An Electrochemical Immunosensor for Sensitive Detection of Exosomes Based on Au/MXenes and AuPtPdCu
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of Ti3C2
2.3. Synthesis of Au/Ti3C2
2.4. Synthesis of AuPtPdCu
2.5. Preparation of AuPtPdCu-Apt Nanoprobe
2.6. Exosome Extraction
2.7. Calculation of Analytical Parameters
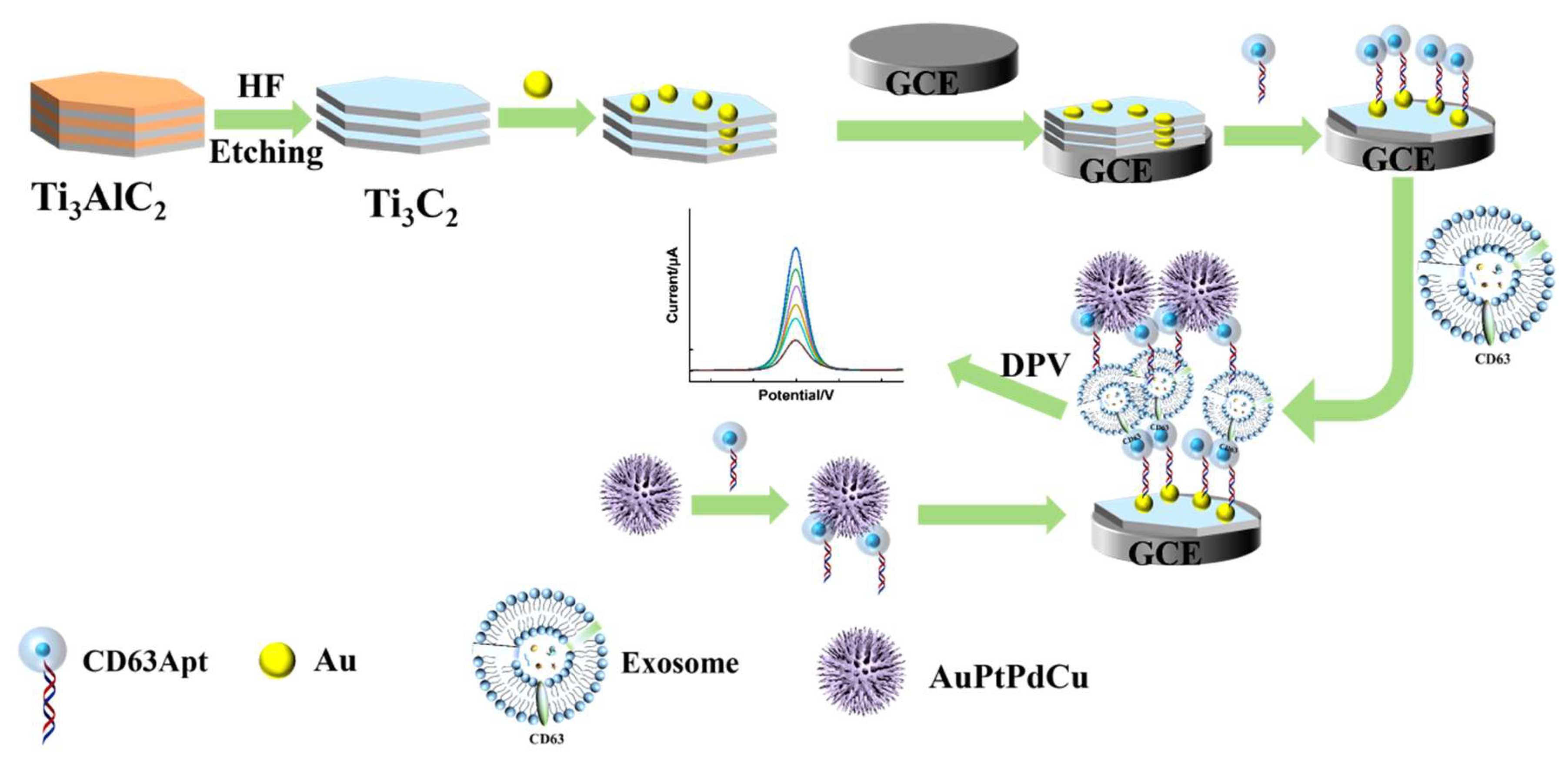
2.8. Construction of the Electrochemical Immunosensor
3. Results and Discussion
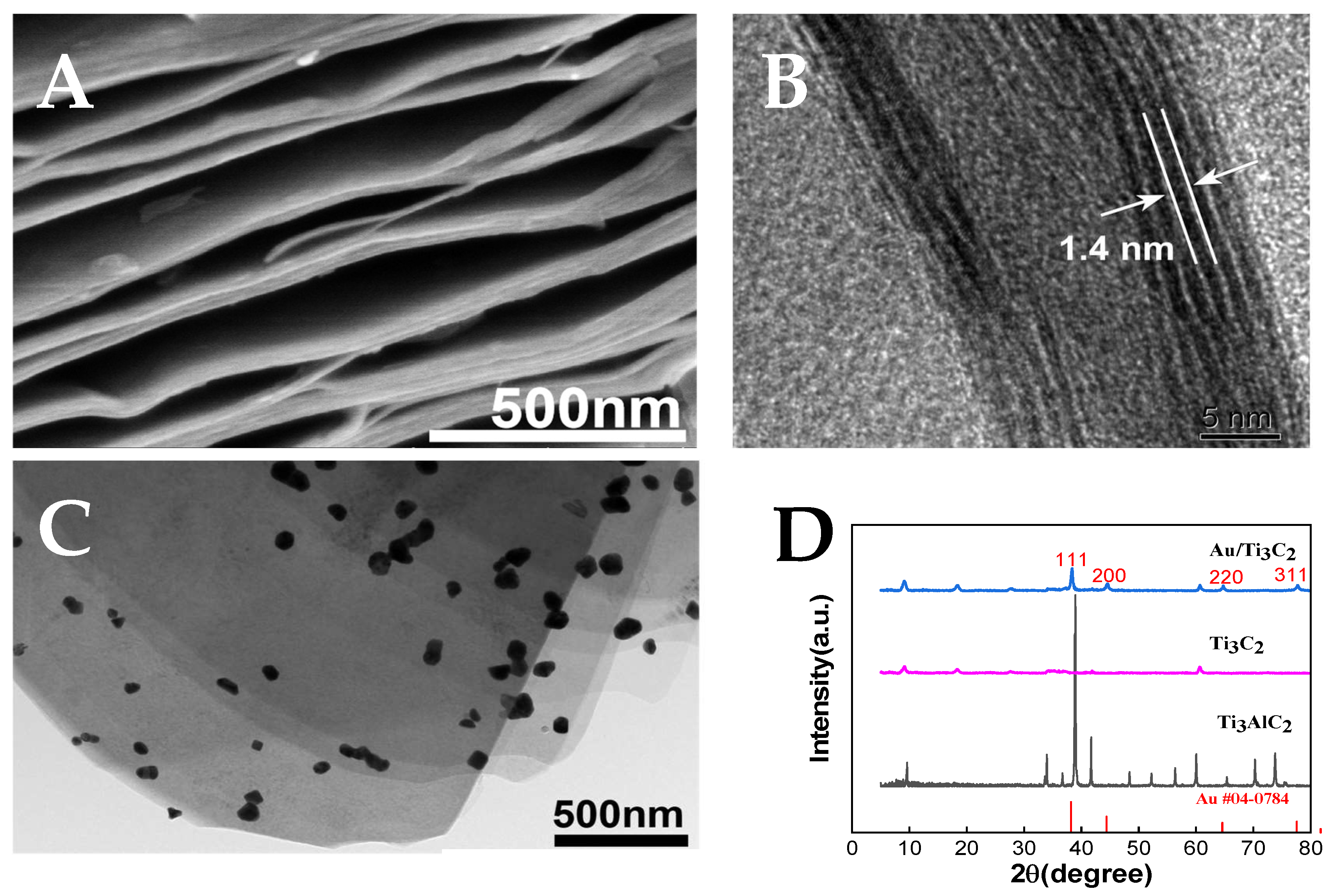
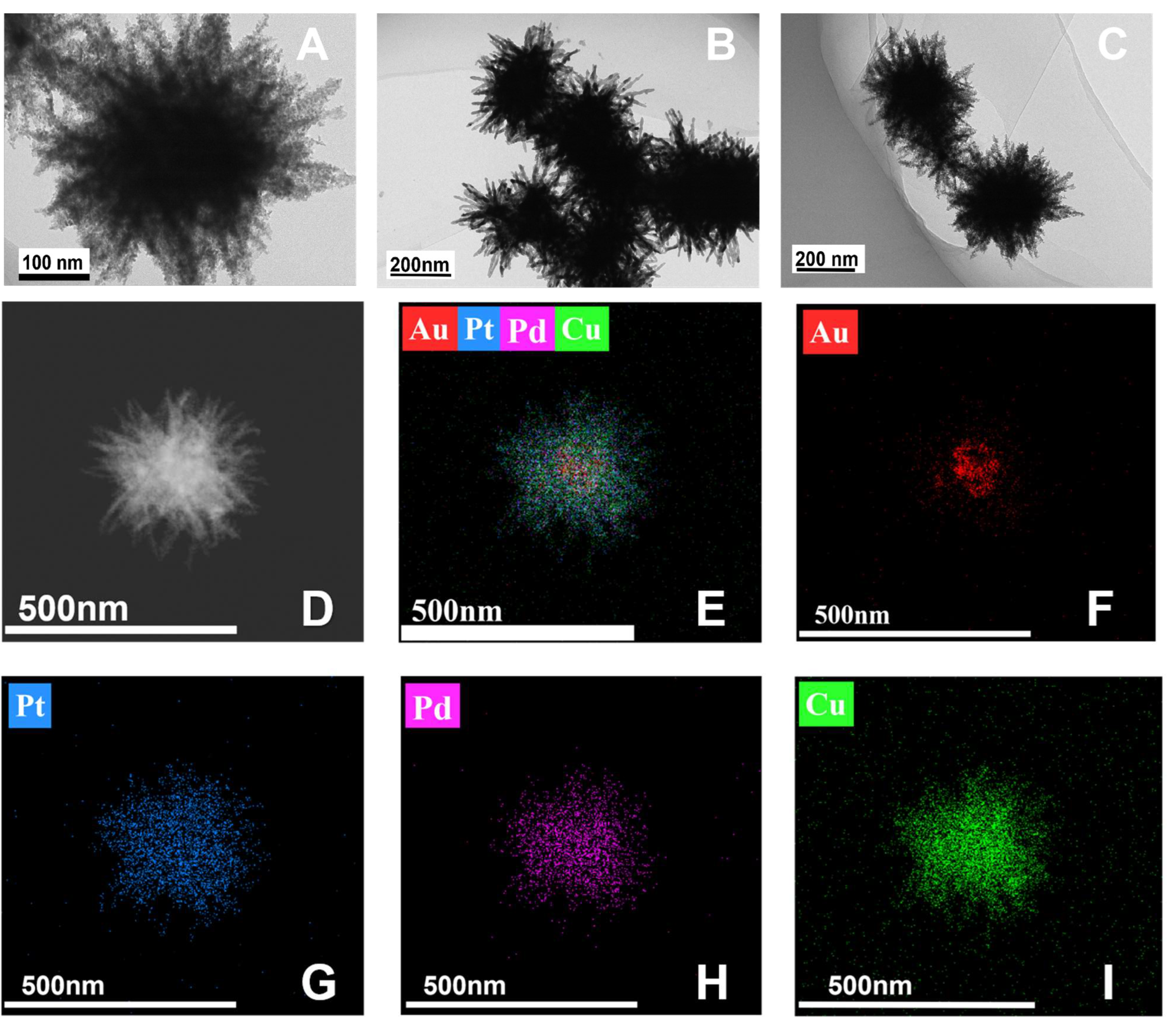
3.1. Characterization of Ti3C2 and Au/Ti3C2
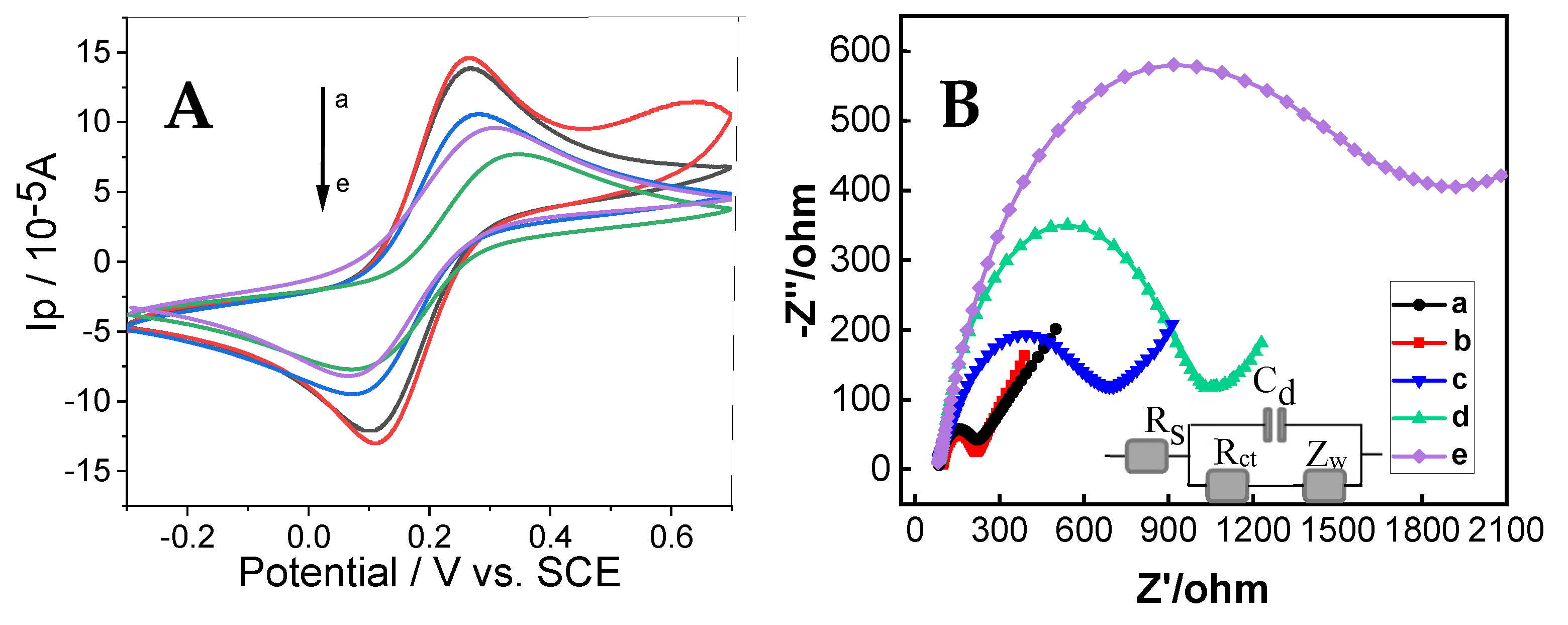
3.2. Electrochemical Properties of the Immunosensor
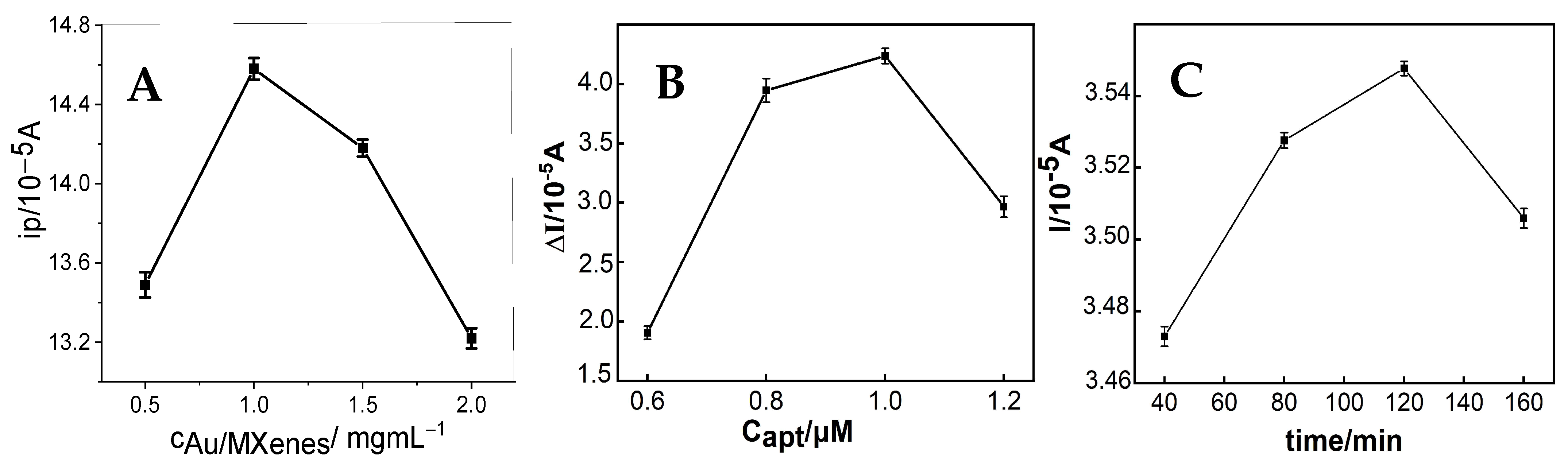
3.3. Optimization of the Detection Conditions
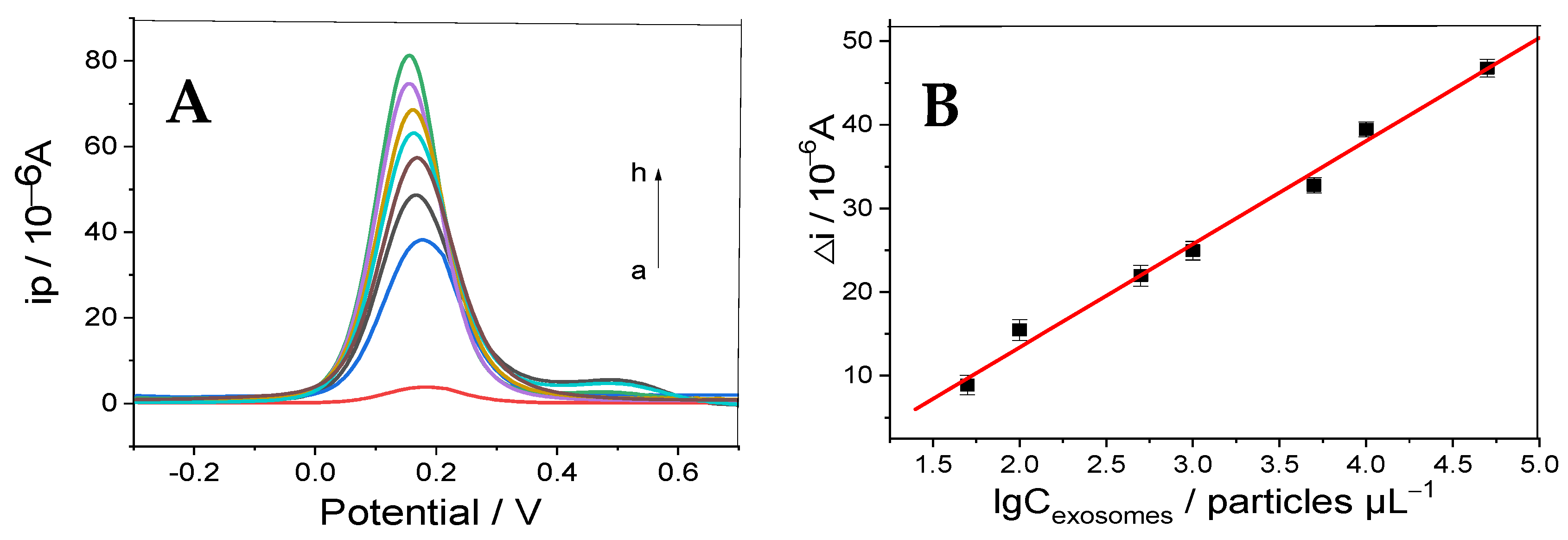
3.4. Quantitative Determination of Exosomes
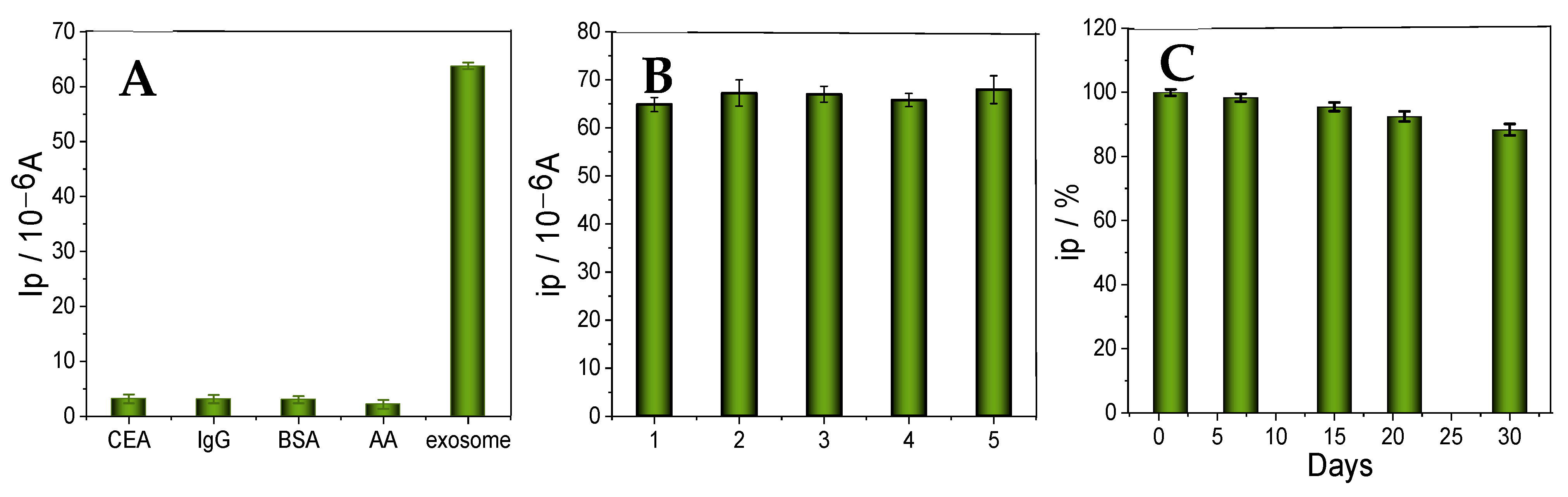
3.5. Specificity, Reproducibility, and Stability of the Immunosensors
3.6. Detection of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Xu, Y.; Wang, Z.; Liao, K.; Zhang, Y.; Sun, Y. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of H2O2 in cancer cells. Talanta 2022, 249, 123612. [Google Scholar] [CrossRef]
- Erridge, S.; Lyratzopoulos, G.; Renzi, C.; Millar, A.; Lee, R. Rapid Diagnostic Centres and early cancer diagnosis. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2021, 71, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. Trac-Trends Anal. Chemistry 2024, 180, 117949. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, W.; Bu, J.; Li, Y.; Li, R.; Nie, R.; Xiao, C.; Ma, K.; Huang, X.; Li, Y. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol. Carcinog. 2019, 58, 674–685. [Google Scholar] [CrossRef]
- Fu, H.; Yang, H.; Zhang, X.; Wang, B.; Mao, J.; Li, X.; Wang, M.; Zhang, B.; Sun, Z.; Qian, H.; et al. Exosomal TRIM3 is a novel marker and therapy target for gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 162. [Google Scholar] [CrossRef]
- Inubushi, S.; Kawaguchi, H.; Mizumoto, S.; Kunihisa, T.; Baba, M.; Kitayama, Y.; Takeuchi, T.; Hoffman, R.M.; Sasaki, R. Oncogenic miRNAs Identified in Tear Exosomes From Metastatic Breast Cancer Patients. Anticancer. Res. 2020, 40, 3091–3096. [Google Scholar] [CrossRef]
