Incubation of Horseradish Peroxidase near 50 Hz AC Equipment Promotes Its Disaggregation and Enzymatic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Enzyme
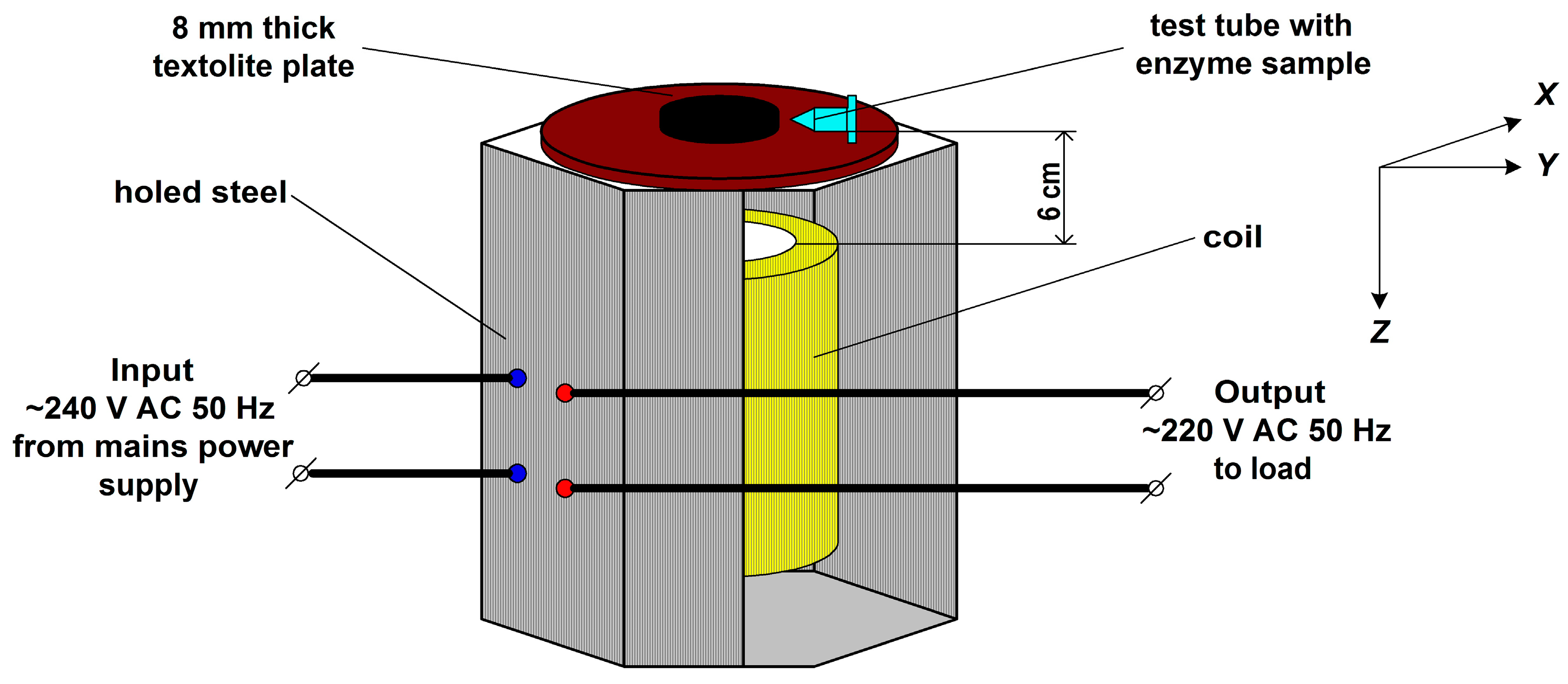
2.2. Experimental Setup and Enzyme Treatment
2.3. Atomic Force Microscopy Measurements
2.4. Spectrophotometry Measurements and Data Processing
2.5. Electromagnetic Field Measurements
3. Results
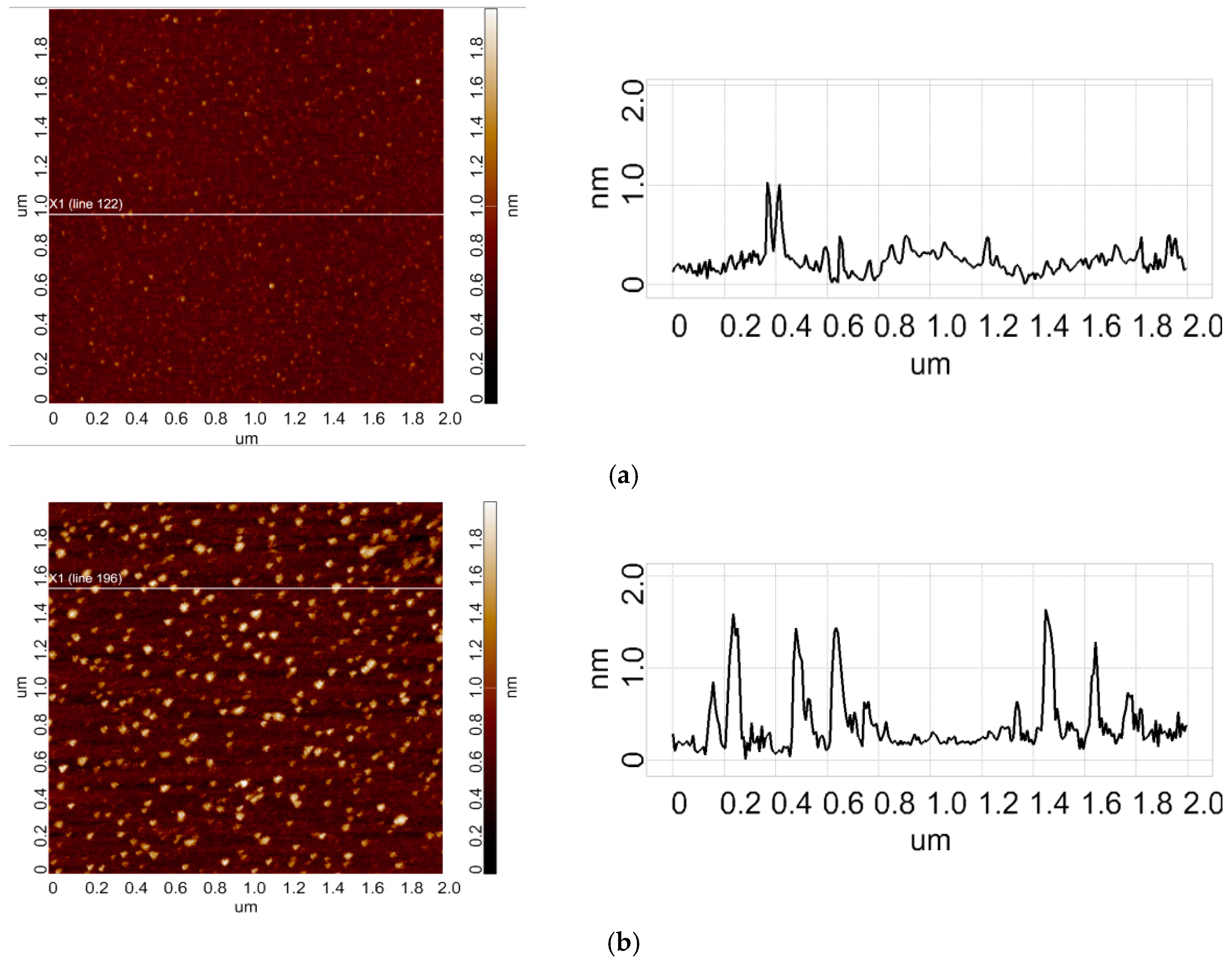
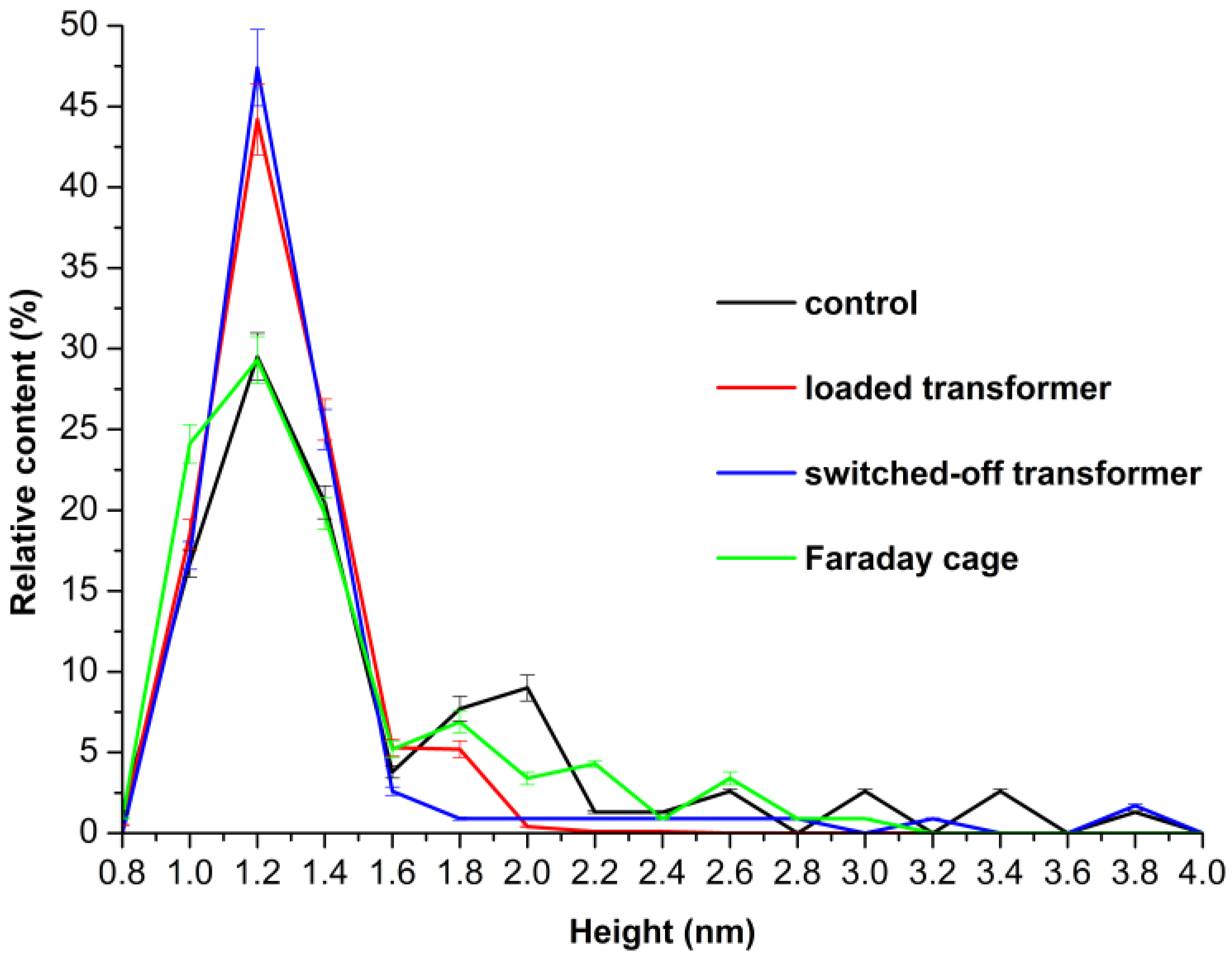
3.1. AFM Analysis of HRP Adsorption and Aggregation Behaviour
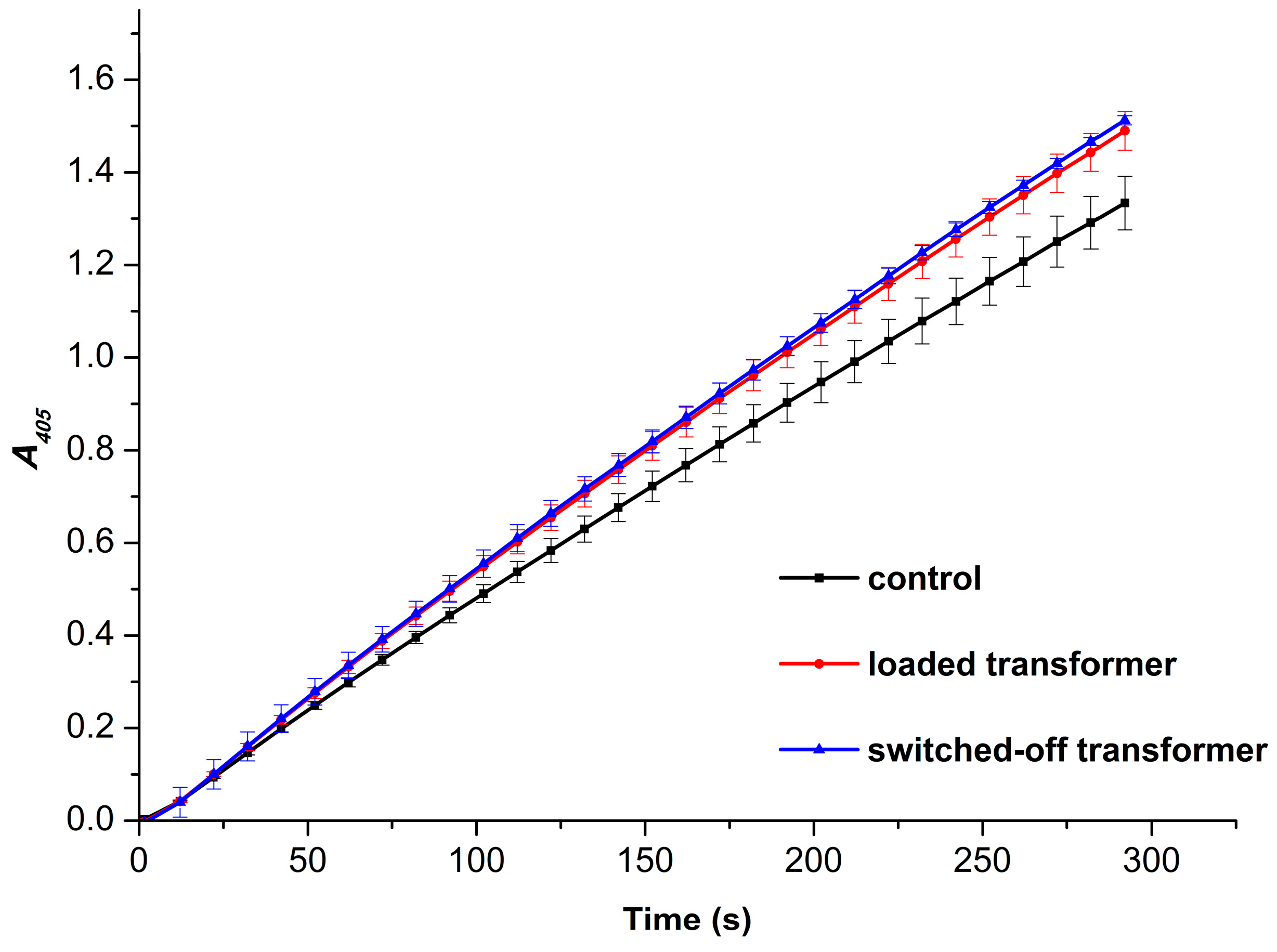
3.2. Spectrophotometric Estimation of Enzymatic Activity
3.3. Results of Electromagnetic Field Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogt, P.; Küchemann, S.; Kuhn, J. The flashing light bulb: A quantitative introduction to the theory of alternating current. Phys. Teach. 2021, 59, 138–139. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. Rotating magnetic field as tool for enhancing enzymes properties - laccase case study. Sci. Rep. 2019, 9, 3707. [Google Scholar] [CrossRef] [PubMed]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Portaccio, M.; De Luca, P.; Durante, D.; Rossi, S.; Bencivenga, U.; Canciglia, P.; Lepore, M.; Mattei, A.; De Maio, A.; Mita, D.G. In vitro studies of the influence of ELF electromagnetic fields on the activity of soluble and insoluble peroxidase. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2003, 24, 449–456. [Google Scholar] [CrossRef]
- Sun, J.; Sun, F.; Xu, B.; Gu, N. The quasi-one-dimensional assembly of horseradish peroxidase molecules in presence of the alternating magnetic field. Coll. Surf. A Physicochem. Eng. Aspects 2010, 360, 94–98. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Jin, Y.; Wang, M.; Gu, N. Magnetically enhanced dielectrophoretic assembly of horseradish peroxidase molecules: Chaining and molecular monolayers. Chem. Phys. Chem. 2008, 9, 1847–1850. