Review on Research Progress of Photoelectrochemical Biosensors
Abstract
1. Introduction
2. Working Principle and Advantages of PEC Biosensors
3. Advances in Photoactive Materials
3.1. Metal Oxides
3.2. Metal Sulfides
3.3. Graphitic Carbon Nitride
3.4. Quantum Dots
3.5. Novel Photoactive Materials
4. Signal Amplification Strategies
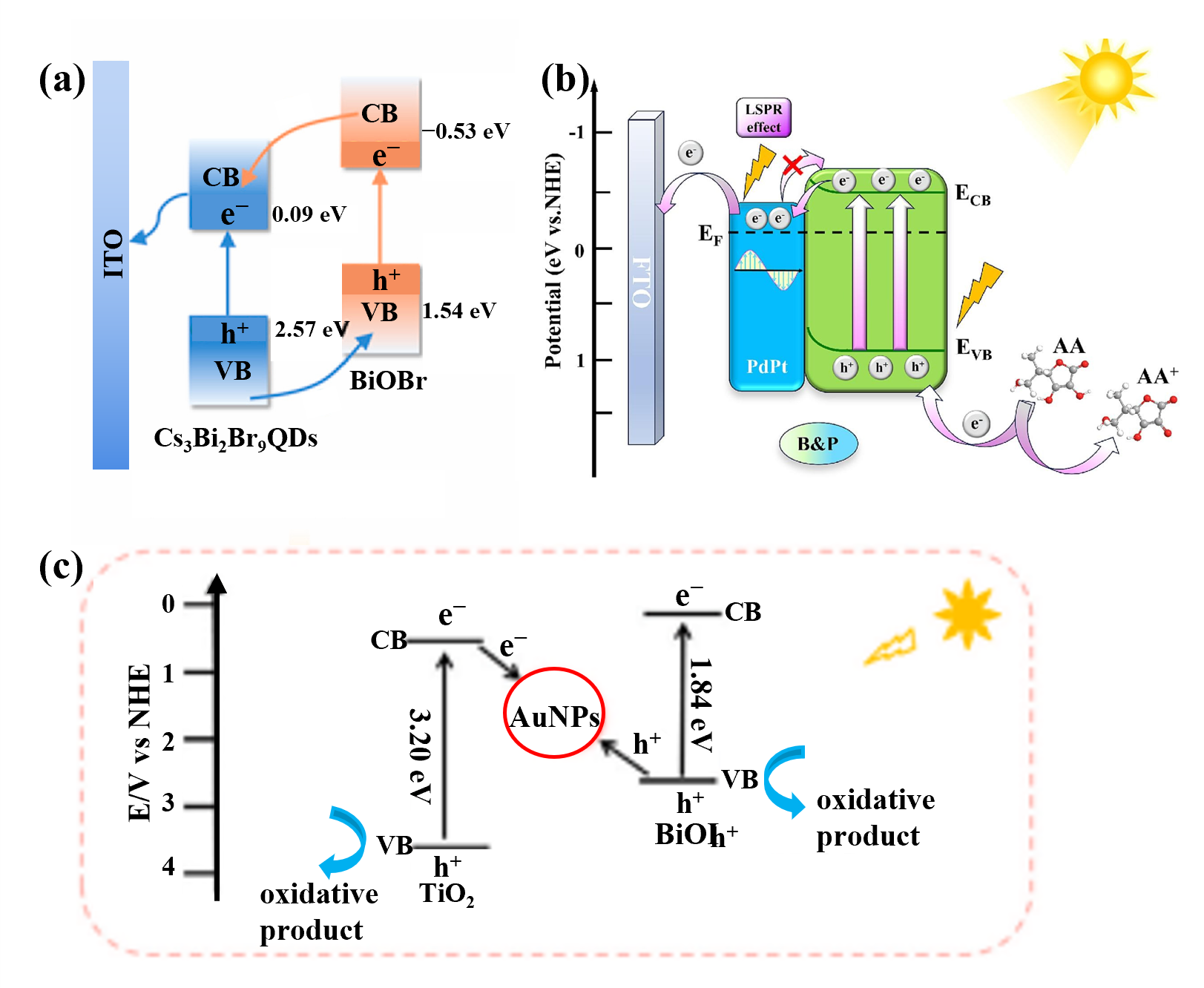
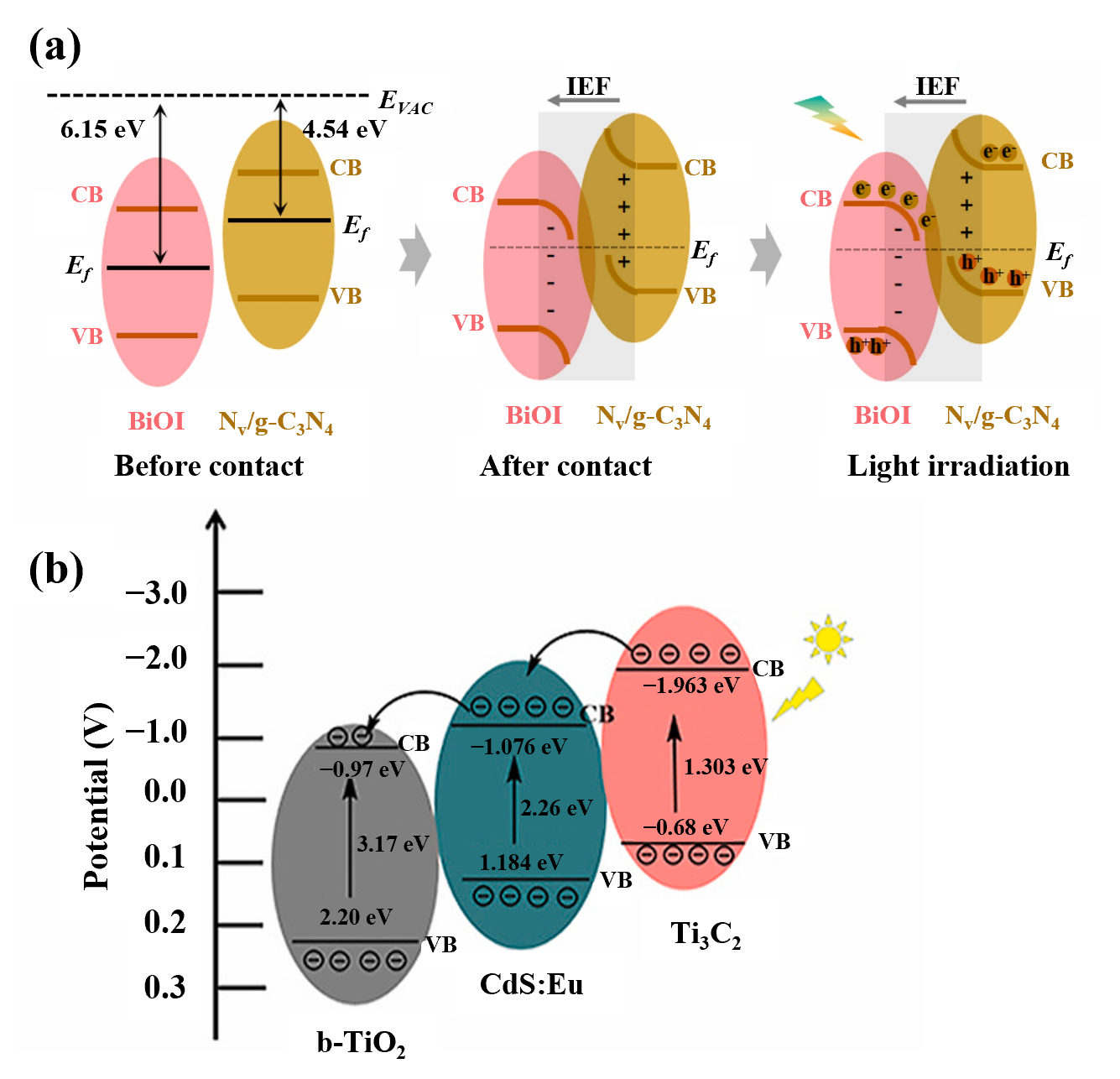
4.1. Construction of Heterojunctions
4.2. Localized Surface Plasmon Resonance
4.3. Electron Donors/Acceptors
4.4. Defect Construction
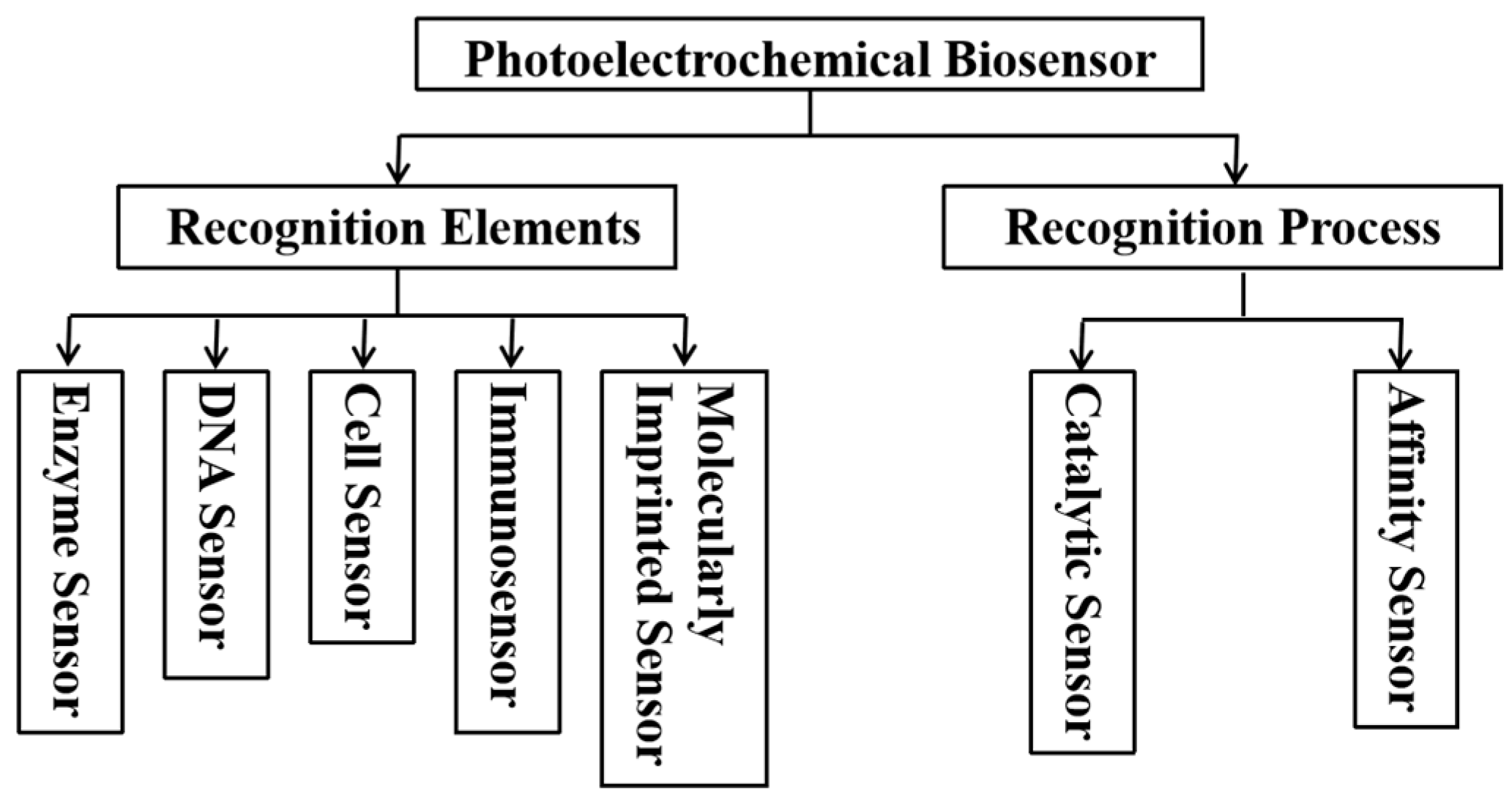
5. Research Progress of PEC Biosensors
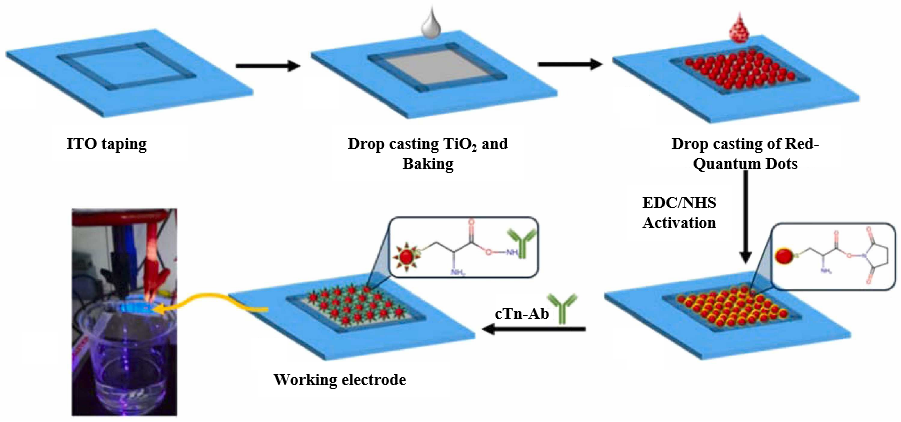
5.1. PEC Immunosensor
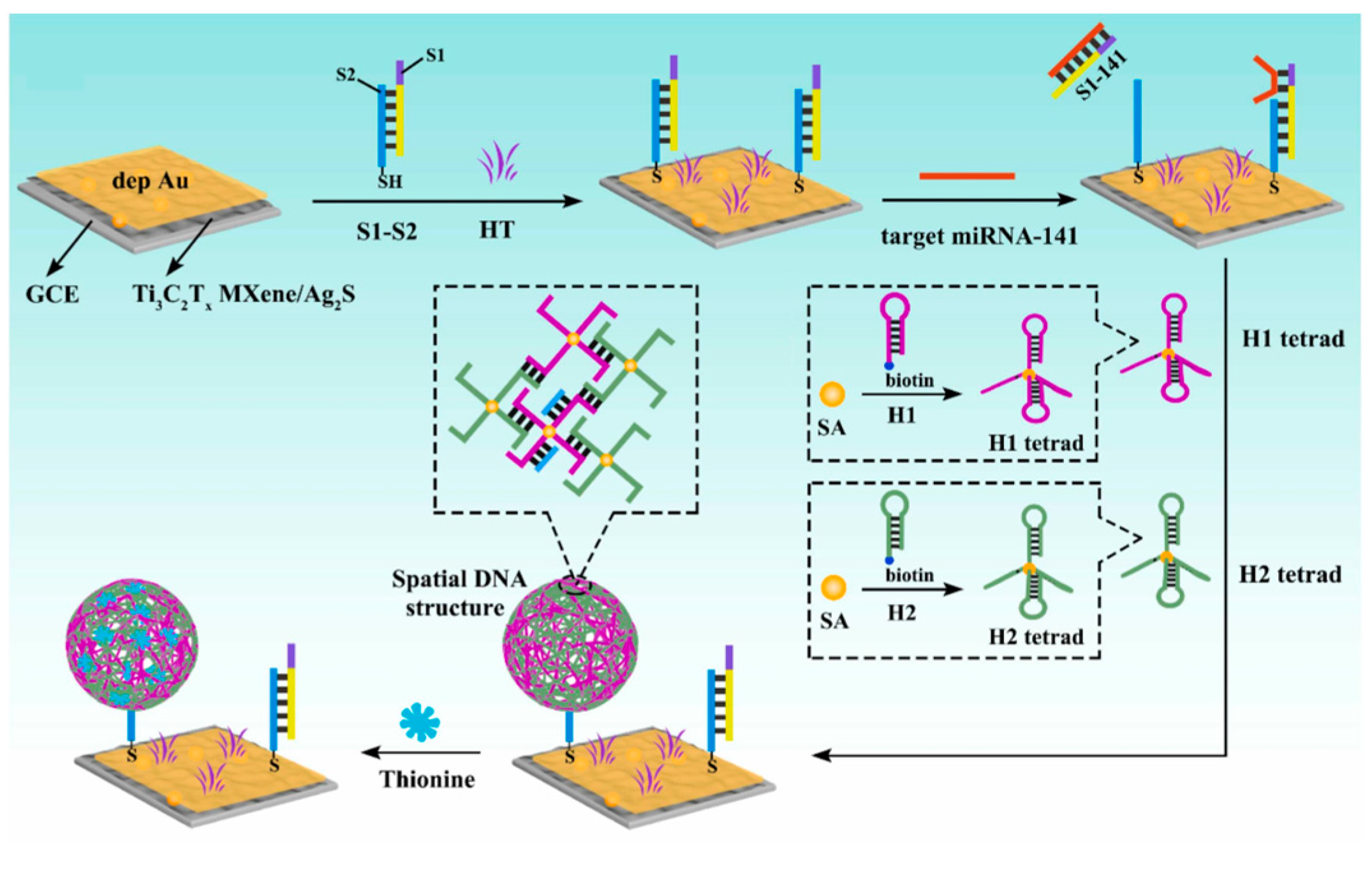
5.2. PEC Gene Sensor
5.3. PEC Microfluidic Biosensors
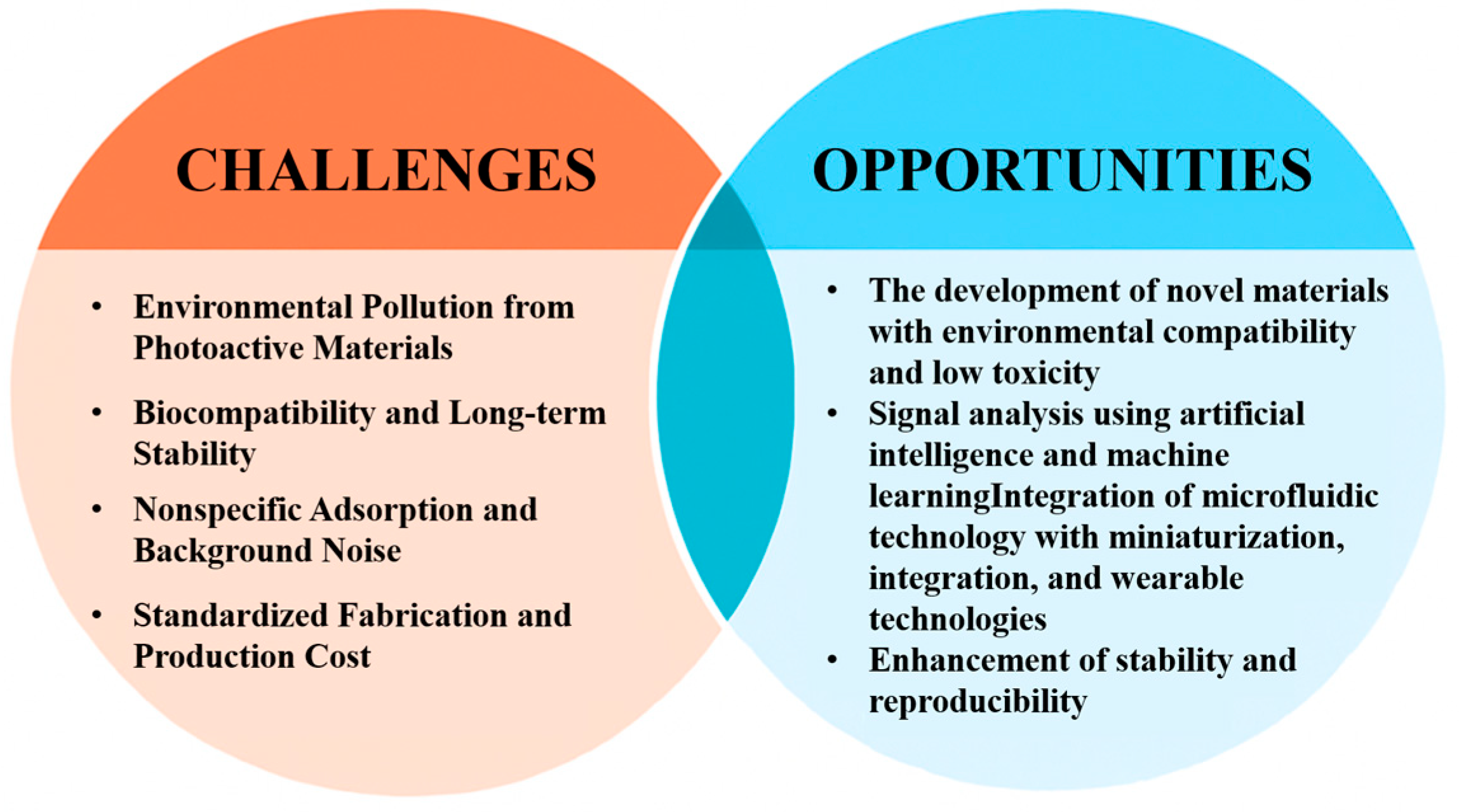
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, X.; Chen, C.; Chen, Y.; Li, Y.; Ye, H.; Wang, B.; Chen, H.; Guo, J.; Ma, X. Magnetic nanorobots as maneuverable immunoassay probes for automated and efficient enzyme linked immunosorbent assay. ACS Nano 2022, 16, 180–191. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Meng, Y.; He, Y.; Xiong, Y.; Ao, K.; Huang, J.; Chen, J.; Xie, Y.; Ying, B. Color and distance two-dimensional visual and homogeneous dual fluorescence analysis of pathogenic bacteria in clinical samples. Sens. Actuators B Chem. 2022, 357, 131422. [Google Scholar] [CrossRef]
- Gil, B.; Keshavarz, M.; Wales, D.; Yeatman, E. Orthogonal Surface-Enhanced Raman Scattering/Field-Effect Transistor Detection of Breast and Colorectal Cancer-Derived Exosomes using Graphene as a Tag-Free Diagnostic Template. Adv. NanoBiomed Res. 2023, 3, 2300055. [Google Scholar] [CrossRef]
- Chandio, I.; Cheung, S.; Wu, L.; Cui, M.; Ai, Y.; Liang, Q. Engineered rGO-AuNPs@ MXene hybrid nanocomposites for highly efficient electrochemical aptasensing of alpha-fetoprotein. Microchem. J. 2025, 214, 113952. [Google Scholar] [CrossRef]
- Yu, R.; Tan, Z.; Xiang, X.; Dan, Y.; Deng, G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer 2017, 17, 608. [Google Scholar] [CrossRef]
- Zheng, J.; Wei, J.; Yang, H.; Xu, F.; Lou, Y.; Song, P.; Wang, A.; Mei, L.; Zhang, L.; Feng, J. Hollow SnO2/CdS QDs/CdCO3 heterostructured nanocubes coupled with hollow PtPd/MnCo-CeO2nanozyme-mediated synergistic amplification for ultrasensitive PEC immunoanalysis of lung cancer biomarker. Biosens. Bioelectron. 2023, 235, 115398. [Google Scholar] [CrossRef]
