Multifunctional Nanoplatforms Bridging Diagnostics and Therapeutics in Cancer
Abstract
1. Introduction
2. Current Barriers in Cancer Therapy
2.1. Limitations of Conventional Therapies
2.2. Tumor Microenvironment-Driven Barriers
2.3. Challenges in Phototherapies and Combination Approaches
2.4. Diagnostic and Imaging Limitations
2.5. Barriers to Translational Nanomedicine
3. Design Strategies for Multifunctional Nanoplatforms
3.1. Carbon-Based Nanostructures
3.2. Metal and Metal Oxide Platforms
3.3. MOFs and Coordination Assemblies
3.4. Silica and Organosilica Systems
3.5. Polymeric and Lipid Nanocarriers
3.6. Polydopamine and Hybrid Shell Architectures
3.7. Biomimetic and Biohybrid Constructs
3.8. Molecular and Supramolecular Agents
3.9. Unifying Design Principles
4. Mechanistic Pathways Driving Nano-System Function
4.1. Tumor Microenvironment (TME) Responsiveness
4.2. Optical Absorption, Emission, and Energy Transfer
4.3. Photothermal Conversion and Hyperthermia
4.4. Reactive Oxygen and Radical Generation
4.5. Targeting and Localization Mechanisms
4.6. Integrated Multifunctional Synergies
5. Advances in Multimodal Tumor Imaging
5.1. Fluorescence Imaging from Visible to NIR-II
5.2. Photoacoustic and Optoacoustic Imaging
5.3. Magnetic Resonance Imaging and Contrast Innovation
5.4. Computed Tomography Imaging
5.5. Ultrasound and Hybrid Imaging
5.6. Multimodal Integration and Functional Diagnostics
6. Synergistic Therapeutic Modalities in Nanomedicine
6.1. Photothermal and Photodynamic Therapy as a Central Hub
6.2. Chemo–Photo Synergies
6.3. Catalytic Pathways: CDT and Ferroptosis
6.4. Immunotherapy Coupled with Local Modalities
6.5. Radiotherapy and Acoustic Synergies
6.6. Gas, Starvation, and Metabolic Therapies
6.7. Theranostic Integration
7. Preclinical Outcomes and Translational Readiness
7.1. Potent Tumor Inhibition and Complete Ablation
7.2. Synergistic and Multimodal Therapeutic Mechanisms
7.3. Immune Activation and Systemic Responses
7.4. Tumor Microenvironment Responsiveness and Resistance Avoidance
7.5. Imaging-Guided Precision and Theranostic Integration
7.6. Safety, Biocompatibility, and Clinical Readiness
7.7. Overall Translational Outlook
8. Persistent Limitations of Nano-Theranostic Platforms
8.1. Biological and Microenvironmental Variability
8.2. Complexity Versus Clinical Practicality
8.3. Safety, Clearance, and Biocompatibility
9. Future Priorities for Clinical Translation
9.1. Adaptive and Feedback-Driven Platforms
9.2. Streamlined Multifunctionality
9.3. Integration with Immunotherapy
9.4. Targeting Metastasis and Resistant Niches
9.5. Scalability and Regulatory Readiness
10. Conclusions
Evidence-to-Practice Roadmap for Nanotheranostics in Oncology
- What we know: Columns 2–6 aggregate proven needs, mechanisms, preclinical signals, and where they fit clinically.
- What we don’t know: Column 8 captures gaps (e.g., patient heterogeneity, metal fate, dosing windows, workflow standardization).
- Future priorities: Column 7 converts gaps into actionable next steps (e.g., closed-loop theranostics, biomarker-led stratification, simplified/GMP-feasible builds).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cao, C.Y.; Si, G.X.; Yang, N.; Wang, W.J.; Zhang, Z.Y.; Zang, F.C.; Song, X.J.; Chen, P.; Dong, X.C. Fe3+-DOX-mediated self-assembled nanolipids for tumor microenvironment activated synergistic ferroptotic-chemo therapy assisted with MR-imaging. Sens. Actuators B-Chem. 2024, 415, 136039. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Chen, Z.; Yan, C.; Zhang, J.; Song, C.; Wang, L. Deformable Smart DNA Nanomachine for Synergistic Intracellular Cancer-Related miRNAs Imaging and Chemo-Gene Therapy of Drug-Resistant Tumors. Small 2024, 20, e2308562. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Su, L.; Chen, Z.; Zhu, K.; Zhang, X.; Wu, Y.; Song, J. A Radio-Pharmaceutical Fluorescent Probe for Synergistic Cancer Radiotherapy and Ratiometric Imaging of Tumor Reactive Oxygen Species. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305744. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yang, M.; Feng, X.; Liao, H.; Zhang, Z.; Du, Y. Multifunctional Theranostic Nanoparticles for Enhanced Tumor Targeted Imaging and Synergistic FUS/Chemotherapy on Murine 4T1 Breast Cancer Cell. Int. J. Nanomed. 2022, 17, 2165–2187. [Google Scholar] [CrossRef]
- Xu, Z.S.; Zhang, L.; Gong, M.F.; Sun, T.; Zhou, C.Y.; Xiao, S.L.; Liu, Y.; Zhang, D. Fe-Mediated Self-Assembled Nanodrug for Tumor Microenvironment Activated Synergistic Ferroptosis-Based-Chemodynamic/Chemo Therapy and Magnetic Resonance Imaging. ACS Mater. Lett. 2024, 6, 656–665. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, M.; Xu, N.; Xie, Y.; Zhang, X. A Tumor Microenvironment Responsive Nanotheranostics Agent for Magnetic Resonance Imaging and Synergistic Photodynamic Therapy/Photothermal Therapy of Liver Cancer. Front. Chem. 2021, 9, 650899. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Cai, Q.P.; Tang, Z.B.; Yang, Y.X.; Yuan, C.H.; Xu, Y.T.; Zeng, B.R.; Luo, W.A.; Kuo, S.W.; et al. Tumor microenvironment-activated self-charge-generable metallosupramolecular polymer nanocapsules for photoacoustic imaging-guided targeted synergistic photothermal-chemotherapy. Chem. Eng. J. 2021, 405, 126690. [Google Scholar] [CrossRef]
- Shu, G.; Wang, H.; Zhao, H.X.; Zhang, X. Microwave-Assisted Synthesis of Black Titanium Monoxide for Synergistic Tumor Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 3323–3333. [Google Scholar] [CrossRef]
- Sun, M.; Guo, J.; Hao, H.; Tong, T.; Wang, K.; Gao, W. Tumour-homing chimeric polypeptide-conjugated polypyrrole nanoparticles for imaging-guided synergistic photothermal and chemical therapy of cancer. Theranostics 2018, 8, 2634–2645. [Google Scholar] [CrossRef]
- Tan, D.; Long, H.; Du, M.; Yu, J.; Sun, X.; Wang, Y.; Zheng, J.; Chen, H.; Gao, Y. Fabrication of a nanoplatform based on chitosan and hyaluronic acid containing alkyne-functionalized gold nanoparticles for tumor targeted synergistic phototherapy. Int. J. Biol. Macromol. 2025, 309, 142974. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, H.X.; Zhao, R.X.; Zhang, Z.J.; Liu, B.; Gong, H.J.; Zhu, Y.L.; Ding, H.; Gai, S.L.; Feng, L.L. Tumor microenvironment activated glutathione self-depletion theranostic nanocapsules for imaging-directed synergistic cancer therapy. Chem. Eng. J. 2022, 450, 138137. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, X.; Ma, Y.; Dai, R.; Wu, S.; Wang, T.; Xing, J.; Gao, J.; Zhang, R. Dual H2O2-Amplified Nanofactory for Simultaneous Self-Enhanced NIR-II Fluorescence Activation Imaging and Synergistic Tumor Therapy. Small 2022, 18, e2203531. