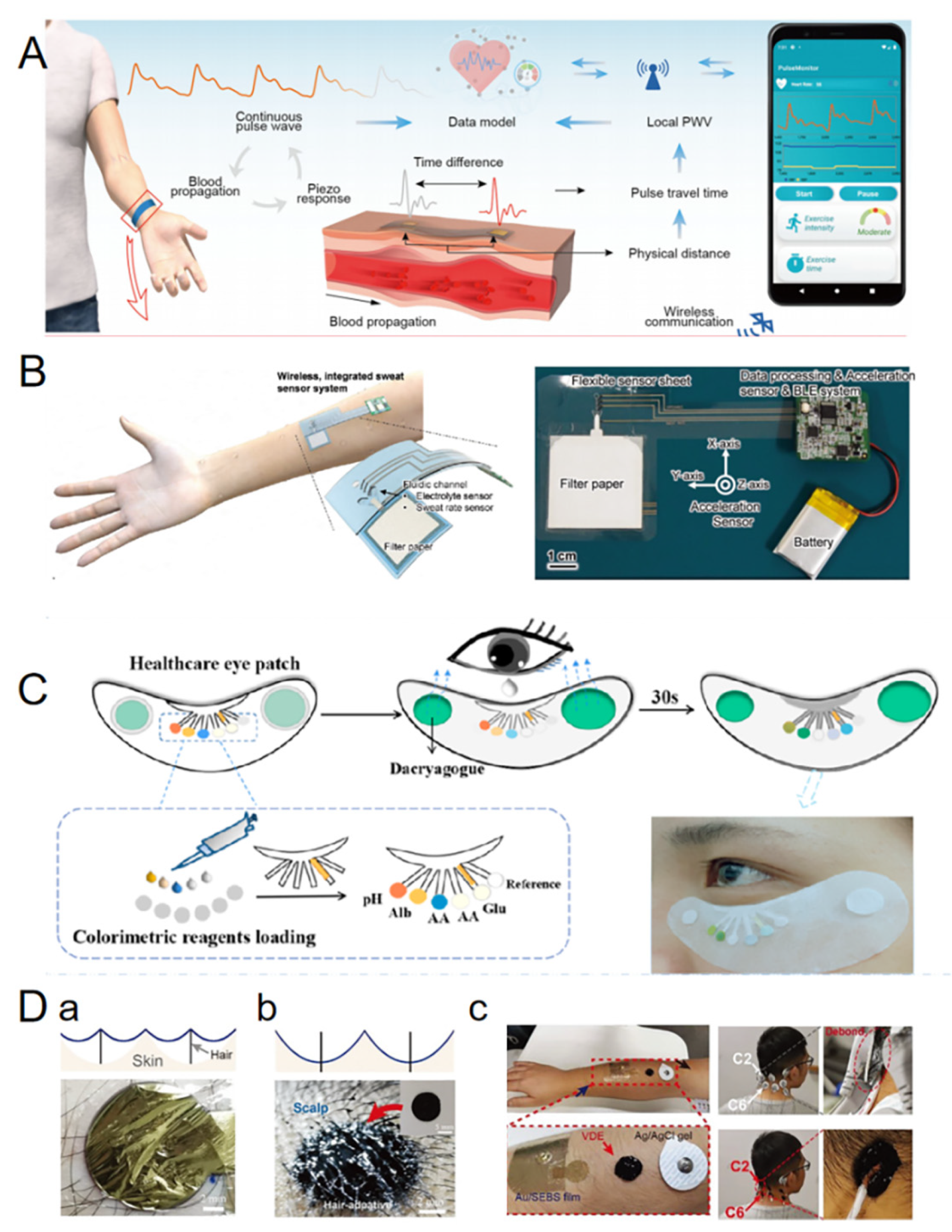
4.1. Disease Diagnosis
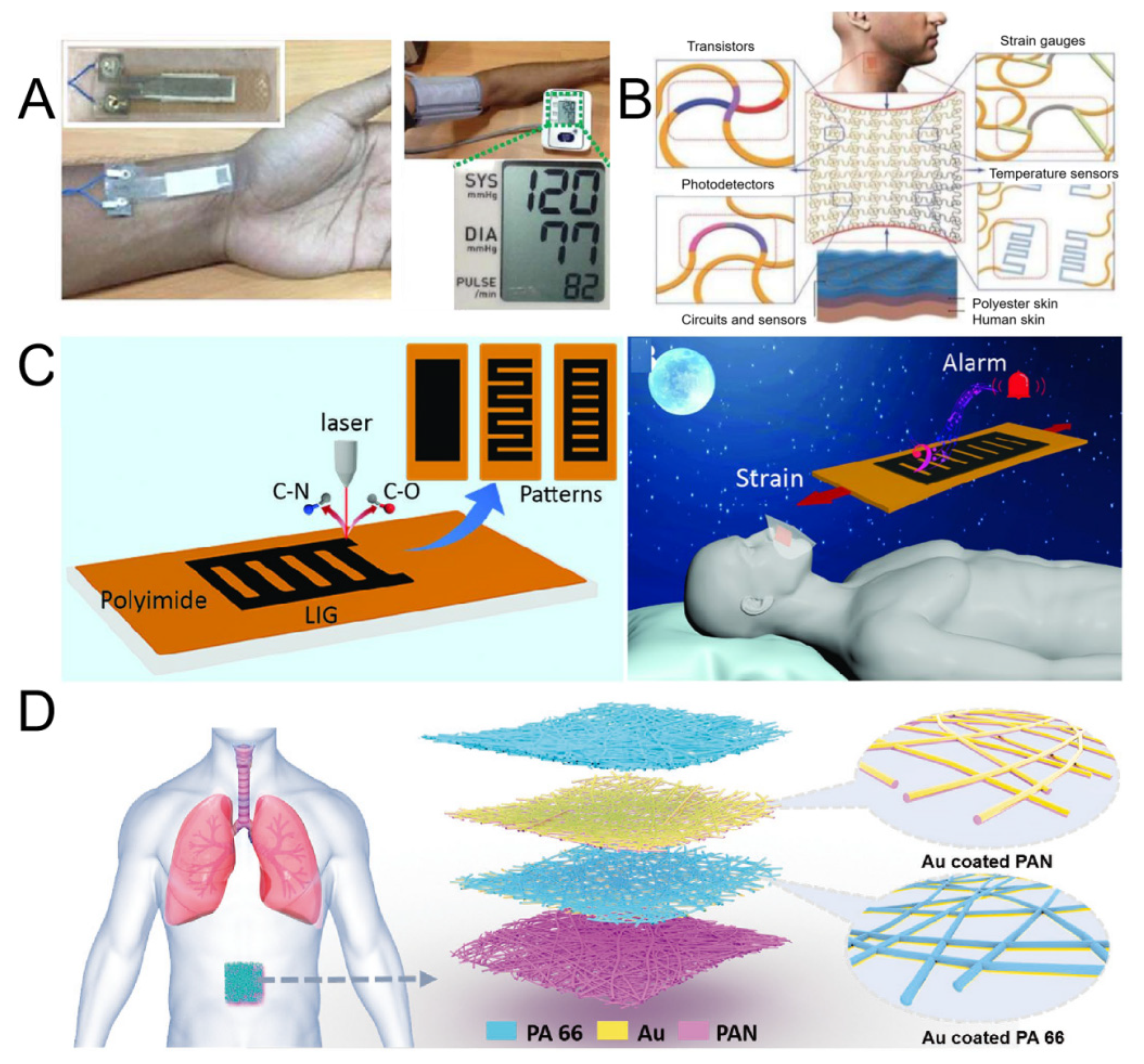
4.2. Monitoring Physical Health
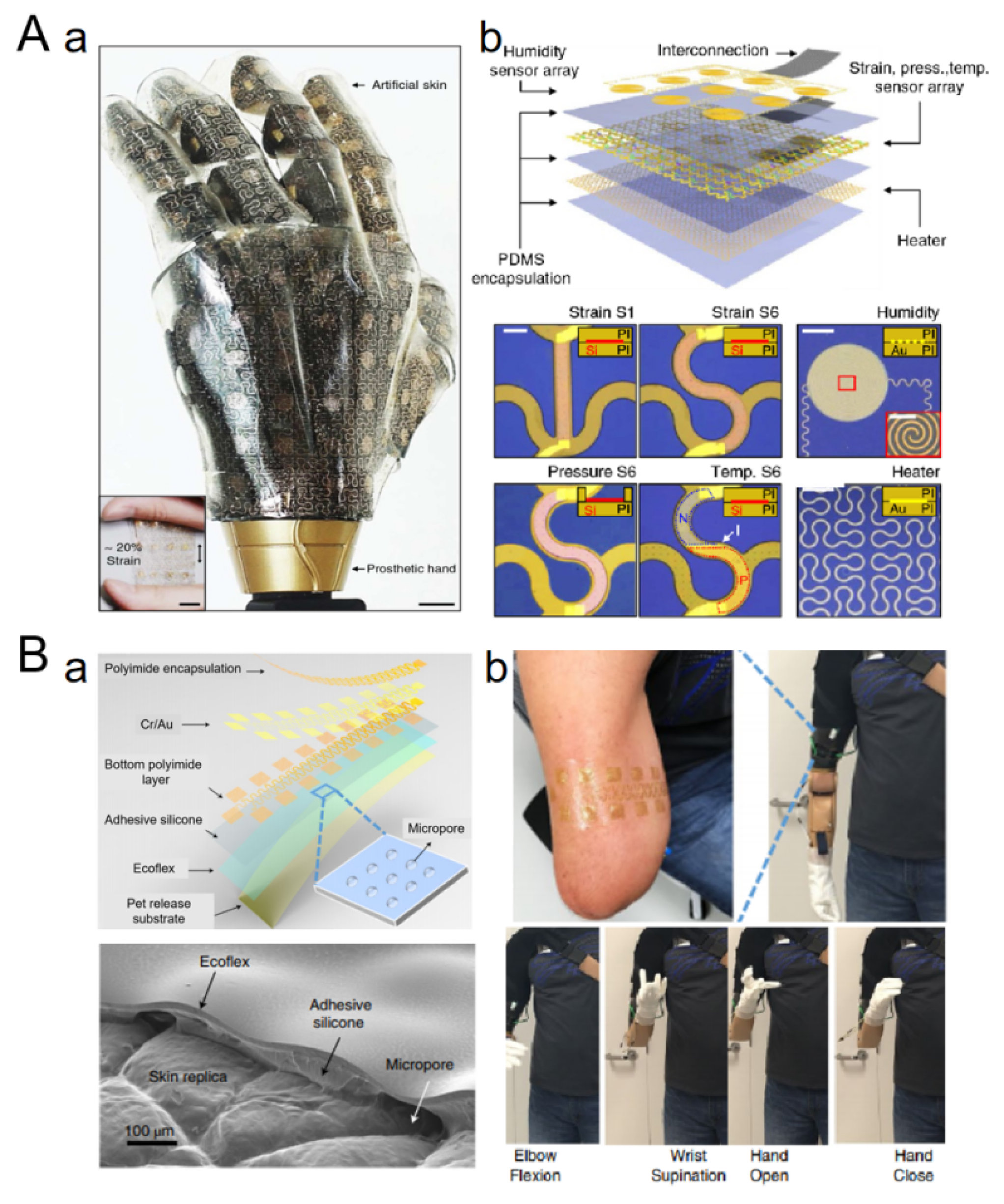
4.3. Interface with Prosthetics/Computer Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Dong, L.; Zhang, H.; Yu, R.; Pan, C.; Wang, Z.L. Recent Progress in Electronic Skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Wang, H.; Cheng, W.; Li, Y.; Pan, L.; Shi, Y. Advanced electronic skin devices for healthcare applications. J. Mater. Chem. B 2019, 7, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Chang, X.; Pan, D.; Song, G.; Guo, Z.; Naik, N. Recent Progress in Essential Functions of Soft Electronic Skin. Adv. Funct. Mater. 2021, 31, 2104686. [Google Scholar] [CrossRef]
- García Núñez, C.; Manjakkal, L.; Dahiya, R. Energy autonomous electronic skin. npj Flex. Electron. 2019, 3, 1. [Google Scholar] [CrossRef]
- Liu, F.; Deswal, S.; Christou, A.; Sandamirskaya, Y.; Kaboli, M.; Dahiya, R. Neuro-inspired electronic skin for robots. Sci. Robot. 2022, 7, eabl7344. [Google Scholar] [CrossRef]
- Shih, B.; Shah, D.; Li, J.; Thuruthel, T.G.; Park, Y.-L.; Iida, F.; Bao, Z.; Kramer-Bottiglio, R.; Tolley, M.T. Electronic skins and machine learning for intelligent soft robots. Sci. Robot. 2020, 5, eaaz9239. [Google Scholar] [CrossRef]
- Xia, X.; Cao, X.; Zhang, B.; Zhang, L.; Dong, J.; Qin, J.; Xuan, P.; Liu, L.; Sun, Y.; Fan, W.; et al. Human Skin-Mimicking Ionogel-Based Electronic Skin for Intelligent Robotic Sorting. Macromol. Rapid Commun. 2024, 2400379. [Google Scholar] [CrossRef]
- Byun, J.; Lee, Y.; Yoon, J.; Lee, B.; Oh, E.; Chung, S.; Lee, T.; Cho, K.-J.; Kim, J.; Hong, Y. Electronic skins for soft, compact, reversible assembly of wirelessly activated fully soft robots. Sci. Robot. 2018, 3, eaas9020. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Mao, Q.; Zhu, R. Multifunctional Electronic Skins Enable Robots to Safely and Dexterously Interact with Human. Adv. Sci. 2022, 9, 2104969. [Google Scholar] [CrossRef]
- Lee, Y.; Nicholls, B.; Sup Lee, D.; Chen, Y.; Chun, Y.; Siang Ang, C.; Yeo, W.-H. Soft Electronics Enabled Ergonomic Human-Computer Interaction for Swallowing Training. Sci. Rep. 2017, 7, 46697. [Google Scholar] [CrossRef]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human–Machine Interfaces. Adv. Mater. 2023, 35, 2203193. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Z.; Han, C.; Cao, Z.; Hu, Y.; Zhao, P.; Wang, Y. A wave-shaped electrode flexible sensor capable of sensitively responding to wrinkle excitation for a multifunctional human–computer interaction system. Nano Res. 2024, 17, 4454–4461. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, R.; Jiang, J.; Wang, H.; Qian, L.; Yu, B.; Zhao, Z.-J. Coral-Inspired Capacitive Pressure Sensor with High Sensitivity and Wide Range for Human–Computer Interaction. ACS Appl. Electron. Mater. 2024, 6, 6726–6737. [Google Scholar] [CrossRef]
- Peter, C.; Urban, B. Emotion in Human-Computer Interaction. In Expanding the Frontiers of Visual Analytics and Visualization; Dill, J., Earnshaw, R., Kasik, D., Vince, J., Wong, P.C., Eds.; Springer: London, UK, 2012; pp. 239–262. [Google Scholar]
- Yang, X.; Chen, W.; Fan, Q.; Chen, J.; Chen, Y.; Lai, F.; Liu, H. Electronic Skin for Health Monitoring Systems: Properties, Functions, and Applications. Adv. Mater. 2024, 36, 2402542. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, J.; Zhu, J.; Yan, Z.; Wu, Q.; Han, S.; Huang, J.; Guan, L. Self-adhesive electronic skin for ultra-sensitive healthcare monitoring. J. Mater. Chem. A 2023, 11, 4977–4986. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, Q.; Ding, Y.; Zhang, C.; Song, X.; Chen, Z.; Zhang, Y.; Mei, Q.; Zhao, X.; Huang, Q.; et al. Wet-Adaptive Electronic Skin. Adv. Mater. 2023, 35, 2305630. [Google Scholar] [CrossRef]