- Xu, F.; Xu, B.; Ai, Q.; Wang, A.; Mei, L.; Song, P.; Feng, J. Synergistic signal amplification of ultrathin nanowire-like PtCoFeRuMo high-entropy nanozyme and Z-scheme WO3/ZnIn2S4 heterostructure for split-typed photoelectrochemical aptasensing of myoglobin. Chem. Eng. J. 2024, 489, 151374. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Hassan, R. Advances in electrochemical nano-biosensors for biomedical and environmental applications: From current work to future perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, B.; Xu, F.; Wang, A.; Mei, L.; Wu, L. Dual amplification for PEC ultrasensitive aptasensing of biomarker HER-2 based on Z-scheme UiO-66/CdIn2S4 heterojunction and flower-like PtPdCu nanozyme. Talanta 2024, 274, 126034. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2019, 92, 363–377. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, J.; Zhou, Q.; Yu, Z.; Wu, D.; Tang, D. Excited-state intramolecular proton transfer-driven photon-gating for photoelectrochemical sensing of CO-releasing molecule-3. Anal. Chem. 2024, 96, 5014–5021. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, J.; Ju, Y.; Xue, H.; Pang, H. Current advances in semiconductor nanomaterial-based photoelectrochemical biosensing. Chem. Eur. J. 2018, 24, 14010–14027. [Google Scholar] [CrossRef]
- Francesca, B.; Palchetti, I. Photoelectrochemical genosensors for the determination of nucleic acid cancer biomarkers. Curr. Opin. Electrochem. 2018, 12, 51–59. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Q.; Li, W.; Xiao, F.; Xu, H. From engineered photoactive materials to detection signal amplification strategies in photoelectrochemical biosensing of pathogens: New horizons and perspectives. Chem. Eng. J. 2024, 480, 147941. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, S.; Dong, H.; Shang, Q.; Zhang, X.; Li, Y.; Wang, S.; Li, Y. Ultrasensitive photoelectrochemical immunosensor based on Dual-Photosensitive electrodes. Bioelectrochemistry 2022, 147, 108169. [Google Scholar] [CrossRef]
- Sandhyarani, N. Surface modification methods for electrochemical biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–75. [Google Scholar]
- Suni, I. Substrate materials for biomolecular immobilization within electrochemical biosensors. Biosensors 2021, 11, 239. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Lu, G.; Xu, L.; Li, H. A photoelectrochemical immunosensor based on cobalt corrole sensitized carbon nitride for human norovirus detection. Sens. Actuators B Chem. 2024, 418, 136348. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, S.; Dong, H.; Zhang, X.; Wang, S.; Li, Y.; Li, Y.; Cui, H.; Wei, Q. Self-powered photoelectrochemical immunosensor with triple enhanced photoelectric response for sensitive detection of cTnI. Sens. Actuators B Chem. 2023, 393, 134234. [Google Scholar] [CrossRef]