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; He, Y.; Li, L.; Luo, X.; Zhao, R.; Yan, X.; Chen, C. Self-enriching nanozyme with photothermal-cascade amplification for tumor microenvironment-responsive synergistic therapy and enhanced photoacoustic imaging. Mater. Today Bio 2025, 34, 102230. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, J.; Wang, S.; Lin, T.; Ye, F.; Zhao, S. Carbon Dots with Absorption Red-Shifting for Two-Photon Fluorescence Imaging of Tumor Tissue pH and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2021, 13, 35365–35375. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole-Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Deng, W.J.; Yang, Y.; Yu, R.Q.; Chu, X. Core-Shell-Shell Multifunctional Nanoplatform for Intracellular Tumor-Related mRNAs Imaging and Near-Infrared Light Triggered Photodynamic-Photothermal Synergistic Therapy. Anal. Chem. 2017, 89, 10321–10328. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Hao, H.L.; Zhao, W.; Zhao, X.; Chen, H.Y.; Xu, J.J. A plasmon-enhanced theranostic nanoplatform for synergistic chemo-phototherapy of hypoxic tumors in the NIR-II window. Chem. Sci. 2021, 12, 10848–10854. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, M.; Zhang, L.; Ha, E.; Hu, X.; Zou, R.; Yan, L.; Hu, J. Tumor Microenvironment Responsive Biodegradable Fe-Doped MoO(x) Nanowires for Magnetic Resonance Imaging Guided Photothermal-Enhanced Chemodynamic Synergistic Antitumor Therapy. Adv. Healthc. Mater. 2021, 10, e2001665. [Google Scholar] [CrossRef]
- Dai, Y.; Du, W.; Gao, D.; Zhu, H.; Zhang, F.; Chen, K.; Ni, H.; Li, M.; Fan, Q.; Shen, Q. Near-infrared-II light excitation thermosensitive liposomes for photoacoustic imaging-guided enhanced photothermal-chemo synergistic tumor therapy. Biomater. Sci. 2022, 10, 435–443. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Kim, J.; Sarkar, S.; Mondal, J.; An, J.M.; Lee, Y.K.; Moniruzzaman, M.; Lim, K.T. Two-photon excitable membrane targeting polyphenolic carbon dots for long-term imaging and pH-responsive chemotherapeutic drug delivery for synergistic tumor therapy. Biomater. Sci. 2022, 10, 1680–1696. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, Z.; Zhuang, Z.; Li, Z.; Yan, C.; Yang, M.; Chen, Q.; Li, X.; Gong, A. Cascade biomimetic intelligent nanotheranostic agents for imaging-guided tumor synergistic therapy. Nanomedicine 2023, 18, 35–52. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, L.; Tang, Y.; Wang, Y.; Li, N.; Wang, D.; Zhang, Z.; Lin, L.; Du, Y.; Ou, X.; et al. US/MR Bimodal Imaging-Guided Bio-Targeting Synergistic Agent for Tumor Therapy. Int. J. Nanomed. 2022, 17, 2943–2960. [Google Scholar] [CrossRef]
- Li, Y.; Hao, L.; Liu, F.; Yin, L.; Yan, S.; Zhao, H.; Ding, X.; Guo, Y.; Cao, Y.; Li, P.; et al. Cell penetrating peptide-modified nanoparticles for tumor targeted imaging and synergistic effect of sonodynamic/HIFU therapy. Int. J. Nanomed. 2019, 14, 5875–5894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Zhang, T.; Wang, C.; Fan, Y.; Wang, C.; Song, N.; Zhou, P.; Yan, C.H.; Tang, Y. Tumor Microenvironment-Regulating Two-Photon Probe Based on Bimetallic Post-Coordinated MOF Facilitating the Dual-Modal and Deep Imaging-Guided Synergistic Therapies. ACS Appl. Mater. Interfaces 2024, 16, 12289–12301. [Google Scholar] [CrossRef]
- Luo, B.; Huang, X.; Ye, Y.; Cai, J.; Feng, Y.; Cai, X.; Wang, X. CuS NP-based nanocomposite with photothermal and augmented-photodynamic activity for magnetic resonance imaging-guided tumor synergistic therapy. J. Inorg. Biochem. 2022, 235, 111940. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Teng, Y.; Wang, S.; Guo, J.; Wang, C. NIR-controlled HSP90 inhibitor release from hollow mesoporous nanocarbon for synergistic tumor photothermal therapy guided by photoacoustic imaging. Nanoscale 2020, 12, 14775–14787. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, A.; Mei, Y.; Xiao, Q.; Xu, X.; Wang, W. NIR Light-Triggered Chemo-Phototherapy by ICG Functionalized MWNTs for Synergistic Tumor-Targeted Delivery. Pharmaceutics 2021, 13, 2145. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Chen, M.; Zhang, J.; Sun, X.; Zhou, H.; Gao, Z. Application of near-infrared-activated and ATP-responsive trifunctional upconversion nano-jelly for in vivo tumor imaging and synergistic therapy. Biosens. Bioelectron. 2024, 250, 116094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G.; Kang, T.; Liu, S.; Wang, L.; Zou, H.; Chong, Y.; Liu, Y. BiVO4/Fe3O4@polydopamine superparticles for tumor multimodal imaging and synergistic therapy. J. Nanobiotechnol. 2021, 19, 90. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, N.; Zhang, T.; Yin, X.; Shen, J. Fabrication of doxorubicin-gated mesoporous polydopamine nanoplatforms for multimode imaging-guided synergistic chemophotothermal therapy of tumors. Drug Deliv. 2020, 27, 367–377. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, R.; Zhao, C.; Sun, N.; Luo, H.; Chen, Y.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. Thermo- and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors. Theranostics 2018, 8, 4097–4115. [Google Scholar] [CrossRef]
- You, S.S.; Ding, G.; Chi, B.; Wang, Z.Y.; Lu, S.; Li, L.; Yu, X.L.; Wang, J. Construction a starving therapy induced photothermal enhanced cascade nanoreactor for imaging guided catalytic synergistic therapy of tumor. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 674, 131941. [Google Scholar] [CrossRef]
- Zeng, X.; Zhuang, H.; Xu, D.; Liang, J.; Jiang, L.; Shao, S.; Xue, P.; Liu, G.; Yan, S. Multifunctional Hollow Bimetallic Sulfide Nanozyme Enables Imaging-Guided Synergistic Ferrotherapy for Tumor Treatment. Nano Lett. 2025, 25, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, W.; Yang, B.; Shi, T.; Liao, S.; Li, Y.; Yin, S. Biomimetic Metallacage Nanoparticles with Aggregation-Induced Emission for NIR-II Fluorescence Imaging-Guided Synergistic Immuno-Phototherapy of Tumors. ACS Appl. Mater. Interfaces 2024, 16, 69028–69044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, Y.; Yang, Z.; Wong, C.Y.; Yang, M. A novel reactive oxygen species nano-amplifier for tumor-targeted photoacoustic imaging and synergistic therapy. J. Colloid. Interface Sci. 2025, 681, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, M.; Wang, J.; Zhou, Y.; Dai, P.; Zhao, M.; Lv, W.; Liu, S.; Zhao, Q. NIR-Triggered On-Demand Nitric Oxide Release for Enhanced Synergistic Phototherapy of Hypoxic Tumor. Bioconjug. Chem. 2023, 34, 1327–1335. [Google Scholar] [CrossRef]
- Zheng, F.; Fan, D.Y.; Yao, H.Y.; Ding, J.P.; Huang, S.; Fang, Y.P.; Dong, J.; Chen, F.; Zeng, W.B. Synergistic Photodynamic and Chemodynamic Therapy for Tumor Treatment Using a Glutathione-Activated Photosensitizer with Near-Infrared (NIR) Imaging. ACS Mater. Lett. 2024, 6, 4673–4681. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Yang, H.; Zheng, G.; Ren, L.; Zhang, Z.; Lin, L.; He, Y.; Wang, Q.; Zou, J. Genetically engineered bio-composite mediated dual-modality imaging-guided synergistic chemo-FUAS for tumor therapy. Mater. Today Bio 2025, 33, 101928. [Google Scholar] [CrossRef]