- Xu, C.; Solomon, S.A.; Gao, W. Artificial intelligence-powered electronic skin. Nat. Mach. Intell. 2023, 5, 1344–1355. [Google Scholar] [CrossRef]
- Yang, W.; Han, W.; Gao, H.; Zhang, L.; Wang, S.; Xing, L.; Zhang, Y.; Xue, X. Self-powered implantable electronic-skin for in situ analysis of urea/uric-acid in body fluids and the potential applications in real-time kidney-disease diagnosis. Nanoscale 2018, 10, 2099–2107. [Google Scholar] [CrossRef]
- Bunea, A.-C.; Dediu, V.; Laszlo, E.A.; Pistriţu, F.; Carp, M.; Iliescu, F.S.; Ionescu, O.N.; Iliescu, C. E-Skin: The Dawn of a New Era of On-Body Monitoring Systems. Micromachines 2021, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, X.; Jian, J.; Wu, Q.; Wei, Y.; Shuai, H.; Hirtz, T.; Zhi, Y.; Deng, G.; Wang, Y.; et al. Substrate-Free Multilayer Graphene Electronic Skin for Intelligent Diagnosis. ACS Appl. Mater. Interfaces 2020, 12, 49945–49956. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-J.; Lai, Q.-T.; Tang, Z.; Tang, X.-G.; Zhao, X.-H.; Roy, V.A.L. Advanced Functional Composite Materials toward E-Skin for Health Monitoring and Artificial Intelligence. Adv. Mater. Technol. 2023, 8, 2201088. [Google Scholar] [CrossRef]
- Iskarous, M.M.; Thakor, N.V. E-Skins: Biomimetic Sensing and Encoding for Upper Limb Prostheses. Proc. IEEE 2019, 107, 2052–2064. [Google Scholar] [CrossRef]
- Franceschi, M.; Seminara, L.; Pinna, L.; Valle, M.; Ibrahim, A.; Dosen, S. Towards the integration of e-skin into prosthetic devices. In Proceedings of the 2016 12th Conference on Ph.D. Research in Microelectronics and Electronics (PRIME), Lisbon, Portugal, 27–30 June 2016; pp. 1–4. [Google Scholar]
- Chen, H.; Dejace, L.; Lacour, S.P. Electronic Skins for Healthcare Monitoring and Smart Prostheses. Annu. Rev. Control Robot. Auton. Syst. 2021, 4, 629–650. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- Rahiminejad, E.; Parvizi-Fard, A.; Iskarous, M.M.; Thakor, N.V.; Amiri, M. A Biomimetic Circuit for Electronic Skin With Application in Hand Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2333–2344. [Google Scholar] [CrossRef]
- Schmitz, J.A.; Sherman, J.M.; Hansen, S.; Murray, S.J.; Balkır, S.; Hoffman, M.W. A Low-Power, Single-Chip Electronic Skin Interface for Prosthetic Applications. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1186–1200. [Google Scholar] [CrossRef]
- Dahiya, R.; Yogeswaran, N.; Liu, F.; Manjakkal, L.; Burdet, E.; Hayward, V.; Jörntell, H. Large-Area Soft e-Skin: The Challenges Beyond Sensor Designs. Proc. IEEE 2019, 107, 2016–2033. [Google Scholar] [CrossRef]
- You, I.; Choi, S.-E.; Hwang, H.; Han, S.W.; Kim, J.W.; Jeong, U. E-Skin Tactile Sensor Matrix Pixelated by Position-Registered Conductive Microparticles Creating Pressure-Sensitive Selectors. Adv. Funct. Mater. 2018, 28, 1801858. [Google Scholar] [CrossRef]
- You, I.; Kim, B.; Park, J.; Koh, K.; Shin, S.; Jung, S.; Jeong, U. Stretchable E-Skin Apexcardiogram Sensor. Adv Mater 2016, 28, 6359–6364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, R.; Zhao, Y.; Liu, H.; Zhang, D.; Shi, X.; Zhang, B.; Liu, C.; Shen, C. Conductive MXene/cotton fabric based pressure sensor with both high sensitivity and wide sensing range for human motion detection and E-skin. Chem. Eng. J. 2021, 420, 127720. [Google Scholar] [CrossRef]