- Wang, B.; Wei, C.; Wang, K.; Fu, B.; Chen, Y.; Han, Y.; Zhang, Z. Fabrication of near infrared light responsive photoelectrochemical immunosensor for in vivo detection of melanoma cells. Biosens. Bioelectron. 2023, 239, 115601. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.; Lu, C.; Wang, Y.; Li, C.; Sun, D. A near-infrared photoelectrochemical immunosensor for CA72-4 sensing based on SnS nanorods integrated with gold nanoparticles. Talanta 2023, 253, 123910. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Peng, Z.; Yang, J.; Li, Y. A novel photoelectrochemical microfluidic chip for multi-index determination of diabetes and its complications. Biosens. Bioelectron. 2022, 217, 114719. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, Q.; Yang, C.; Wang, J.; Xu, X.; Nie, G. Electrochemiluminescence and photoelectrochemistry dual-signal immunosensor based on Ru (bpy)32+-functionalized MOF for prostate-specific antigen sensitive detection. Sens. Actuators B Chem. 2023, 379, 133269. [Google Scholar] [CrossRef]
- Bo, Y.; Li, L.; Miao, P.; Li, C.; Chang, J.; Zhang, Y.; Lv, Y.; Yang, X.; Zhang, J.; Yan, M. 2D Z-scheme ZnIn2S4/g-C3N4 heterojunction based on photoelectrochemical immunosensor with enhanced carrier separation for sensitive detection of CEA. Biosens. Bioelectron. 2024, 247, 115926. [Google Scholar]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Pei, F.; Feng, S.; Hu, W.; Hao, Q.; Liu, B.; Mu, X.; Lei, W.; Tong, Z. A signal-off photoelectrochemical sandwich-type immunosensor based on WO3/TiO2 Z-scheme heterojunction. Microchim. Acta 2023, 190, 384. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Q.; Li, Q.; Zheng, L.; Yang, X.; Wang, X.; Nie, G. A “signal-off” type photoelectrochemical immunosensor for detecting carcinoembryonic antigen based on TiO2 NRs/BiOI heterojunction and SiO2/PDA-Au inhibitor. Microchem. J. 2022, 182, 107888. [Google Scholar]
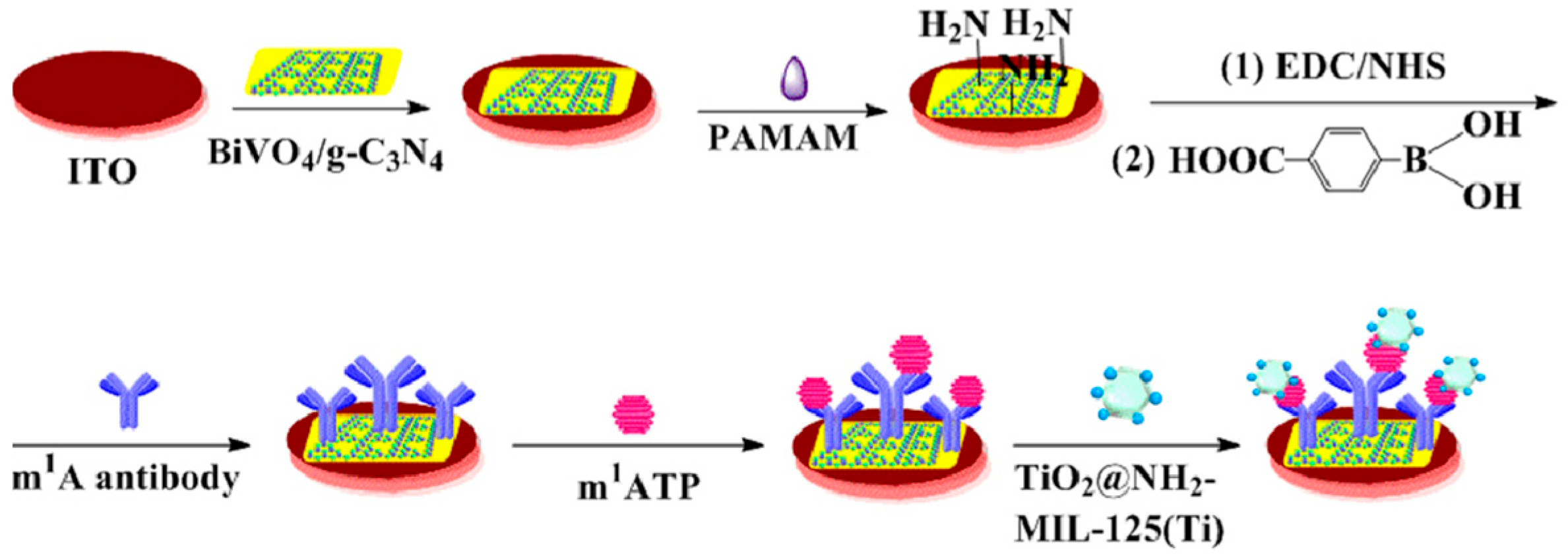
- Zhou, Y.; Wang, Y.; Li, S.; Fang, X.; Yin, H.; Wang, P.; Ai, S. A novel photoelectrochemical immunosensor for N1-methyladenine detection based on BiVO4/g-C3N4 heterojunction with signal amplification of TiO2@NH2-MIL-125(Ti). Sens. Actuators B Chem. 2020, 318, 128310. [Google Scholar] [CrossRef]
- Khan, M.; Ansari, S.; Pradhan, D.; Ansari, M.; Han, D.; Lee, J.; Cho, M. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar]
- Toe, C.; Zhou, S.; Gunawan, M.; Lu, X.; Ng, Y.; Amal, R. Recent advances and the design criteria of metal sulfide photocathodes and photoanodes for photoelectrocatalysis. J. Mater. Chem. A 2021, 9, 20277–20319. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.; Du, M.; Zeng, X.; Zhong, G.; Qiu, B.; Zhang, J. Recent progress of metal sulfide photocatalysts for solar energy conversion. Adv. Mater. 2022, 34, 2202929. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, J.; Wang, C.; Wang, T.; Zhao, F.; Zeng, B. CdS/Bi2S3/NiS ternary heterostructure-based photoelectrochemical immunosensor for the sensitive detection of carbohydrate antigen 125. Anal. Chim. Acta 2024, 1312, 342765. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, T.; Cui, Q.; Qu, Z.; Zhang, Y.; Ma, H.; Wei, Q. ReS2@Au NPs as signal labels quenching steady photocurrent generated by NiCo2O4/CdIn2S4/In2S3 heterojunction for sensitive detection of CYFRA 21-1. Biosens. Bioelectron. 2023, 222, 114992. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, X.; Lu, L.; Tang, D. Snowflake-like CdS@ZnIn2S4 heterojunction-based photocatalyst-electrolyte effect: An innovative mode for photoelectrochemical immunoassay. Biosens. Bioelectron. 2022, 216, 114679. [Google Scholar] [CrossRef]
- Ibrahim, I.; Lim, H.; Zawawi, R.; Tajudin, A.; Ng, Y.; Guo, H.; Huang, N. A review on visible-light induced photoelectrochemical sensors based on CdS nanoparticles. J. Mater. Chem. B 2018, 6, 4551–4568. [Google Scholar] [CrossRef]
- Yao, J. A multiple signal amplification photoelectrochemical biosensor based on biotin-avidin system for kanamycin sensing in fish and milk via synergism of g-C3N4 and Ru@SiO2. Anal. Chim. Acta 2024, 1288, 342141. [Google Scholar] [CrossRef]
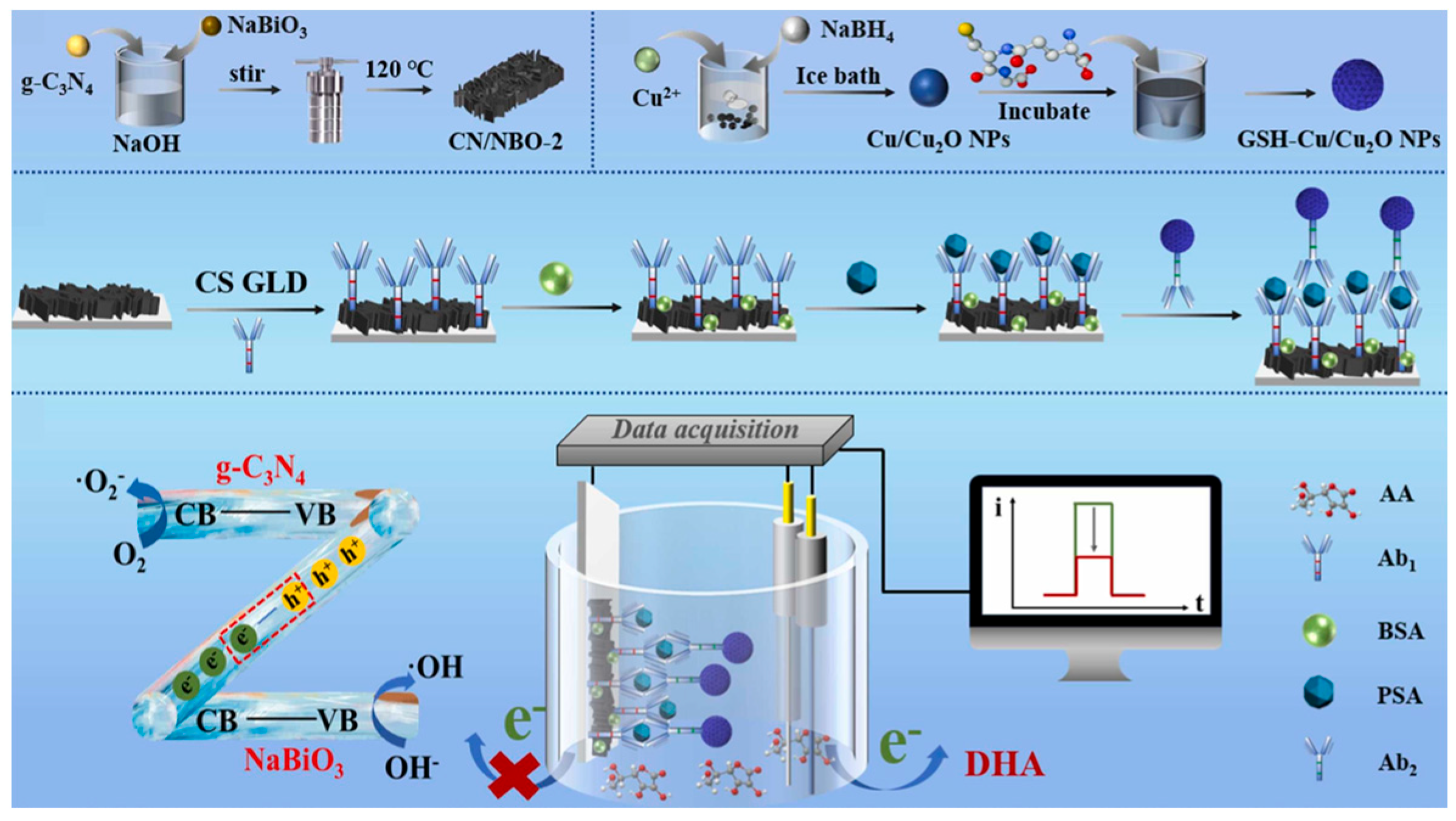
- Wu, P.; Wang, Y.; Cao, K.; Ma, S.; Cao, J.; Liu, Y. Nanozyme-mediated signal amplification on g-C3N4/NaBiO3 Z-scheme heterojunction photoelectrode toward ultrasensitive photoelectrochemical immunoassay for prostate-specific antigen. Sens. Actuators B Chem. 2024, 418, 136353. [Google Scholar] [CrossRef]
- Zou, X.; Sun, Z.; Hu, Y. g-C3N4-based photoelectrodes for photoelectrochemical water splitting: A review. J. Mater. Chem. A 2020, 8, 21474–21502. [Google Scholar] [CrossRef]
- Liu, S.; Long, S.; Zhao, Y.; Chen, Z.; Shen, J.; Fu, G.; Zhang, J.; Kang, J. Potassium and phosphorus co-doped g-C3N4 with improved carrier migration for efficient photocatalytic degradation of 2, 4-dichlorophenoxyacetic acid under visible light. Environ. Res. 2025, 286, 122958. [Google Scholar]
- Yu, W.; Chamkouri, H.; Chen, L. Recent advancement on quantum dot-coupled heterojunction structures in catalysis: A review. Chemosphere 2024, 357, 141944. [Google Scholar] [CrossRef]
- Memon, R.; Shaheen, I.; Qureshi, A.; Niazi, J. Enhanced detection of cardiac troponin-I using CdSe/CdS/ZnS core-shell quantum dot/TiO2 heterostructure photoelectrochemical sensor. J. Alloys Compd. 2024, 1008, 176592. [Google Scholar] [CrossRef]
