Study on the Electro-Fenton Chemomechanical Removal Behavior in Single-Crystal GaN Pin–Disk Friction Wear Experiments
Abstract
1. Introduction
2. Experimental Materials and Principles
2.1. Experimental Materials
2.2. Experimental Principles and Characterization Methods
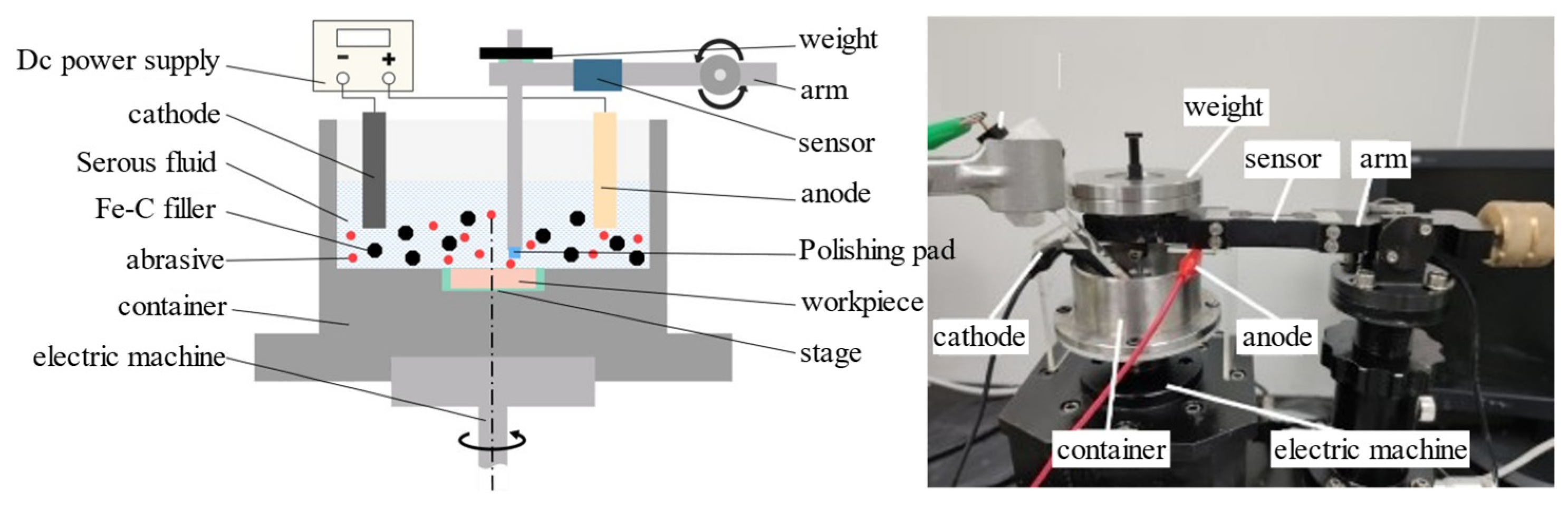
2.3. Experimental Method and Design
- (1)
- Preparation of the electro-Fenton solution: A specified amount of anhydrous Na2SO4 electrolyte was dissolved in deionized water, followed by the addition of citric acid to adjust the pH to 3. The