- Li, W.-D.; Ke, K.; Jia, J.; Pu, J.-H.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Bai, L.; Zhang, K.; Yang, M.-B.; et al. Recent Advances in Multiresponsive Flexible Sensors towards E-skin: A Delicate Design for Versatile Sensing. Small 2022, 18, 2103734. [Google Scholar] [CrossRef]
- Jiang, S.; Li, L.; Xu, H.; Xu, J.; Gu, G.; Shull, P.B. Stretchable e-Skin Patch for Gesture Recognition on the Back of the Hand. IEEE Trans. Ind. Electron. 2020, 67, 647–657. [Google Scholar] [CrossRef]
- Ma, J.; Yang, K.; Jiang, Y.; Shen, L.; Ma, H.; Zhang, W.; Zhang, J.; Zhu, N. Foot-scale MXene film of ultrathin electronic skin for wearable motion sensors. Cell Rep. Phys. Sci. 2022, 3, 101013. [Google Scholar] [CrossRef]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired flexible electronics for smart E-skin. Acta Biomater. 2022, 139, 280–295. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, C.; He, H.; Guan, H.; Zhong, T.; Xing, L.; Xue, X. A self-powered biosensing electronic-skin for real-time sweat Ca2+ detection and wireless data transmission. Smart Mater. Struct. 2019, 28, 085015. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, T.; He, H.; Zhong, T.; Guan, H.; Xing, L.; Liu, B.; Xue, X. A self-powered electronic-skin for detecting CRP level in body fluid based on the piezoelectric-biosensing coupling effect of GaN nanowire. Smart Mater. Struct. 2019, 28, 105001. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, M.K.; Lee, C.H. Skin-Mountable Biosensors and Therapeutics: A Review. Annu. Rev. Biomed. Eng. 2019, 21, 299–323. [Google Scholar] [CrossRef]
- Zhao, R.; Luo, J.; Ke, T.; Zhang, J.; Astruc, D.; Zhou, J.; Gu, H. Ultra-Tough, highly stable and Self-Adhesive Goatskin-Based intelligent Multi-Functional organogel e-skin as Temperature, Humidity, Strain, and bioelectric four-mode sensors for health monitoring. Chem. Eng. J. 2024, 485, 149816. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, X.; Gao, S.; Yue, W.; Li, Y.; Shen, G. Recent Advances in Carbon Material-Based Multifunctional Sensors and Their Applications in Electronic Skin Systems. Adv. Funct. Mater. 2021, 31, 2104288. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Dai, F.; Zhao, J.; Li, S.; Shi, Y.; Li, W.; Geng, L.; Ye, M.; Chen, X.; et al. Wearable five-finger keyboardless input system based on silk fibroin electronic skin. Nano Energy 2022, 103, 107764. [Google Scholar] [CrossRef]
- Kar, E.; Ghosh, P.; Pratihar, S.; Tavakoli, M.; Sen, S. Nature-Driven Biocompatible Epidermal Electronic Skin for Real-Time Wireless Monitoring of Human Physiological Signals. ACS Appl. Mater. Interfaces 2023, 15, 20372–20384. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Liu, Y.; Leng, J. Metamaterial-Based Electronic Skin with Conformality and Multisensory Integration. Adv. Funct. Mater. 2024, 34, 2406789. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Yao, K.; Liu, S.; He, J.; Su, J.; Qu, Q.a.; Gao, Y.; Song, Z.; Yiu, C.; et al. Touch IoT enabled by wireless self-sensing and haptic-reproducing electronic skin. Sci. Adv. 2022, 8, eade2450. [Google Scholar] [CrossRef]
- Chen, X.; Luo, F.; Yuan, M.; Xie, D.; Shen, L.; Zheng, K.; Wang, Z.; Li, X.; Tao, L.-Q. A Dual-Functional Graphene-Based Self-Alarm Health-Monitoring E-Skin. Adv. Funct. Mater. 2019, 29, 1904706. [Google Scholar] [CrossRef]
