A Study on the Impact of Vanadium Doping on the Structural, Optical, and Optoelectrical Properties of ZnS Thin Films for Optoelectronic Applications
Abstract
1. Introduction
2. Materials and Methods
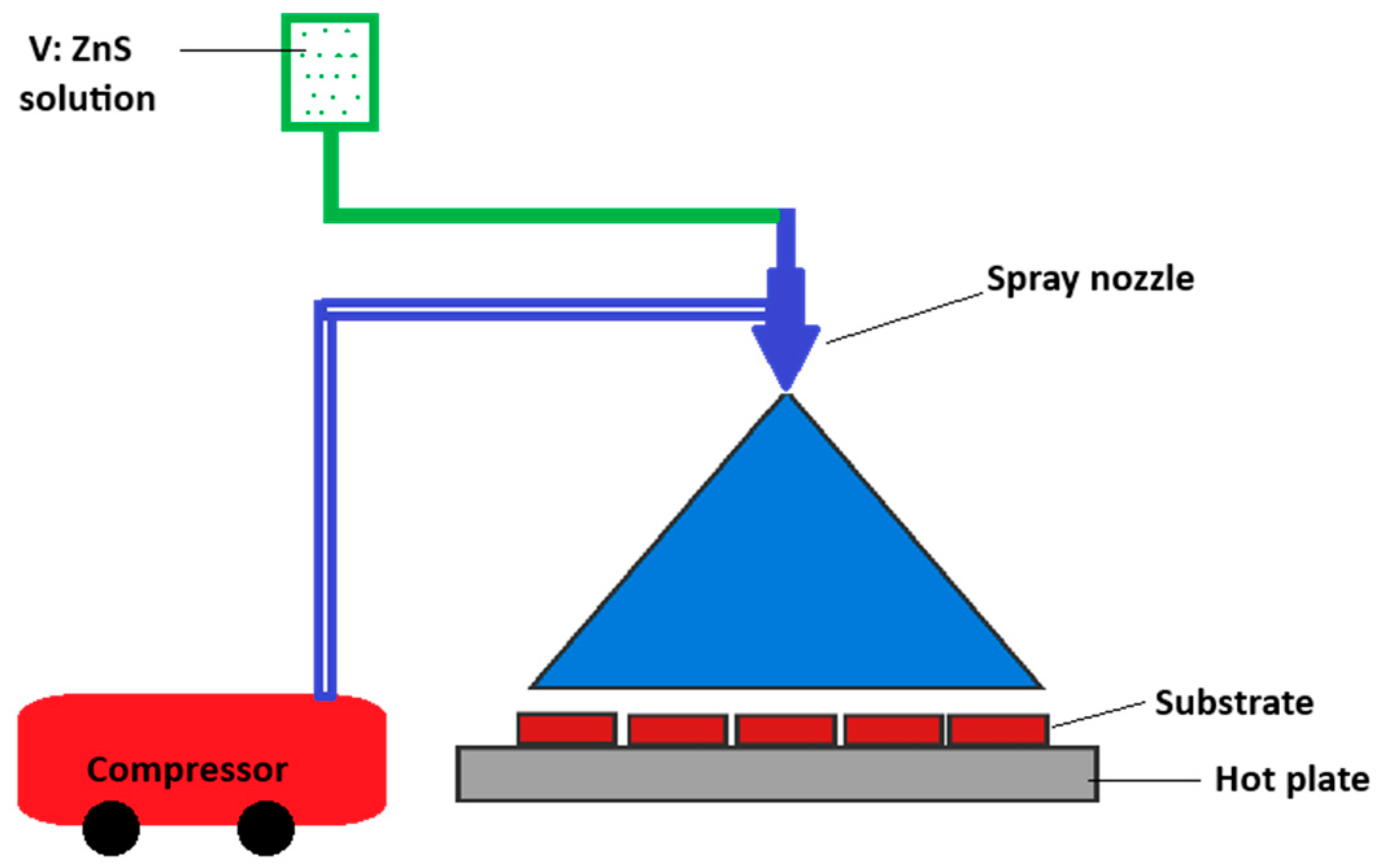
2.1. Synthesis of Vanadium-Doped ZnS Films
2.2. Characterization of Vanadium-Doped ZnS Layers
3. Results and Discussion
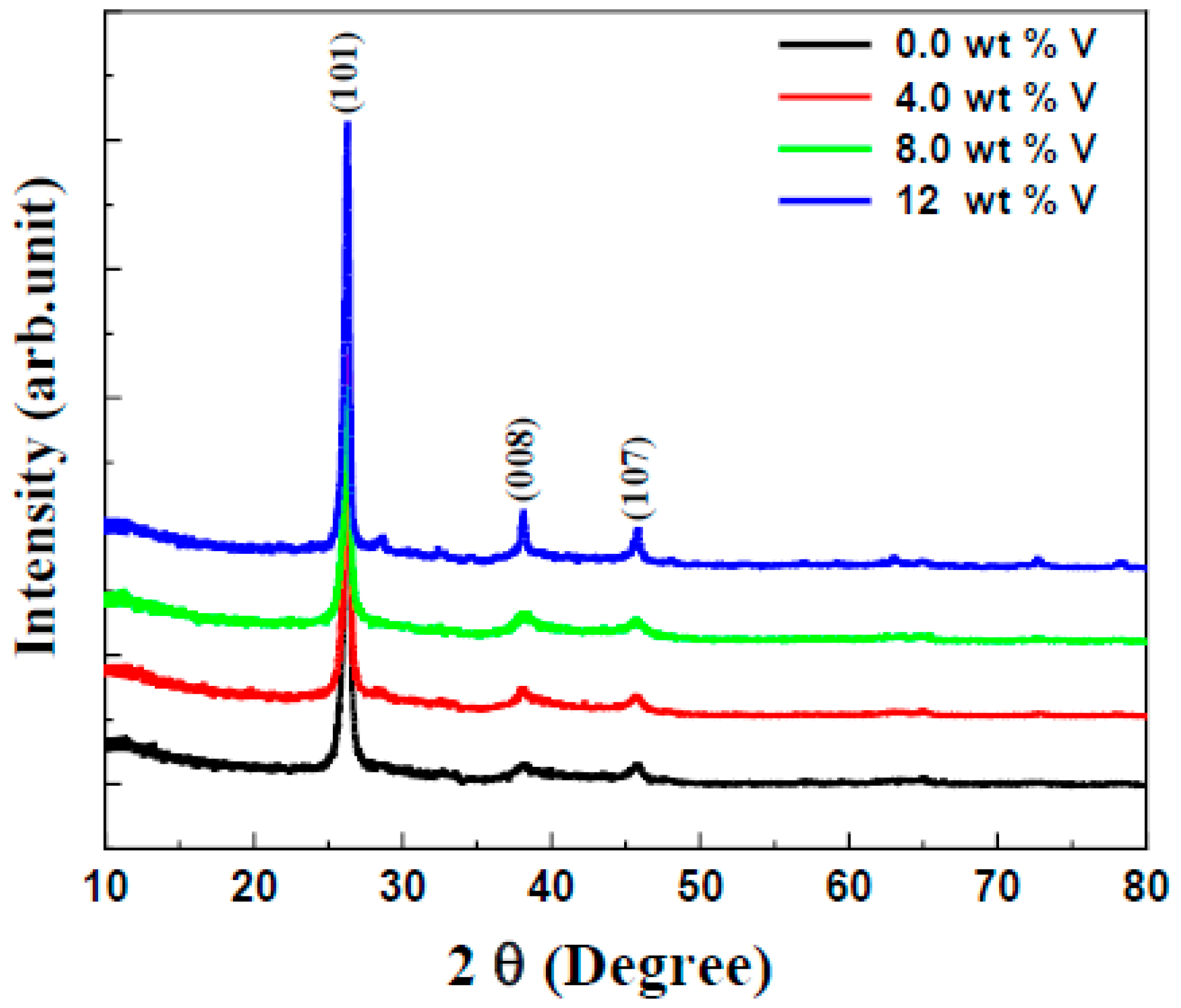
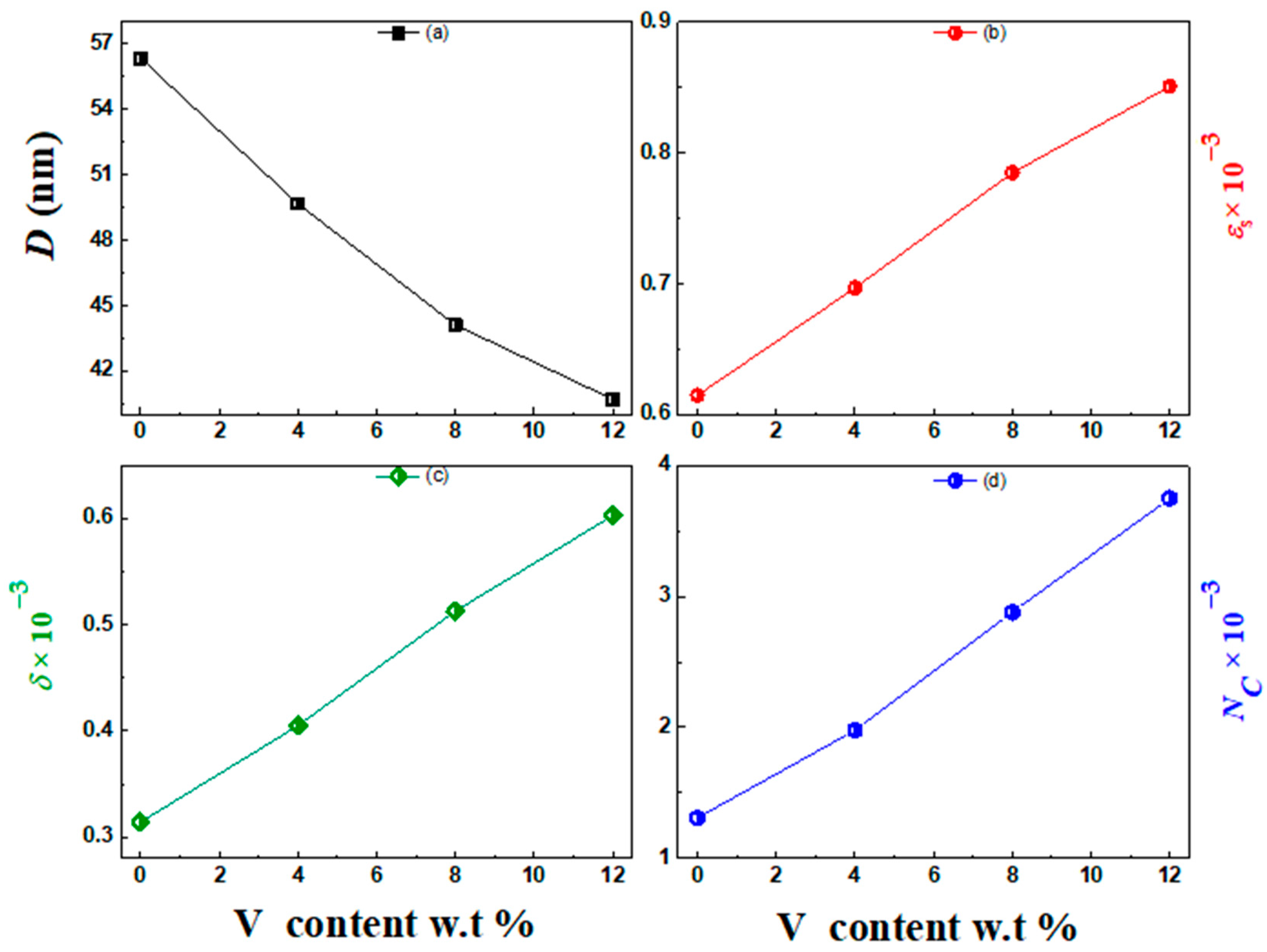
3.1. Structural Investigation
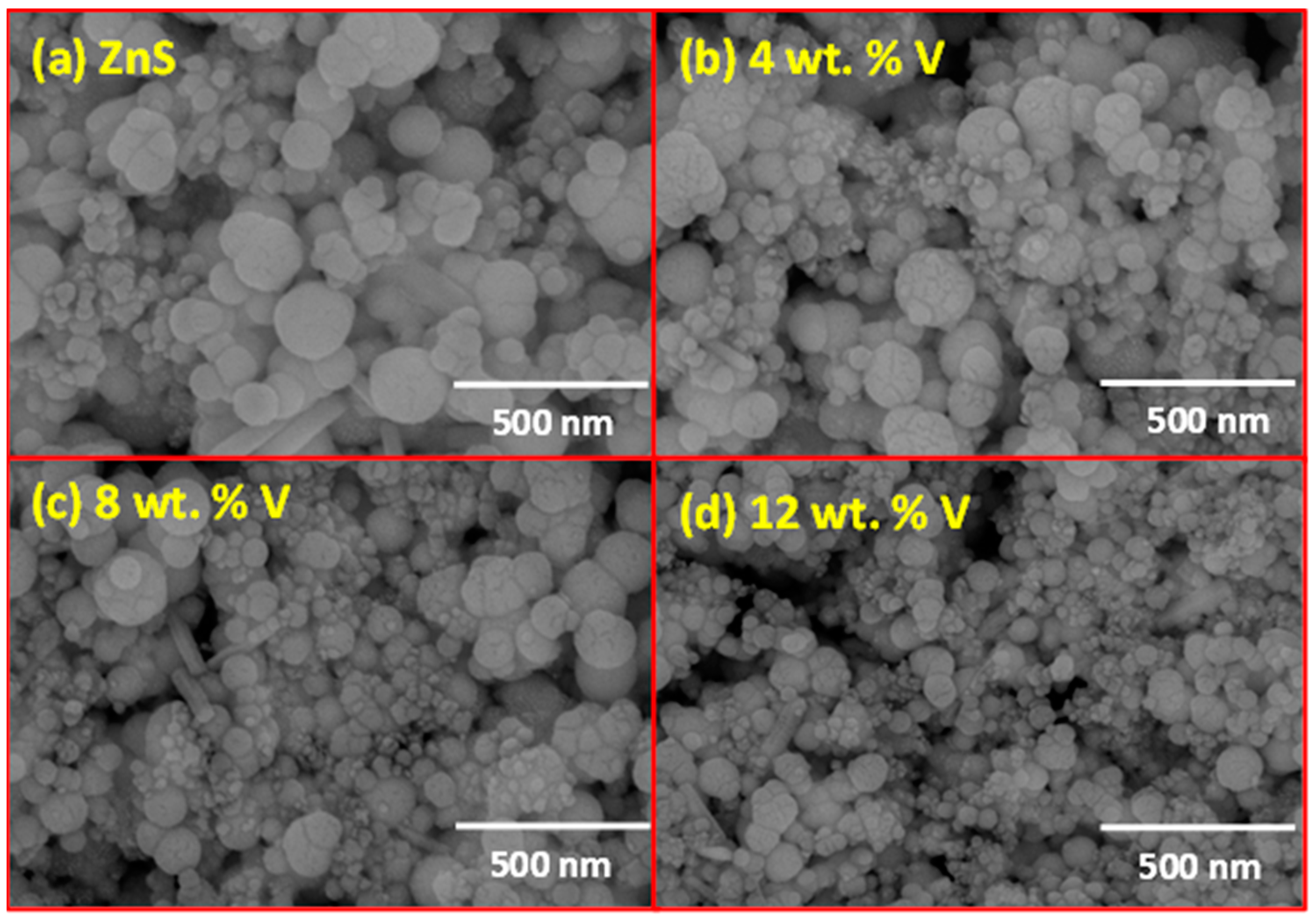
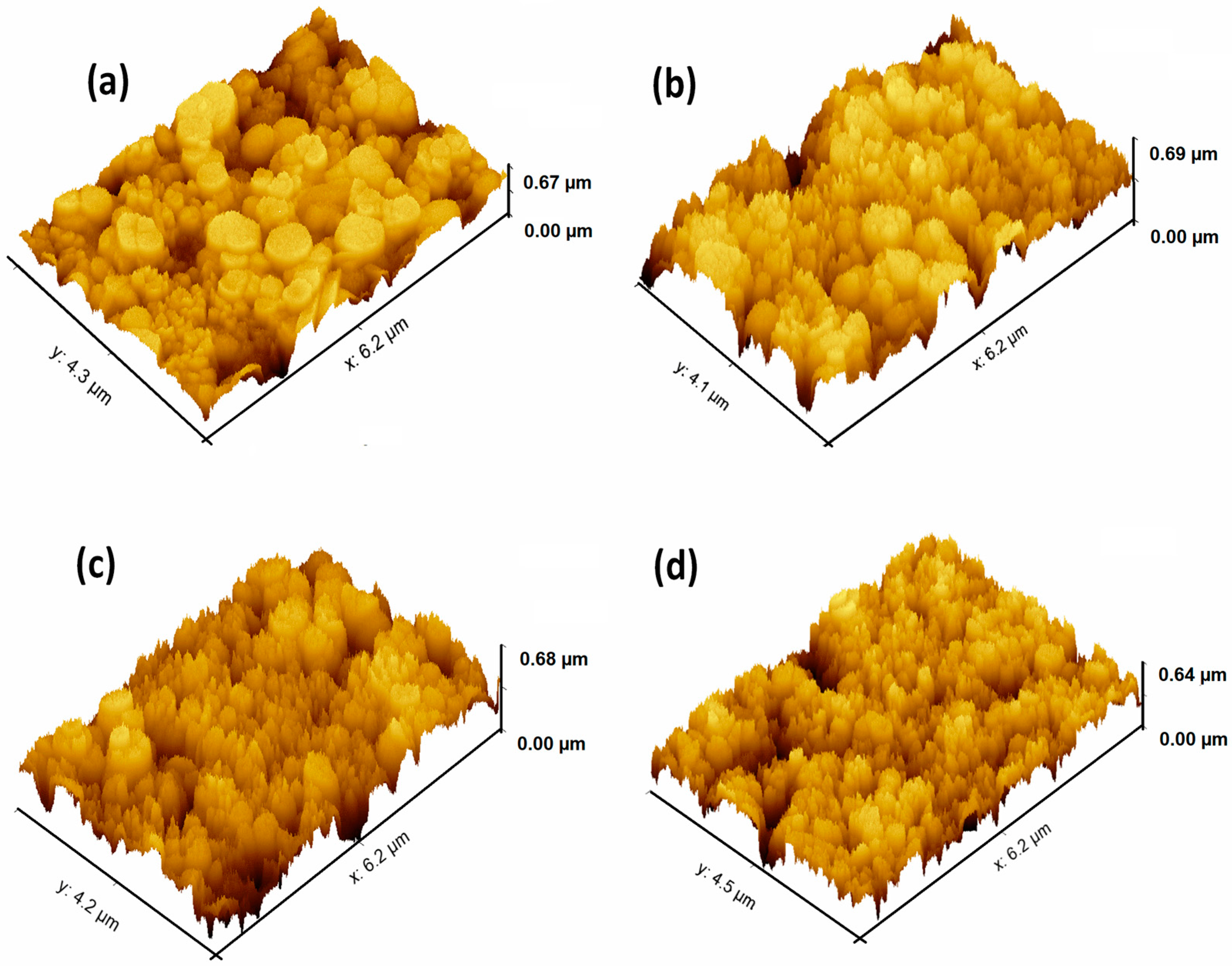
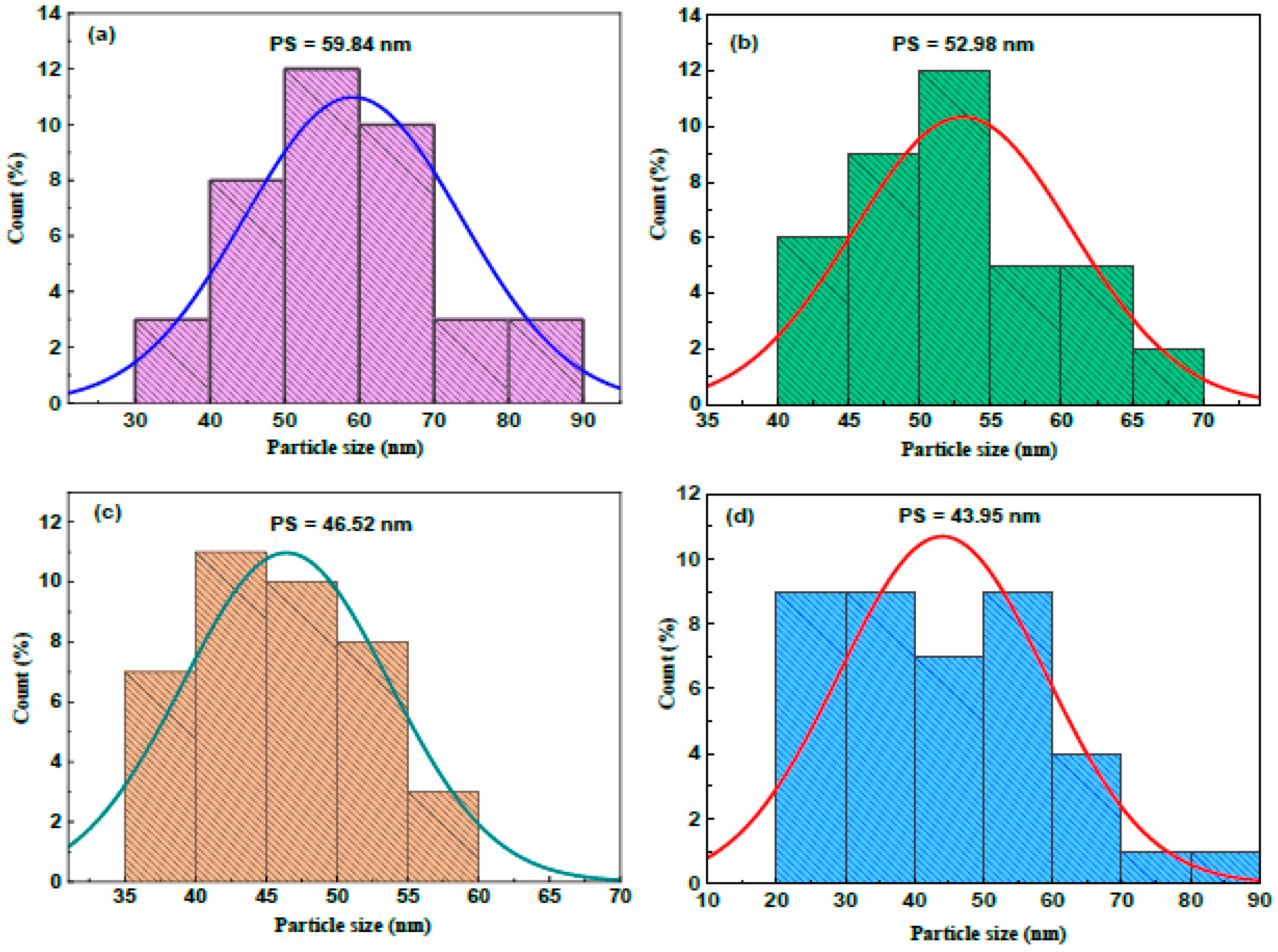
3.2. Microstructural Study
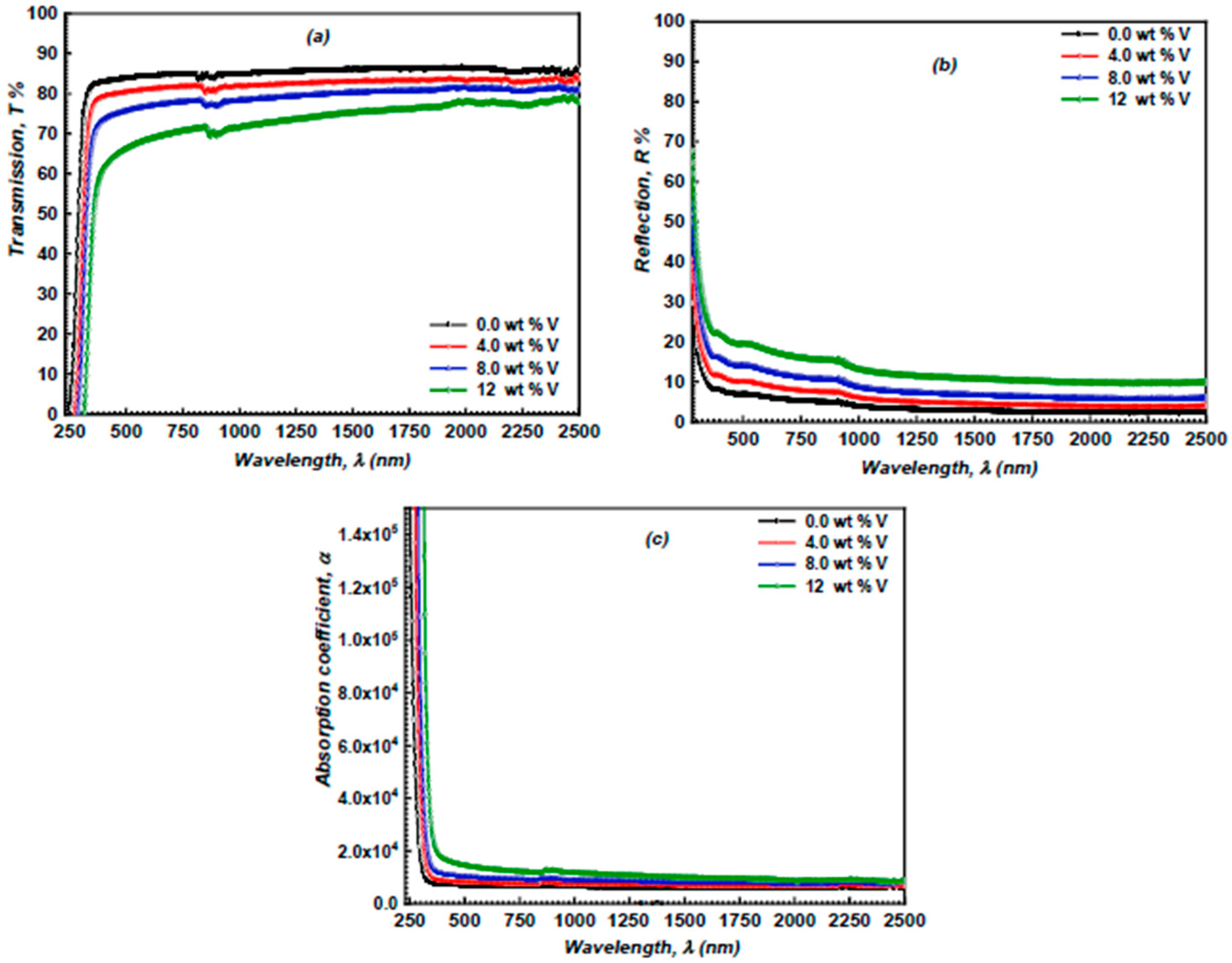
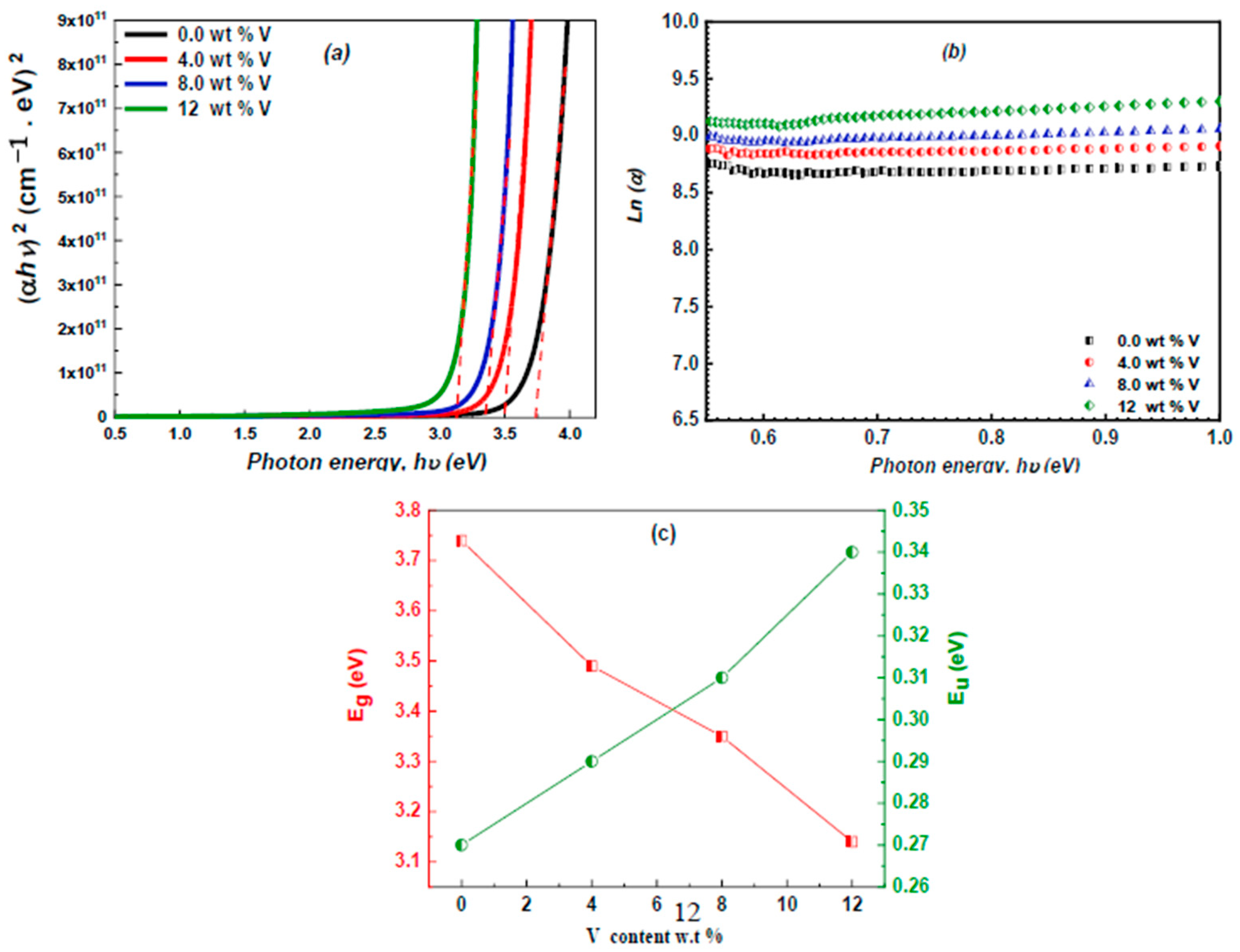
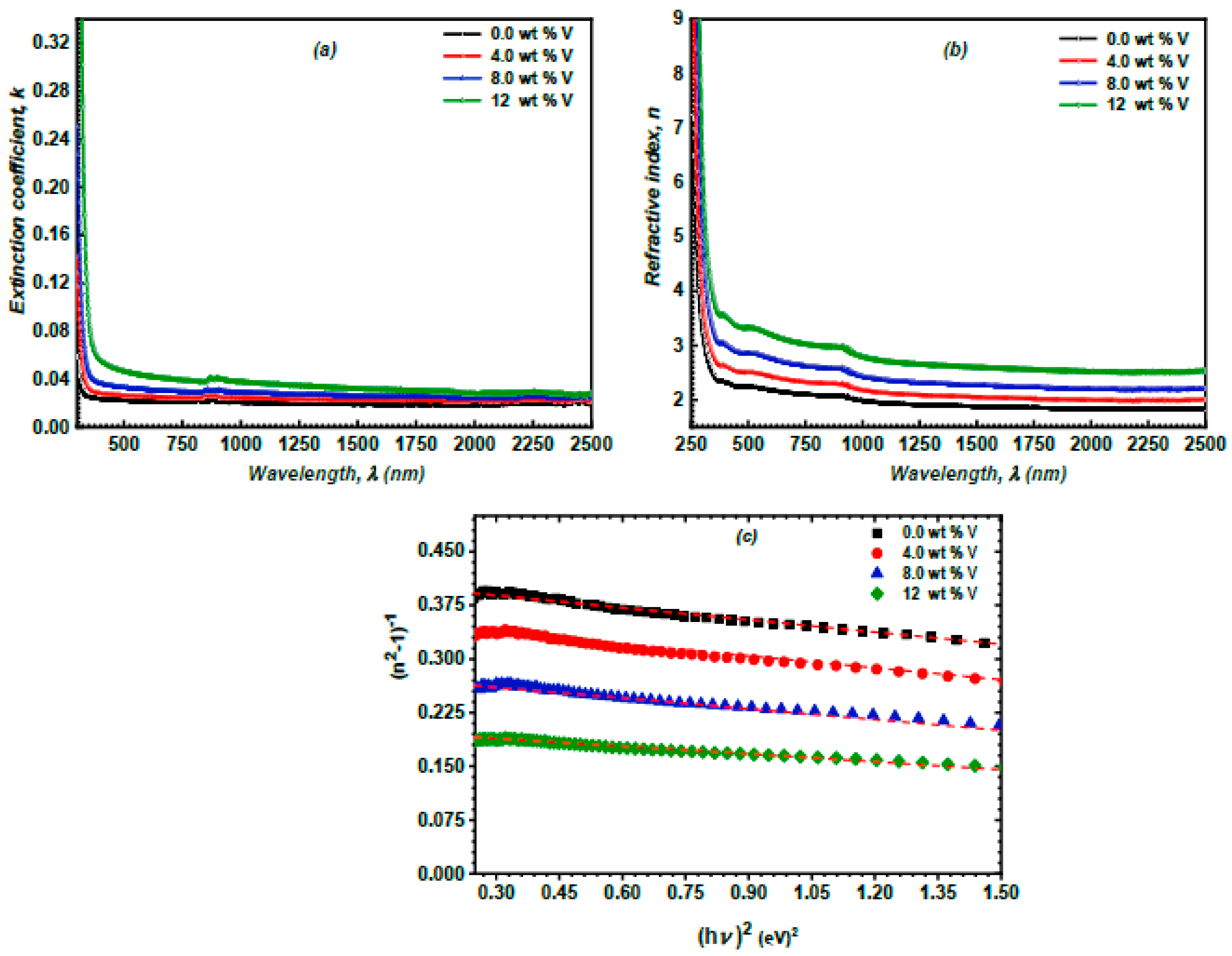
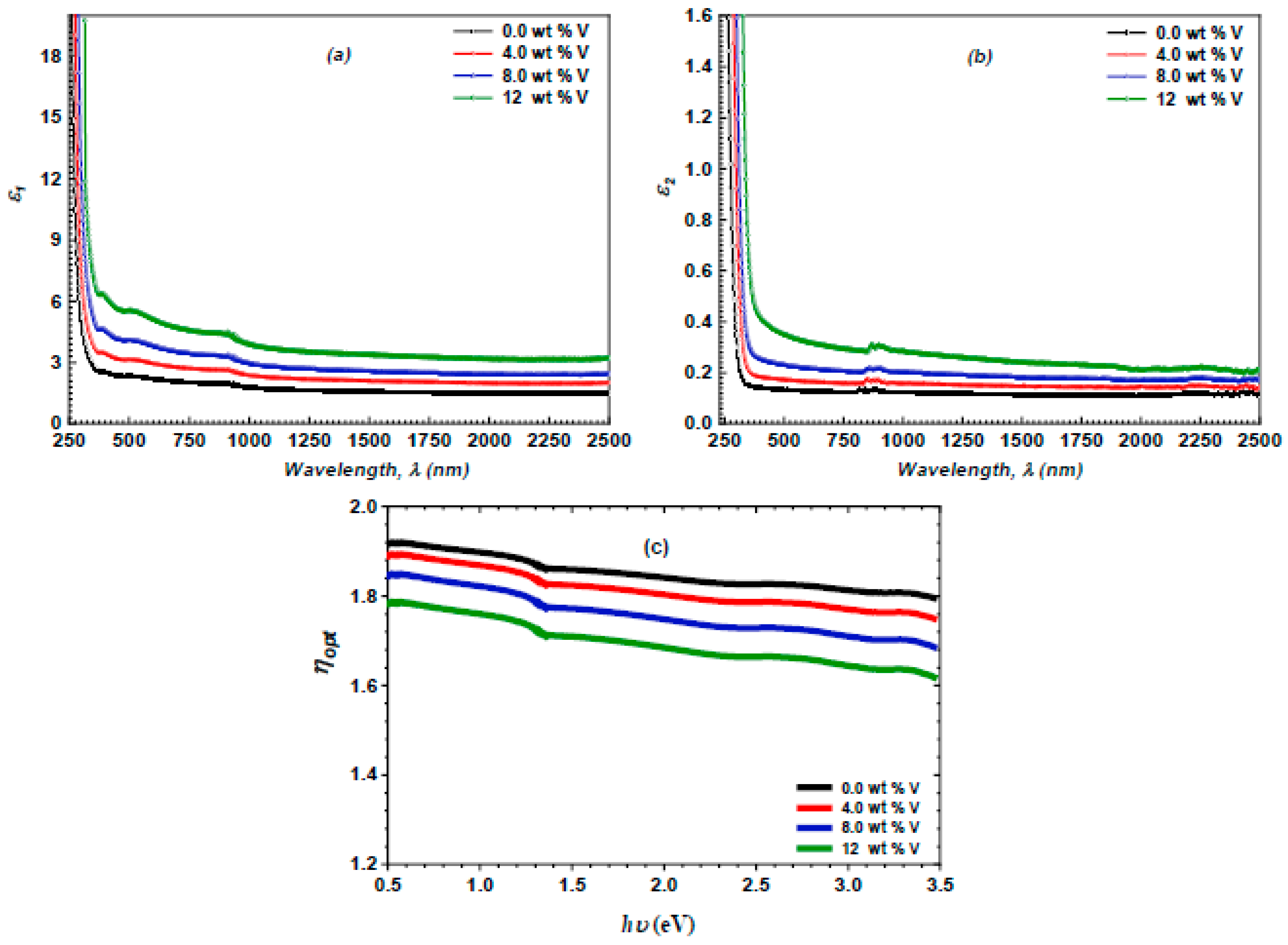
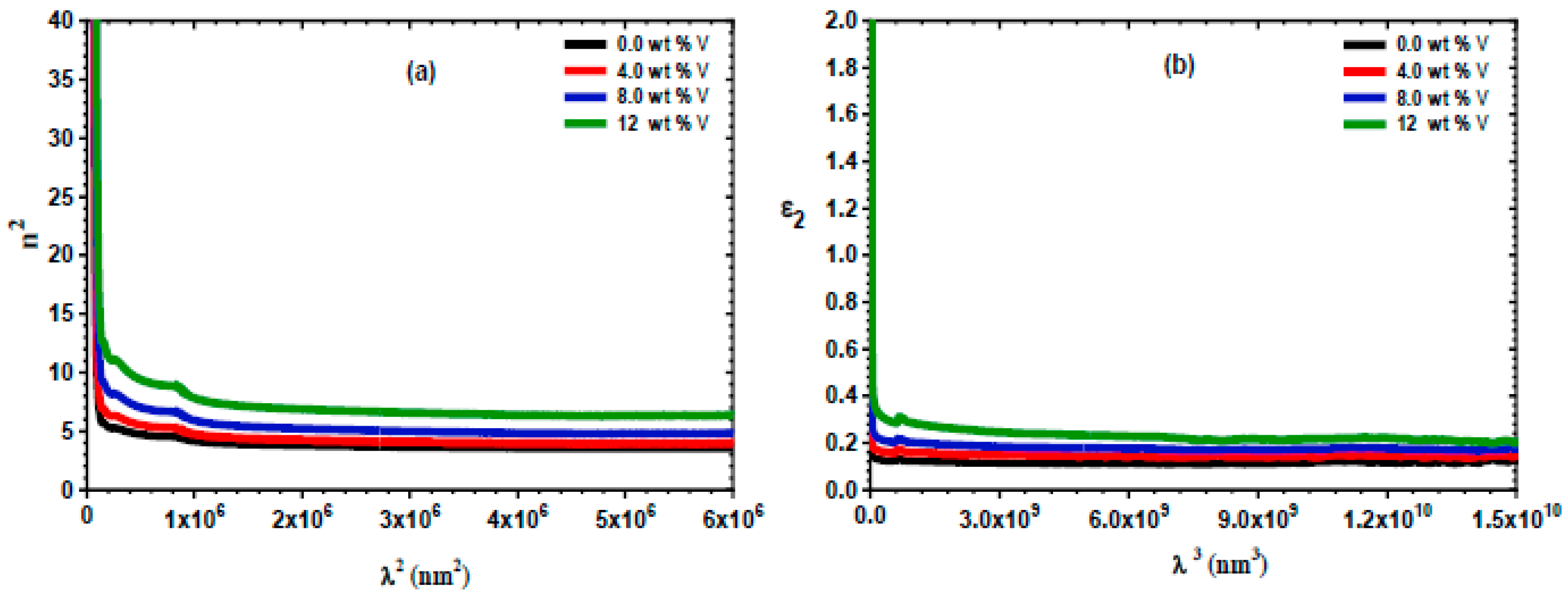
3.3. Linear Optical Properties
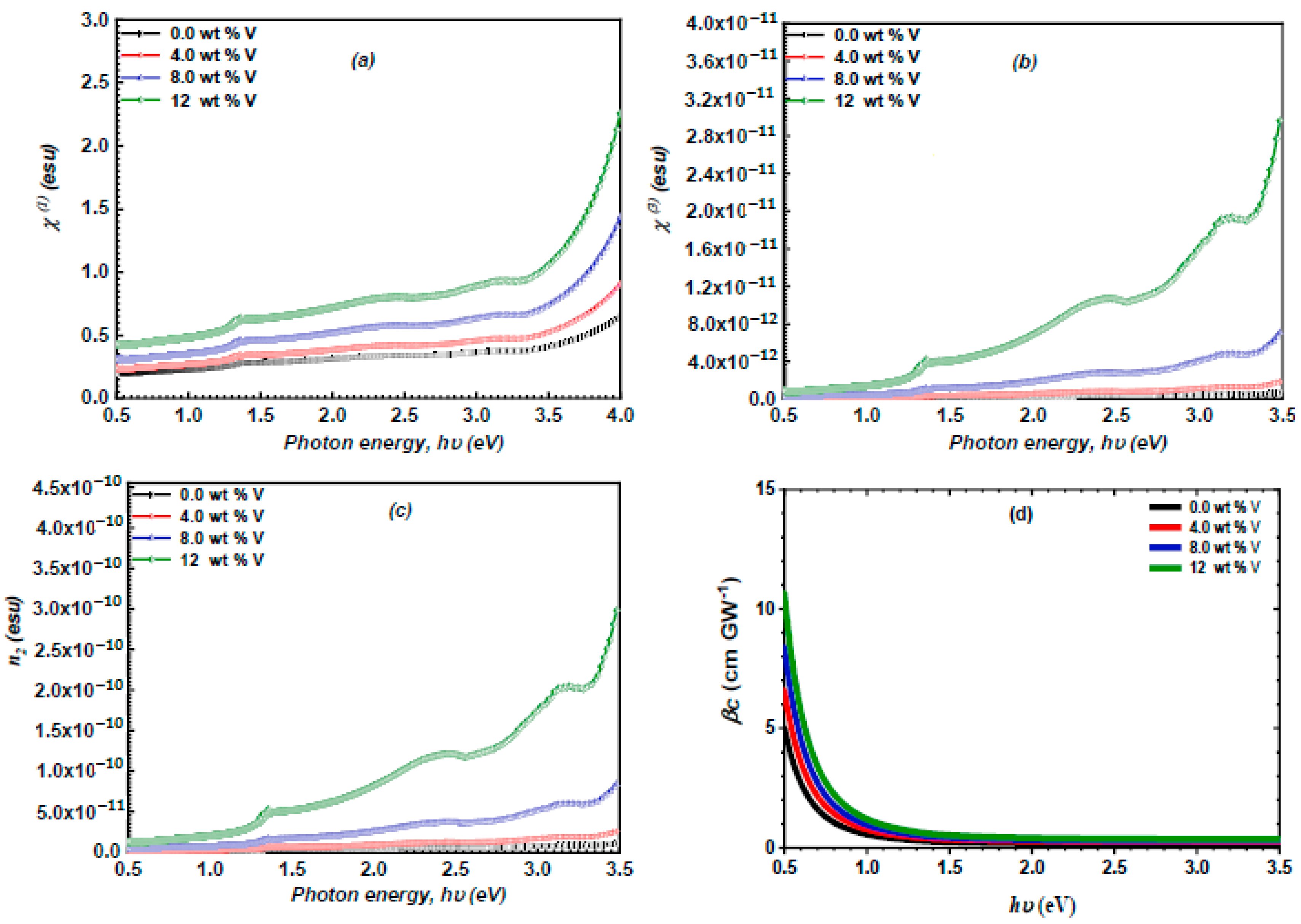
3.4. Nonlinear Optical Study
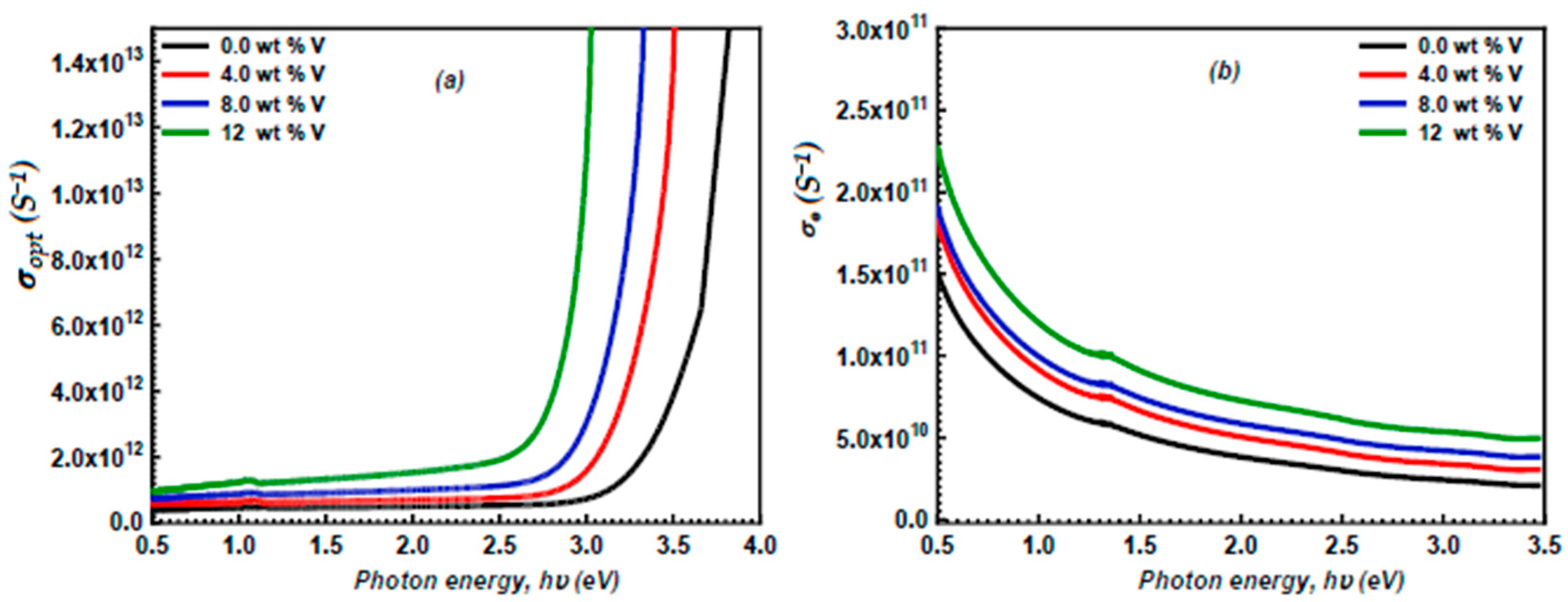
3.5. Optoelectrical Parameters
3.6. The Detection of the Semiconductor Type and Charge Compensation Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saparov, B. Next Generation Thin-Film Solar Absorbers Based on Chalcogenides. Chem. Rev. 2022, 122, 10575–10577. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Zang, Z.; Zhang, Y.; Zheng, Y.; Zhang, Y. The suppressing of excitonic effects in Cu-chalcogenides for solar cell applications. Sol. Energy 2022, 247, 255–260. [Google Scholar] [CrossRef]
- Isotta, E.; Andrade-Arvizu, J.; Syafiq, U.; Jiménez-Arguijo, A.; Navarro-Güell, A.; Guc, M.; Saucedo, E.; Scardi, P. Towards Low Cost and Sustainable Thin Film Thermoelectric Devices Based on Quaternary Chalcogenides. Adv. Funct. Mater. 2022, 32, 2202157. [Google Scholar] [CrossRef]
- Olivier-Fourcade, J.; Ibanez, A.; Jumas, J.C.; Maurin, M.; Lefebvre, I.; Lippens, P.; Lannoo, M.; Allan, G. Chemical bonding and electronic properties in antimony chalcogenides. J. Solid State Chem. 1990, 87, 366–377. [Google Scholar] [CrossRef]
- Kormath Madam Raghupathy, R.; Wiebeler, H.; Kühne, T.D.; Felser, C.; Mirhosseini, H. Database screening of ternary chalcogenides for p-type transparent conductors. Chem. Mater. 2018, 30, 6794–6800. [Google Scholar] [CrossRef]
- Martínez-Escobar, D.; Ramachandran, M.; Sánchez-Juárez, A.; Rios, J.S.N. Optical and electrical properties of SnSe2 and SnSe thin films prepared by spray pyrolysis. Thin Solid Films 2013, 535, 390–393. [Google Scholar] [CrossRef]