- Tang, K.; Wang, W.; Song, Z.; Luo, X. Multifunctional nano-biosensor based on metal-organic framework for enhanced fluorescence imaging of intracellular miRNA-122 and synergistic chemo-photothermal therapy of tumor cells. Anal. Chim. Acta 2021, 1176, 338779. [Google Scholar] [CrossRef]
- Yu, C.; Kong, L.; Tian, J.; Zhang, Y.; Jia, X.; Dang, W.; Xing, B.; Zhang, Q.; Pang, X.; Hu, Z.; et al. Photoacoustic imaging-guided triple-responsive nanoparticles with tumor hypoxia relief for improving chemotherapy/ photothermal/photodynamic synergistic therapy against breast cancer. Biomed. Pharmacother. 2023, 164, 114928. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, X.; Li, J.; Shi, M.; Yao, Y.; Guo, L.; Zhi, N.; Zhang, T. Totally Caged Type I Pro-Photosensitizer for Oxygen-Independent Synergistic Phototherapy of Hypoxic Tumors. Adv. Sci. 2024, 11, e2400462. [Google Scholar] [CrossRef]
- Zhao, J.K.; Dai, D.S.; Zhou, L.F.; Yu, Z.P.; Ma, J.P.; Yang, M.; Yi, C.Q. A tumor microenvironment responsive mesoporous polydopamine theranostic probe embedded with Gd/I-doped carbon nanodots for CT/MR/ FL imaging and chemo/photothermal synergistic therapy. Carbon 2024, 224, 119065. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J.; Cubeddu, L.X. Quantum Dot-Enabled Biosensing for Prostate Cancer Diagnostics. Nanomaterials 2025, 15, 1162. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Cubeddu, L.X. Quantum Dot Research in Breast Cancer: Challenges and Prospects. Materials 2024, 17, 2152. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Dey Chowdhury, S. Advances in photothermal and photodynamic nanotheranostics for precision cancer treatment. J. Nanotheranostics 2024, 5, 228–252. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J.; Cubeddu, L.X. Conjugate Nanoparticles in Cancer Theranostics. J. Nanotheranostics 2025, 6, 24. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Wang, W.; Long, F.; Li, Q.; Gan, D.; Li, X.; Li, B.; Kong, X.; Li, D.; et al. Synergistic Comprehensive Activation Methods for Dual-Modality PDT and Hypoxia-Triggered Chemotherapy Guided by NIR-II Imaging beyond 1700 nm in Deep Tumors. Small 2025, 21, e2500553. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, J.; Zhang, R.; Tian, F.; Zhang, Y.; Zhang, L.; Yang, M. Tumor microenvironment-activated and near-infrared light-driven free radicals amplifier for tetra-modal cancer imaging and synergistic treatment. J. Colloid. Interface Sci. 2025, 689, 137208. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, X.; Guo, J.; Wang, J.; Liu, S.; Wang, M.; Peng, Q.; Jiang, N. Aptamer and Peptide-Engineered Polydopamine Nanospheres for Target Delivery and Tumor Perfusion in Synergistic Chemo-Phototherapy of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2023, 15, 16539–16551. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Lin, L.; Zhao, W.; Zhu, X.; Pang, D.W.; Liu, A.A. Redox Imbalance Triggered Intratumoral Cascade Reaction for Tumor “turn on” Imaging and Synergistic Therapy. Small 2023, 19, e2206272. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yu, L.; Yang, Z.; Ding, J.; Wang, K.N.; Zhang, Y. Monocomponent Nanodots with Dichromatic Output Regulated by Synergistic Dual-Stimuli for Cervical Cancer Tissue Imaging and Photodynamic Tumor Therapy. Anal. Chem. 2022, 94, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xu, W.; Fei, Y.; Li, D.; Jia, X.; Wang, J.; Wang, E. Mn2+/Ir3+-Doped and CaCO3-Covered Prussian Blue Nanoparticles with Indocyanine Green Encapsulation for Tumor Microenvironment Modulation and Image-Guided Synergistic Cancer Therapy. Adv. Healthc. Mater. 2023, 12, e2301413. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; Han, X.; Yan, J.; Wu, Y.; Song, P.; Wang, Y.; Li, X.; Zhang, H. Functional 2D Iron-Based Nanosheets for Synergistic Immunotherapy, Phototherapy, and Chemotherapy of Tumor. Adv. Healthc. Mater. 2022, 11, e2200776. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Zhu, Y.; Cheng, Y.; Wang, Y.; Wang, S.; Tan, F.; Lian, F.; Li, N. Biomodal Tumor-Targeted and Redox-Responsive Bi2Se3 Hollow Nanocubes for MSOT/CT Imaging Guided Synergistic Low-Temperature Photothermal Radiotherapy. Adv. Healthc. Mater. 2019, 8, e1900250. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Cheng, H.; Cheng, Y.; Zhao, Z.; Xu, Y.; Shen, Y.; Zhu, M. A multi-responsive Au NCs@PMLE/Ca(2+) antitumor hydrogel formed in situ on the interior/surface of tumors for PT imaging-guided synergistic PTT/O(2)-enhanced PDT effects. Nanoscale 2022, 14, 7372–7386. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.Q.; Wei, J.S.; Li, X.; Wang, H.; Zhao, Y.D. Intelligent gold nanostars for in vivo CT imaging and catalase-enhanced synergistic photodynamic & photothermal tumor therapy. Theranostics 2019, 9, 5424–5442. [Google Scholar] [CrossRef]
- He, M.; Cheng, Z.; Wang, Z.; Li, M.; Liang, H.; Liu, H.; Yu, L.; Zhao, L.; Yu, F. Controllable Regulation of Ag2S Quantum-Dot-Mediated Protein Nanoassemblies for Imaging-Guided Synergistic PDT/PTT/Chemotherapy against Hypoxic Tumor. Adv. Healthc. Mater. 2023, 12, e2300752. [Google Scholar] [CrossRef]
- Yun, X.; Feng, G.; Zhu, M.; Liu, Y.; Li, B.; Yang, R.; He, H.; Qian, N.; Zheng, B.; Bai, Y. Near infrared-emitting persistent luminescent imaging-guided gene/optothermal synergistic therapy for enhancing tumor cell damage. J. Colloid. Interface Sci. 2025, 702, 138815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, T.; Guan, M.; Zhen, M.; Chen, D.; Guan, X.; Han, H.; Wang, C.; Shu, C. Synergistic Effect of Human Serum Albumin and Fullerene on Gd-DO3A for Tumor-Targeting Imaging. ACS Appl. Mater. Interfaces 2016, 8, 11246–11254. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Y.; Chen, Y.; Fang, H.; Yuan, H.; Shi, X.; Yang, B.; Chen, Z.; He, W.; Guo, Z. A novel luminescent Ir(iii) complex for dual mode imaging: Synergistic response to hypoxia and acidity of the tumor microenvironment. Chem. Commun. 2020, 56, 8055–8058. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, S.; Hu, A.; Zhang, Q.; Shao, Z.; Tian, B.; Lin, Q. Ultrabright contrast agents with synergistic Raman enhancements for precise intraoperative imaging and photothermal ablation of orthotopic tumor models. J. Nanobiotechnol. 2025, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Li, C.; Yang, X.Q.; An, J.; Cheng, K.; Xuan, Y.; Shi, X.M.; Gao, M.J.; Song, X.L.; Zhao, Y.D.; et al. Graphene oxide coating core-shell silver sulfide@mesoporous silica for active targeted dual-mode imaging and chemo-photothermal synergistic therapy against tumors. J. Mater. Chem. B 2018, 6, 4808–4820. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, R.; Hao, J.; Yang, Y.; Zou, H.; Wang, Z. Mesoporous composite nanoparticles for dual-modality ultrasound/magnetic resonance imaging and synergistic chemo-/thermotherapy against deep tumors. Int. J. Nanomed. 2017, 12, 7273–7289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jin, S.; Jiao, M.; Wang, W.; Zhou, X.; Xu, J.; Wang, Y.; Dou, P.; Jin, Z.; Wu, C.; et al. Tumor-targeted Gd-doped mesoporous Fe3O4 nanoparticles for T1/T2 MR imaging guided synergistic cancer therapy. Drug Deliv. 2021, 28, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Luo, Y.; Xiong, J.; Tang, Y.; Yang, H.; Wang, L.; Jiang, F.; Gao, X.; Xu, D.; et al. Experimental Study of Tumor Therapy Mediated by Multimodal Imaging Based on a Biological Targeting Synergistic Agent. Int. J. Nanomed. 2020, 15, 1871–1888. [Google Scholar] [CrossRef]