- Dr, A.S.G. Bridging the Touch Gap: Developing E-Skin for Long-Distance Physical Connection. Partn. Univers. Innov. Res. Publ. 2023, 1, 67–81. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Wang, H.; Gao, X.; Zhou, Z.; Tao, R.; Pan, L.; Shi, Y. Skin-Inspired Electronics and Its Applications in Advanced Intelligent Systems. Adv. Intell. Syst. 2019, 1, 1900063. [Google Scholar] [CrossRef]
- Kim, J.J.; Wang, Y.; Wang, H.; Lee, S.; Yokota, T.; Someya, T. Skin Electronics: Next-Generation Device Platform for Virtual and Augmented Reality. Adv. Funct. Mater. 2021, 31, 2009602. [Google Scholar] [CrossRef]
- Soni, M.; Dahiya, R. Soft eSkin: Distributed touch sensing with harmonized energy and computing. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 378, 20190156. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Park, G.; Park, S.; Khan, S.; Kim, J.; Kim, W. Energy harvesting using thermoelectricity for IoT (Internet of Things) and E-skin sensors. J. Phys. Energy 2019, 1, 042001. [Google Scholar] [CrossRef]
- Yin, L.; Sandhu, S.S.; Liu, R.; Khan, M.I.; Wicker, C.; Garcia-Gradilla, V.; Zhou, J.; Chang, A.-Y.; Wu, S.; Moon, J.-M.; et al. Wearable E-Skin Microgrid with Battery-Based, Self-Regulated Bioenergy Module for Epidermal Sweat Sensing. Adv. Energy Mater. 2023, 13, 2203418. [Google Scholar] [CrossRef]
- Zhu, M.; Lou, M.; Yu, J.; Li, Z.; Ding, B. Energy autonomous hybrid electronic skin with multi-modal sensing capabilities. Nano Energy 2020, 78, 105208. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Tahernia, M.; Yang, J.H.; Koh, A.; Choi, S. Biopower-on-Skin: Electricity generation from sweat-eating bacteria for self-powered E-Skins. Nano Energy 2020, 75, 104994. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, X.; Nathan, A. Flexible Ultralow-Power Sensor Interfaces for E-Skin. Proc. IEEE 2019, 107, 2084–2105. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, R.; Li, G. Self-Powered Electronic Skin with Multisensory Functions Based on Thermoelectric Conversion. Adv. Mater. Technol. 2020, 5, 2000419. [Google Scholar] [CrossRef]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef]
- Lee, G.; Son, J.H.; Lee, S.; Kim, S.W.; Kim, D.; Nguyen, N.N.; Lee, S.G.; Cho, K. Fingerpad-Inspired Multimodal Electronic Skin for Material Discrimination and Texture Recognition. Adv. Sci. 2021, 8, 2002606. [Google Scholar] [CrossRef]
- Wang, S.; Gong, L.; Shang, Z.; Ding, L.; Yin, G.; Jiang, W.; Gong, X.; Xuan, S. Novel Safeguarding Tactile e-Skins for Monitoring Human Motion Based on SST/PDMS–AgNW–PET Hybrid Structures. Adv. Funct. Mater. 2018, 28, 1707538. [Google Scholar] [CrossRef]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, 1901408. [Google Scholar] [CrossRef]
- dos Santos, A.; Fortunato, E.; Martins, R.; Águas, H.; Igreja, R. E-Skin Piezoresistive Pressure Sensor Combining Laser Engraving and Shrinking Polymeric Films for Health Monitoring Applications. Adv. Mater. Interfaces 2021, 8, 2100877. [Google Scholar] [CrossRef]
- Dahiya, R. Electronic Skin. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–4. [Google Scholar]
- Pradhan, G.B.; Jeong, S.; Sharma, S.; Lim, S.; Shrestha, K.; Lee, Y.; Park, J.Y. A Breathable and Strain-Insensitive Multi-Layered E-Skin Patch for Digital Healthcare Wearables. Adv. Funct. Mater. 2024, 34, 2407978. [Google Scholar] [CrossRef]
- Dai, W.; Lei, M.; Dai, Z.; Ding, S.; Wang, F.; Fang, D.; Wang, R.; Qi, B.; Zhang, G.; Zhou, B. Self-Adhesive Electronic Skin with Bio-Inspired 3D Architecture for Mechanical Stimuli Monitoring and Human-Machine Interactions. Small 2024, 20, 2406564. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Yang, X.; Zhang, Z.; Zhang, H.; Cui, X. Thermogalvanic hydrogel-based e-skin for self-powered on-body dual-modal temperature and strain sensing. Microsyst. Nanoeng. 2024, 10, 55. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Tudu, B.; Mandal, D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar] [CrossRef]