- Vinotha, K.; Jayasutha, B.; Abel, M.J.; Vinoth, K. In3+-doped CuS thin films: Physicochemical characteristics and photocatalytic property. J. Mater. Sci. Mater. Electron. 2022, 33, 22862–22882. [Google Scholar] [CrossRef]
- Wang, L.; Tan, X.; Zhu, Q.; Dong, Z.; Wu, X.; Huang, K.; Xu, J. The universality applications of MoS2@ MnS heterojunction hollow microspheres for univalence organic or multivalence aqueous electrolyte energy storage device. J. Power Sources 2022, 518, 230747. [Google Scholar] [CrossRef]
- Derbali, A.; Saidi, H.; Attaf, A.; Benamra, H.; Bouhdjer, A.; Attaf, N.; Ezzaouia, H.; Derbali, L.; Aida, M.S. Solution flow rate influence on ZnS thin films properties grown by ultrasonic spray for optoelectronic application. J. Semicond. 2018, 39, 93001. [Google Scholar] [CrossRef]
- Afifi, H.H.; Mahmoud, S.A.; Ashour, A. Structural study of ZnS thin films prepared by spray pyrolysis. Thin Solid Films 1995, 263, 248–251. [Google Scholar] [CrossRef]
- Mohamed, S.H.; El-Hagary, M.; Emam-Ismail, M. Thickness and annealing effects on the optoelectronic properties of ZnS films. J. Phys. D Appl. Phys. 2010, 43, 75401. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C. Fabrication of wurtzite ZnS nanobelts via simple thermal evaporation. Appl. Phys. Lett. 2003, 83, 359–361. [Google Scholar] [CrossRef]
- Simandan, I.-D.; Sava, F.; Buruiana, A.-T.; Burducea, I.; Becherescu, N.; Mihai, C.; Velea, A.; Galca, A.-C. The effect of the deposition method on the structural and optical properties of ZnS thin films. Coatings 2021, 11, 1064. [Google Scholar] [CrossRef]
- Elidrissi, B.; Addou, M.; Regragui, M.; Bougrine, A.; Kachouane, A.; Bernede, J.C. Structure, composition and optical properties of ZnS thin films prepared by spray pyrolysis. Mater. Chem. Phys. 2001, 68, 175–179. [Google Scholar] [CrossRef]
- Echendu, O.K.; Weerasinghe, A.R.; Diso, D.G.; Fauzi, F.; Dharmadasa, I.M. Characterization of n-type and p-type ZnS thin layers grown by an electrochemical method. J. Electron. Mater. 2013, 42, 692–700. [Google Scholar] [CrossRef]
- Khadayeir, A.A.; Jasim, R.I.; Jumaah, S.H.; Habubi, N.F.; Chiad, S.S. Influence of Substrate Temperature on Physical Properties of Nanostructured ZnS Thin Films. J. Phys. Conf. Ser. 2020, 1664, 12009. [Google Scholar] [CrossRef]
- Choubey, R.K.; Kumar, S.; Lan, C.W. Shallow chemical bath deposition of ZnS buffer layer for environmentally benign solar cell devices. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 25015. [Google Scholar] [CrossRef]