- Qureshi, A.; Shaikh, T.; Niazi, J. Semiconductor quantum dots in photoelectrochemical sensors from fabrication to biosensing applications. Analyst 2023, 148, 1633–1652. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, Z.; Wang, C.; Song, R.; Li, Z. Transferring the released component of Z-scheme heterojunction onto opposite photoelectrode: A dual-electrode-cooperating signal amplification strategy in photo fuel cell for self-powered biosensing. Biosens. Bioelectron. 2025, 287, 117732. [Google Scholar] [CrossRef]
- Xiao, K.; Zhu, R.; Du, C.; Zheng, H.; Zhang, X.; Chen, J. Zinc–air battery-assisted self-powered PEC sensors for sensitive assay of PTP1B activity based on perovskite quantum dots encapsulated in vinyl-functionalized covalent organic frameworks. Anal. Chem. 2022, 94, 9844–9850. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, R.; Liu, J.; Liu, L.; Wu, Y. Two-dimensional Ti2C MXene-induced photocurrent polarity switching photoelectrochemical biosensing platform for ultrasensitive and selective detection of soluble CD146. Sens. Actuators B Chem. 2022, 350, 130859. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Zheng, H.; Peng, Z.; Hu, Z.; Zhou, Y.; Wang, B. An ultrasensitive MXene-based electrochemical immunosensor for the detection and species identification of archaeological silk microtraces. Biosens. Bioelectron. 2023, 238, 115581. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.; Wang, L.; Wang, K.; Chen, H.; Zheng, H.; Zhou, Y.; Hu, Z.; Peng, Z.; Wan, J.; et al. A visible and near-infrared light dual-responsive PEC immunosensor for archaeological silk microtrace detection based on in situ growth of Ag2S on ZnO-MXene nanocomposites. Chem. Eng. J. 2023, 469, 143926. [Google Scholar] [CrossRef]
- Huang, M.; Deng, H.; Cheng, M.; Li, H.; Yuan, R.; Wei, S. A photoelectrochemical biosensor based on Ti3C2Tx MXene/Ag2S as a novel photoelectric material for ultrasensitive detection of microRNA-141. Sens. Actuators B Chem. 2023, 394, 134343. [Google Scholar] [CrossRef]
- Dmytriv, T.; Lushchak, V. Potential biosafety of Mxenes: Stability, biodegradability, toxicity and biocompatibility. Chem. Rec. 2024, 24, e202300338. [Google Scholar] [CrossRef]
- Wei, X.; Akbar, M.; Raza, A.; Li, G. A review on bismuth oxyhalide based materials for photocatalysis. Nanoscale Adv. 2021, 3, 3353–3372. [Google Scholar] [CrossRef]
- Wang, H.; Wan, X.; Wang, X.; Li, M.; Tang, D. Ultrathin mesoporous BiOCl nanosheets-mediated liposomes for photoelectrochemical immunoassay with in-situ signal amplification. Biosens. Bioelectron. 2023, 239, 115628. [Google Scholar] [CrossRef]
- Chang, H.; Zhu, Q.; Wu, Y. A sensitive label-free biosensor based on Ag2S-sensitived Bi2WO6/BiOBr heterojunction for photoelectrochemical immunoassay of prostate specific antigen. Talanta 2023, 257, 124343. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, Y.; Wang, H.; Ren, X.; Wei, Q.; Wu, D. Construction of a photoelectrochemical immunosensor based on CuInS2 photocathode and BiVO4/BiOI/Ag2S photoanode and sensitive detection of NSE. Biosens. Bioelectron. 2022, 211, 114368. [Google Scholar] [CrossRef]