- Wang, B.; Mao, J.-H.; Wang, B.-Y.; Wang, L.-X.; Wen, H.-Y.; Xu, L.-J.; Fu, J.-X.; Yang, H. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 2020, 489, 87–99. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.-M.; An, Y.; Geng, X.; Zhao, K.; Qu, L.; Li, Z. Structure-switching aptamer triggering hybridization displacement reaction for label-free detection of exosomes. Talanta 2020, 209, 120510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dai, X.; Zhao, S.; Cui, X.; Gong, T.; Song, Z.; Meng, H.; Zhang, X.; Yu, B. Aptamer-based fluorescent sensors for the detection of cancer biomarkers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Xie, Q.; Chu, Z.; Zhang, F.; Wang, Q. An electrochemical aptasensor for exosomes based on strand displacement amplification and hybridization chain reaction amplification. Sens. Actuators B Chem. 2023, 393, 134273. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yue, S.; Lu, Y.; Yang, C.; Fang, J.; Xu, Z. Sensitive Multicolor Visual Detection of Exosomes via Dual Signal Amplification Strategy of Enzyme-Catalyzed Metallization of Au Nanorods and Hybridization Chain Reaction. ACS Sens. 2019, 4, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, J.; Liu, Y.; Li, L.; Yan, L.; Xia, Y.; Wu, F.; Li, C.; Li, S.; Chen, J. A paper-supported aptasensor based on upconversion luminescence resonance energy transfer for the accessible determination of exosomes. Biosens. Bioelectron. 2018, 102, 582–588. [Google Scholar] [CrossRef]
- Conteduca, D.; Brunetti, G.; Barth, I.; Quinn, S.D.D.; Ciminelli, C.; Krauss, T.F.F. Multiplexed Near-Field Optical Trapping Exploiting Anapole States. ACS Nano 2023, 17, 16695–16702. [Google Scholar] [CrossRef]
- An, Y.; Li, R.; Zhang, F.; He, P. A ratiometric electrochemical sensor for the determination of exosomal glycoproteins. Talanta 2021, 235, 122790. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Yang, M.; Pang, S.W. Highly sensitive detection of exosomes by 3D plasmonic photonic crystal biosensor. Nanoscale 2018, 10, 19927–19936. [Google Scholar] [CrossRef]