- Vidal, J.; Vigil, O.; De Melo, O.; Lopez, N.; Zelaya-Angel, O. Influence of NH3 concentration and annealing in the properties of chemical bath deposited ZnS films. Mater. Chem. Phys. 1999, 61, 139–142. [Google Scholar] [CrossRef]
- Dona, J.M.; Herrero, J. Process and film characterization of chemical-bath-deposited ZnS thin films. J. Electrochem. Soc. 1994, 141, 205. [Google Scholar] [CrossRef]
- Oladeji, I.O.; Chow, L. Synthesis and processing of CdS/ZnS multilayer films for solar cell application. Thin Solid Films 2005, 474, 77–83. [Google Scholar] [CrossRef]
- Dai, S.; Wang, T.; Li, R.; Wang, Q.; Ma, Y.; Tian, L.; Su, J.; Wang, Y.; Zhou, D.; Zhang, X. Preparation and electrical properties of N-doped ZnSnO thin film transistors. J. Alloys Compd. 2018, 745, 256–261. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Malik, M.A.; Alghamdi, Y.G.; Ahmad, K.S.; Riaz, S.; Naseem, S. Chemical bath deposition of Fe-doped ZnS thin films: Investigations of their ferromagnetic and half-metallic properties. Mater. Sci. Semicond. Process. 2015, 39, 283–291. [Google Scholar] [CrossRef]
- Jrad, A.; Naouai, M.; Ammar, S.; Turki-Kamoun, N. Investigation of molybdenum dopant effect on ZnS thin films: Chemical composition, structural, morphological, optical and luminescence surveys. Mater. Sci. Semicond. Process. 2021, 130, 105825. [Google Scholar] [CrossRef]
- Mohamed, N.E.; Amer, M.I.; Moustafa, S.H.; Hashem, H.; Emam-Ismail, M.; Shaaban, E.R.; El-Hagary, M. Improvements of multifunctional spintronic and optoelectronic applications of nanostructured Eu-doped ZnS thin films through magnetic and linear, nonlinear optical investigations. Mater. Chem. Phys. 2024, 319, 129314. [Google Scholar] [CrossRef]
- Mosavi, S.M.; Kafashan, H. Physical properties of Cd-doped ZnS thin films. Superlattices Microstruct. 2019, 126, 139–149. [Google Scholar] [CrossRef]
- Shaili, H.; Salmani, E.M.; Essajai, R.; Beraich, M.; Battal, W.; Ouafi, M.; Elhat, A.; Rouchdi, M.; Ez-Zahraouy, H.; Hassanain, N. Structural, electronic and optical properties of Cu-doped ZnS thin films deposited by the ultrasonic spray method-DFT study. Opt. Quantum Electron. 2021, 53, 300. [Google Scholar] [CrossRef]
- Jebathew, A.J.; Karunakaran, M.; Ade, R.; Jayram, N.D.; Ganesh, V.; Bitla, Y.; Vinoth, S.; Algarni, H.; Yahia, I.S. Optical manipulation of nebulizer spray pyrolysed ZnS thin films for photodetector applications: Effect of Al, Sn and Sb doping. Opt. Mater. 2021, 117, 111177. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Altowairqi, Y.; Mohamed, M.B. Enhancing the linear and nonlinear optical properties by ZnS/V-doped polyvinyl alcohol/carboxymethyl cellulose/polyethylene glycol polymeric nanocomposites for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 25127–25138. [Google Scholar] [CrossRef]