- Shahbazi, M.; Faghfouri, L.; Ferreira, M.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Zheng, L.; Zhang, J.; Liu, D.; Nie, G. A novel signal-on photoelectrochemical immunosensor based on anthocyanin-sensitized poly (indole-5-carboxylic acid) for ultrasensitive detection of CEA. Sens. Actuators B Chem. 2024, 422, 136681. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Zhu, L.; Liu, K.; Jing, L.; Xie, L.; Ge, S.; Yu, J. Cs3Bi2Br9 QDs/BiOBr Heterojunction Photoelectrochemical Biosensor with APE1 Enzyme-driven Bipedal DNA Walker Signal Amplification for miRNA-320d Detection. Anal. Chim. Acta 2025, 1379, 344713. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wu, H.; Yang, Y.; Wang, Q.; Jia, B.; Zheng, J. Research progress on zinc oxide-based heterojunction photocatalysts. J. Mater. Chem. A 2024, 12, 20838–20867. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, Z.; Yao, H.; Liu, W.; Miao, Y.; Zhang, Z.; Wu, D. Au-mediated Z-scheme TiO2-Au-BiOI photoelectrode for sensitive and selective photoelectrochemical detection of L-cysteine. Sens. Actuators B Chem. 2023, 393, 134285. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Kong, Q.; Long, R.; Wang, C.; Jiang, J.; Xiong, Y. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of plasmonic effect and schottky junction via interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, C.; Wang, H.; Gao, M.; Zhao, L. Designing Schottky junction constructed from porous PdPt nanospheres embedded in Bi2S3 nanorods for ultrasensitive photoelectrochemical immunoassay of acute myocardial infarction biomarkers. Chem. Eng. J. 2024, 502, 157966. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Huang, S.; Wu, Q.; Liu, J.; Duan, H.; Chen, H. Constructing BiOI@ Nv/g-C3N4 with S-scheme heterojunction for enhanced photoelectrochemical performances towards highly sensitive and selective detection of trace chlorpyrifos. Anal. Chim. Acta 2025, 1359, 344102. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Liu, X.; Mao, C.; Jin, B. A photoelectrochemical biosensor based on b-TiO2/CdS: Eu/Ti3C2 heterojunction for the ultrasensitive detection of miRNA-21. Talanta 2023, 253, 123601. [Google Scholar] [CrossRef]
- Ji, R.; Liu, J.; Zhang, T.; Peng, Y.; Li, Y.; Chen, D.; Xu, Q.; Lu, J. Construction of a ternary Z-scheme In2S3@Au@P3HT photocatalyst for the degradation of phenolic pollutants under visible light. Sep. Purif. Technol. 2021, 272, 118787. [Google Scholar] [CrossRef]