- di Toma, A.; Brunetti, G.; Colapietro, P.; Ciminelli, C. High-Resolved Near-Field Sensing by Means of Dielectric Grating With a Box-Like Resonance Shape. IEEE Sens. J. 2024, 24, 6045–6053. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, W.; Kimber, M.; Lu, M.; Dong, L. Rapid Differentiation of Host and Parasitic Exosome Vesicles Using Microfluidic Photonic Crystal Biosensor. ACS Sens. 2018, 3, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xu, P.; Wang, M.; Wang, G.; Li, G.; Jing, A. Electrochemical Micro-Immunosensor of Cubic AuPt Dendritic Nanocrystals/Ti3C2-MXenes for Exosomes Detection. Micromachines 2023, 14, 138. [Google Scholar] [CrossRef]
- Cancelliere, R.; Mele, P.; Bartolucci, L.; Cordiner, S.; Freitas, W.d.S.; Mazzuca, C.; Mecheri, B.; Micheli, L.; Mulone, V.; Paialunga, E.; et al. Mutual interaction of pyrolysis operating conditions and surface morphology for the electrochemical performance of biochar-modified screen-printed electrodes. J. Environ. Chem. Eng. 2025, 13, 115477. [Google Scholar] [CrossRef]
- Saraswathi, K.A.; Reddy, M.S.B.; Jayarambabu, N.; Rao, K.V.; Aich, S.; Rao, T.V. Non-Invasive Disposable 2D Ti3C2Tx based Enzyme Free Electrochemical Sweat Glucose Biosensor. Microchem. J. 2024, 205, 111302. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Zhuang, T.; Liu, J.; Sojic, N.; Wang, Z. Sensitive electrochemiluminescence biosensing of polynucleotide kinase using the versatility of two-dimensional Ti3C2TX MXene nanomaterials. Anal. Chim. Acta 2022, 1191, 339346. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124, 184–190. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Lu, L.; Yang, X.; Xia, J.; Zhang, F.; Wang, Z. Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin1. Anal. Chim. Acta 2020, 1094, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, S.; Zhang, H.; Yang, W.; Yi, Z.; Yi, Y.; Wang, J.; Ahmad, S.; Raza, R. Ultrathin broadband terahertz metamaterial based on single-layer nested patterned graphene. Phys. Lett. A 2025, 534, 130262. [Google Scholar] [CrossRef]
- Navitski, I.; Ramanaviciute, A.; Ramanavicius, S.; Pogorielov, M.; Ramanavicius, A. MXene-Based Chemo-Sensors and Other Sensing Devices. Nanomaterials 2024, 14, 447. [Google Scholar] [CrossRef]