solution was mixed uniformly under ultrasonic agitation before incorporating a designated quantity of Fe-C Fenton catalyst for subsequent use.
- (2)
- Setup and parameter configuration of the apparatus: The pre-treated single-crystal GaN was securely mounted in a container, which was then placed on the load-bearing platform of the apparatus. The support arm was adjusted to a horizontal position using the elevation stage, while the experimental friction radius was set using the forward-and-backward movement knob, which was subsequently tightened. Electrodes were installed, with modified amino-MWCNT graphite felt serving as the cathode and foam nickel as the anode. Relevant experimental parameters were configured, including dynamic zeroing before equipment activation. Files were saved after stopping rotation before disassembling the workpiece.
- (3)
- Cleaning procedure for the workpiece: The disassembled workpiece underwent ultrasonic cleaning in isopropyl alcohol for one minute and was marked for subsequent analysis.
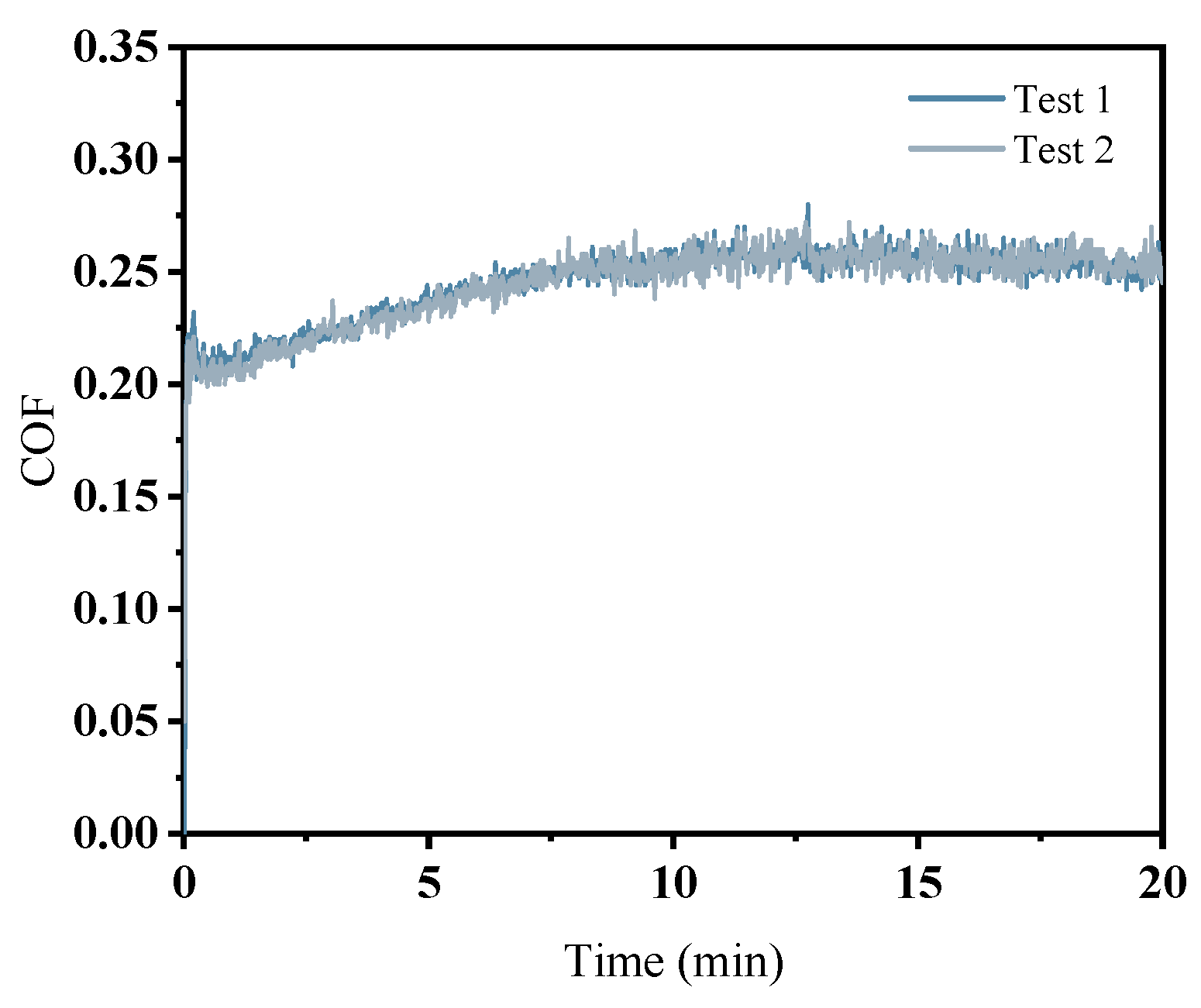
2.4. Experimental Feasibility Verification