- Cai, G.; Yu, Z.; Ren, R.; Tang, D. Exciton-plasmon interaction between AuNPs/graphene nanohybrids and CdS quantum dots/TiO2 for photoelectrochemical aptasensing of prostate-specific antigen. ACS Sens. 2018, 3, 632–639. [Google Scholar] [CrossRef]
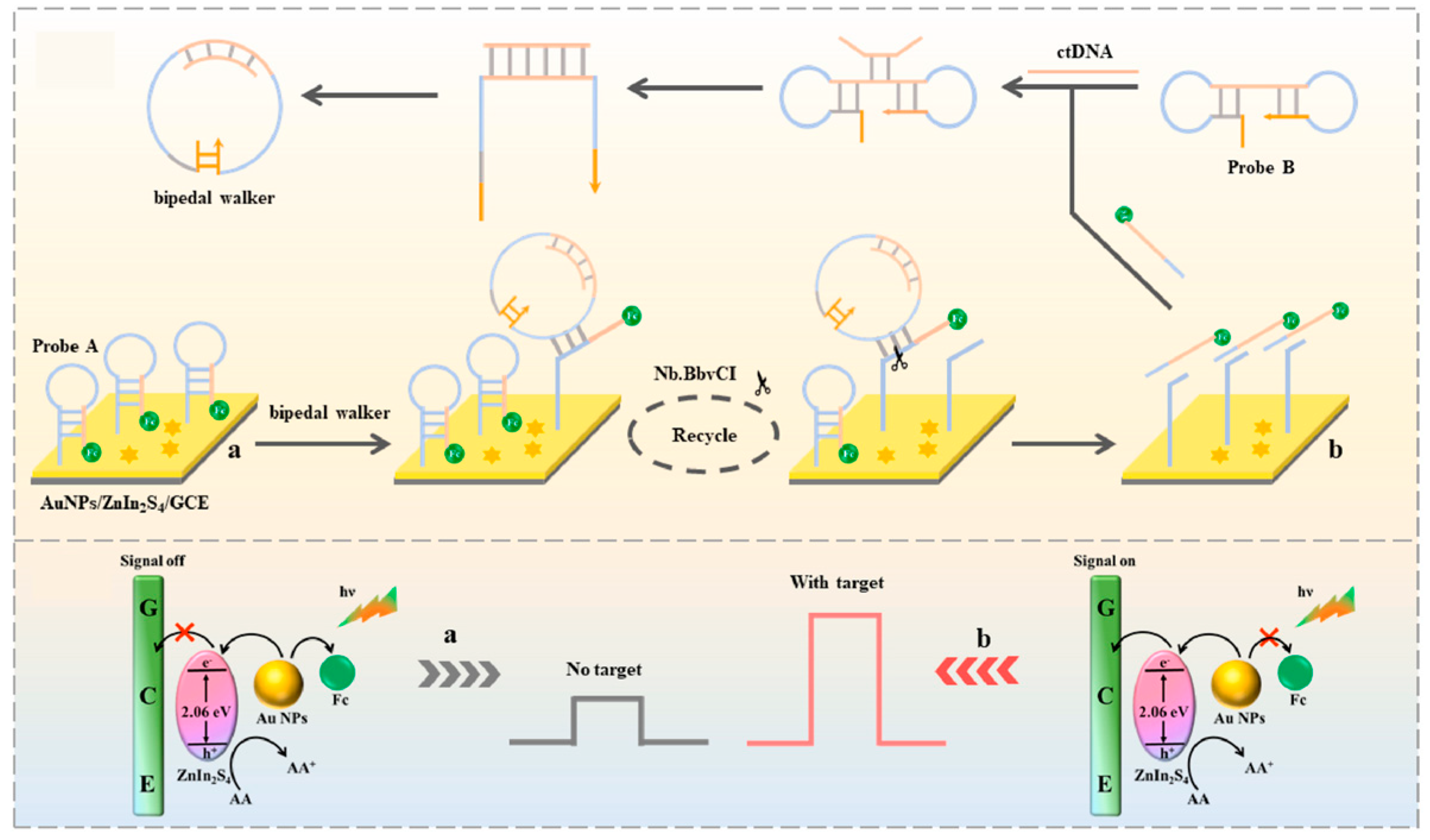
- Cui, Y.; Yan, H.; Sun, Z.; Ling, Y.; Luo, H.; Li, N. A photoelectrochemical biosensor based on ZnIn2S4@AuNPs coupled with circular bipedal DNA walker for signal-on detection of circulating tumor DNA. Biosens. Bioelectron. 2023, 231, 115295. [Google Scholar] [CrossRef]
- Wang, G.; Jiao, H.; Liu, K.; Wu, X.; Dong, Y.; Li, Z.; Zhang, C. A novel strategy for the construction of photoelectrochemical sensors based on quantum dots and electron acceptor: The case of dopamine detection. Electrochem. Commun. 2014, 41, 47–50. [Google Scholar] [CrossRef]
- Liu, S.; Chi, D.; Chen, R.; Ma, Y.; Fang, H.; Liu, B. N-doped C layer boost Z-scheme interfacial charge transfer in TiO2/ZnIn2S4 heterojunctions for enhance photocatalytic hydrogen evolution. Renew. Energy 2023, 219, 119494. [Google Scholar] [CrossRef]
- Cui, H.; Yao, C.; Cang, Y.; Liu, W.; Zhang, Z.; Miao, Y.; Xin, Y. Oxygen vacancy-regulated TiO2 nanotube photoelectrochemical sensor for highly sensitive and selective detection of tetracycline hydrochloride. Sens. Actuators B Chem. 2022, 359, 131564. [Google Scholar] [CrossRef]
- Zhu, J.; Mei, L.; Wang, A.; Song, Y.; Feng, J. Integration of phosphate functionalized Pt/TiO2 and Ru(bpy)32+ sensitization for ultrasensitive assay of adenosine deaminase activity on a novel split-typed PEC aptasensor. Biosens. Bioelectron. 2023, 226, 115141. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Li, B.; Zhu, H.; Shi, J. 3D interconnected nanoporous Ta3N5 films for photoelectrochemical water splitting: Thickness-controlled synthesis and insights into stability. Sci. China Mater. 2021, 64, 1876–1888. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, S.; Yang, X.; Uiiah, A.; Feng, W. Double enhancement photoelectrochemical immunosensors based on BiOBr/ZnCdS heterojunction and LSPR for liver cancer detection. IEEE Sens. J. 2024, 24, 33929–33936. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Ding, L.; You, Q.; Wang, K. Perovskite-type BiFeO3/ultrathin graphite-like carbon nitride nanosheets pn heterojunction: Boosted visible-light-driven photoelectrochemical activity for fabricating ampicillin aptasensor. Biosens. Bioelectron. 2019, 124, 33–39. [Google Scholar] [CrossRef]