- Sun, P.J.; Li, G.J.; Li, Y.Y.; Yang, J.L.; Chen, T.; Wang, Y.B.; Zhou, Y.M. Long wavelength AIE enzyme prodrug-photosensitizer with dual cationic structure for fluorescence imaging of tumor cells and synergistic photodynamic therapy. Sens. Actuators B-Chem. 2025, 441, 138031. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Liao, Y.; Hu, D.; He, Y.; Feng, N.; Kwok, R.T.K.; Lam, J.W.Y.; Zhang, J.; He, B.; et al. Strategically Designed Mitochondria-Targeting AIEgens for Effective Eradication of Primary and Metastatic Tumors via Synergistic Phototherapy and Induced Immunogenic Cell Death. Adv. Healthc. Mater. 2025, 14, e2500513. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza-BODIPY for Imaging-Guided Synergistic Photodynamic and Photothermal Tumor Therapy. Adv. Healthc. Mater. 2018, 7, e1800606. [Google Scholar] [CrossRef]
- Zhang, X.G.; Chen, X.M.; Zhang, P.; Li, M.T.; Feng, M.; Zhang, Y.Q.; Cheng, L.L.; Tang, J.J.; Xu, L.T.; Liu, Y.D.; et al. A novel two-dimensional nanoheterojunction via facilitating electron-hole pairs separation for synergistic tumor phototherapy and immunotherapy. Nano Res. 2022, 16, 7148–7163. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Jiang, H.; Mo, X.; Liu, C.; Liu, X.; Feng, T.; Zhou, Y. Near-infrared and pH-responsive carbon dots/bergenin for biological imaging and chemo-photothermal synergistic tumor therapy. J. Colloid. Interface Sci. 2025, 694, 137679. [Google Scholar] [CrossRef]
- Thirumurugan, S.; Dash, P.; Sakthivel, R.; Lin, Y.C.; Sun, Y.S.; Lin, C.P.; Wang, A.N.; Liu, X.; Dhawan, U.; Chung, R.J. Gold nanoparticles decorated on MOF derived Cu5Zn8 hollow porous carbon nanocubes for magnetic resonance imaging guided tumor microenvironment-mediated synergistic chemodynamic and photothermal therapy. Biomater. Adv. 2024, 158, 213778. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Jiang, X.; Shen, J. alpha-Fe2O3@Au-PEG-Ce6-Gd Nanoparticles as Acidic H2O2-Driven Oxygenators for Multimodal Imaging and Synergistic Tumor Therapy. Langmuir 2023, 39, 5333–5341. [Google Scholar] [CrossRef]
- You, Q.; Zhang, K.; Liu, J.; Liu, C.; Wang, H.; Wang, M.; Ye, S.; Gao, H.; Lv, L.; Wang, C.; et al. Persistent Regulation of Tumor Hypoxia Microenvironment via a Bioinspired Pt-Based Oxygen Nanogenerator for Multimodal Imaging-Guided Synergistic Phototherapy. Adv. Sci. 2020, 7, 1903341. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Z.; Xu, H.; Huang, H.; Tao, X.; Hu, Y.; He, Y.; Zhang, Z.; Huang, X. Redox-Activatable Magnetic Nanoarchitectonics for Self-Enhanced Tumor Imaging and Synergistic Photothermal-Chemodynamic Therapy. Small Methods 2024, 8, e2301099. [Google Scholar] [CrossRef]
- Zheng, S.H.; Dou, P.P.; Jin, S.; Jiao, M.; Wang, W.J.; Jin, Z.; Wang, Y.; Li, J.J.; Xu, K. Tumor microenvironment/NIR-responsive carbon monoxide delivery with hollow mesoporous CuS nanoparticles for MR imaging guided synergistic therapy. Mater. Des. 2021, 205, 109731. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, X.; Chen, Q.; Ji, Y.; Zhang, X.; Zhu, X.; Zhang, Z. A multi-functional nanoplatform for tumor synergistic phototherapy. Nanotechnology 2016, 27, 085104. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, Y.; Xu, M.; Foda, M.F.; Han, H. Sequential assembled chimeric peptide for precise synergistic phototherapy and photoacoustic imaging of tumor apoptosis. Chem. Eng. J. 2022, 427, 130775. [Google Scholar] [CrossRef]
- Chang, T.; Qiu, Q.; Ji, A.; Qu, C.; Chen, H.; Cheng, Z. Organic single molecule based nano-platform for NIR-II imaging and chemo-photothermal synergistic treatment of tumor. Biomaterials 2022, 287, 121670. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, F.; Chen, K.; Sun, Z.; Wang, Z.; Xue, Y.; Li, M.; Fan, Q.; Shen, Q.; Zhao, Q. An Activatable Phototheranostic Nanoplatform for Tumor Specific NIR-II Fluorescence Imaging and Synergistic NIR-II Photothermal-Chemodynamic Therapy. Small 2023, 19, e2206053. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Hu, H.; Sun, Z.; He, P.; Zhu, D.; Xu, Y.; Liu, B.; Wei, G. Assembling Ag2S quantum dots onto peptide nanosheet as a biomimetic two-dimensional nanoplatform for synergistic near infrared-II fluorescent imaging and photothermal therapy of tumor. J. Colloid. Interface Sci. 2024, 663, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, B.; Liu, F.; Li, N.; Wang, M.; Wu, P.; Wu, G.L.; Fang, H.; He, Y.; Zhou, W.; et al. NIR-II Imaging-Guided Mitochondrial-Targeting Organic Nanoparticles for Multimodal Synergistic Tumor Therapy. Small 2023, 19, e2207995. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Wang, W.; Zheng, X.; Zhang, C.; Jin, Y.; Meng, S.; Li, J.; Dai, R.; Kang, W.; et al. A novel NIR-II FL/ PA imaging-guided synergistic photothermal-immune therapy: Biomineralizing nanosystems integrated with anti-tumor and bone repair. Mater. Today Bio 2024, 26, 101052. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Q.; Tang, Z.; Liu, C.; Li, Z.; Wu, A.; Lin, H. Tumor Microenvironment Stimuli-Responsive Fluorescence Imaging and Synergistic Cancer Therapy by Carbon-Dot-Cu2+ Nanoassemblies. Angew. Chem. Int. Ed. Engl. 2020, 59, 21041–21048. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Zhong, H.; Niu, S.; Ding, C.; Lv, S. Iridium oxide nanoparticles-based theranostic probe for in vivo tumor imaging and synergistic chem/photothermal treatments of cancer cells. Chem. Eng. J. 2022, 430, 132675. [Google Scholar] [CrossRef]
- Li, D.J.; Zhang, T.; Min, C.W.; Huang, H.; Tan, D.H.; Gu, W.G. Biodegradable theranostic nanoplatforms of albumin-biomineralized nanocomposites modified hollow mesoporous organosilica for photoacoustic imaging guided tumor synergistic therapy. Chem. Eng. J. 2020, 388, 124253. [Google Scholar] [CrossRef]