3. Experimental Results and Discussion
3.1. Effect of Abrasive Type on the Removal Behavior of Single-Crystal GaN Materials
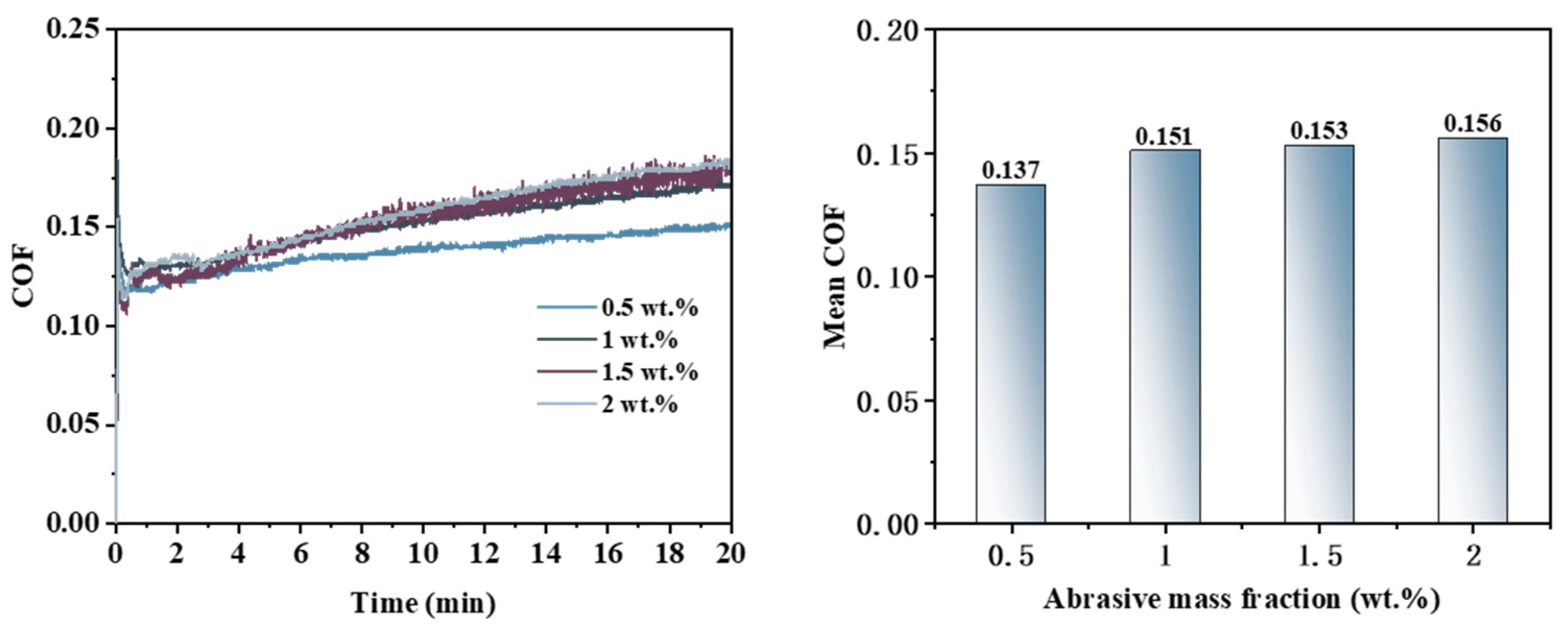
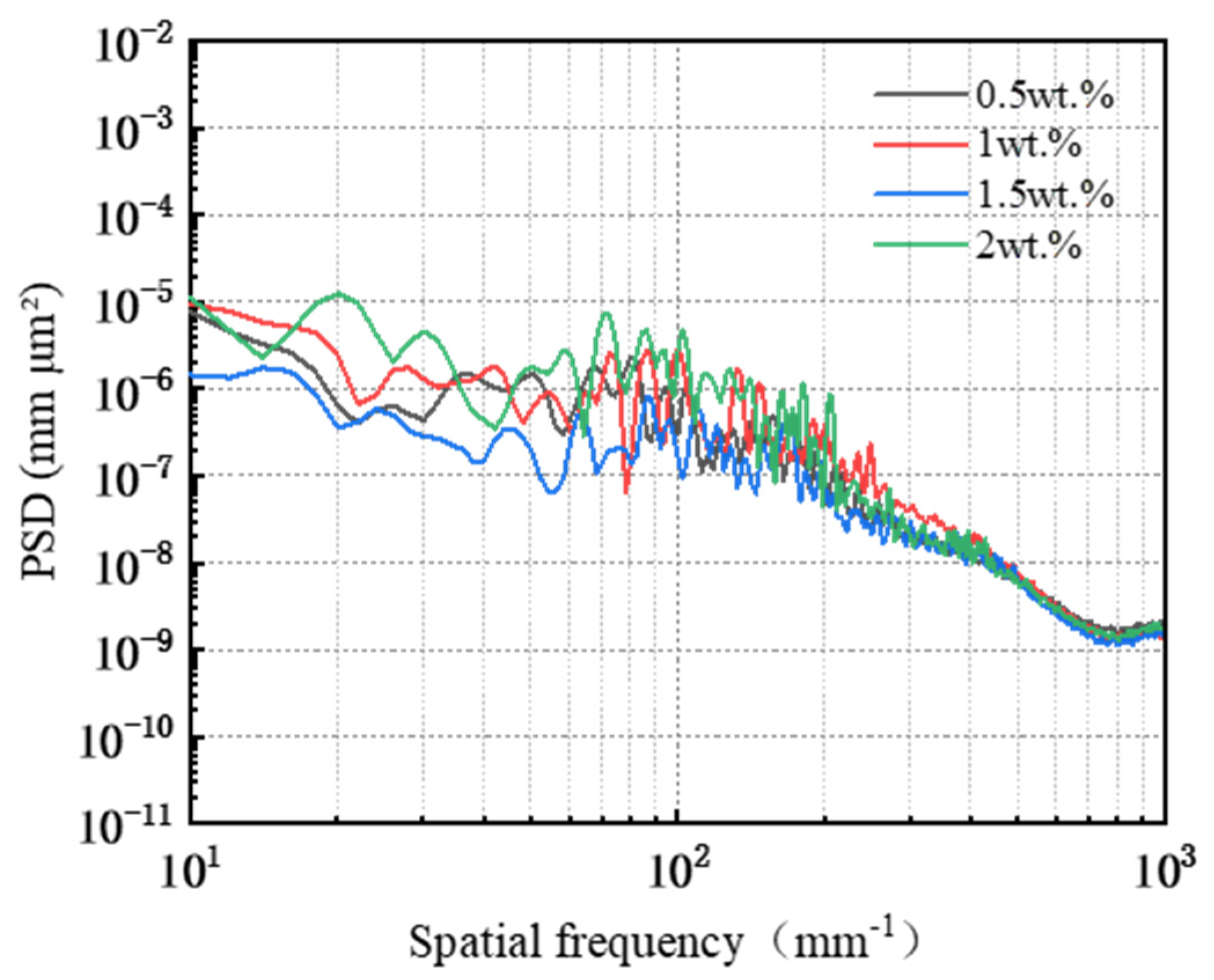
3.2. Effect of Abrasive Concentration on the Removal Behavior of Single-Crystal GaN Materials
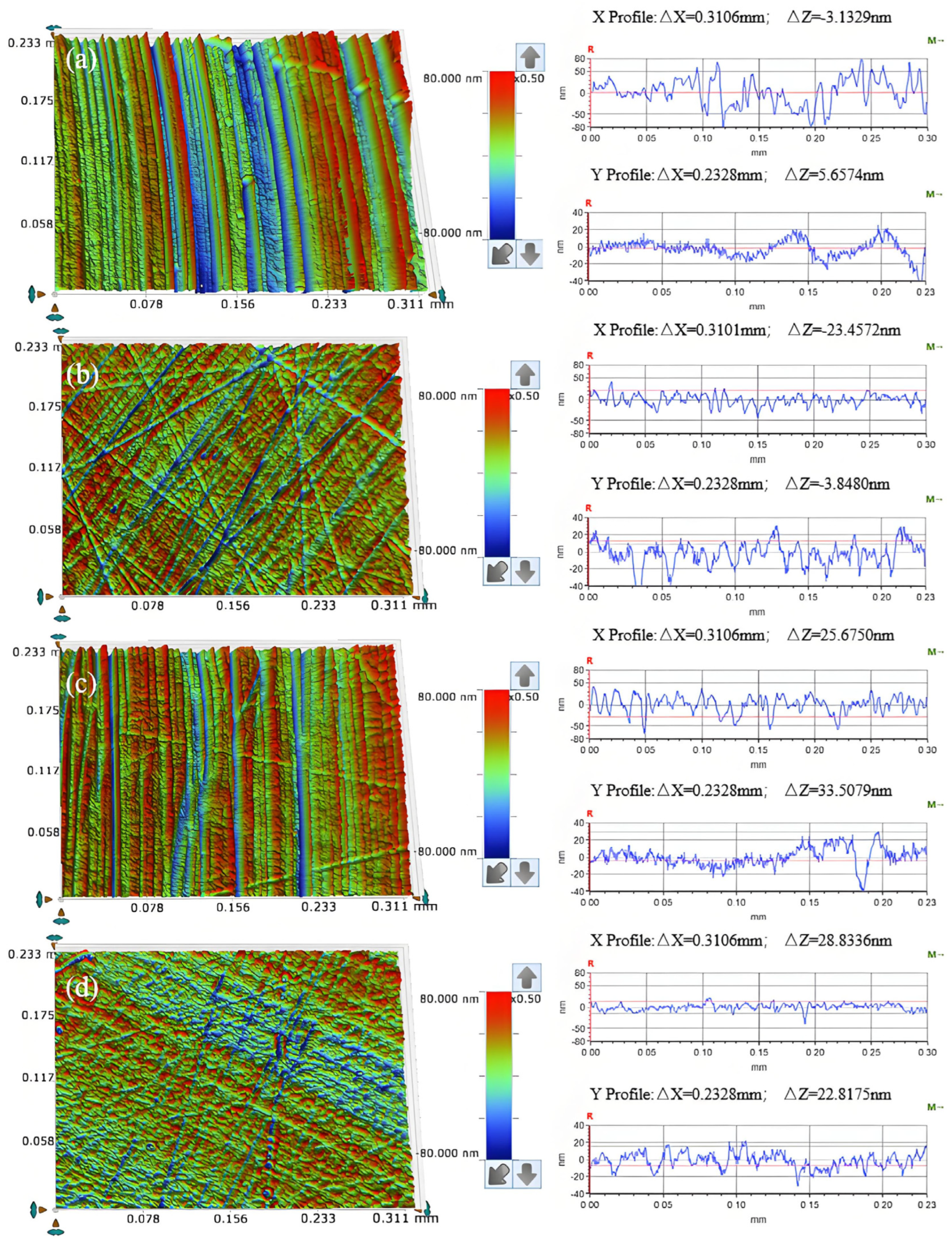
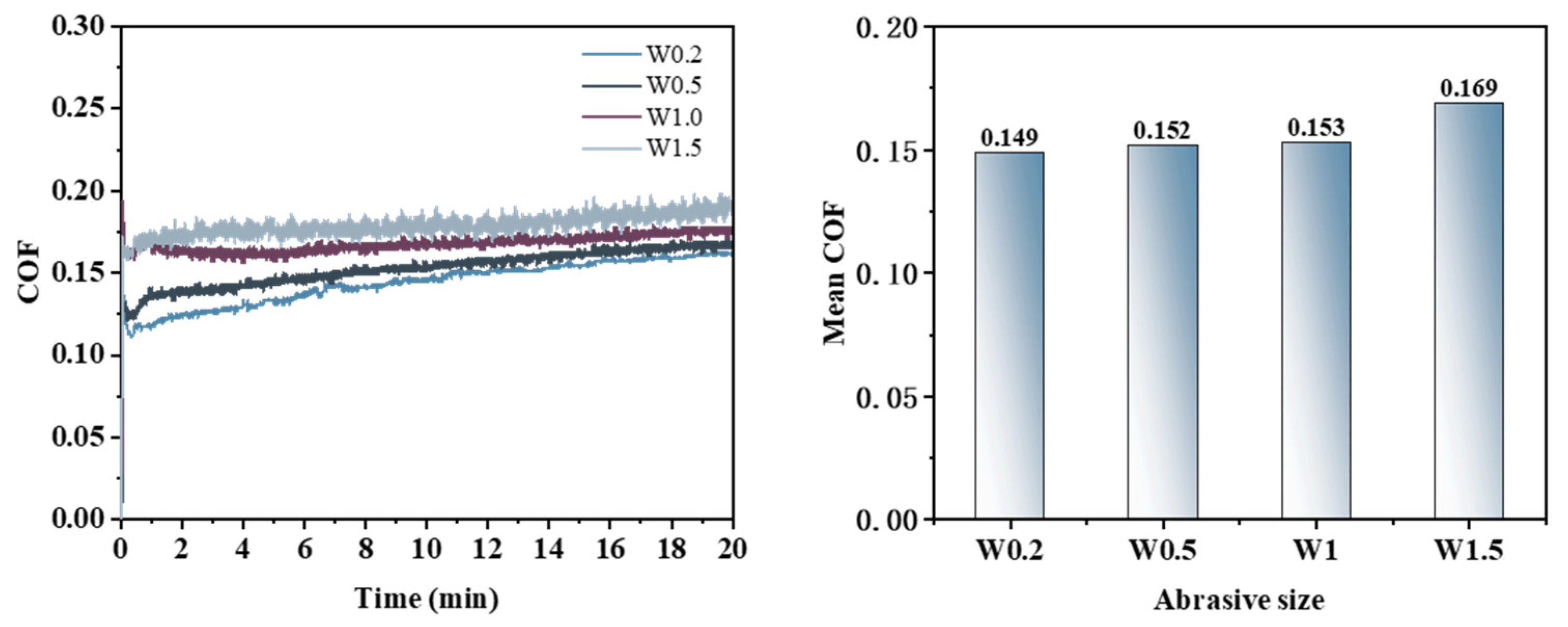
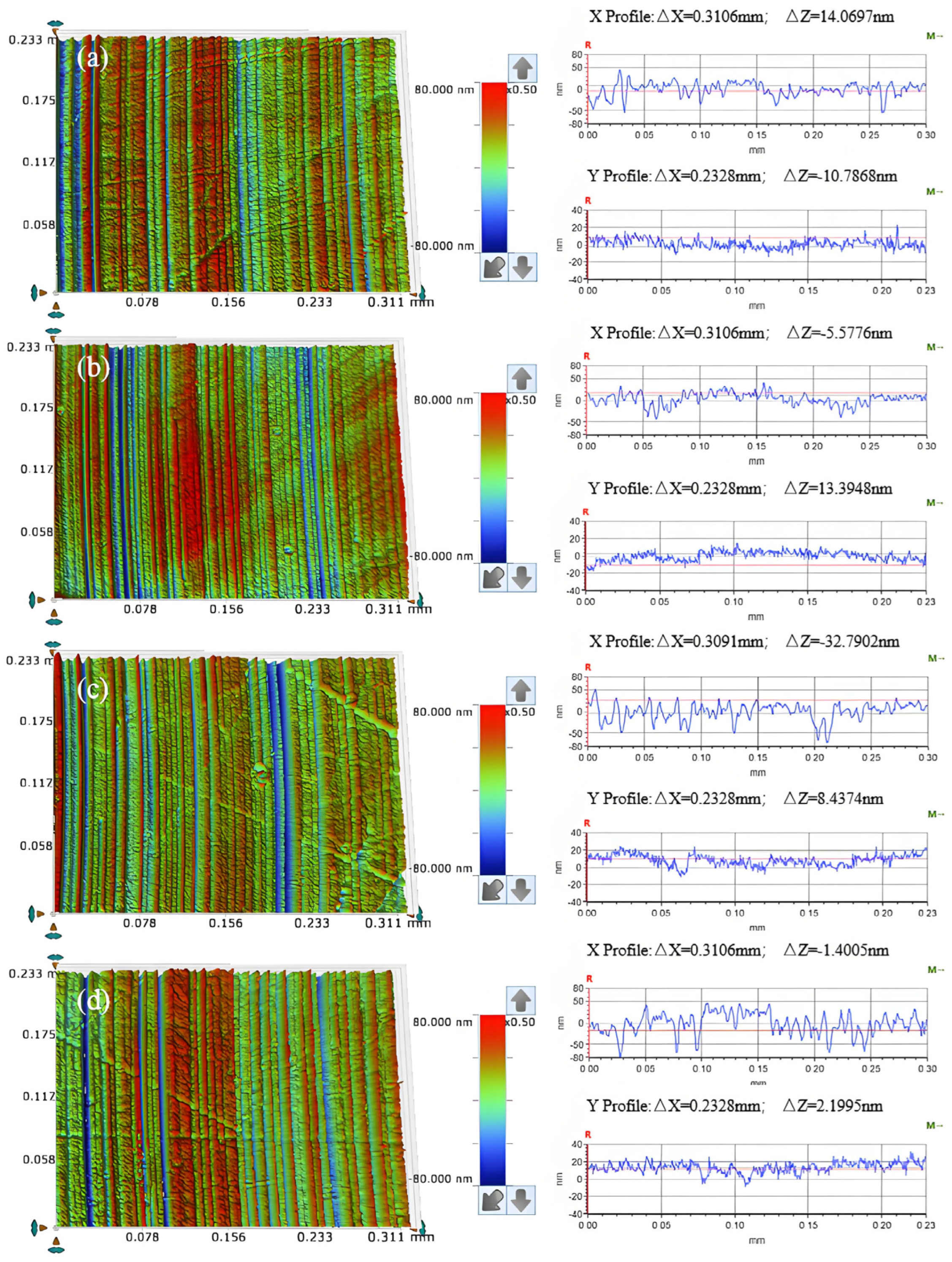
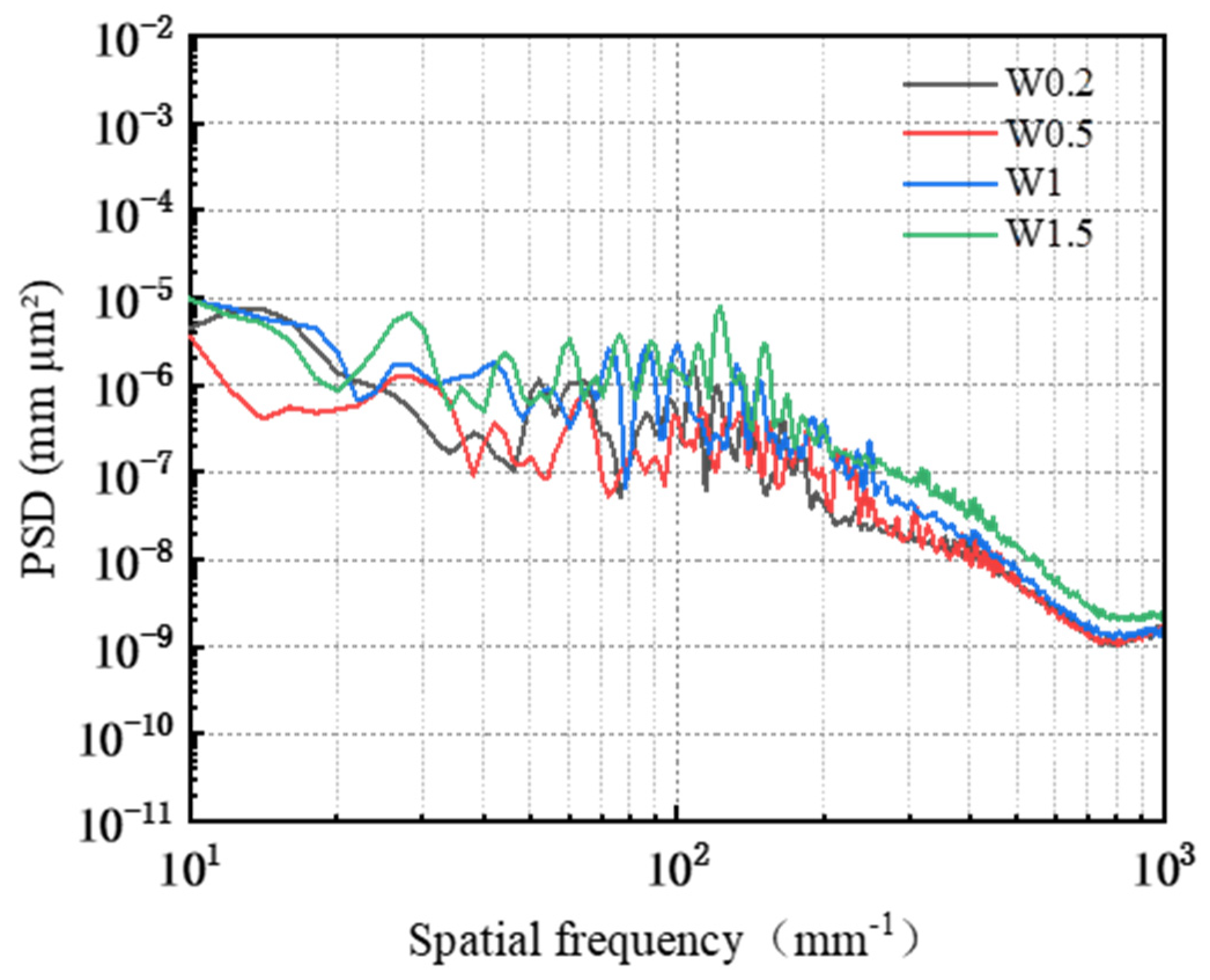
3.3. Effect of Abrasive Grain Size on the Removal Behavior of Single-Crystal GaN Materials