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Lopez-Ramirez, N.; Volke-Sepulveda, T.; Gaime-Perraud, I.; Saucedo-Castañeda, G.; Favela-Torres, E. Effect of stirring on growth and cellulolytic enzymes production by Trichoderma harzianum in a novel bench-scale solid-state fermentation bioreactor. Bioresour. Technol. 2018, 265, 291–298. [Google Scholar] [CrossRef]
- Metzler, D.E. Biochemistry, the Chemical Reactions of Living Cells, 1st ed.; Academic Press: Cambridge, UK, 1977. [Google Scholar]
- Calabrò, E.; Magazù, S. Electromagnetic Fields Effects on the Secondary Structure of Lysozyme and Bioprotective Effectiveness of Trehalose. Adv. Phys. Chem. 2012, 2012, 970369. [Google Scholar] [CrossRef]
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Glynn, C.; O’Connell, A.M.; Ennis, R.; Lowery, A.J.; et al. Microwave imaging in breast cancer–results from the first-in-human clinical investigation of the wavelia system. Acad. Radiol. 2022, 29 (Suppl. 1), S211–S222. [Google Scholar] [CrossRef]
- Vojisavljevic, V.; Pirogova, E.; Cosic, I. Influence of Electromagnetic Radiation on Enzyme Kinetics. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5021–5024. [Google Scholar] [CrossRef]
- Jumaat, H.; Ping, K.H.; Abd Rahman, N.H.; Yon, H.; Redzwan, F.N.M.; Awang, R.A. A compact modified wideband antenna with CBCPW, stubline and notch-staircase for breast cancer microwave imaging application. AEU-Int. J. Electron. Commun. 2021, 129, 153492. [Google Scholar] [CrossRef]
- Karam, S.A.S.; O’Loughlin, D.; Oliveira, B.L.; O’Halloran, M.; Asl, B.M. Weighted delay-and-sum beamformer for breast cancer detection using microwave imaging. Measurement 2021, 177, 109283. [Google Scholar] [CrossRef]