- Akl, A.A.; El Radaf, I.M.; Hassanien, A.S. An extensive comparative study for microstructural properties and crystal imperfections of Novel sprayed Cu3SbSe3 Nanoparticle-thin films of different thicknesses. Optik 2021, 227, 165837. [Google Scholar] [CrossRef]
- Ben Aissa, M.A.; Khezami, L.; Taha, K.; Elamin, N.; Mustafa, B.; Al-Ayed, A.S.; Modwi, A. Yttrium oxide-doped ZnO for effective adsorption of basic fuchsin dye: Equilibrium, kinetics, and mechanism studies. Int. J. Environ. Sci. Technol. 2021, 19, 9901–9914. [Google Scholar] [CrossRef]
- Akl, A.A.; El Radaf, I.M.; Hassanien, A.S. Intensive comparative study using X-Ray diffraction for investigating microstructural parameters and crystal defects of the novel nanostructural ZnGa2S4 thin films. Superlattices Microstruct. 2020, 143, 106544. [Google Scholar] [CrossRef]
- Elamin, N.; Modwi, A.; Aissa, M.A.B.; Taha, K.K.; Al-Duaij, O.K.; Yousef, T.A. Fabrication of Cr–ZnO photocatalyst by starch-assisted sol–gel method for photodegradation of congo red under visible light. J. Mater. Sci. Mater. Electron. 2021, 32, 2234–2248. [Google Scholar] [CrossRef]
- Kraidy, A.F.; El Radaf, I.M.; Zeinert, A.; Lahmar, A.; Pelaiz-Barranco, A.; Gagou, Y. Optoelectrical properties of the ternary chalcogenide SnSb2S5 as a new absorber layer for photovoltaic application. J. Phys. D Appl. Phys. 2024, 57, 205102. [Google Scholar] [CrossRef]
- Shkir, M.; Ganesh, V.; AlFaify, S.; Yahia, I.S.; Zahran, H.Y. Tailoring the linear and nonlinear optical properties of NiO thin films through Cr3+ doping. J. Mater. Sci. Mater. Electron. 2018, 29, 6446–6457. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Sharma, I.; Akl, A.A. Physical and optical properties of a-Ge-Sb-Se-Te bulk and film samples: Refractive index and its association with electronic polarizability of thermally evaporated a-Ge15−xSbxSe50Te35 thin-films. J. Non-Cryst. Solids 2020, 531, 119853. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Alkallas, F.H.; Trabelsi, A.B.G.; Shkir, M.; Hakami, J.; Khan, A.; Ali, H.E.; Awwad, N.S.; AlFaify, S. Improved linear and nonlinear optical properties of PbS thin films synthesized by spray pyrolysis technique for optoelectronics: An effect of Gd3+ doping concentrations. Phys. B Condens. Matter 2022, 641, 414099. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Sharma, I. Band-gap engineering, conduction and valence band positions of thermally evaporated amorphous Ge15−xSbxSe50Te35 thin films: Influences of Sb upon some optical characterizations and physical parameters. J. Alloys Compd. 2019, 798, 750–763. [Google Scholar] [CrossRef]