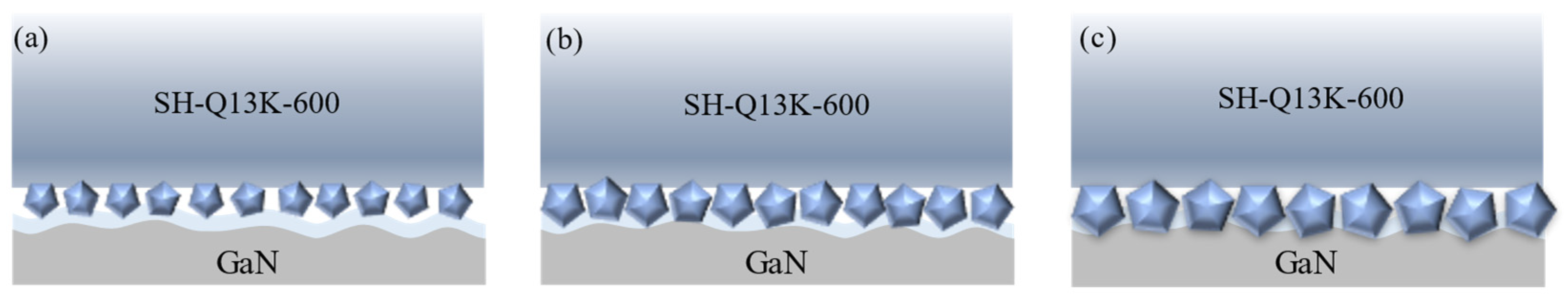
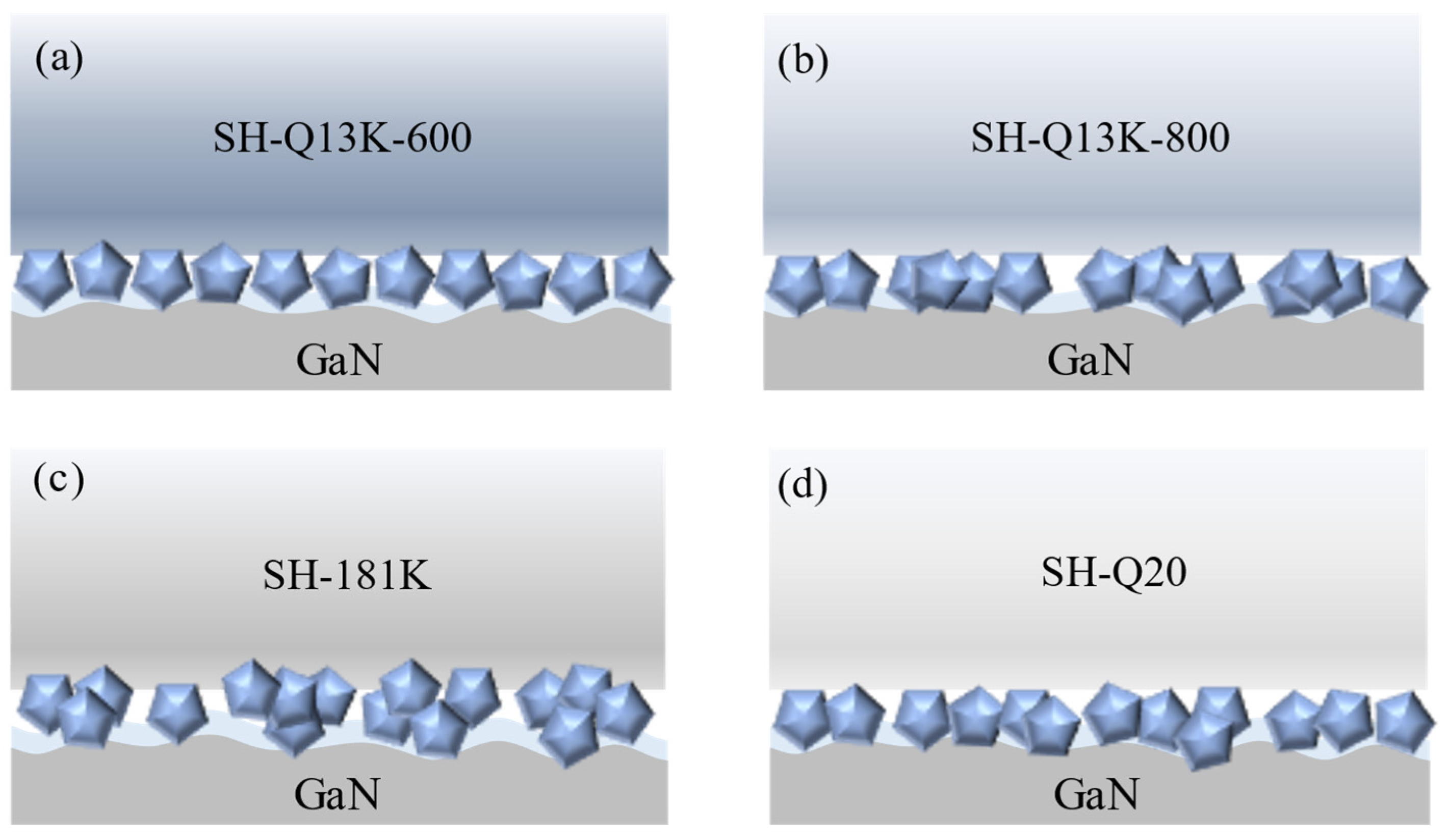
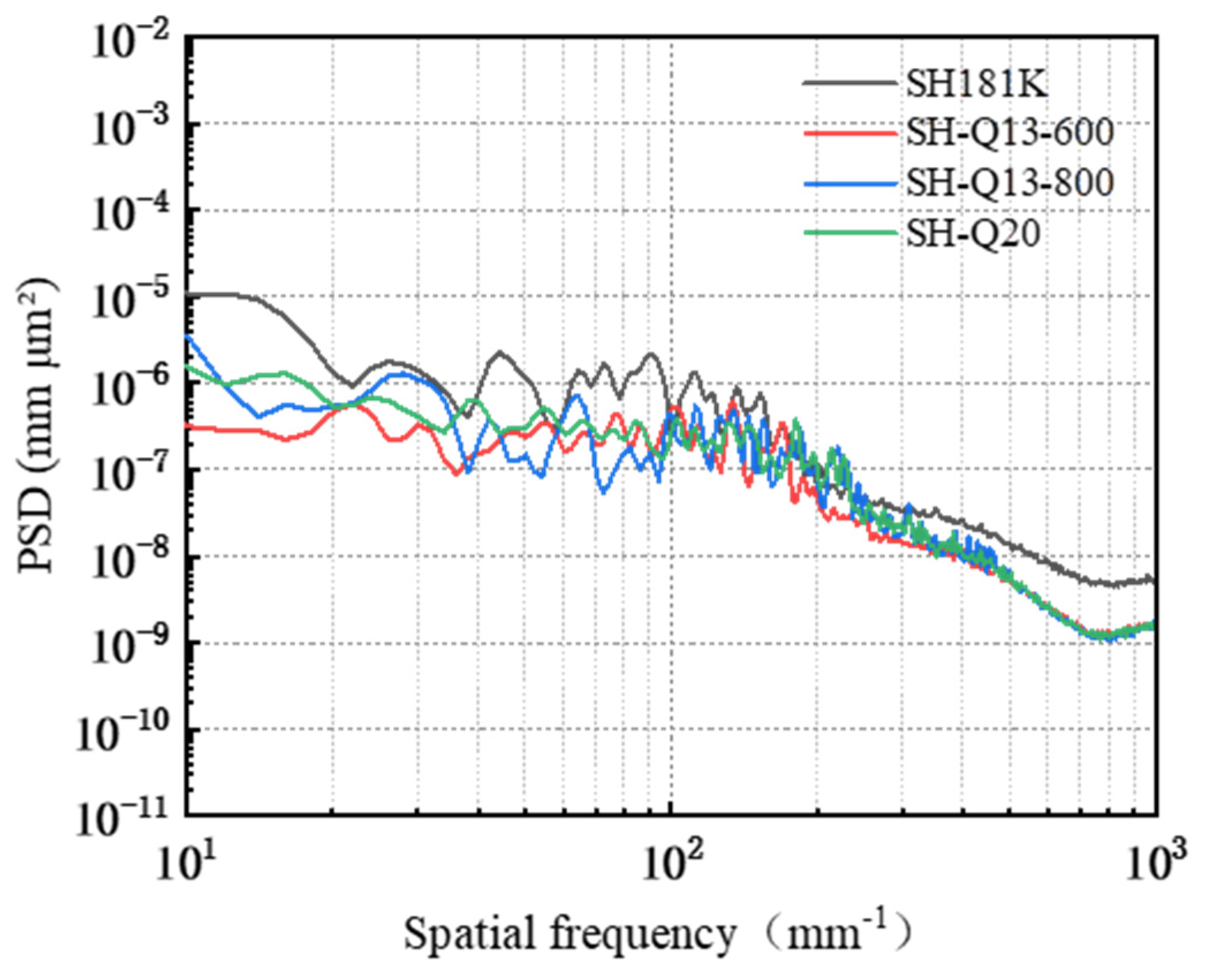
3.4. Effect of Polishing Pad Type on the Removal Behavior of Single-Crystal GaN Materials
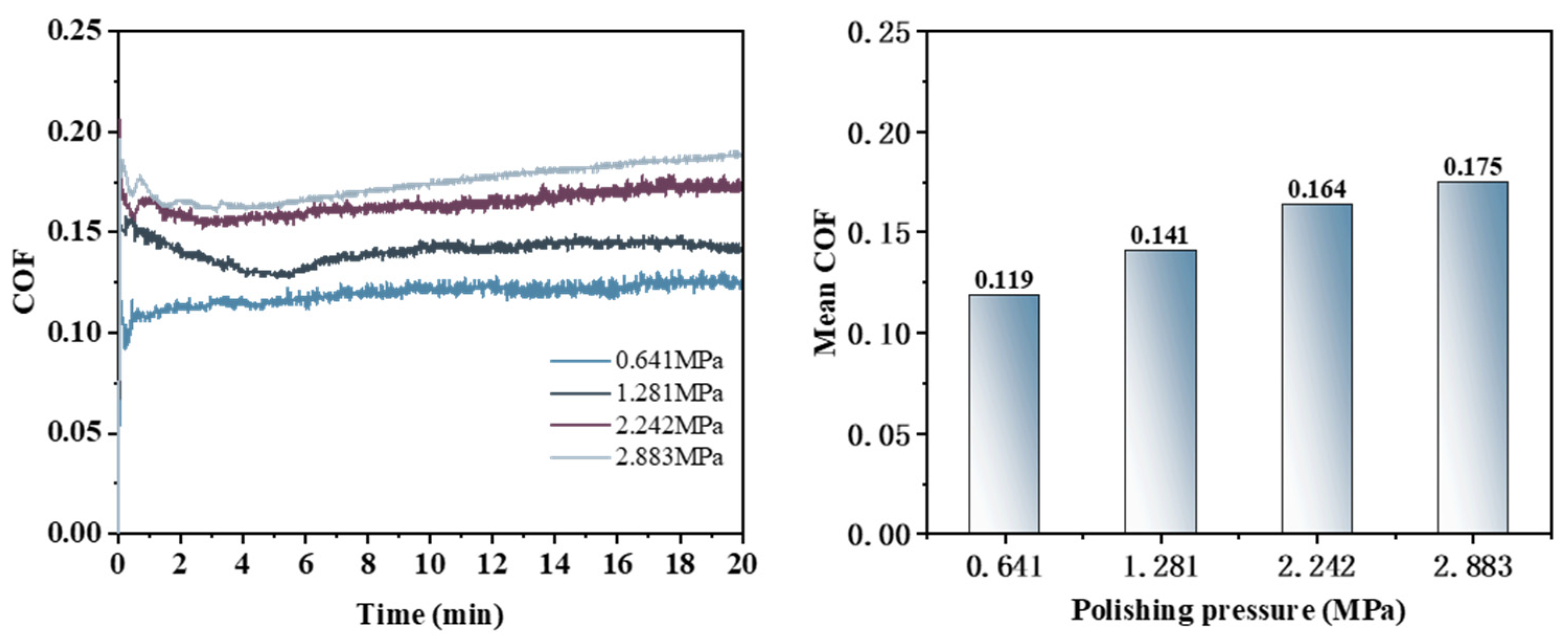
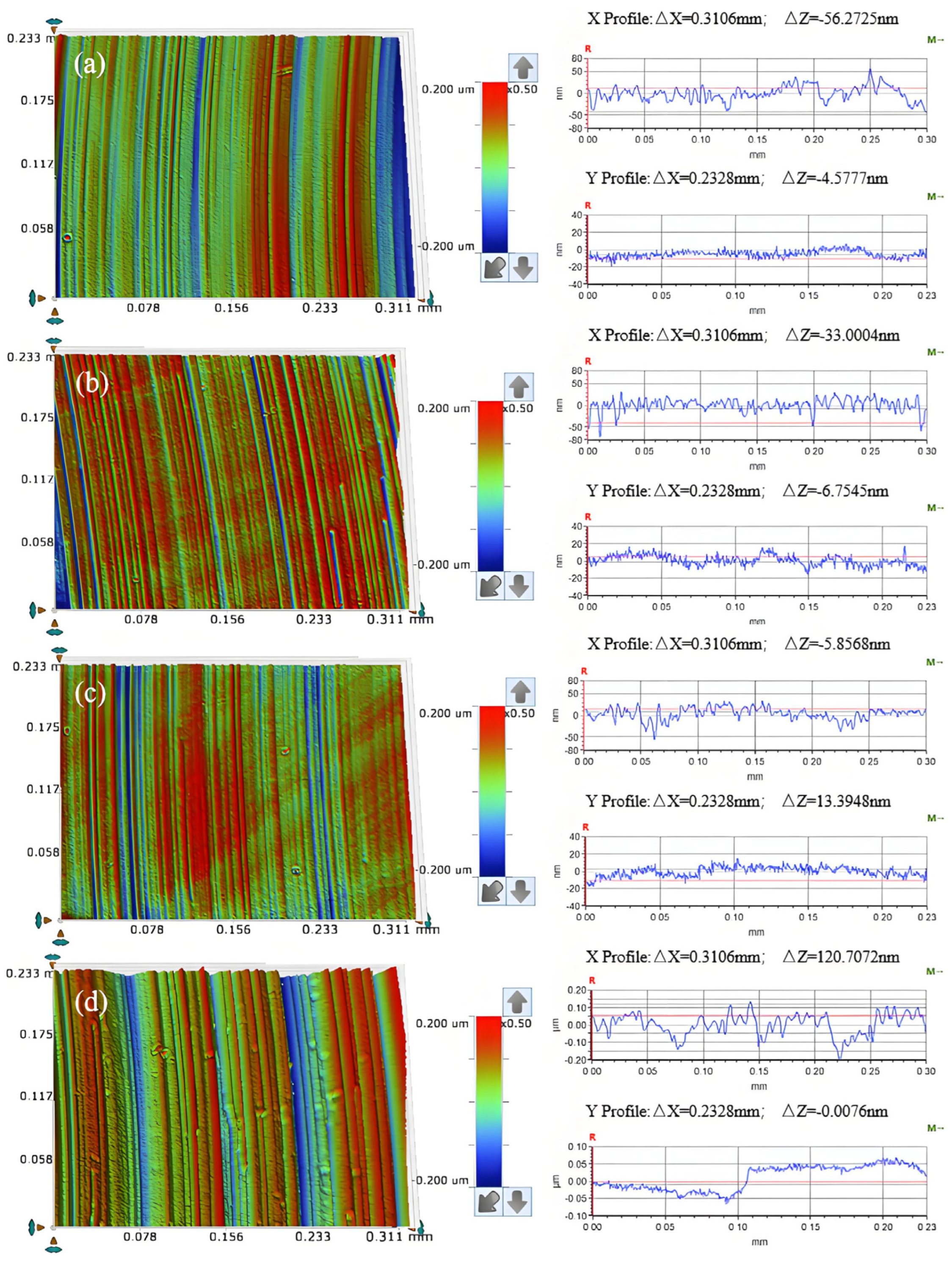
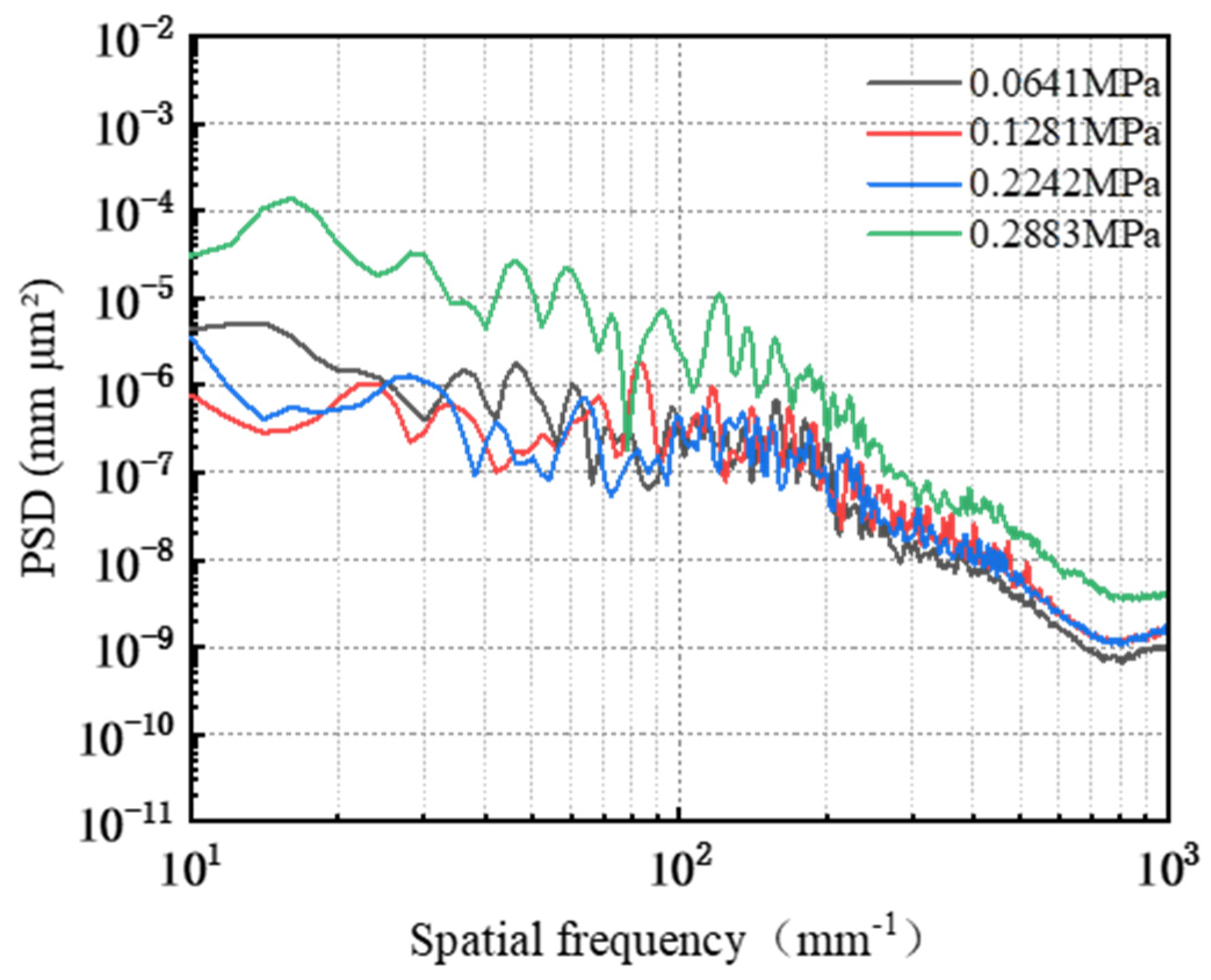
3.5. Effect of Polishing Pressure on the Removal Behavior of Single-Crystal GaN Materials
4. Conclusions
- (1)
- Different types of abrasives exhibit different removal capabilities during electro-Fenton chemical mechanical polishing. Compared to other abrasives, diamond has significant advantages in terms of stability and material removal ability. The grain size, concentration, and polishing pressure of the abrasive modulate the removal of the oxide layer, mainly by influencing the depth of its embedding in the oxide layer on the surface of the workpiece. In addition, the type of polishing pad affects the material removal capability on the workpiece surface by changing the holding power of the abrasive and the fit between the workpiece and the polishing pad.