- Warille, A.A.; Altun, G.; Elamin, A.A.; Kaplan, A.A.; Mohamed, H.; Yurt, K.K.; Elhaj, A.E. Skeptical approaches concerning the effect of exposure to electromagnetic fields on brain hormones and enzyme activities. J. Microsc. Ultrastruct. 2017, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zinoviev, S.V.; Evdokimov, A.N.; Sakharov, K.Y.; Turkin, V.A.; Aleshko, A.I.; Ivanov, A.V. Determination of therapeutic value of ultra-wideband pulsed electromagnetic microwave radiation on models of experimental oncology. Meditsinskaya Fiz. Med. Phys. 2015, 3, 62–67. [Google Scholar]
- Emamdadi, N.; Gholizadeh, M.; Housaindokht, M.R. Investigation of static magnetic field effect on horseradish peroxidase enzyme activity and stability in enzymatic oxidation process. Int. J. Biol. Macromol. 2021, 170, 189–195. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arıca, M.Y. Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: Horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2023, 398, 133875. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite-Minkstimiene, A.; Morkvenaite-Vilkonciene, I.; Genys, P.; Mikhailova, R.; Semashko, T.; Voronovic, J.; Ramanaviciene, A. Biofuel cell based on glucose oxidase from Penicillium funiculosum 46.1 and horseradish peroxidase. Chem. Eng. J. 2015, 264, 165–173. [Google Scholar] [CrossRef]
- Chung, Y.; Tannia, D.C.; Kwon, Y. Glucose biofuel cells using bienzyme catalysts including glucose oxidase, horseradish peroxidase and terephthalaldehyde crosslinker. Chem. Eng. J. 2018, 334, 1085–1092. [Google Scholar] [CrossRef]
- Abreau, C.; Nedellec, Y.; Ondel, O.; Buret, F.; Cosnier, S.; Le Goff, A.; Holzinger, M. Glucose oxidase bioanodes for glucose conversion and H2O2 production for horseradish peroxidase biocathodes in a flow through glucose biofuel cell design. J. Power Sources 2018, 392, 176–180. [Google Scholar] [CrossRef]