- Chen, B.; Cao, Y.; Li, Q.; Yan, Z.; Liu, R.; Zhao, Y.; Zhang, X.; Wu, M.; Qin, Y.; Sun, C.; et al. Liquid metal-tailored gluten network for protein-based e-skin. Nat. Commun. 2022, 13, 1206. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Zhang, J.; Sun, X.; Chang, H.; Yuan, B.; Guo, R.; Duan, M.; Liu, J. Printed Conformable Liquid Metal e-Skin-Enabled Spatiotemporally Controlled Bioelectromagnetics for Wireless Multisite Tumor Therapy. Adv. Funct. Mater. 2019, 29, 1907063. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Ginnaram, S.; Lin, S.-P.; Hsu, F.-C.; Lu, T.-C.; Lu, M.-H. Breathable and Stretchable Multifunctional Triboelectric Liquid-Metal E-Skin for Recovering Electromagnetic Pollution, Extracting Biomechanical Energy, and as Whole-Body Epidermal Self-Powered Sensors. Adv. Funct. Mater. 2024, 34, 2312443. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, R.; Zhai, H.; Sun, J. Stretchable electronic skin based on silver nanowire composite fiber electrodes for sensing pressure, proximity, and multidirectional strain. Nanoscale 2017, 9, 3834–3842. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Jian, J.; Li, M.; Jiang, G.; Li, X.; Deng, G.; Ji, S.; Wei, Y.; Pang, Y.; et al. Multifunctional and high-performance electronic skin based on silver nanowires bridging graphene. Carbon 2020, 156, 253–260. [Google Scholar] [CrossRef]
- Wang, J.; Jiu, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; He, P.; Suganuma, K. A highly sensitive and flexible pressure sensor with electrodes and elastomeric interlayer containing silver nanowires. Nanoscale 2015, 7, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Qian, Z.; Song, G.; Zhuang, Z.; Sun, X.; Ma, S.; Liang, Y.; Ren, L.; Ren, L. Gas-permeable and stretchable on-skin electronics based on a gradient porous elastomer and self-assembled silver nanowires. Chem. Eng. J. 2023, 463, 142350. [Google Scholar] [CrossRef]
- Wang, H. Development of a conformable electronic skin based on silver nanowires and PDMS. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012040. [Google Scholar] [CrossRef]
- Won, P.; Park, J.J.; Lee, T.; Ha, I.; Han, S.; Choi, M.; Lee, J.; Hong, S.; Cho, K.-J.; Ko, S.H. Stretchable and Transparent Kirigami Conductor of Nanowire Percolation Network for Electronic Skin Applications. Nano Lett. 2019, 19, 6087–6096. [Google Scholar] [CrossRef]
- Chen, G.; Liang, X.; Men, X.; Liu, L.; Wang, F.; Bao, X.; Zhang, H. Enhancing thermal conductivity and chemical protection of bacterial cellulose/silver nanowires thin-film for high flexible electronic skin. Int. J. Biol. Macromol. 2023, 229, 422–431. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Tian, H.; Li, M.; Jian, J.; Wei, Y.; Tian, Y.; Wang, D.-Y.; Pang, Y.; Geng, X.; et al. Multilayer Graphene Epidermal Electronic Skin. ACS Nano 2018, 12, 8839–8846. [Google Scholar] [CrossRef]