- (2)
- A comprehensive examination of the workpiece surface topographies, cross-section curves, and PSD analysis results after the friction wear experiments show that the mechanical removal effect promotes the generation of the oxide layer on the surface of the workpiece best when using W0.5 diamond with a concentration of 1.5 wt% and a polyurethane pad (SH-Q13K-600) as the polishing pad, and applying a polishing pressure of 0.2242 MPa; at the same time the abrasive shows good material removal capability.
- (3)
- In the initial phase of the cutting-disk friction and wear experiment, the abrasive’s scraping action on the workpiece surface results in pronounced scratches, leading to a rapid increase in the friction force and consequently a sharp rise in the friction coefficient. As the reaction progresses, these scratches expand the contact area with ·OH, further facilitating oxide layer formation. Simultaneously, both the abrasive and the polishing pad effectively remove the existing oxide layers from the workpiece surface while accelerating the development of new oxide layers. When there is a balance between the generation rate and the removal rate of the oxide layer on the workpiece surface, material removal stabilizes, resulting in the gradual stabilization observed in the COF curve.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murata, J.; Sadakuni, S.; Okamoto, T.; Hattori, A.N.; Yagi, K.; Sano, Y.; Arima, K.; Yamauchi, K. Structural and chemical characteristics of atomically smooth GaN surfaces prepared by abrasive-free polishing with Pt catalyst. J. Cryst. Growth 2012, 349, 83–88. [Google Scholar] [CrossRef]
- Xu, X.; Vaudo, R.P.; Brandes, G.R. Fabrication of GaN wafers for electronic and optoelectronic devices. Opt. Mater. 2003, 23, 1–5. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M.; Nagahama, S. InxGa(1−x)N/InyGa(1−y)N superlattices grown on GaN films. J. Appl. Phys. 2024, 74. [Google Scholar] [CrossRef]
- Liu, Z.; Chong, W.C.; Wong, K.M.; Lau, K.M. GaN-based LED micro-displays for wearable applications. Microelectron. Eng. 2015, 148, 98–103. [Google Scholar] [CrossRef]
- Wong, M.S.; Nakamura, S.; DenBaars, S.P. Review—Progress in High Performance III-Nitride Micro-Light-Emitting Diodes. ECS J. Solid State Sci. Technol. 2020, 9, 015012. [Google Scholar] [CrossRef]
- Liu, Z.J.; Chong, W.C.; Wong, K.M.; Lau, K.M. 360 PPI Flip-Chip Mounted Active Matrix Addressable Light Emitting Diode on Silicon (LEDoS) Micro-Displays. J. Disp. Technol. 2013, 9, 678–682. [Google Scholar] [CrossRef]
- Cok, R.S.; Meitl, M.; Rotzoll, R.; Melnik, G.; Fecioru, A.; Trindade, A.J.; Raymond, B.; Bonafede, S.; Gomez, D.; Moore, T.; et al. Inorganic light-emitting diode displays using micro-transfer printing. J. Soc. Inf. Disp. 2017, 25, 589–609. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Huang, Y.-M.; Gunawan, W.H.; Chang, G.-H.; Liou, F.-J.; Chow, C.-W.; Kuo, H.-C.; Liu, Y.; Yeh, C.-H. 4.343-Gbit/s Green Semipolar (20-21) μ-LED for High Speed Visible Light Communication. IEEE Photonics J. 2021, 13, 1–4. [Google Scholar] [CrossRef]
- Lin, G.-R.; Kuo, H.-C.; Cheng, C.-H.; Wu, Y.-C.; Huang, Y.-M.; Liou, F.-J.; Lee, Y.-C. Ultrafast 2 × 2 green micro-LED array for optical wireless communication beyond 5 Gbit/s. Photon. Res. 2021, 9, 2077–2087. [Google Scholar] [CrossRef]
- Murata, J.; Okamoto, T.; Sadakuni, S.; Hattori, A.N.; Yagi, K.; Sano, Y.; Arima, K.; Yamauchi, K. Atomically Smooth Gallium Nitride Surfaces Prepared by Chemical Etching with Platinum Catalyst in Water. J. Electrochem. Soc. 2012, 159, H417–H420. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Pan, G.; Lu, X. Effect of photocatalytic oxidation technology on GaN CMP. Appl. Surf. Sci. 2016, 361, 18–24. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Yang, Y.; Hu, S.; Song, J.; Ji, J.; Song, J. A synergistic polishing technology by mixed abrasives with photocatalysis and fenton reaction. Mater. Sci. Semicond. Process. 2023, 166, 107734. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H. Highly efficient and damage-free polishing of GaN (0 0 0 1) by electrochemical etching-enhanced CMP process. Appl. Surf. Sci. 2020, 514, 145957. [Google Scholar] [CrossRef]
- Guan, H.; Niu, S.; Wang, Y.; Lu, X.; Ding, Z.; Liu, W.; Zhao, D. Synergetic effect of H2O2 and PTA on the microscratch and indentation of GaN wafer with electricity. Tribol. Int. 2021, 158, 106941. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Chen, F. Improving the catalytic activity of CeO2/H2O2 system by sulfation pretreatment of CeO2. J. Mol. Catal. A Chem. 2014, 381, 38–45. [Google Scholar] [CrossRef]
- Pan, J.; Zhuo, Z.; Zhang, Q.; Zheng, Q.; Yan, Q. Friction and wear mechanisms for single crystal GaN based on an electro-Fenton enhanced chemical reaction. Wear 2022, 498–499, 204315. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, J.; Wang, H.; Shen, Z.; Luan, T.; Yan, Q. Study of corrosion rate control mechanism based on magnetorheological electro-Fenton composite polishing of single-crystal GaN wafers. J. Solid State Electrochem. 2023, 27, 2163–2176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Zuo, S.; Zhou, M.; Pan, Y.; Ren, G.; Li, Y.; Zhang, Y. A highly efficient flow-through electro-Fenton system enhanced with nitrilotriacetic acid for phenol removal at neutral pH. Sci. Total Environ. 2019, 697, 134173. [Google Scholar] [CrossRef]