- Matsui, T.; Hori, M.; Shizawa, N.; Nakayama, H.; Shinmyo, A.; Yoshida, K. High-efficiency secretory production of peroxidase C1a using vesicular transport engineering in transgenic tobacco. J. Biosci. Bioeng. 2006, 102, 102–109. [Google Scholar] [CrossRef]
- Krainer, F.W.; Glieder, A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Repnikov, V.V.; et al. Effect of Spherical Elements of Biosensors and Bioreactors on the Physicochemical Properties of a Peroxidase Protein. Polymers 2021, 13, 1601. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ershova, M.O.; Ivanova, I.A.; Ableev, A.N.; Tatur, V.Y.; Lukyanitsa, A.A.; Ivanova, N.D.; et al. Atomic Force Microscopy Study of the Long-Term Effect of the Glycerol Flow, Stopped in a Coiled Heat Exchanger, on Horseradish Peroxidase. Micromachines 2024, 15, 499. [Google Scholar] [CrossRef]
- Lewis, D.F. Guide to Cytochromes P450: Structure and Function; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Archakov, A.I.; Bachmanova, G.I. Cytochrome P450 and Active Oxygen; Taylor & Francis: New York, NY, USA, 1990. [Google Scholar]
- Gui, F.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Inactivation and structural change of horseradish peroxidase treated with supercritical carbon dioxide. Food Chem. 2006, 97, 480–489. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Meneses, L.; Duarte, A.R.C.; Paiva, A.; Held, C. Assessing the Influence of Betaine-Based Natural Deep Eutectic Systems on Horseradish Peroxidase. ACS Sustain. Chem. Eng. 2022, 10, 12873–12881. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Gómez-López, V.M. Pulsed light inactivation of horseradish peroxidase and associated structural changes. Food Chem. 2017, 237, 632–637. [Google Scholar] [CrossRef]