- Ho, D.; Sun, Q.; Kim, S.; Han, J.; Kim, D.; Cho, J. Stretchable and Multimodal All Graphene Electronic Skin. Adv. Mater. 2016, 28, 2601–2608. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- An, J.; Le, T.-S.D.; Huang, Y.; Zhan, Z.; Li, Y.; Zheng, L.; Huang, W.; Sun, G.; Kim, Y.-J. All-Graphene-Based Highly Flexible Noncontact Electronic Skin. ACS Appl. Mater. Interfaces 2017, 9, 44593–44601. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Yi, F.-L.; Liu, J.-R.; Li, Y.-Q.; Wang, Z.-Y.; Yang, G.; Huang, P.; Hu, N.; Fu, S.-Y. Super soft but strong E-Skin based on carbon fiber/carbon black/silicone composite: Truly mimicking tactile sensing and mechanical behavior of human skin. Compos. Sci. Technol. 2020, 186, 107910. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Wen, Z.; Cheng, P.; Zheng, H.; Shao, H.; Xia, Y.; Chen, C.; Lan, H.; Xie, X.; et al. Liquid-Metal-Based Super-Stretchable and Structure-Designable Triboelectric Nanogenerator for Wearable Electronics. ACS Nano 2018, 12, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, O.B.; Majidi, C. EGaIn–Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Haridas Cp, A.; Pillai, S.K.; Naskar, S.; Mondal, T.; Naskar, K. Polyurethane/Carbon Nanotube-Based ThermoSense Electronic Skin: Perception to Decision Making Aided by Internet of Things Brain. ACS Appl. Mater. Interfaces 2024, 16, 48211–48222. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, H.; He, X.; Hu, Y.; Zhu, E.; Gao, Y.; Liu, D.; Shi, Z.; Li, J.; Yang, Q.; et al. Flexible electronic skin sensor based on regenerated cellulose/carbon nanotube composite films. Cellulose 2020, 27, 10199–10211. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Peng, B.; Li, X.; Fang, T.; Liu, S.; Liu, J.; Li, B.; Li, F. Stretchable, conductive, breathable and moisture-sensitive e-skin based on CNTs/graphene/GelMA mat for wound monitoring. Biomater. Adv. 2022, 143, 213172. [Google Scholar] [CrossRef]
- Ghosh, R. Recent progress in piezotronic sensors based on one-dimensional zinc oxide nanostructures and its regularly ordered arrays: From design to application. Nano Energy 2023, 113, 108606. [Google Scholar] [CrossRef]
- Panth, M.; Cook, B.; Zhang, Y.; Ewing, D.; Tramble, A.; Wilson, A.; Wu, J. High-Performance Strain Sensors Based on Vertically Aligned Piezoelectric Zinc Oxide Nanowire Array/Graphene Nanohybrids. ACS Appl. Nano Mater. 2020, 3, 6711–6718. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, D. Soft robotic glove with integrated sEMG sensing for disabled people with hand paralysis. In Proceedings of the 2016 IEEE International Conference on Robotics and Biomimetics (ROBIO), Qingdao, China, 3–7 December 2016; pp. 714–718. [Google Scholar]
- Che Lah, N.A. Seamless on-skin and self-powered hybrid ZnO-based thin films: Progress and perspective. Surf. Interfaces 2024, 48, 104312. [Google Scholar] [CrossRef]
- He, H.; Fu, Y.; Zang, W.; Wang, Q.; Xing, L.; Zhang, Y.; Xue, X. A flexible self-powered T-ZnO/PVDF/fabric electronic-skin with multi-functions of tactile-perception, atmosphere-detection and self-clean. Nano Energy 2017, 31, 37–48. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef] [PubMed]
- Shyu, T.C.; Damasceno, P.F.; Dodd, P.M.; Lamoureux, A.; Xu, L.; Shlian, M.; Shtein, M.; Glotzer, S.C.; Kotov, N.A. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat. Mater. 2015, 14, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, Y.; Xu, W. Recent Process of Flexible Transistor-Structured Memory. Small 2021, 17, 1905332. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, W.; Ni, Y.; Xu, Z.; Cui, B.; Liu, J.; Wei, H.; Xu, W. Stretchable Neuromorphic Transistor That Combines Multisensing and Information Processing for Epidermal Gesture Recognition. ACS Nano 2022, 16, 2282–2291. [Google Scholar] [CrossRef]
- Meivel, s. E-Health Real-Time Monitoring System using IoT Sensor (ESKIN) Methodology. Int. J. Adv. Technol. Eng. Explor. 2019, 8, 307–312. [Google Scholar]