- Rana, M.S.; Das, S.K.; Rahman, M.O.; Ahmed, F.; Hossain, M.A. Vanadium doped ZnS nanoparticles: Effect of vanadium concentration on structural, optical and electrical properties. Trans. Electr. Electron. Mater. 2021, 22, 612–621. [Google Scholar] [CrossRef]
- Lavanya, S.; Kumar, T.R.; Gunavathy, K.V.; Vibha, K.; Shkir, M.; Hakami, J.; Ali, H.E.; Ubaidullah, M. A noticeable improvement in opto-electronic properties of nebulizer sprayed In2S3 thin films for stable-photodetector applications. Micro Nanostructures 2022, 169, 207337. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Neffati, R.; Aly, K.A. Impact of Cd-addition upon optical properties and dispersion parameters of thermally evaporated CdxZn1−xSe films: Discussions on bandgap engineering, conduction and valence band positions. Optik 2020, 212, 164681. [Google Scholar] [CrossRef]
- Kotbi, A.; El Radaf, I.M.; Alaoui, I.H.; Cantaluppi, A.; Zeinert, A.; Lahmar, A. Structural and Optical Characterization of Porous NiV2O6 Films Synthesized by Nebulizer Spray Pyrolysis for Photodetector Applications. Micromachines 2024, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Jebathew, A.J.; Karunakaran, M.; Kumar, K.D.A.; Valanarasu, S.; Ganesh, V.; Shkir, M.; Yahia, I.S.; Zahran, H.Y.; Kathalingam, A. An effect of Gd3+ doping on core properties of ZnS thin films prepared by nebulizer spray pyrolysis (NSP) method. Phys. B Condens. Matter 2019, 574, 411674. [Google Scholar] [CrossRef]
- Lahmar, A.; Benchaabane, A.; Aderdour, M.; Zeinert, A.; Es-Souni, M. Temperature influence on microstructure and optical properties of TiO2–Au thin films. Appl. Phys. A 2016, 122, 137. [Google Scholar] [CrossRef]
- Shkir, M.; Khan, A.; Imran, M.; Khan, M.A.; Zargar, R.A.; Alshahrani, T.; Kumar, K.D.A.; Mohanraj, P.; Chandekar, K.V.; AlFaify, S. Spray pyrolysis developed Nd doped Co3O4 nanostructured thin films and their structural, and opto-nonlinear properties for optoelectronics applications. Opt. Laser Technol. 2022, 150, 107959. [Google Scholar] [CrossRef]
- Al-Zahrani, H.Y.S.; Alsulami, A. Effects of Cr doping on the structural, optical and electrical characterizations of spray-deposited ZnSnO3 thin films. Appl. Phys. A 2023, 129, 783. [Google Scholar] [CrossRef]