- Ni, Z.; Zheng, S.; Chen, G.; Fan, Q.; Zhang, X.; Zhang, H.; Li, J.; Bian, D.; Qian, S. Enhancement Mechanism of Chemical Mechanical Polishing for GaN Based on Electro-Fenton Reaction. ECS J. Solid State Sci. Technol. 2023, 12, 024005. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, J.; Zhuo, Z.; Xiang, M.; Yan, Q. Abrasion behavior of TiO2 catalyzing H2O2 to synergistically remove single crystal 6H-SiC under ultraviolet irradiation. Surf. Interfaces 2023, 38, 102781. [Google Scholar] [CrossRef]
- Guo, J.; Gong, J.; Shi, P.; Xiao, C.; Jiang, L.; Chen, L.; Qian, L. Study on the polishing mechanism of pH-dependent tribochemical removal in CMP of CaF2 crystal. Tribol. Int. 2020, 150, 106370. [Google Scholar] [CrossRef]
- Totolin, V.; Göcerler, H.; Ripoll, M.R.; Jech, M. Tribo-electrochemical study of stainless steel surfaces during chemical–mechanical polishing. Lubr. Sci. 2016, 28, 363–380. [Google Scholar] [CrossRef]
- Belkhir, N.; Aliouane, T.; Bouzid, D. Correlation between contact surface and friction during the optical glass polishing. Appl. Surf. Sci. 2014, 288, 208–214. [Google Scholar] [CrossRef]
- Suratwala, T.I.; Feit, M.D.; Steele, W.A. Toward Deterministic Material Removal and Surface Figure During Fused Silica Pad Polishing. J. Am. Ceram. Soc. 2010, 93, 1326–1340. [Google Scholar] [CrossRef]
- Chen, X. Research on Chemical Mechanical Polishing Experiments and Material Removal Mechanism. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2014. [Google Scholar]
- Wu, Y.; Jiang, L.; Li, W.; Zheng, J.; Chen, Y.; Qian, L. Two material removal modes in chemical mechanical polishing: Mechanical plowing vs. chemical bonding. Friction 2024, 12, 897–905. [Google Scholar] [CrossRef]
- Ban, X.; Tian, Z.; Zhu, J.; Duan, T.; Zheng, S.; Wang, N.; Han, S.; Qiu, H.; Li, Z. Compound mechanical and chemical-mechanical polishing processing technique for single-crystal silicon carbide. Precis. Eng. 2024, 86, 160–169. [Google Scholar] [CrossRef]
- Ma, L.; Peng, X.; Dai, Y. The Role of Fenton-like Reaction in the Polishing of Silicon Carbide Optical Materials. Aeronaut. Precis. Manuf. Technol. 2012, 48, 9–11, 62. [Google Scholar]
- Bokare, A.D.; Choi, W. Zero-Valent Aluminum for Oxidative Degradation of Aqueous Organic Pollutants. Environ. Sci. Technol. 2009, 43, 7130–7135. [Google Scholar] [CrossRef]
- HLiang, Z.; Yan, Q.S.; Lu, J.B.; Gao, W.Q. Experiment on Chemical Magnetorheological Finishing of SiC Single Crystal Wafer. Mater. Sci. Forum 2016, 874, 407–414. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, H.; Wu, Y.; Chen, Z.; Yan, Q.; Pan, J. New skin corrosion effect of magnetorheological electro-Fenton polishing investigated by friction and wear experiments. Mater. Sci. Semicond. Process. 2024, 184, 108759. [Google Scholar] [CrossRef]









| Factors | Levels | Remarks |
|---|---|---|
| Base solution | 0.1 mol/L Na2SO4 | Basic parameters of electro-Fenton solution preparation |
| Electro-Fenton catalyst | 3 wt% Fe-C | |
| H2O2 | Start with 5 wt% and refill at 0.3 g/min | |
| pH | 3 | |
| Pin | Polishing pad φ3 mm | Pin–Disk type simulated CMP friction base test conditions |
| Disk | 10 × 10 mm2 GaN-Ga surface | |
| Friction radius | 2 mm | |
| Rotational speed | 150 rpm | |
| The current intensity | 20 mA | |
| Time | 20 min |
| Serial Number | Factors | ||||
|---|---|---|---|---|---|
| Type of Abrasive | Abrasive Concentration (wt%) | Abrasive Particle Size | Type of Polishing Pad | Polishing Pressure (MPa) | |
| A1 | Diamond | 5 | W1 (≈0.7 μm) | SH-Q13K-600 | 0.2242 |
| A2 | Alumina | 5 | W1 | SH-Q13K-600 | 0.2242 |
| A3 | Silicon carbide | 5 | W1 | SH-Q13K-600 | 0.2242 |
| A4 | Silica Sol | 5 | 120 nm | SH-Q13K-600 | 0.2242 |
| A5 | Diamond | 0.5 | W1 | SH-Q13K-600 | 0.2242 |
| A6 | Diamond | 1 | W1 | SH-Q13K-600 | 0.2242 |
| A7 | Diamond | 1.5 | W1 | SH-Q13K-600 | 0.2242 |
| A8 | Diamond | 2 | W1 | SH-Q13K-600 | 0.2242 |
| A9 | Diamond | 1 | W0.2 (≈0.2 μm) | SH-Q13K-600 | 0.2242 |
| A10 | Diamond | 1 | W0.5 (≈0.5 μm) | SH-Q13K-600 | 0.2242 |
| A11 | Diamond | 1 | W1.5 (≈1.5 μm) | SH-Q13K-600 | 0.2242 |
| A12 | Diamond | 1 | W0.5 | SH-Q13K-800 | 0.2242 |
| A13 | Diamond | 1 | W0.5 | SH-Q20 | 0.2242 |
| A14 | Diamond | 1 | W0.5 | SH-181K | 0.2242 |
| A15 | Diamond | 1 | W0.5 | SH-Q13K-600 | 0.0641 |
| A16 | Diamond | 1 | W0.5 | SH-Q13K-600 | 0.1281 |
| A17 | Diamond | 1 | W0.5 | SH-Q13K-600 | 0.2883 |
| Model Number | Hardness (JIS-A) | Density (g/cm3) | Compression Ratio | Thickness (mm) |
|---|---|---|---|---|
| SH-Q13K-600 | 78 | 0.47 | 6.3 | 1.4 |
| SH-Q13K-800 | 90 | 0.53 | 5.5 | 1.4 |
| SH-181K | 70 | 0.49 | 5.8 | 1.4 |
| SH-Q20 | 90 | 0.56 | 6.7 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.; Shen, Z.; Xie, J.; Pan, J. Study on the Electro-Fenton Chemomechanical Removal Behavior in Single-Crystal GaN Pin–Disk Friction Wear Experiments. Micromachines 2025, 16, 210. https://doi.org/10.3390/mi16020210
Ou Y, Shen Z, Xie J, Pan J. Study on the Electro-Fenton Chemomechanical Removal Behavior in Single-Crystal GaN Pin–Disk Friction Wear Experiments. Micromachines. 2025; 16(2):210. https://doi.org/10.3390/mi16020210
Chicago/Turabian StyleOu, Yangting, Zhuoshan Shen, Juze Xie, and Jisheng Pan. 2025. "Study on the Electro-Fenton Chemomechanical Removal Behavior in Single-Crystal GaN Pin–Disk Friction Wear Experiments" Micromachines 16, no. 2: 210. https://doi.org/10.3390/mi16020210
APA StyleOu, Y., Shen, Z., Xie, J., & Pan, J. (2025). Study on the Electro-Fenton Chemomechanical Removal Behavior in Single-Crystal GaN Pin–Disk Friction Wear Experiments. Micromachines, 16(2), 210. https://doi.org/10.3390/mi16020210