- Hassani, L.; Ranjbar, R.; Khajeh, K.; Naderi-Manesh, H.; Naderi-Manesh, M.; Sadeghi, M. Horseradish peroxidase thermostabilization: The combinatorial effects of the surface modification and the polyols. Enz. Microbial Technol. 2006, 38, 118–125. [Google Scholar] [CrossRef]
- Çelebi, M.; Özdemir, Z.Ö.; Topuzoğullari, M. Microwave-assisted rapid conjugation of horseradish peroxidase-dextran aldehyde with Schiff base reaction and decolorization of Reactive Blue 19. Turk. J. Chem. 2022, 46, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wei, X.; Pang, H.; Wang, K.; Liu, Q.; Fu, H.; Chen, X.; Wang, Y. Effects of radio-frequency energy on peroxidase inactivation and physiochemical properties of stem lettuce and the underlying cell morphology mechanism. Food Chem. 2020, 322, 126753. [Google Scholar] [CrossRef]
- de Barcelos Costa, H.C.; Siguemoto, É.C.; Cavalcante, T.A.B.B.; de Oliveira Silva, D.; Vieira, L.G.M.; Gut, J.A.W. Effect of microwave-assisted processing on polyphenol oxidase and peroxidase inactivation kinetics of açai-berry (Euterpe oleracea) pulp. Food Chem. 2021, 341, 128287. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, C.P.; Mok, J.H.; Heskitt, B.F.; Sastry, S.K. Impact of intermittent and continuous electric fields on peroxidase inactivation in orange juice: An experimental and molecular dynamics analysis. J. Food Eng. 2024, 367, 111890. [Google Scholar] [CrossRef]
- Brochier, B.; Hertz, P.F.; Marczak, L.D.F.; Mercali, G.D. Influence of ohmic heating on commercial peroxidase and sugarcane juice peroxidase inactivation. J. Food Eng. 2020, 284, 110066. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Influence of moderate electric field on inactivation kinetics of peroxidase and polyphenol oxidase and on phenolic compounds of sugarcane juice treated by ohmic heating. LWT Food Sci. Technol. 2016, 74, 396–403. [Google Scholar] [CrossRef]