- Maji, D.; Oh, D.; Gautam, K.S.; Zhou, M.; Zhang, H.; Kao, J.; Giblin, D.; Smith, M.; Lim, J.; Lee, S.; et al. Copper-Catalyzed Covalent Dimerization of Near-Infrared Fluorescent Cyanine Dyes: Synergistic Enhancement of Photoacoustic Signals for Molecular Imaging of Tumors. Anal. Sens. 2022, 2, e202100045. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Tu, W.; Yu, X.; Ahmad, F.; Zhang, X.; Wu, W.; An, X.; Chen, X.; Li, W. W-doped TiO2 nanoparticles with strong absorption in the NIR-II window for photoacoustic/CT dual-modal imaging and synergistic thermoradiotherapy of tumors. Theranostics 2019, 9, 5214–5226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.H.; Pan, P.; Hu, J.J.; Cheng, S.X.; Zhang, X.Z. Tumor-Microenvironment-Triggered Ion Exchange of a Metal-Organic Framework Hybrid for Multimodal Imaging and Synergistic Therapy of Tumors. Adv. Mater. 2020, 32, e2001452. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Wang, Y.; Ren, Q.; Guo, H.; Matson, J.B.; Chen, X.; Nie, Z. Enzyme-induced in vivo assembly of gold nanoparticles for imaging-guided synergistic chemo-photothermal therapy of tumor. Biomaterials 2019, 223, 119460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.N.; Wang, Q.H.; Cao, Z.Y.; Yu, C.; Zhang, R.K.; Xiang, K.Y.; Wu, H.G.; Li, K.; Ni, Q.Q.; Ma, Q.Y.; et al. Multifunctional H2S-activated metal-organic framework systems for targeted colorectal cancer imaging and synergistic copper-induced tumor regression. Bmemat 2025. early view. [Google Scholar] [CrossRef]
- Liu, D.; He, H.L.; Kong, F.; Cao, Y.X.; Zang, F.C.; Ma, M.; Gu, N.; Zhang, Y. A versatile metal-organic nanoplatform in combination with CXCR4 antagonist and PD-L1 inhibitor for multimodal synergistic cancer therapy and MRI-guided tumor imaging. Nano Today 2022, 47, 101689. [Google Scholar] [CrossRef]
- Yin, J.; Chen, D.; Wu, S.; Li, C.; Liu, L.; Shao, Y. Tumor-targeted nanoprobes for enhanced multimodal imaging and synergistic photothermal therapy: Core-shell and dumbbell Gd-tailored gold nanorods. Nanoscale 2017, 9, 16661–16673. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, D.; Chen, L.; Liu, K.; Zheng, Y.; Li, L.; Li, J.; Lin, H.; Gao, J. Fluorinated Iron Metal-Organic Frameworks for Activatable 19F Magnetic Resonance Imaging and Synergistic Therapy of Tumors. Nano Lett. 2023, 23, 11989–11998. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, X.J.; Zhou, Y.; Xu, S.Y.; James, T.D.; Wang, L.Y. Nanoplatforms with synergistic redox cycles and rich defects for activatable image-guided tumor-specific therapy. Chem 2022, 8, 2498–2513. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, W.; Xu, X.; Lin, Z.; Xie, C.; Zhang, Y.; Zhang, T.; Li, L.; Lu, Y.; Li, Q. AgBiS2 nanoparticles with synergistic photodynamic and bioimaging properties for enhanced malignant tumor phototherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110324. [Google Scholar] [CrossRef]
- Hu, T.; Jia, L.; Li, H.; Yang, C.; Yan, Y.; Lin, H.; Zhang, F.; Qu, F.; Guo, W. An Intelligent and Soluble Microneedle Composed of Bi/BiVO4 Schottky Heterojunction for Tumor Ct Imaging and Starvation/Gas Therapy-Promoted Synergistic Cancer Treatment. Adv. Healthc. Mater. 2024, 13, e2303147. [Google Scholar] [CrossRef]
- Jia, P.; Tu, J.; Shen, H.; Jiang, Y.; Zhang, Q.; Xue, W.; Liu, M.; Liu, J.; Miao, Y.; Ouyang, R.; et al. Defect-engineered magnetic bismuth nanomedicine for dual-modal imaging and synergistic lung tumor therapy. Mater. Today Bio 2025, 32, 101680. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S.; Wang, Z.; Feng, L.; Sun, Q.; Chen, G.; He, F.; Liu, S.; Li, W.; Yang, P. Multimode Imaging-Guided Photothermal/Chemodynamic Synergistic Therapy Nanoagent with a Tumor Microenvironment Responded Effect. ACS Appl. Mater. Interfaces 2020, 12, 52479–52491. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Tang, Y.; Wang, F.; Du, Y.; Ou, X.; Lin, L.; Zhang, Z.; Ding, Y.; Wu, M.; et al. US/PA/MR multimodal imaging-guided multifunctional genetically engineered bio-targeted synergistic agent for tumor therapy. J. Nanobiotechnol. 2024, 22, 615. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Xue, Y.; Lin, J.; Fu, Y.; Xia, Z.; Pan, D.; Zhang, J.; Qiao, K.; Zhang, Z.; et al. Reversing cold tumors to hot: An immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy. Bioact. Mater. 2021, 6, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.S.; Li, T.; Wang, W.X.; Wang, Z.Q.; Liu, Q.R.; Mao, G.J.; Li, Y.F.; Li, C.Y. A tumor-targeting fluorescent probe for ratiometric imaging of pH and improving PDT/PTT synergistic therapy. Sens. Actuators B-Chem. 2023, 393, 134287. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Ma, Q.; Liu, Y. Near-infrared nanoparticles based on indocyanine green-conjugated albumin: A versatile platform for imaging-guided synergistic tumor chemo-phototherapy with temperature-responsive drug release. Onco Targets Ther. 2018, 11, 8517–8528. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Y.; Yuan, Q.; Hu, H.; Hu, X.; Qian, Z.; Rolle, J.K.; Gu, Y.; Li, S. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano 2020, 14, 3414–3425. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, L.; Hao, L.; Wang, K.; Yang, W.; Liu, Z.; Lei, Z.; Zhang, Y.; Li, W.; Jiang, L.; et al. Upconversion Nanoplatform Enables Multimodal Imaging and Combinatorial Immunotherapy for Synergistic Tumor Treatment and Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 21766–21780. [Google Scholar] [CrossRef]
- Du, L.; Qin, H.; Ma, T.; Zhang, T.; Xing, D. In Vivo Imaging-Guided Photothermal/Photoacoustic Synergistic Therapy with Bioorthogonal Metabolic Glycoengineering-Activated Tumor Targeting Nanoparticles. ACS Nano 2017, 11, 8930–8943. [Google Scholar] [CrossRef]
- Liu, X.; Meng, C.; Ji, G.Q.; Liu, J.; Zhu, P.; Qian, J.Q.; Zhu, S.X.; Zhang, Y.N.; Ling, Y. Tumor microenvironment-activatable boolean logic supramolecular nanotheranostics based on a pillar[6]arene for tumor hypoxia imaging and multimodal synergistic therapy. Mater. Chem. Front. 2021, 5, 5846–5856. [Google Scholar] [CrossRef]
- Qin, Y.F.; Zheng, Z.L.; Chen, X.J.; Liu, Q.; Ren, S.L.; Zhang, W.W.; Duan, A.L.; Zhang, R.P. Tumor Microenvironment-Activated Nanosystem with High Aggregation and On-Demand Degradation for Imaging-Guided Synergistic Hydrogenothermal Therapy. Adv. Ther. 2022, 5, 2200056. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Y.; Li, J.; Li, L.; Liang, Q.; Huang, S.; Yang, C.; Li, Z.; Yao, H. Near-infrared-II-driven Z-scheme heterojunction Polyglycolated MoS2/CoFe2O4 amplified edge potential for dual-mode imaging guided tumor synergistic therapy. J. Colloid. Interface Sci. 2025, 683, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Huang, X.; Wang, Y.; Chen, D.; Ou, C.; Zhang, Q.; Shao, J.; Huang, W.; Dong, X. Tumor-Microenvironment-Responsive Nanoconjugate for Synergistic Antivascular Activity and Phototherapy. ACS Nano 2018, 12, 11446–11457. [Google Scholar] [CrossRef]