- Sakli, A.; Lahmar, A.; Gamra, D.; Clin, M.; Bouchriha, H.; Lejeune, M. Effect of thermal annealing on microstructure and optical properties of silver-carbon nanocomposite thin films. Mater. Today Proc. 2022, 51, 543–548. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, P.; Hassanien, A.S. Optical properties and optoelectrical parameters of the quaternary chalcogenide amorphous Ge15SnxS35−xTe50 films. J. Non. Cryst. Solids 2022, 590, 121673. [Google Scholar] [CrossRef]
- Khan, A.; Shkir, M.; Manthrammel, M.A.; Ganesh, V.; Yahia, I.S.; Ahmed, M.; El-Toni, A.M.; Aldalbahi, A.; Ghaithan, H.; AlFaify, S. Effect of Gd doping on structural, optical properties, photoluminescence and electrical characteristics of CdS nanoparticles for optoelectronics. Ceram. Int. 2019, 45, 10133–10141. [Google Scholar] [CrossRef]
- El Radaf, I.M.; Al-Zahrani, H.Y.S.; Hassanien, A.S. Novel synthesis, structural, linear and nonlinear optical properties of p-type kesterite nanosized Cu2MnGeS4 thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 8336–8348. [Google Scholar] [CrossRef]
- Paulraj, K.; Ramaswamy, S.; Shkir, M.; Yahia, I.S.; Hamdy, M.S.; AlFaify, S. Analysis of neodymium rare earth element doping in PbS films for opto-electronics applications. J. Mater. Sci. Mater. Electron. 2020, 31, 1817–1827. [Google Scholar] [CrossRef]
- Al-Zahrani, H.Y.S.; Alsulami, A. Structural and optical features of neoteric Ag2BaGeS4 thin films synthesized by a chemical bath deposition process. J. Mater. Sci. Mater. Electron. 2023, 34, 1550. [Google Scholar] [CrossRef]
- Jebathew, A.J.; Karunakaran, M.; Shkir, M.; Algarni, H.; AlFaify, S.; Khan, A.; Alotaibi, N.; Alshahrani, T. High sensitive samarium-doped ZnS thin films for photo-detector applications. Opt. Mater. 2021, 122, 111649. [Google Scholar] [CrossRef]
- Alzaid, M.; Qasem, A.; Shaaban, E.R.; Hadia, N.M.A. Extraction of thickness, linear and nonlinear optical parameters of Ge20+xSe80−x thin films at normal and slightly inclined light for optoelectronic devices. Opt. Mater. 2020, 110, 110539. [Google Scholar] [CrossRef]
- Hassanien, A.S. Intensive linear and nonlinear optical studies of thermally evaporated amorphous thin Cu-Ge-Se-Te films. J. Non. Cryst. Solids 2022, 586, 121563. [Google Scholar] [CrossRef]
- Khan, M.T.; Shkir, M.; Yahia, I.S.; Almohammedi, A.; AlFaify, S. An impact of Cr-doping on physical properties of PbI2 thin films facilely deposited by spin coating technique. Superlattices Microstruct. 2020, 138, 106370. [Google Scholar] [CrossRef]