- Ren, M.; Li, J.; Zhao, Y.; Zhai, W.; Zhou, K.; Yu, Y.; Wang, S.; Dai, K.; Liu, C.; Shen, C. Highly strain-sensitive and stretchable multilayer conductive composite based on aligned thermoplastic polyurethane fibrous mat for human motion monitoring. Compos. Commun. 2024, 46, 101840. [Google Scholar] [CrossRef]
- Xue, R.; Huang, Y.; Zhang, J.; Wang, T.; Wu, Y.; Shi, Q. High sensitivity flexible piezoelectric nanogenerator based on multi-layer piezoelectric composite fiber for human motion energy harvesting. J. Appl. Polym. Sci. 2024, 141, e56179. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wang, N.; Li, C. Highly stretchable and transparent triboelectric nanogenerator based on multilayer structured stable electrode for self-powered wearable sensor. Nano Energy 2020, 78, 105385. [Google Scholar] [CrossRef]
- Iravani, S. Role of MXenes in advancing soft robotics. Soft Matter 2023, 19, 6196–6212. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, Y.-B.; Chen, K.; Wang, Q.; Li, Y.; Xing, X.; Ru, J.; Meng, L.-G.; Shu, J.; Shpigel, N.; et al. Super-Stretchable and High-Energy Micro-Pseudocapacitors Based on MXene Embedded Ag Nanoparticles. Adv. Mater. 2024, 36, 2401271. [Google Scholar] [CrossRef]
- Ghani, A.A.; Kim, B.; Nawaz, M.; Devarayapalli, K.C.; Lim, Y.; Kim, G.; Lee, D.S. Adsorption and electrochemical regeneration of 2D magnetic MXene nanosheets loaded with tetracycline. Chem. Eng. J. 2023, 467, 143473. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Xu, W.; Zeng, R.; Weng, J.; Sun, L. A label-free colorimetric biosensor utilizing natural material for highly sensitive exosome detection. Talanta 2024, 275, 126182. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Feng, W.; Wang, M.; Zhang, L.; Liang, G.; Jing, A. New Ultrasensitive Sandwich-Type Immunoassay of Dendritic Tri-Fan Blade-like PdAuCu Nanoparticles/Amine-Functionalized Graphene Oxide for Label-Free Detection of Carcinoembryonic Antigen. Micromachines 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhuang, L.; Chang, Z.; Ge, M.; Mei, Q.; Yang, L.; Dong, W.F. Hierarchical Au nanoarrays functionalized 2D Ti2CTX MXene membranes for the detection of exosomes isolated from human lung carcinoma cells. Biosens. Bioelectron. 