- Peng, X.; Dong, K.; Ning, C.; Cheng, R.; Yi, J.; Zhang, Y.; Sheng, F.; Wu, Z.; Wang, Z.L. All-Nanofiber Self-Powered Skin-Interfaced Real-Time Respiratory Monitoring System for Obstructive Sleep Apnea-Hypopnea Syndrome Diagnosing. Adv. Funct. Mater. 2021, 31, 2103559. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Zhou, J.; Huang, X.; Xu, L.; Jia, S.; Gao, Z.; Yao, K.; Li, D.; Zhang, B.; et al. Thin, soft, wearable system for continuous wireless monitoring of artery blood pressure. Nat. Commun. 2023, 14, 5009. [Google Scholar] [CrossRef]
- Honda, S.; Tanaka, R.; Matsumura, G.; Seimiya, N.; Takei, K. Wireless, Flexible, Ionic, Perspiration-Rate Sensor System with Long-Time and High Sweat Volume Functions Toward Early-Stage, Real-Time Detection of Dehydration. Adv. Funct. Mater. 2023, 33, 2306516. [Google Scholar] [CrossRef]
- Xu, J.; Tao, X.; Liu, X.; Yang, L. Wearable Eye Patch Biosensor for Noninvasive and Simultaneous Detection of Multiple Biomarkers in Human Tears. Anal. Chem. 2022, 94, 8659–8667. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhao, H.; Wang, X.; Jiang, Y.; Zhu, M.; Yelemulati, H.; Xie, R.; Li, Q.; Su, R.; Cao, Z.; et al. Hairy-Skin-Adaptive Viscoelastic Dry Electrodes for Long-Term Electrophysiological Monitoring. Adv. Mater. 2023, 35, 2211236. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Z.; Qin, S.; Hu, J.; Yuan, S.; Wang, Z.L.; Chen, X. A wearable triboelectric impedance tomography system for noninvasive and dynamic imaging of biological tissues. Sci. Adv. 2024, 10, eadr9139. [Google Scholar]
- Du, W.; Zhang, L.; Suh, E.; Lin, D.; Marcus, C.; Ozkan, L.; Ahuja, A.; Fernandez, S.; Shuvo, I.I.; Sadat, D.; et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. 2023, 9, eadh5325. [Google Scholar] [CrossRef]
- Lauretti, C.; Davalli, A.; Sacchetti, R.; Guglielmelli, E.; Zollo, L. Fusion of M-IMU and EMG signals for the control of trans-humeral prostheses. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1123–1128. [Google Scholar]
- Zhou, H.; Alici, G. Non-Invasive Human-Machine Interface (HMI) Systems with Hybrid On-Body Sensors for Controlling Upper-Limb Prosthesis: A Review. IEEE Sens. J. 2022, 22, 10292–10307. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef]
- Norton, J.J.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.-Y.; Cheng, H.; Jeong, J.-W.; Akce, A. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef]
| Materials | Advantages | Disadvantages | |
|---|---|---|---|
| Substrate material | High-molecular polymer | -Good flexibility -Biocompatibility -Chemical stability | -Limited sensitivity |
| Nanofiber fabric | -High specific surface area -Effective adsorption and transfer of substances -Good flexibility and breathability | -Low mechanical strength | |
| Conductive materials | Silver nanowires (AgNWs) | -Excellent electrical conductivity -High transparency -Good flexibility | -Easy oxidation in the air -High cost |
| Carbon nanomaterials | -Excellent electrical, mechanical and thermal properties -High chemical stability | -Poor dispersion | |
| Liquid metal (LM) | -Good liquidity -Good electrical conductivity and stretchability | -Poor biocompatibility | |
| Sensing material | Carbon materials | -High sensitivity -Quick response -High chemical stability | -Poor dispersion |
| Metal oxides | -Good piezoelectricity, gas sensitivity and optical properties -Good chemical stability | -Weak electrical conductivity | |