- Elez-Martinez, P.; Aguilo´-Aguayo, I.; Martin-Belloso, O. Inactivation of orange juice peroxidase by high-intensity pulsed electric fields as influenced by process parameters. J. Sci. Food Agric. 2006, 86, 71–81. [Google Scholar] [CrossRef]
- Yang, J.; Heogh, W.; Ju, H.; Kang, S.; Jang, T.-S.; Jung, H.-D.; Jahazi, M.; Han, S.C.; Park, S.J.; Kim, H.S.; et al. Functionally graded structure of a nitride-strengthened Mg2Si-based hybrid composite. J. Magnes. Alloys 2024, 12, 1239–1256. [Google Scholar] [CrossRef]
- Shumov, I.D.; Kanashenko, S.L.; Ziborov, V.S.; Ivanov, Y.D.; Archakov, A.I.; Pleshakova, T.O. Magnetron sputtering deposition of ultra-thin metal coatings for the visualization of protein-containing objects of nanometer size by electron microscopy. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1058, 012048. [Google Scholar] [CrossRef]
- Nogal, B.; Bowman, C.A.; Ward, A.B. Time-course, negative-stain electron microscopy–based analysis for investigating protein–protein interactions at the single-molecule level. J. Biol. Chem. 2017, 292, 19400–19410. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.; Nield, J. Determining the structure of biological macromolecules by transmission electron microscopy, single particle analysis and 3D reconstruction. Prog. Biophys. Mol. Biol. 2001, 75, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Kiselyova, O.I.; Yaminsky, I.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Certificate of Analysis. Peroxidase from Horseradish. Type VI-A, Essentially Salt-Free, Lyophilized Powder, ≥250 units/mg Solid (Using Pyrogallol), 950–2000 units/mg Solid (Using ABTS). Product Number P6782, Batch Number SLCK8071. Available online: https://www.sigmaaldrich.com/certificates/COFA/P6/P6782/P6782-BULK________SLCK8071__.pdf (accessed on 21 March 2024).