- Yang, Y.; Yun, K.; Li, Y.; Zhang, L.; Zhao, W.; Zhu, Z.; Tian, B.; Chen, F.; Pan, W. Self-assembled multifunctional polymeric micelles for tumor-specific bioimaging and synergistic chemo-phototherapy of cancer. Int. J. Pharm. 2021, 602, 120651. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Lin, L.; Yan, N.; Yang, W.H.; Cai, K.Y.; Tian, H.Y.; Chen, X.S. Anion receptor-mediated multicomponent synergistic self-assembly of porphyrin for efficient phototherapy to elicit tumor immunotherapy. Nano Today 2022, 46, 101579. [Google Scholar] [CrossRef]
- Tao, L.L.; Cheng, G.; Lv, F.N.; Wang, R.Q.; Yang, N.; Xing, Z.H.; Gu, B.Y.; Meng, S.Y.; Xu, W.; Huo, M.R. Synergistic strategy based on mild phototherapy and deep tumor hypoxia reversal comprehensively remodels the tumor microenvironment for improved immunotherapy. Chem. Eng. J. 2023, 472, 145092. [Google Scholar] [CrossRef]
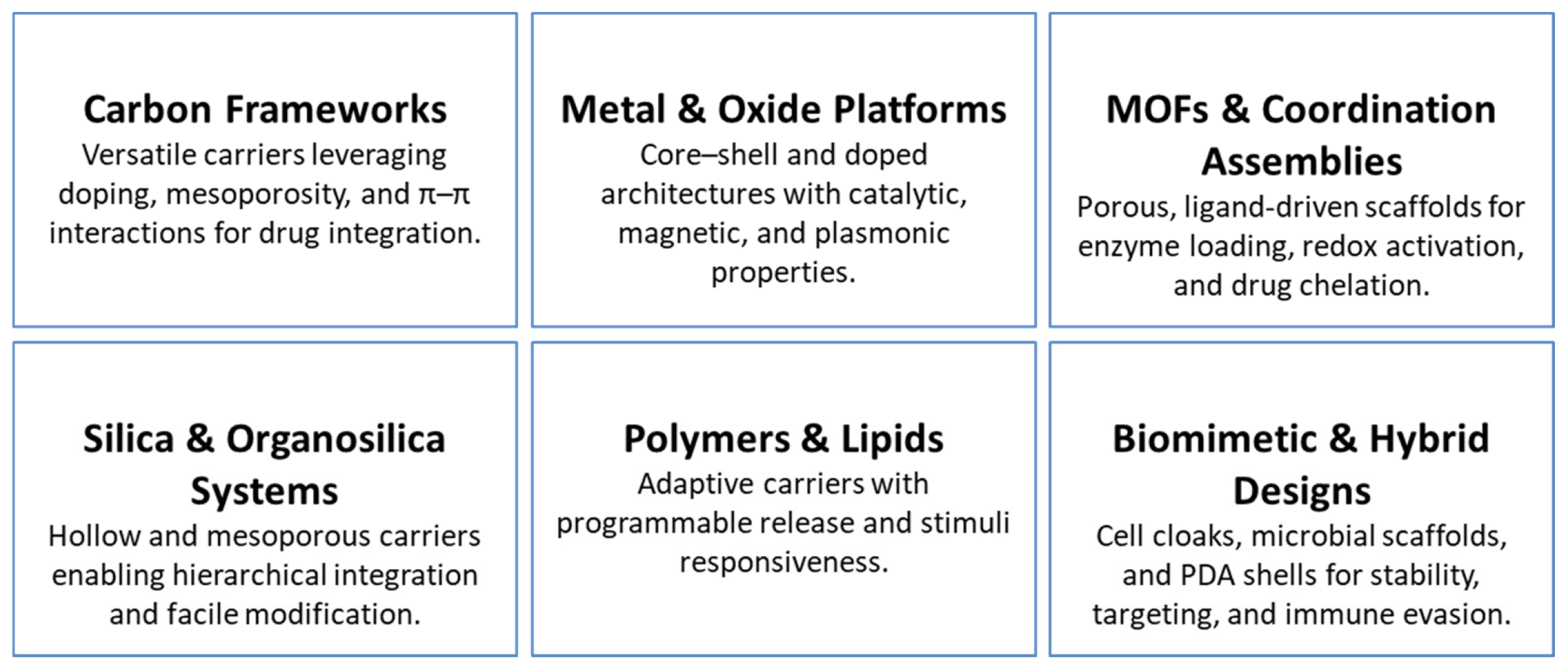
| Nanomaterial/System Design & Fabrication | Examples & Structural Features | Expert Insights | Representative Refs. |
|---|---|---|---|
| Carbon-Based Nanostructures | S, N-CDs (hydrothermal); g-CQDs (acid-assisted synthesis); HMCS (PEGylated, GA-loaded); MWNT-based hybrids. | Heteroatom doping, mesoporosity, and π–π interactions enhance optical properties, loading capacity, and molecular specificity. | [14,20,26,27] |
| Metal & Metal Oxide Platforms | Pt-tipped Au@ZIF-8; Fe-doped MoOx nanowires; Gd-MFe3O4 mesoporous nanoparticles; MnO2-coated GO/ICG & DOX systems. | Alloying, heteroatom doping, and surface-shell engineering enable tunable redox reactivity, controlled biodegradation, and enhanced catalytic/plasmonic functions. | [17,18,21,64] |
| MOFs & Coordination Assemblies | Fe/Cu-MOF-199@PDA; UiO-67 CIDF; ZIF-67-derived FCS; DOX@FL coordination networks. | MOF porosity and modular coordination chemistry support high loading capacity, redox activation, and direct assembly of therapeutic agents. | [5,24,32,33] |
| Silica & Organo-silica Systems | UCNJ tri-layer (core–shell–hydrogel); HMONs with ultrasmall CuS/DSF/3-AT; GOx–Gd–CuS@MSNs. | Silica frameworks enable hierarchical integration of multiple agents with robust porosity control and facile surface functionalization. | [25,28,35] |
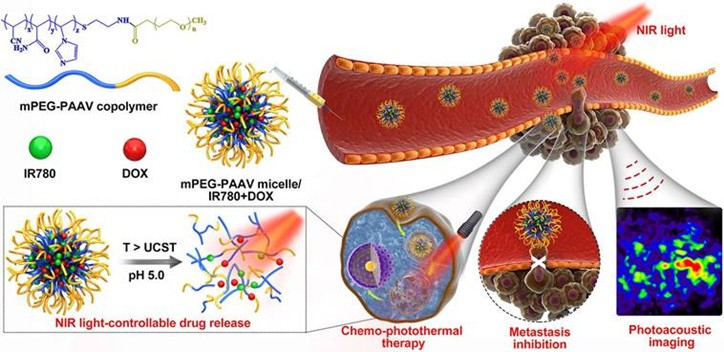
| Polymeric & Lipid Systems | Thermosensitive liposomes (DG@TLs); PLGA@MB/Gd nanoparticles; UCST micelles (mPEG-PAAV); P1-CapNO polymers. | Polymer and lipid matrices offer tunable size, responsive release behavior, and programmable encapsulation for combination therapy. | [19,23,31,36] |
| PDA & Hybrid Shells | UCNP@SiO2-MB@PDA; PFP@MPDA-DOX; BiVO4/Fe3O4@PDA supra particle. | PDA coatings provide universal adhesion, photothermal functionality, biodegradability, and pH-responsive drug regulation. | [16,29,30] |
| Biomimetic & Biohybrid Platforms | Bifidobacterium–nanoparticle hybrids; membrane-cloaked MCNPs; engineered E. coli@PDA-DOX. | Microbial scaffolds and cell-membrane cloaks support homotypic targeting, immune evasion, and enhanced intratumoral accumulation. | [22,34,38,65] |
| Molecular & Supramolecular Agents | DPP-TPA nanoparticles; ferrocene–disulfide PS (HFP-SS-Fc); benzo phenothiazine pro-PS (BPN); AIE systems (TPETTBI, TPN-Cb). | Molecular-level engineering enables intrinsic responsiveness (AIE, redox activation, caging) without reliance on inorganic scaffolds. | [15,37,41,66,67] |
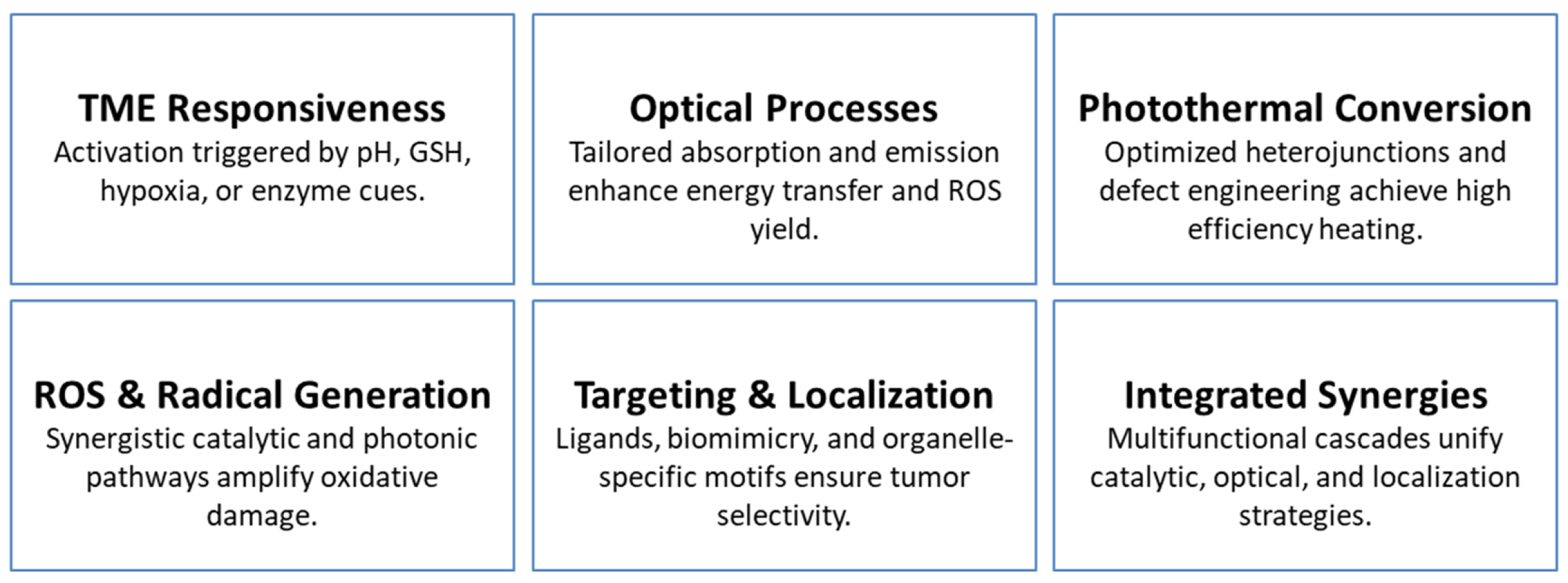
| Mechanistic Functional Properties | Examples & Explanations | Expert Insights | Representative Refs. |
|---|---|---|---|
| pH-Responsive Activation | MPDA undergoes decomposition in acidic/GSH-rich TME, enabling drug release and fluorescence activation; ZIF-8 MOFs disassemble under acidic pH, releasing Zn2+ and enhancing AIE output. | Acidic TME conditions are leveraged for both structural disassembly and real-time signal amplification. | [39,42] |
| GSH-Responsive Redox Cycling | MoOx platforms deplete intracellular GSH while generating 1O2 and ·OH; Cu nanodots—with 3-AT–mediated catalase suppression—accelerate Cu+-driven Fenton chemistry under NIR-II. | GSH depletion simultaneously weakens tumor antioxidant defenses and sustains redox-driven therapeutic cycling. | [35,40] |
| Hypoxia-Triggered Activation | NTR-activated pro-photosensitizers switch on fluorescence and initiate oxygen-independent Type I PDT in hypoxic regions. | Hypoxia acts as a biochemical gate, enabling selective PDT with minimal background activation. | [41] |
| Optical Energy Transfer | UCNJs emit 660 nm light to excite methylene blue, generating singlet oxygen. | Precise spectral coupling maximizes excitation efficiency and enhances PDT performance. | [28] |
| Heavy-Atom Effect for ROS | Iodine-substituted aza-BODIPY increases 1O2 yield by 1.57× compared to bromide analogs and achieves 34.8% PCE. | Heavy-atom substitution enhances intersystem crossing and boosts ROS formation. | [68] |
| Heterojunction Charge Transfer | MoS2–Ti3C2 Z-scheme heterojunction exhibits 59.1% PCE and robust O2−· generation via directional charge separation. | Efficient charge separation strengthens both photothermal output and photocatalytic ROS generation. | [69] |
| Photothermal Conversion Efficiencies | Carbon dots (54.9%); MoOx (51.5%); Cu2+ catalytic NPs (57.45%); MPDA (45.6%); polymeric IR780 micelles (23.8%). | Highest PCE values typically arise from heterojunction structures and defect-engineered nanomaterials. | [30,36,40,69,70,71] |
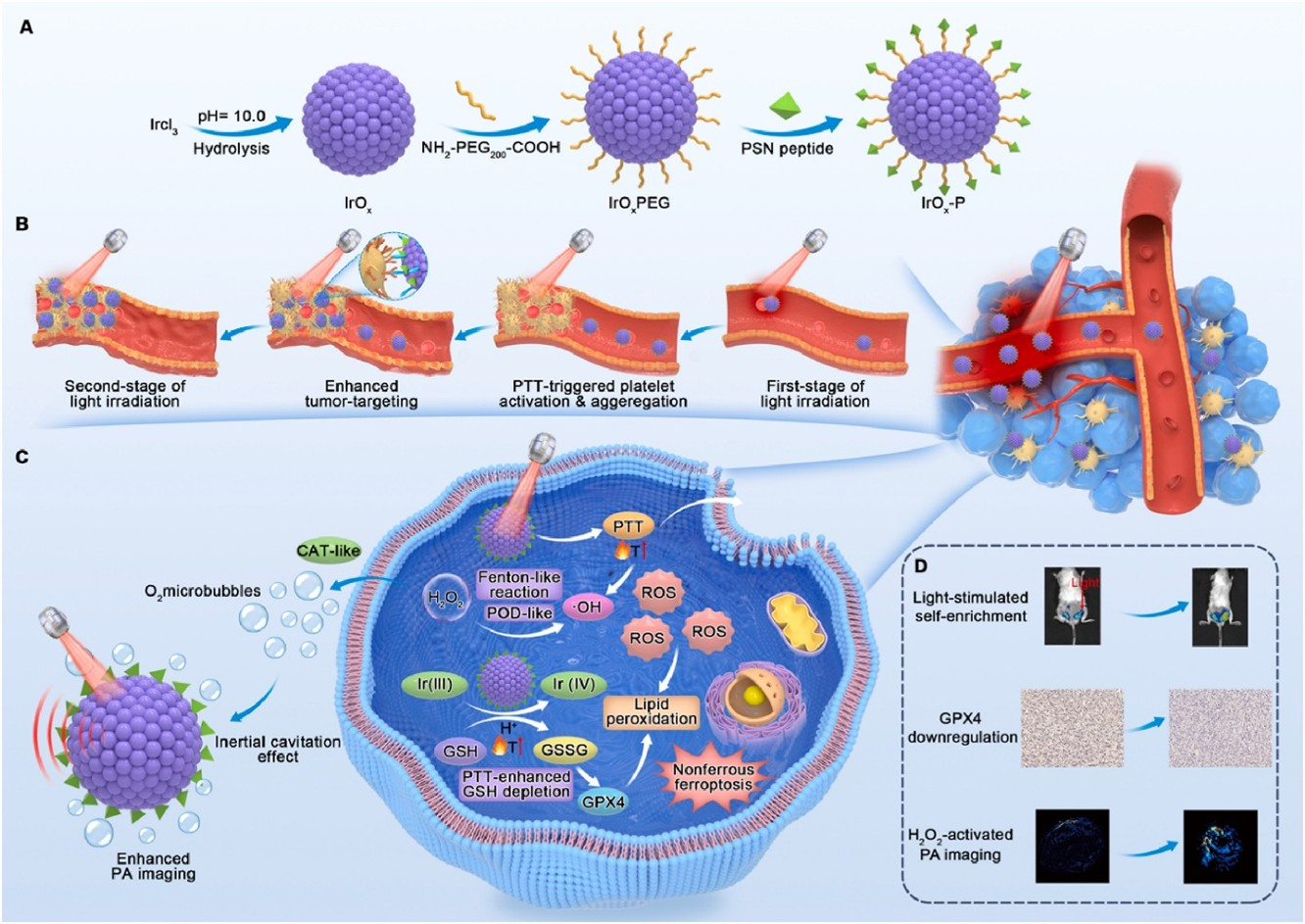
| Catalase-Like Activity | Pt-decorated constructs catalyze H2O2 to O2; IrOX nanozymes simultaneously deplete GSH and catalyze ·OH and O2 production. | Catalase-mimetic functions enhance intratumoral O2 availability while amplifying ROS-mediated therapy. | [13,73] |
| Fenton & Fenton-Like Catalysis | Fe-doped sulfides enhance electron transfer to boost ·OH generation; CuS nanodots combine NIR-II photothermal heating with Cu+ Fenton catalysis. | Transition-metal doping narrows band gaps and accelerates catalytic reaction kinetics. | [33,35,74] |
| Gas Therapeutics | NO-releasing platforms enable thermally activated NO delivery (lifetime ~2 s, diffusion ~200 µm); Mn carbonyl complexes release CO under H2O2/NIR and degrade to Mn2+. | Gas transmitters expand therapeutic mechanisms beyond ROS, enabling O2-independent modalities. | [36,75] |
| Targeting Mechanisms | HA for CD44 recognition; c(RGDyK) for integrin binding; TPP-modified MoS2–Ti3C2 for mitochondrial localization; cancer cell membrane cloaks; bacterial colonization of hypoxia enabling cavitation. | Multilevel targeting integrates ligand affinity, biomimetic interfaces, and organelle-specific localization. | [34,38,56,69,76] |
| Integrated Synergies | MoOx (PCE 51.5%, O2 production, GSH depletion, 1O2/·OH generation); Fe-doped sulfides (NIR-II PTT + Fenton + ferroptosis); AIE agents combining emission, ROS, PTT, and mitochondrial targeting. | Multifunctional constructs support self-reinforcing mechanistic cascades that amplify therapeutic outcome. | [33,40,67] |