2022, 216, 114647. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, T.; Xiong, Y.; Qiu, L.; Yu, H.; Liang, F. Highly efficient blackberry-like trimetallic PdAuCu nanoparticles with optimized Pd content for ethanol electrooxidation. Nanoscale 2021, 13, 9960–9970. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, J.; Yu, R.; Chen, S.; Deng, X.; Chen, A.; Qiu, J. PdPtRu trimetallic nanozymes and application to electrochemical immunosensor for sensitive SARS-COV-2 antigen detection. Talanta 2023, 260, 124604. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.-D.; Wang, A.-J.; Xue, Y.; Wu, L.; Feng, J.-J. A new label-free electrochemical immunosensor based on dendritic core-shell AuPd@Au nanocrystals for highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, S.; Yi, Z.; Zhang, H.; Song, Q.; Hao, Z.; Sun, T.; Wu, P.; Zeng, Q.; Raza, R. Advanced optical reinforcement materials based on three-dimensional four-way weaving structure and metasurface technology. Appl. Phys. Lett. 2025, 126, 033503. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.-J.; Yuan, P.-X.; Luo, X.; Xue, Y.; Feng, J.-J. Three dimensional sea-urchin-like PdAuCu nanocrystals/ferrocene-grafted-polylysine as an efficient probe to amplify the electrochemical signals for ultrasensitive immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 132, 294–301. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TX MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, G.; Ji, Q.; Zhang, Y.; Li, J. Synergistic Electrocatalytic Nitrogen Reduction Enabled by Confinement of Nanosized Au Particles onto a Two-Dimensional Ti3C2 Substrate. ACS Appl. Mater. Interfaces 2019, 11, 25758–25765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, L.-P.; Feng, J.-J.; Yuan, P.-X.; Luo, X.; Wang, A.-J. Simple one-pot aqueous synthesis of 3D superstructured PtCoCuPd alloyed tripods with hierarchical branches for ultrasensitive immunoassay of cardiac troponin I. Biosens. Bioelectron. 2019, 145, 111638. [Google Scholar] [CrossRef]