- Ronzhina, N.L.; Zorina, E.S.; Zavialova, M.G.; Legina, O.K.; Naryzhny, S.N. Variability of haptoglobin beta-chain proteoforms. Biomeditsinskaya Khimiya 2024, 70, 114–124. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Tatur, V.Y.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; et al. The Effect of a Dodecahedron-Shaped Structure on the Properties of an Enzyme. J. Funct. Biomater. 2022, 13, 166. [Google Scholar] [CrossRef]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7). Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzyme-activity-assays/enzymatic-assay-of-peroxidase (accessed on 18 February 2022).
- Lavery, C.B.; MacInnis, M.C.; MacDonald, M.J.; Williams, J.B.; Spencer, C.A.; Burke, A.A.; Irwin, D.J.G.; D’Cunha, G.B. Purification of Peroxidase from Horseradish (Armoracia rusticana) Roots. J. Agric. Food Chem. 2010, 58, 8471–8476. [Google Scholar] [CrossRef]
- Wentao, T.; Zutao, X.; Bin, Z.; Xiuyou, S.; Yuanyuan, Z.; Kuan, L. Study on Factors Affecting Residual Magnetism of Phase Selection of Extreme High Voltage Transformer and Its Calculation Method. J. Electr. Electron. Eng. 2019, 7, 57–63. [Google Scholar] [CrossRef]
- Ekuwe, A.O.; Rawn, B. Investigations into the transformer inrush current problem. Niger. J. Technol. 2018, 37, 1058–1064. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Rakshit, S.; Mitra, R.K.; Pal, S.K. Role of hydration on the functionality of a proteolytic enzyme α-chymotrypsin under crowded environment. Biochimie 2011, 93, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bowman, J.; Tu, S.; Nykypanchuk, D.; Kuksenok, O.; Minko, S. Polyethylene glycol Crowder’s effect on enzyme aggregation, thermal stability, and residual catalytic activity. Langmuir 2021, 37, 8474–8485. [Google Scholar] [CrossRef]
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein aggregation in bacteria. FEMS Microbiol. Rev. 2020, 44, 54–72. [Google Scholar] [CrossRef]
- Colombie, S.; Gaunand, A.; Lindet, B. Lysozyme inactivation and aggregation in stirred-reactor. J. Mol. Catal. B Enzym. 2001, 11, 559–565. [Google Scholar] [CrossRef]
- Vitagliano, L.; Berisio, R.; De Simone, A. Role of Hydration in Collagen Recognition by Bacterial Adhesins. Biophys. J. 2011, 100, 2253–2261. [Google Scholar] [CrossRef]
- Beaufils, C.; Man, H.-M.; de Poulpiquet, A.; Mazurenko, I.; Lojou, E. From Enzyme Stability to Enzymatic Bioelectrode Stabilization Processes. Catalysts 2021, 11, 497. [Google Scholar] [CrossRef]
- Fritz, P.A.; Bera, B.; van den Berg, J.; Visser, I.; Kleijn, J.M.; Boom, R.M.; Schroën, C.G.P.H. Electrode Surface Potential-Driven Protein Adsorption and Desorption through Modulation of Electrostatic, van der Waals, and Hydration Interactions. Langmuir 2021, 37, 6549–6555. [Google Scholar] [CrossRef]
- Trefalt, G.; Szilagyi, I.; Borkovec, M. Poisson–Boltzmann description of interaction forces and aggregation rates involving charged colloidal particles in asymmetric electrolytes. J. Colloid Interface Sci. 2013, 406, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Duinhoven, S.; Poort, R.; van der Voet, G.; Agterof, W.G.M.; Norde, W.; Lyklema, J. Driving forces of enzyme adsorption at solid-liquid interfaces. J. Colloid Interface Sci. 1995, 170, 340–350. [Google Scholar] [CrossRef]