- Alsulami, A.; Al-Zahrani, H.Y.S. Preparation and characterization of innovative VSbO4 thin films via nebulized spray pyrolysis technique. Opt. Quantum Electron. 2023, 55, 1191. [Google Scholar] [CrossRef]
- Alagarasan, D.; Hegde, S.S.; Kumar, A.; Shanmugavelu, B.; Murahari, P.; Ganesan, R.; Shetty, H.D.; Naik, R.; Ubaidullah, M.; Gupta, M. Influence of La3+ doping on nebulizer spray pyrolysed In2S3 thin film for enhanced photodetector performance. J. Photochem. Photobiol. A Chem. 2023, 444, 114941. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Sharma, I. Physicochemical, optical, and dielectric studies of physically vapor deposited amorphous thin Cu25−x (ZnGe)25−xSe50+2x films. J. Non. Cryst. Solids 2024, 630, 122887. [Google Scholar] [CrossRef]
- Berra, S.; Mahroug, A.; Hamrit, S.; Ahmad, M.; Zoukel, A.; Berri, S.; Selmi, N. Experimental and DFT study of structural and optical properties of Ni-doped ZnO nanofiber thin films for optoelectronic applications. Opt. Mater. 2022, 134, 113188. [Google Scholar] [CrossRef]
- Ahmoum, H.; Chelvanathan, P.; Su’ait, M.S.; Boughrara, M.; Li, G.; Al-Waeli, A.H.A.; Sopian, K.; Kerouad, M.; Amin, N. Impact of preheating environment on microstructural and optoelectronic properties of Cu2ZnSnS4 (CZTS) thin films deposited by spin-coating. Superlattices Microstruct. 2020, 140, 106452. [Google Scholar] [CrossRef]
- Alsulami, A.; Alsalme, A. Synthesis, Structural, Optical and Optoelectrical Properties of the Chemically Deposited Cu2BaGeS4 Thin Films. ECS J. Solid State Sci. Technol. 2023, 12, 123006. [Google Scholar] [CrossRef]
- Hossain, M.F.; Shah, M.A.H.; Islam, M.A.; Hossain, M.S. Transparent conducting SnO2 thin films synthesized by nebulized spray pyrolysis technique: Impact of Sb doping on the different physical properties. Mater. Sci. Semicond. Process. 2021, 121, 105346. [Google Scholar] [CrossRef]
- Hassanien, A.S.; El Radaf, I.M. Effectiveness of Sn-addition on optical properties and physicochemical parameters of SnxSb2−xSe3 thin films. Mater. Chem. Phys. 2023, 303, 127827. [Google Scholar] [CrossRef]
- Shaaban, E.R.; Hassaan, M.Y.; Moustafa, M.G.; Qasem, A.; Ali, G.A.M. Optical constants, dispersion parameters and non-linearity of different thickness of As40S45Se15 thin films for optoelectronic applications. Optik 2019, 186, 275–287. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Sharma, I.; Aly, K.A. Linear and nonlinear optical studies of thermally evaporated chalcogenide a-Pb-Se-Ge thin films. Phys. B Condens. Matter 2021, 613, 412985. [Google Scholar] [CrossRef]