- Jing, A.; Zhang, C.; Liang, G.; Feng, W.; Tian, Z.; Jing, C. Hyaluronate-Functionalized Graphene for Label-Free Electrochemical Cytosensing. Micromachines 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Tinno, A.D.; Cataldo, A.; Bellucci, S.; Kumbhat, S.; Micheli, L. Nafion-based Label-free immunosensor as a reliable warning system: The case of AFB1 detection in cattle feed. Microchem. J. 2023, 191, 108868. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. 3D Porous Oxidation-Resistant MXene/Graphene Architectures Induced by In Situ Zinc Template toward High-Performance Supercapacitors. Adv. Funct. Mater. 2021, 31, 2101087. [Google Scholar] [CrossRef]
- Liu, J.; Tang, D. Dopamine-loaded Liposomes-amplified Electrochemical Immunoassay based on MXene (Ti3C2)-AuNPs. Electroanalysis 2022, 34, 1329–1337. [Google Scholar] [CrossRef]
- Feng, J.-J.; Lin, X.-X.; Chen, S.-S.; Huang, H.; Wang, A.-J. Thymine-directed synthesis of highly branched gold-palladium alloy nanobrambles as a highly active surface-enhanced Raman scattering substrate. Sens. Actuators B Chem. 2017, 247, 490–497. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide viaL-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-effective and disposable label-free voltammetric immunosensor for sensitive detection of interleukin-6. Biosens. Bioelectron. 2022, 213, 114467. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. In Situ Formation of Gold Nanoparticles Decorated Ti3C2 MXenes Nanoprobe for Highly Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins. Anal. Chem. 2020, 92, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yue, X.; Li, Y.; Wu, F.; Meng, M.; Yin, Y.; Xi, R. A novel electrochemical aptasensor for exosomes determination and release based on specific host-guest interactions between cucurbit 7 uril and ferrocene. Talanta 2021, 232, 122451. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Huang, Z.; Li, T.; Deng, A.; Kong, J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 2018, 184, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhu, L.; Hu, J.; Pang, D.-W.; Zhang, Z.-L. Simple and rapid extracellular vesicles quantification via membrane biotinylation strategy coupled with fluorescent nanospheres-based lateral flow assay. Talanta 2019, 200, 408–414. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Q.; Zhao, C.; Feng, Q. Stimuli-responsive DNA microcapsules for homogeneous electrochemiluminescence sensing of tumor exosomes. Sens. Actuators B Chem. 2021, 329, 129136. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Chen, J.; Chen, X.; Yang, W. Ultrasensitive electrochemiluminescence biosensor for the detection of tumor exosomes based on peptide recognition and luminol-AuNPs@g-C3N4 nanoprobe signal amplification. Talanta 2021, 221, 121379. [Google Scholar] [CrossRef]
- Ning, C.-F.; Wang, L.; Tian, Y.-F.; Yin, B.-C.; Ye, B.-C. Multiple and sensitive SERS detection of cancer-related exosomes based on gold-silver bimetallic nanotrepangs. Analyst 2020, 145, 2795–2804. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, J.; Bu, J.; Li, R.; Li, X.; Fan, S.; Ling, K.; Jiang, H. Mn2+-modified black phosphorus nanosensor for detection of exosomal microRNAs and exosomes. Microchim. Acta 2023, 190, 295. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Chen, S.; Chen, Y.; Cheng, L.; Dai, H.; Gao, L. Electrochemiluminescence enhanced by molecular engineering linear π-conjugated polymer: An ingenious ECL emitter for the construction of exosome sensing platform. Talanta 2024, 277, 126405. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Maeng, S.-W.; Mok, J.W.; Choi, M.; Cha, H.J.; Joo, C.-K.; Hahn, S.K. Hydrogel Microneedles Extracting Exosomes for Early Detection of Colorectal Cancer. Biomacromolecules 2023, 24, 1445–1452. [Google Scholar] [CrossRef]
| Fabrication Step | CV | EIS | ||
|---|---|---|---|---|
| ΔE/V | K0/cm s−1 | Rct/KΏ | K0/cm s−1 | |
| Bare | 0.18 ± 0.02 | — | 0.15 ± 0.2 | — |
| Au/Ti3C2 | 0.17 ± 0.02 | 2.7 (±0.1) × 10−2 | 0.11 ± 0.2 | 3.13 ± (0.2) × 10−3 |
| Apt/Au/Ti3C2 | 0.20 ± 0.02 | — | 0.5 ± 0.4 | — |
| Exosome/Apt/Au/Ti3C2 | 0.22 ± 0.02 | — | 1.1 ± 0.5 | — |
| Apt/AuPtPdCu/exosome/Apt/Au/Ti3C2 | 0.24 ± 0.01 | — | 1.8 ± 0.2 | — |
| Method | Matrix | Detection Range (Particles μL −1) | Detection Limit (Particles μL−1) | Refs. |
|---|---|---|---|---|