- Roth, C.M.; Lenhoff, A.M. Electrostatic and van der Waals Contributions to Protein Adsorption: Computation of Equilibrium Constants. Langmuir 1993, 9, 962–972. [Google Scholar] [CrossRef]
- Roth, C.M.; Lenhoff, A.M. Electrostatic and van der Waals Contributions to Protein Adsorption: Comparison of Theory and Experiment. Langmuir 1995, 11, 3500–3509. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bolotskova, P.N.; Bondarchuk, E.V.; Gryaznov, V.G.; Gudkov, S.V.; Kozlov, V.A.; Okuneva, M.A.; Ovchinnikov, O.V.; Smoliy, O.P.; Turkanov, I.F. Long-Term Effect of Low-Frequency Electromagnetic Irradiation in Water and Isotonic Aqueous Solutions as Studied by Photoluminescence from Polymer Membrane. Polymers 2021, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, S.O.; Shkirin, A.V.; Ninham, B.W.; Sychev, A.A.; Babenko, V.A.; Penkov, N.V.; Kryuchkov, N.P.; Bunkin, N.F. Ionspecific and thermal effects in the stabilization of the gas nanobubble phase in bulk aqueous electrolyte solutions. Langmuir 2016, 32, 11245–11255. [Google Scholar] [CrossRef] [PubMed]
- Bunkin, N.F.; Shkirin, A.V.; Suyazov, N.V.; Babenko, V.A.; Sychev, A.A.; Penkov, N.V.; Belosludtsev, K.N.; Gudkov, S.V. Formation and dynamics of ion-stabilized gas nanobubbles in the bulk of aqueous NaCl solutions. J. Phys. Chem. B 2016, 120, 1291–1303. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bunkin, F.V. Bubston structure of water and electrolyte aqueous solutions. Physics-Uspekhi 2016, 59, 846. [Google Scholar] [CrossRef]


| Detector’s Antenna Orientation Axis * | Electric Field Strength (V/m) | Magnetic Induction (µT) |
|---|---|---|
| X | 97.4 ± 14.7 | 21.4 ± 10.9 |
| Y | 110.6 ± 3.5 | 80.6 ± 5.4 |
| Z | 179.0 ± 11.0 | 9.3 ± 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Shumov, I.D.; Kozlov, A.F.; Ableev, A.N.; Vinogradova, A.V.; Nevedrova, E.D.; Afonin, O.N.; Zhdanov, D.D.; Tatur, V.Y.; Lukyanitsa, A.A.; et al. Incubation of Horseradish Peroxidase near 50 Hz AC Equipment Promotes Its Disaggregation and Enzymatic Activity. Micromachines 2025, 16, 344. https://doi.org/10.3390/mi16030344
Ivanov YD, Shumov ID, Kozlov AF, Ableev AN, Vinogradova AV, Nevedrova ED, Afonin ON, Zhdanov DD, Tatur VY, Lukyanitsa AA, et al. Incubation of Horseradish Peroxidase near 50 Hz AC Equipment Promotes Its Disaggregation and Enzymatic Activity. Micromachines. 2025; 16(3):344. https://doi.org/10.3390/mi16030344
Chicago/Turabian StyleIvanov, Yuri D., Ivan D. Shumov, Andrey F. Kozlov, Alexander N. Ableev, Angelina V. Vinogradova, Ekaterina D. Nevedrova, Oleg N. Afonin, Dmitry D. Zhdanov, Vadim Y. Tatur, Andrei A. Lukyanitsa, and et al. 2025. "Incubation of Horseradish Peroxidase near 50 Hz AC Equipment Promotes Its Disaggregation and Enzymatic Activity" Micromachines 16, no. 3: 344. https://doi.org/10.3390/mi16030344
APA StyleIvanov, Y. D., Shumov, I. D., Kozlov, A. F., Ableev, A. N., Vinogradova, A. V., Nevedrova, E. D., Afonin, O. N., Zhdanov, D. D., Tatur, V. Y., Lukyanitsa, A. A., Ivanova, N. D., Yushkov, E. S., Enikeev, D. V., Konev, V. A., & Ziborov, V. S. (2025). Incubation of Horseradish Peroxidase near 50 Hz AC Equipment Promotes Its Disaggregation and Enzymatic Activity. Micromachines, 16(3), 344. https://doi.org/10.3390/mi16030344