- Golan, G.; Axelevitch, A.; Gorenstein, B.; Manevych, V. Hot-probe method for evaluation of impurities concentration in semiconductors. Microelectron. J. 2006, 37, 910–915. [Google Scholar] [CrossRef]
- Jubeer, E.M.; Manthrammel, M.A.; Subha, P.A.; Shkir, M.; Biju, K.P.; AlFaify, S.A. Defect engineering for enhanced optical and photocatalytic properties of ZnS nanoparticles synthesized by hydrothermal method. Sci. Rep. 2023, 13, 16820. [Google Scholar] [CrossRef]
| V Content (wt.%) | ||||
|---|---|---|---|---|
| 0.0 | 56.35 | 0.615 | 0.314 | 1.31 |
| 4.0 | 49.67 | 0.697 | 0.405 | 1.98 |
| 8.0 | 44.12 | 0.785 | 0.513 | 2.88 |
| 12 | 40.71 | 0.851 | 0.603 | 3.75 |
| V Content (wt.%) | Eo (eV) | Ed (eV) | no | εs | f | ||
|---|---|---|---|---|---|---|---|
| 0.0 | 0.27 | 3.74 | 3.69 | 9.48 | 1.89 | 3.56 | 34.98 |
| 4.0 | 0.29 | 3.49 | 3.25 | 10.56 | 2.01 | 4.06 | 36.43 |
| 8.0 | 0.31 | 3.35 | 2.91 | 11.34 | 2.18 | 4.45 | 37.31 |
| 12 | 0.34 | 3.15 | 2.17 | 12.76 | 2.23 | 5.01 | 40.57 |
| V Content (wt.%) | Hz | |||||
|---|---|---|---|---|---|---|
| 0.0 | 1.49 | 4.83 | 13.93 | 3.45 | 4.34 | 0.18 |
| 4.0 | 1.71 | 5.04 | 12.24 | 5.06 | 4.07 | 0.31 |
| 8.0 | 1.84 | 5.46 | 9.32 | 6.35 | 3.97 | 0.44 |
| 12 | 2.15 | 5.80 | 8.59 | 7.61 | 3.67 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, H.Y.S.; El Radaf, I.M.; Lahmar, A. A Study on the Impact of Vanadium Doping on the Structural, Optical, and Optoelectrical Properties of ZnS Thin Films for Optoelectronic Applications. Micromachines 2025, 16, 337. https://doi.org/10.3390/mi16030337
Al-Zahrani HYS, El Radaf IM, Lahmar A. A Study on the Impact of Vanadium Doping on the Structural, Optical, and Optoelectrical Properties of ZnS Thin Films for Optoelectronic Applications. Micromachines. 2025; 16(3):337. https://doi.org/10.3390/mi16030337
Chicago/Turabian StyleAl-Zahrani, H. Y. S., I. M. El Radaf, and A. Lahmar. 2025. "A Study on the Impact of Vanadium Doping on the Structural, Optical, and Optoelectrical Properties of ZnS Thin Films for Optoelectronic Applications" Micromachines 16, no. 3: 337. https://doi.org/10.3390/mi16030337
APA StyleAl-Zahrani, H. Y. S., El Radaf, I. M., & Lahmar, A. (2025). A Study on the Impact of Vanadium Doping on the Structural, Optical, and Optoelectrical Properties of ZnS Thin Films for Optoelectronic Applications. Micromachines, 16(3), 337. https://doi.org/10.3390/mi16030337