| Fluorescence | Graphene oxide–DNA aptamer | 3.0 × 104 to 6.0 × 105 | 2.1 × 104 | [56] |
| Fluorescence | Biotin-functionalized phosphatidylethanolamine | 4.0 × 103 to 2.0 × 105 | 2.0 × 103 | [57] |
| Electrochemiluminescence | CdS quantum dots in the inner pores of DNA microcapsules | 2.0 × 102 to 7.5 × 104 | 60 | [58] |
| Electrochemiluminescence | Lum-AuNPs@g-C3N4 | 102 to 107 | 39 | [59] |
| Electrochemistry | Cucurbit [7] uril modified gold and ferrocene | 5.0 × 102 to 5.0 × 103 | 4.82 × 102 | [55] |
| SERs | Gold–silver–silver core–shell–shell nanotrepangs | 1 to 1.0 × 107 | 35 | [60] |
| Fluorescence | Black phosphorus (BP)@Mn2+/DNA | 1.0 × 105 to 1.0 × 10⁶ | 2.5 × 104 | [61] |
| Electrochemiluminescence | Zirconium-based conjugated polymers and polyethyleneimine | 102 to 108 | 33 | [62] |
| Hydrogel microneedles extraction | Hydrogel microneedles | 102 to 106 | 100 | [63] |
| Electrochemistry | Au/MXenes and AuPtPdCu | 5.0 × 101 to 5.0 × 104 | 19 | this work |
| Sample Number | Added (LgC particles μL−1) | Found (LgC particles μL−1) | Recovery (%) |
|---|---|---|---|
| 1 | 1.69 | 1.698 | 100.4 |
| 2 | 2 | 1.895 | 94.7 |
| 3 | 2.69 | 2.624 | 97.5 |
| 4 | 3 | 3.047 | 101.5 |
| 5 | 3.39 | 3.510 | 103.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yang, R.; Zhu, X.; Shi, J.; Wang, S.; Jing, A. An Electrochemical Immunosensor for Sensitive Detection of Exosomes Based on Au/MXenes and AuPtPdCu. Micromachines 2025, 16, 280. https://doi.org/10.3390/mi16030280
Gao J, Yang R, Zhu X, Shi J, Wang S, Jing A. An Electrochemical Immunosensor for Sensitive Detection of Exosomes Based on Au/MXenes and AuPtPdCu. Micromachines. 2025; 16(3):280. https://doi.org/10.3390/mi16030280
Chicago/Turabian StyleGao, Jie, Rong Yang, Xiaorui Zhu, Jiling Shi, Sufei Wang, and Aihua Jing. 2025. "An Electrochemical Immunosensor for Sensitive Detection of Exosomes Based on Au/MXenes and AuPtPdCu" Micromachines 16, no. 3: 280. https://doi.org/10.3390/mi16030280
APA StyleGao, J., Yang, R., Zhu, X., Shi, J., Wang, S., & Jing, A. (2025). An Electrochemical Immunosensor for Sensitive Detection of Exosomes Based on Au/MXenes and AuPtPdCu. Micromachines, 16(3), 280. https://doi.org/10.3390/mi16030280