| Mechanisms | Principle | Sensing Range | Response Speed | Self-Powered | Environmental Sensitivity | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Piezoelectric sensing | Positive piezoelectric effect | 0.1 Pa–100 MPa | μs | Yes | Temperature | -No external power supply required. -Quick. response. | -Weak output signal. -Limited material. selection. |
| Piezoresistive sensing | Resistivity/geometric change | 0.01–1000% strain | ms | No | Humidity, temperature | -Simple structure. -Low cost. -Easy to implement. | -Limited sensitivity. -Greatly affected by environmental factors. |
| Triboelectric sensing | Contact electric/electrostatic induction | 0.1 N–100 N | ms | Yes | Humidity | -Low cost. -Self-electricity. | -Unstable charge generation. -Greatly affected by the friction mode and environmental humidity. |
| Capacitive sensing | Dielectric constant/distance variation | 0.1–500% strain | μs | No | Temperature | -High sensitivity. -Fast response speed. -Sensitive to small pressure changes. | -Susceptible to electromagnetic interference. -High production process requirements. |
| Applications | Functions | Challenges | Examples |
|---|---|---|---|
| Disease diagnosis | Monitors the concentration of bioelectrical signals and chemical substances in the body to assist in the early diagnosis of diseases. | -Biocompatibility challenges to prevent triggering an immune response. -Accurate signal interpretation and analysis are complicated. | Implantable cancer marker monitoring chips. |
| Monitoring physical health | Real-time monitoring of physiological parameters such as heart rate, blood pressure, body temperature and sweat composition to assess overall health. | -Long-term wearing comfort is poor. -Signals are susceptible to motion and environmental interference. | Smartwatch with an E-skin patch that continuously monitors heart rate and records steps and calories burned. |
| Interface with prosthetics | Prosthetic limb is endowed with tactile perception, so that the user can feel the grip strength, the surface texture of the object, and improve operation’s accuracy. | -Efficient integration with the human nervous system is difficult. -Precision of tactile feedback needs to be improved. | Bionic arms with E-skin can accurately sense and respond to the state of grasping objects. |
| Interface with computer systems | Enhances the interactive experience of electronic products, such as pressure-sensing operation for mobile phones and accurate recognition of touch gestures for computers. | -Structure integration is difficult. -High cost. | Some high-end mobile phones use E-skin technology to support heavy pressure and light touch to achieve different functional operations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, Y.; Yuan, Y.; Wang, Y.; Liu, A.; Jia, S.; Yang, W. Wearable Medical Devices: Application Status and Prospects. Micromachines 2025, 16, 394. https://doi.org/10.3390/mi16040394
Wang X, Gao Y, Yuan Y, Wang Y, Liu A, Jia S, Yang W. Wearable Medical Devices: Application Status and Prospects. Micromachines. 2025; 16(4):394. https://doi.org/10.3390/mi16040394
Chicago/Turabian StyleWang, Xiaowen, Yingnan Gao, Yueze Yuan, Yaping Wang, Anqin Liu, Sen Jia, and Wenguang Yang. 2025. "Wearable Medical Devices: Application Status and Prospects" Micromachines 16, no. 4: 394. https://doi.org/10.3390/mi16040394
APA StyleWang, X., Gao, Y., Yuan, Y., Wang, Y., Liu, A., Jia, S., & Yang, W. (2025). Wearable Medical Devices: Application Status and Prospects. Micromachines, 16(4), 394. https://doi.org/10.3390/mi16040394