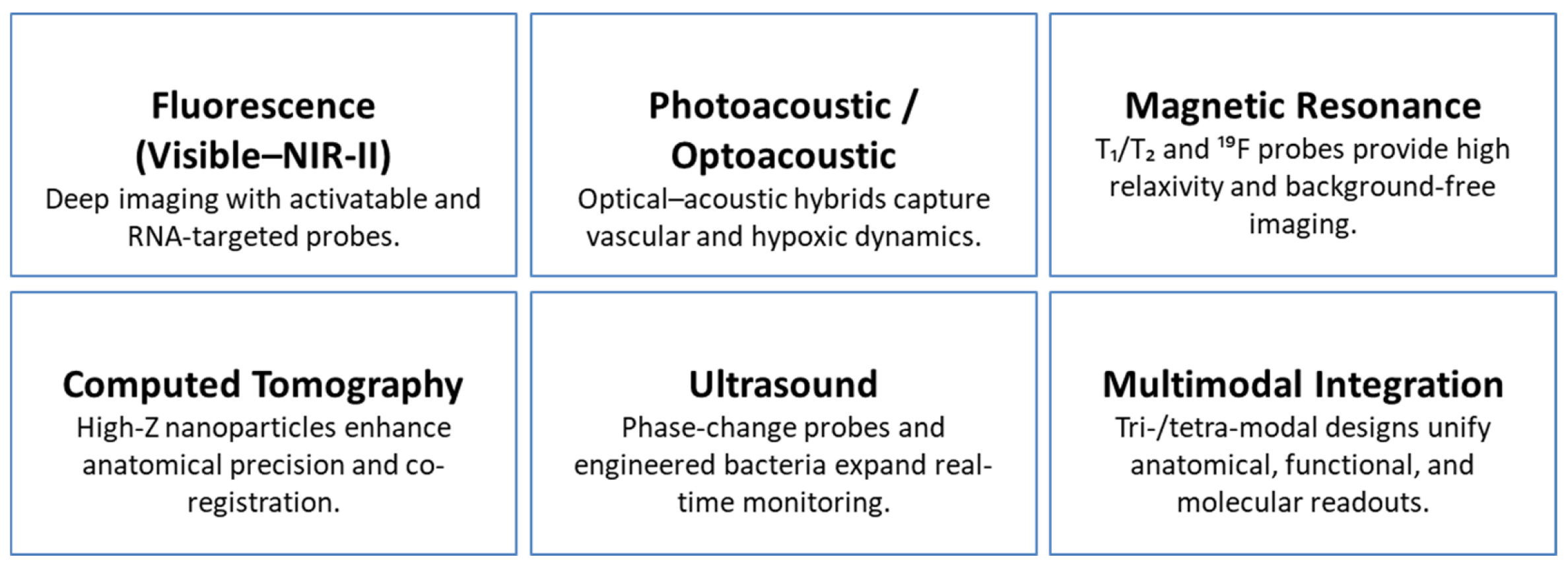
| Diagnostic & Imaging Capabilities | Representative Examples | Expert Insights | Representative Refs. |
|---|---|---|---|
| Fluorescence Imaging (FL, NIR-II, Two-Photon, Lifetime) | NIR-II vascular mapping and bone-targeted diagnostics; apoptosis tracking via two-photon and lifetime FL; activatable probes responsive to pH, GSH, H2O2, or hypoxia; RNA-targeted nano sensors enabling mRNA/miRNA discrimination. | NIR-II imaging enhances penetration depth and tumor–background contrast; activatable probes provide stimulus-specific selectivity; RNA-level FL sensors add molecular-resolution precision to tumor characterization. | [2,12,14,16,24,34,37,39,50,78,79,81,82,83,84] |
| Photoacoustic/Optoacoustic Imaging (PA, PAM, MSOT) | NIR-II PAI and multispectral optoacoustic tomography for high-resolution tumor delineation; monitoring of apoptosis and vascular remodeling; TME-responsive probes activated by MMPs, NTR, or H2S. | PA combines optical absorption with ultrasound detection to achieve deep, high-contrast imaging; biochemical activation improves specificity for hypoxia, enzymatic activity, and redox gradients. | [13,15,31,35,41,54,77,82,85,86,87,88,89,90] |
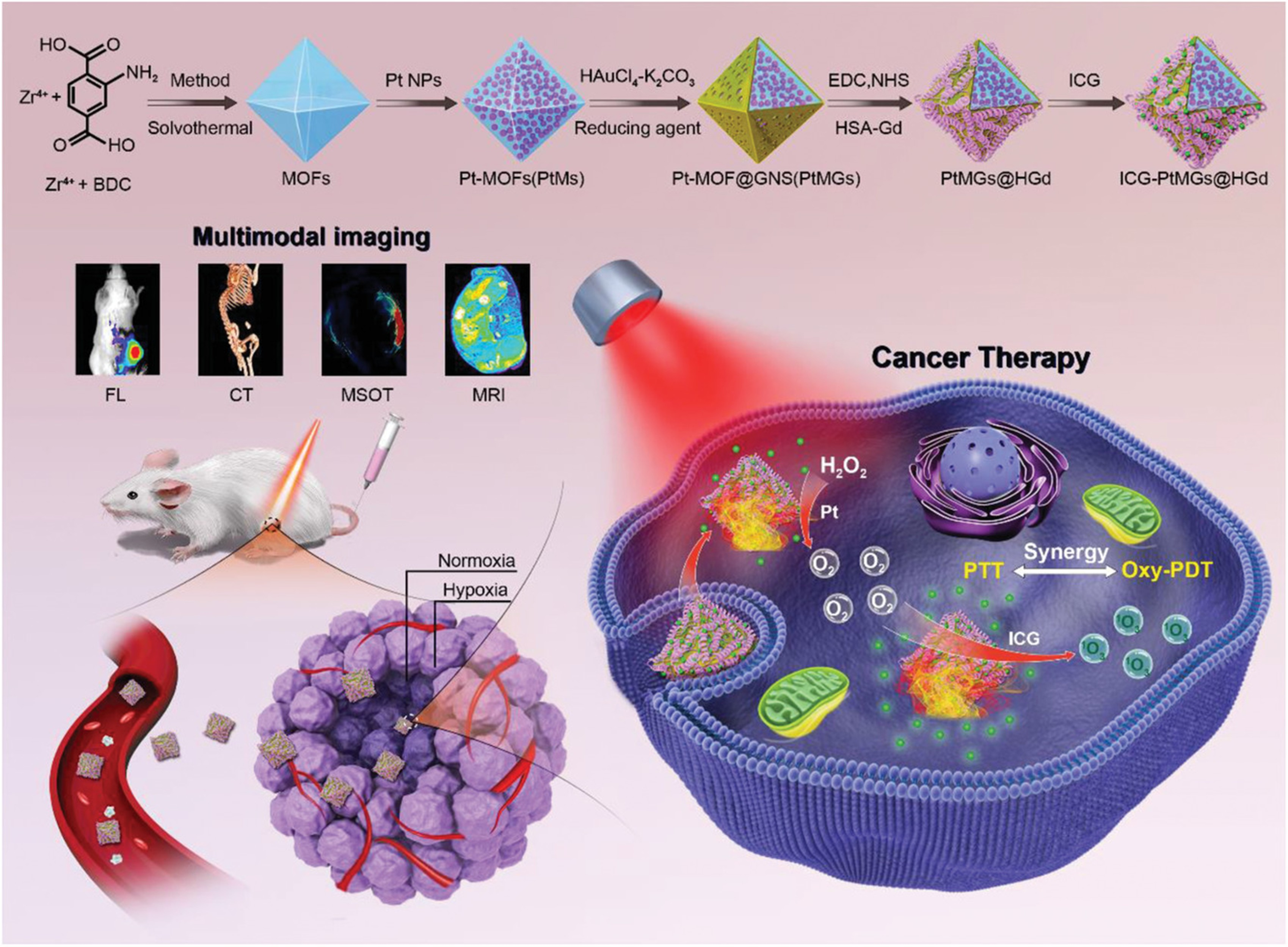
| Magnetic Resonance Imaging (MRI: T1, T2, Dual, 19F) | Mn-, Fe-, and Gd-based T1/T2 contrast enhancers; dual-mode T1/T2 agents; activatable probes responsive to pH, GSH, and redox state; 19F MRI permitting background-free quantification. | Dual-mode probes minimize diagnostic ambiguity; 19F imaging offers high specificity without endogenous interference; elevated relaxivity values surpass those of standard clinical formulations. | [5,6,18,32,59,64,74,75,91,92,93,94] |
| Computed Tomography (CT) | High-Z nanomaterials (Au, Pt, Bi, W) for improved X-ray attenuation; CT combined with MRI and FL for structural–molecular integration. | CT provides precise anatomical mapping; high-Z nano agents frequently double as therapeutic photothermal or radio sensitizing components, enabling integrated diagnostics and therapy. | [17,29,42,56,73,87,95,96,97] |
| Ultrasound Imaging (US, HIFU Guidance) | PFH-loaded, aptamer-modified nanodroplets; bubble-generating constructs; engineered bacteria expressing gas vesicles for endogenous US contrast. | US gains functional depth when coupled with PA/MR or biologically engineered contrast sources; supports real-time therapeutic navigation, including HIFU ablation. | [4,22,30,38,63,99] |
| Multimodal Imaging Integration | Tri- and tetra-modal platforms combining MRI, CT, FL, PA, and US within a single construct. | Multimodal systems unify anatomical, molecular, and functional imaging, offering comprehensive intraoperative and longitudinal diagnostic guidance. | [29,42,48,73,98,100] |
| Functional & Molecular Diagnostics | Imaging of apoptosis, hypoxia, perfusion, ROS, GSH, H2O2, H2S; mRNA/miRNA-responsive nanoprobes for molecular fingerprinting. | Diagnostics evolve from passive contrast to active biosensing, enabling real-time mapping of metabolic stress, microenvironmental gradients, and gene-level markers. | [3,12,14,25,39,41,60,77,84,90,94,101] |
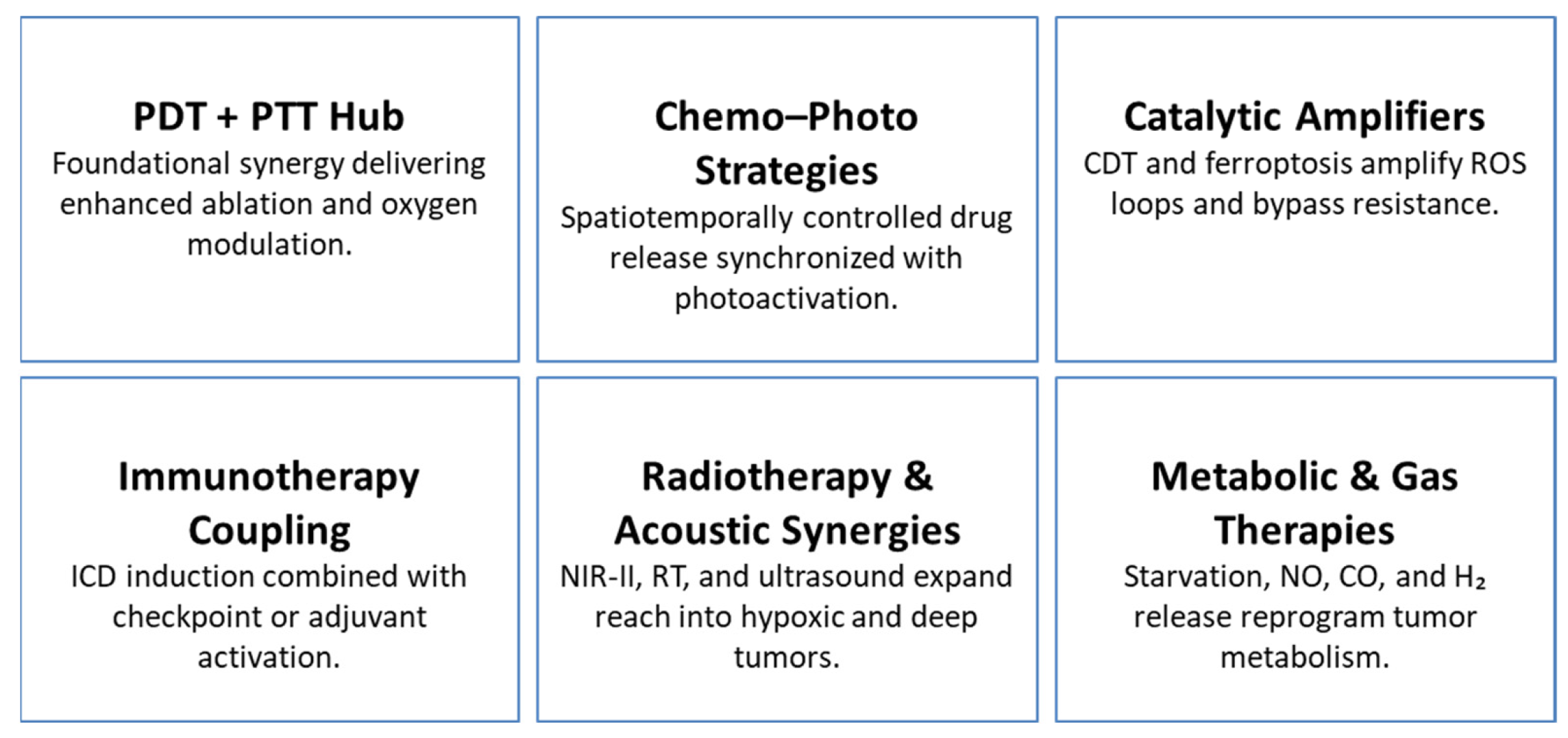
| Therapeutic Capability | Representative Examples | Expert Insights | Representative Refs. |
|---|---|---|---|
| PDT + PTT Synergy | S, N-doped CDs; HA-TiO2@MWCNTs/HMME; iodinated aza-BODIPY nanoparticles | PDT/PTT combinations strengthen localized cytotoxicity, while catalytic components help sustain PDT efficacy under hypoxia. | [6,14,56,68,76] |
| Chemo–Photo Combinations | DOX-Pt@Au@ZIF-8; MMP-responsive Au NPs; polymeric micelles | Co-delivery and stimulus-triggered release enable spatially regulated chemo–photo activation and reduced systemic toxicity. | [17,30,42,89,102] |
| CDT and Ferroptosis | PBAM MOFs; Fe-doped MoOx; Cu/CC nanocomposites; IrOX-P cascades | Redox-catalytic ROS amplification enhances phototherapy, while iron-dependent ferroptosis provides irreversible lipid damage. | [1,13,18,83,88] |
| Immunotherapy + Local Modalities | ICG-CpG@MOF; FYH-PDA-DOX; biomimetic metallacages | Local ICD induction primes systemic antitumor immunity and complements immune checkpoint blockade strategies. | [34,53,69,100,104] |
| Radiotherapy & Acoustic Synergies | PEGylated W-TiO2; BiVO4/Fe3O4@PDA; F3-PLGA@MB/Gd | Thermo-radiotherapy improves RT efficacy in hypoxic tissues, while acoustic platforms integrate SDT, PTT, and mechanical drug release. | [23,29,38,87,105] |
| Metabolic & Gas Therapies | GOx-based nano factories; P1-CapNO; MnCO@CuS | Metabolic disruption and gas signaling (NO, CO, H2) modulate the TME and synergize with oxidative and photothermal therapies. | [12,36,75,106,107] |
| Theranostic Integration | DCDM nanoparticles; DUPM constructs; persistent luminescence probes | Embedded imaging functions provide real-time feedback, enabling adaptive, stimulus-responsive therapeutic control. | [3,40,58,73,98,108] |
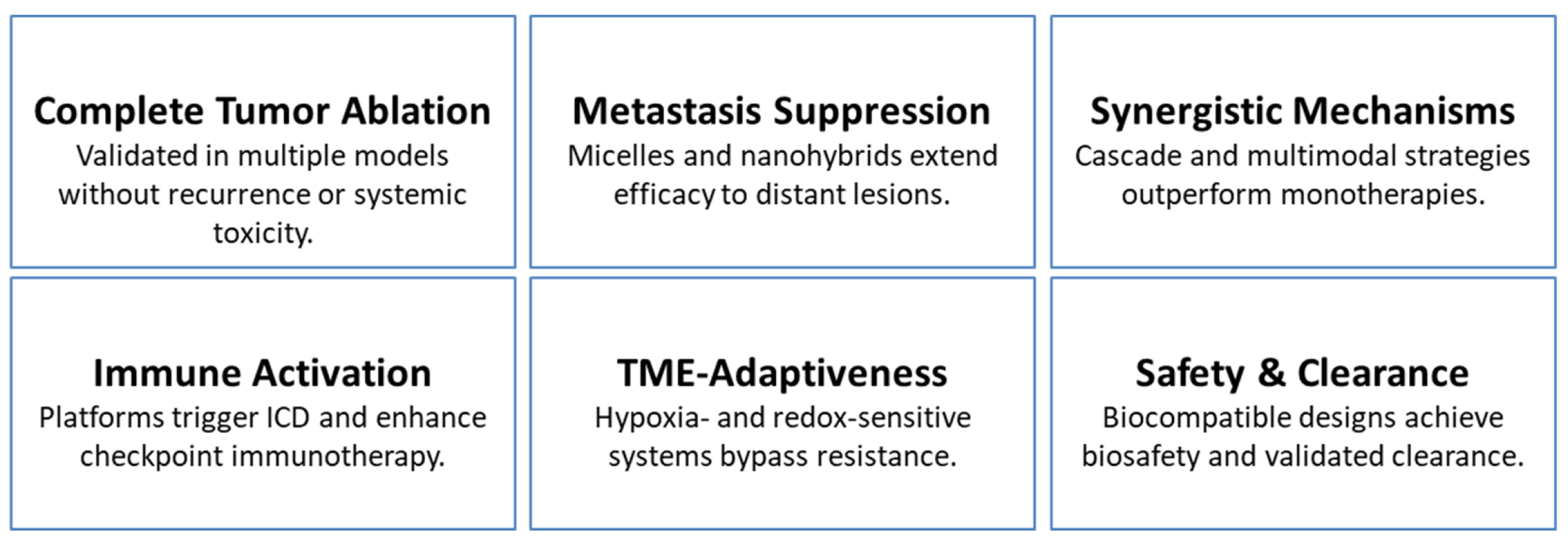
| In Vivo Outcomes & Translational Significance | Examples/Explanations | Expert Insights | Representative Refs. |
|---|---|---|---|
| Complete tumor ablation & recurrence prevention | NM-NPs achieved total tumor elimination; DAA nanoparticles prevented relapse; DOX/PPy-ELP-F3 ablated tumors without systemic toxicity. | Complete tumor clearance is attainable through optimized nanoplatform engineering—an essential benchmark for durable, relapse-free cancer therapy. | [9,78,109] |
| Metastasis suppression & systemic control | mPEG-PAAV micelles eradicated primary tumors and suppressed lung metastases; ABI Nys inhibited bone destruction and metastasis; m@MTT suppressed both local and distant lesions. | Demonstrating efficacy against metastatic progression strengthens translational relevance, addressing the most fatal dimension of cancer. | [31,69,82] |
| Multimodal & synergistic therapies | DOX-Pt-tipped Au@ZIF-8 enabled potent chemo-phototherapy; Fe3+-DOX nanoliposomes triggered apoptosis + ferroptosis; Cu/CC assemblies enabled trimodal ROS-based therapy. | Synergy-driven designs overcome resistance mechanisms and achieve deeper, more durable tumor regression than monotherapies. | [1,17,83] |
| Immune activation & checkpoint synergy | FYH-PDA-DOX induced robust T-cell responses; TAPP-GCP@TCPP@BSA promoted ICD and synergized with PD-L1 blockade; Albumin-based nanocomposites regressed primary and metastatic TNBC lesions. | Nanoplatforms can function as powerful immune sensitizers, amplify checkpoint blockade efficacy and enable systemic tumor control. | [104,111,112] |
| TME responsiveness & resistance avoidance | H2S-activatable MOFs selectively ablated orthotopic colon tumors; P1-CapNO overcame hypoxia-induced PDT resistance; AUC-GOx/Cel nano factories enhanced CDT via catalytic feedback. | Leveraging TME-driven activation enhances precision, reduces off-target toxicity, and helps overcome adaptive resistance in heterogeneous tumors. | [12,36,90] |
| Imaging-guided precision & theranostic integration | UCNP@SiO2-MB@PDA enabled NIR-guided PDT/PTT; PC61BA-(Gd-DO3A)/HSA improved MRI-guided surgical precision; MnCO@CuS provided MRI-guided gas/photo-chemo dynamic therapy. | Integrating diagnostics with therapy transforms imaging into a real-time decision tool, enhancing spatial accuracy and treatment adaptability. | [16,59,75] |
| Safety, biocompatibility & clearance | PEGylated WTO nanoparticles were completely cleared within 30 days; IABN nanoparticles demonstrated low systemic toxicity; DUPM nanomedicine achieved 94.43% inhibition with strong biocompatibility. | Favorable biodistribution, biodegradability, and low toxicity establish a strong foundation for clinical translation. | [40,68,87] |
| Therapeutic/Platform Theme | Rationale (Clinical Need) | Mechanistic Core (How It Works) | Current Evidence (Preclinical) | Candidate Populations/Contexts | Outcome Signals (What We See) | Practice-Directed Next Steps | Future Gaps & Priorities | Representative Refs. |
|---|---|---|---|---|---|---|---|---|
| PDT + PTT Synergy Hub | Overcome chemo/radio-resistance; precise local control with minimal systemic toxicity | Light-driven ROS (PDT) + hyperthermia (PTT); catalytic O2 supply via catalase, MOx, or PDA-hybrid shells to offset hypoxia | Complete tumor ablation in murine models; sustained ROS generation in MPDA/MON/PDA-ICG constructs | TNBC, hepatic tumors, hypoxic & heterogeneous solid masses | Full preclinical eradication with low toxicity | Standardize NIR/NIR-II parameters; integrate intraoperative imaging guidance | Depth constraints; inorganic residue long-term safety; thermal gradients | [6,14,56,68,76] |
| Chemo–Photo Platforms (PTT/PDT + Drug) | Minimize systemic chemotherapy burden via on-demand localized release | Heat/pH/MMP-responsive release; co-localized chemo + photoablation | Single-laser chemo–photo synergy; ligand-directed artemisinin delivery; robust micelle & mesoporous control | Large solid tumors; lesions near sensitive anatomy | Enhanced inhibi-tion vs. mono-therapy; strong cooperative effects | Optimize release–light timing; image-guided dose planning | Scale-up issues; EPR variability; risk of premature activation | [17,30,31,42,89] |
| CDT & Ferroptosis Catalytic Amplifiers | Address hypoxia and redox-rich, drug-resistant tumors | Fenton/Fenton-like ·OH generation; GSH depletion; lipid peroxidation (ferroptosis); NIR-II enhanced catalysis | MRI-guided CDT; trimodal ROS cascades; ferroptosis + apoptosis/ICD | Drug-resistant, hypoxic tumors with high antioxidant buffering | Potentiated tumor kill; catalytic self-reinforcement | Develop ferroptosis biomarkers; pair with low-dose PDT/PTT | Off-target oxidative injury; metal fate & persistence; variable redox microenvironments | [1,13,18,33,40,74,83,88] |
| Immunotherapy + Local Ablation | Translate local ablation into systemic immune control | ICD induction; checkpoint synergy (PD-L1); CpG or adjuvant-reinforced immune activation; macrophage repolarization | Distant tumor suppression; cold-tumor reprogramming; improved checkpoint outcomes | Metastatic TNBC; immunologically “cold” phenotypes | Systemic T-cell activation; abscopal-like responses | Synchronize ICD with checkpoint dosing; integrate immune monitoring | Durability across heterogeneous immune states; autoimmune risks | [34,53,69,100,104,111,112] |
| RT & Acoustic Synergies (SDT, HIFU, FUAS) | Treat deep, poorly perfused lesions; combine mechanical & thermal modalities | SDT ROS formation; thermo-radiotherapy; HIFU-triggered drug release; biological cavitation via engineered microbes | NIR-II PTT + RT synergy; SDT/HIFU platforms with activatable delivery | Deep-seated tumors; bone/brain-adjacent malignancies | Enhanced ablation; multi-modal image guidance | Co-register RT/US with MRI/PAI; define thermal–dose thresholds | Workflow complexity; cavitation unpredictability; tissue-specific acoustics | [23,29,38,87,105] |
| Metabolic & Gas Therapies (Starvation/NO/CO/H2) | Reprogram TME metabolism and Vaso dynamics to overcome resistance | GOx-mediated glucose depletion + H2O2 amplification; NO/CO/H2 release regulating perfusion, stress pathways, or inflammation | Dual H2O2-amplifying nano factories; NO-augmented PTT; CO-integrated PTT/CDT | Hypoxic, glycolytic tumors; metastatic niches | Strong synergy with PDT/PTT/CDT; reduced inflammatory damage | TME-mapping for patient stratification; safety thresholds for gas donors | Tumor metabolic plasticity; gas diffusion control; vascular off-target effects | [12,36,75,106,107] |
| Theranostics & Imaging-Integrated Platforms | Need real-time monitoring, adaptive dosing, surgical margin clarity | MRI/CT/PA/FL/NIR-II integrated with ROS/O2/GSH-responsive activation; persistent luminescence for excitation-free tracking | NIR-II/CT/MRI-guided PDT/PTT/CDT; dynamic adaptive imaging | Candidates requiring surgical navigation or ambiguous margins | Higher spatial precision; multimodal real-time feedback | Define imaging–response endpoints; develop closed-loop control algorithms | Scanner variability; relaxivity–safety trade-offs; standardization barriers | [3,16,40,58,59,73,75,94,98,108] |
| Materials & Manufacturability (Cross-Cutting Theme) | Scale, safety, and regulatory feasibility | Intrinsic multifunctionality via doping/defects; PDA/PLGA; green synthesis; reduced component complexity | High PCE and catalytic efficiency with simplified constructs; 30-day clearance in some inorganic systems | Outpatient & OR-compatible systems; repeat-dose settings | Strong biocompatibility; low dark toxicity | Prioritize single-molecule or few-component platforms; GMP-ready synthesis | Chronic biodistribution; patient variability in EPR; regulatory complexity | [20,23,29,30,40,67,68,69,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Gill, E.J. Multifunctional Nanoplatforms Bridging Diagnostics and Therapeutics in Cancer. Micromachines 2025, 16, 1323. https://doi.org/10.3390/mi16121323
Omidian H, Gill EJ. Multifunctional Nanoplatforms Bridging Diagnostics and Therapeutics in Cancer. Micromachines. 2025; 16(12):1323. https://doi.org/10.3390/mi16121323
Chicago/Turabian StyleOmidian, Hossein, and Erma J. Gill. 2025. "Multifunctional Nanoplatforms Bridging Diagnostics and Therapeutics in Cancer" Micromachines 16, no. 12: 1323. https://doi.org/10.3390/mi16121323
APA StyleOmidian, H., & Gill, E. J. (2025). Multifunctional Nanoplatforms Bridging Diagnostics and Therapeutics in Cancer. Micromachines, 16(12), 1323. https://doi.org/10.3390/mi16121323