- Li, R.; Tu, W.; Wang, H.; Dai, Z. Near-infrared light excited and localized surface plasmon resonance-enhanced photoelectrochemical biosensing platform for cell analysis. Anal. Chem. 2018, 90, 9403–9409. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, D.; Zhang, Y.; Luo, R.; Ma, J.; Lei, J.; Ju, H. Electron donor coordinated metal-organic framework to enhance photoelectrochemical performance. Angew. Chem. Int. Ed. 2023, 62, e202308514. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, S.; Yuan, R.; Liu, H. Defective Se-doped In2S3 nanomaterial-based photoelectrochemical biosensor for the ultrasensitive detection of chloramphenicol. Sens. Actuators B Chem. 2022, 373, 132705. [Google Scholar] [CrossRef]
- Zhang, B.; An, Z.; Li, M.; Guo, L. Synthesis; functionalization and photoelectrochemical immunosensing application of WO3-based semiconductor materials. Trends Anal. Chem. 2023, 165, 117149. [Google Scholar] [CrossRef]
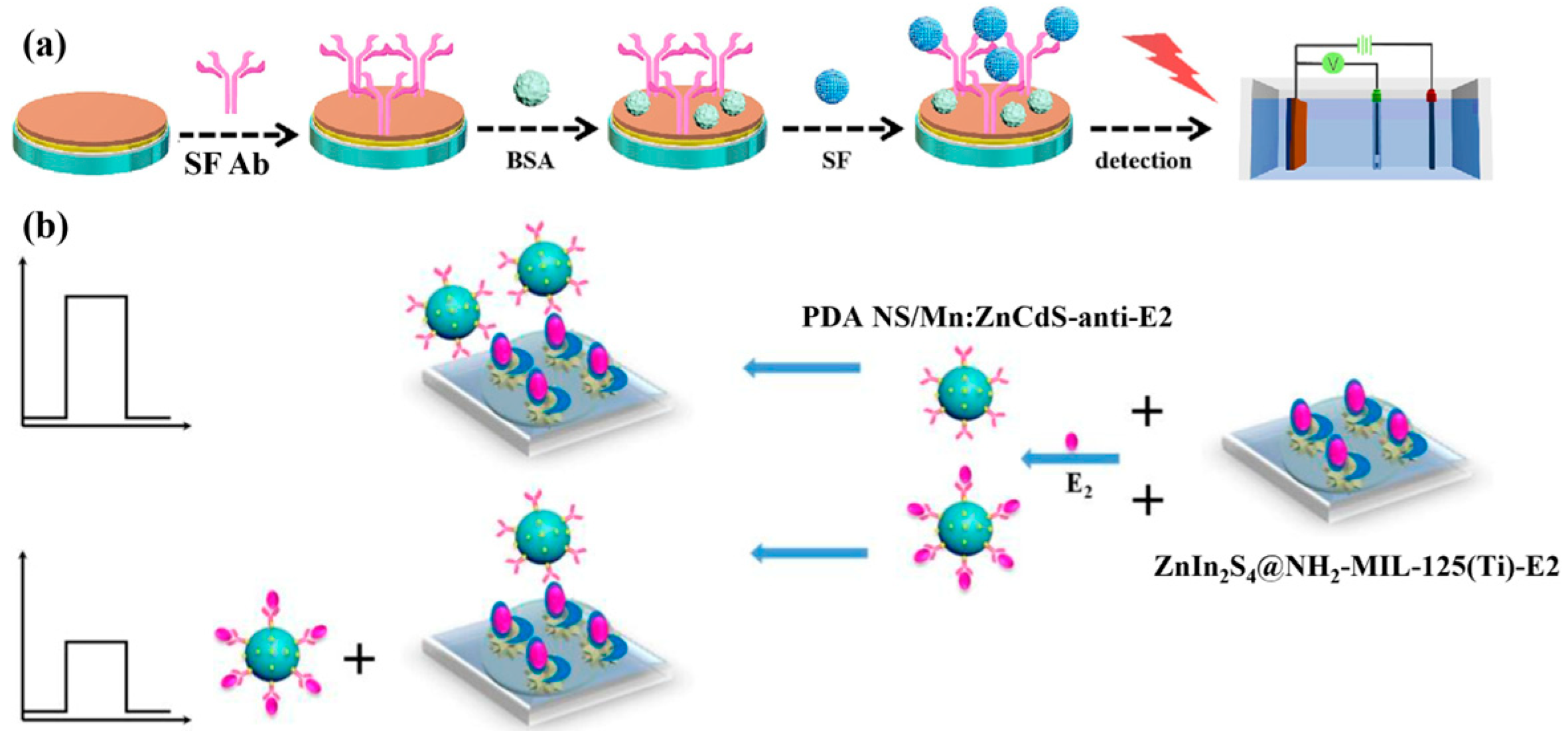
- Yan, T.; Wu, T.; Wei, S.; Wang, H.; Sun, M.; Yan, L.; Wei, Q.; Ju, H. Photoelectrochemical competitive immunosensor for 17β-estradiol detection based on ZnIn2S4@NH2-MIL-125 (Ti) amplified by PDA NS/Mn: ZnCdS. Biosens. Bioelectron. 2020, 148, 111739. [Google Scholar] [CrossRef]
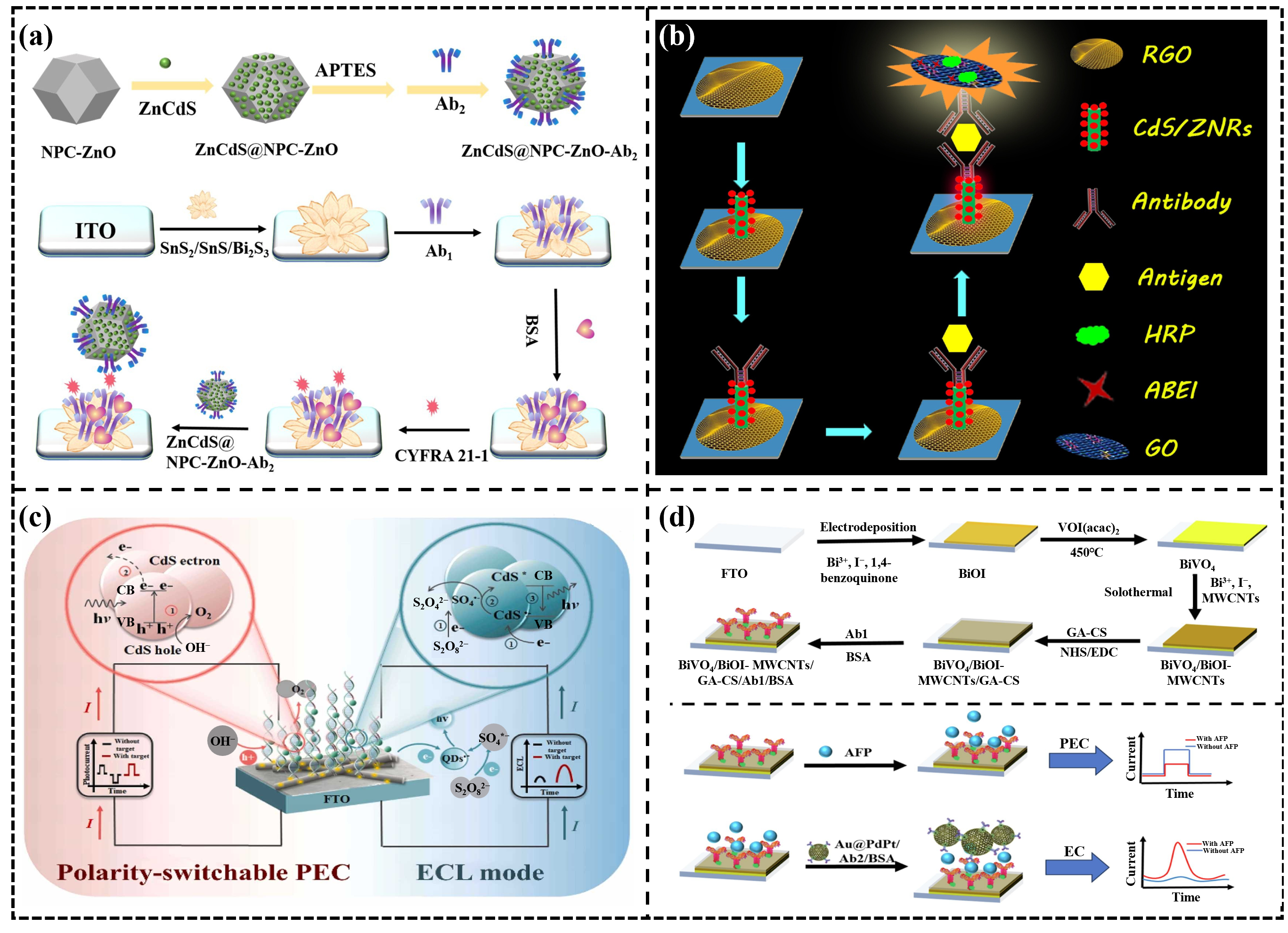
- Liu, D.; Qian, Y.; Xu, R.; Zhang, Y.; Ren, X.; Ma, H.; Wei, Q. A dual-signal amplification photoelectrochemical immunosensor for ultrasensitive detection of CYFRA 21-1 based on the synergistic effect of SnS2/SnS/Bi2S3 and ZnCdS@NPC-ZnO. Sens. Actuators B Chem. 2021, 346, 130456. [Google Scholar] [CrossRef]
- Ge, S.; Liang, L.; Lan, F.; Zhang, Y.; Wang, Y.; Yan, M.; Yu, J. Photoelectrochemical immunoassay based on chemiluminescence as internal excited light source. Sens. Actuators B Chem. 2016, 234, 324–331. [Google Scholar] [CrossRef]
- Li, X.; Yuan, H.; Yuan, X.; Li, Y.; Zhang, F.; Xie, J.; Li, L.; Zhang, Q.; Li, C.; Xue, Q. An anti-interference PEC-ECL biosensing for cancer-related gene based on self-supporting semi-encapsulated heterojunction modulated interface polarity-switching. Sens. Actuators B Chem. 2025, 425, 136962. [Google Scholar] [CrossRef]
- Wu, H.; Yang, X. Biofunctional photoelectrochemical/electrochemical immunosensor based on BiVO4/BiOI-MWCNTs and Au@ PdPt for alpha-fetoprotein detection. Bioelectrochemistry 2024, 160, 108773. [Google Scholar] [CrossRef]
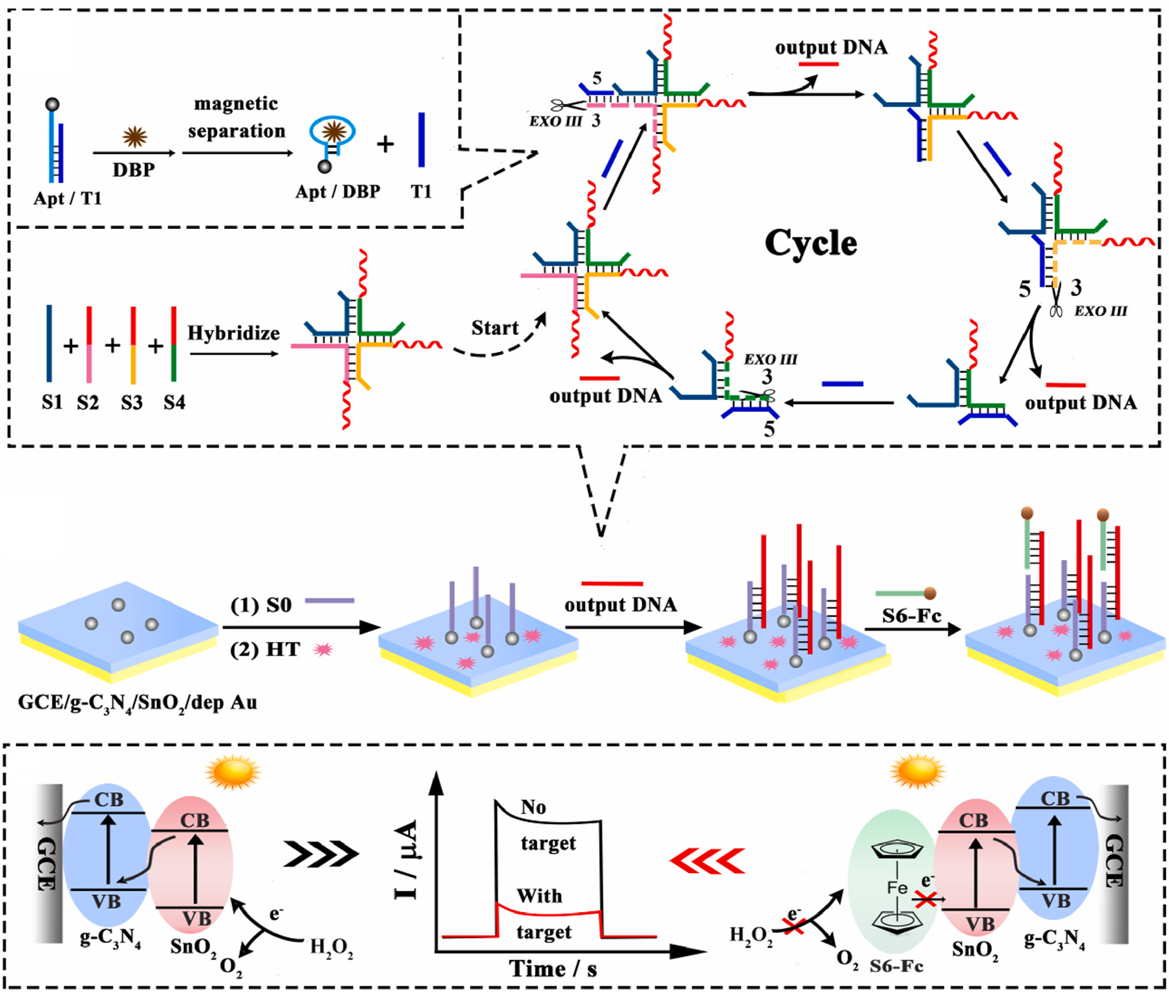
- Yang, J.; Zeng, H.; Chai, Y.; Yuan, R.; Liu, H. Ultrasensitive photoelectrochemical biosensor amplified by target induced assembly of cruciform DNA nanostructure for the detection of dibutyl phthalate. Anal. Chim. Acta 2023, 1262, 341242. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, D.; Zhang, Y.; Yang, X.; Man, C.; Jiang, Y.; Zhang, W.; Zhang, X. Biosensing meets photoelectrochemistry: A promising analytical tool to be integrated with microfluidic device for on-site food safety analysis. Chem. Eng. J. 2025, 519, 165398. [Google Scholar] [CrossRef]
- Wu, T.; Lv, L.; Zhang, N.; Fu, J.; Du, Y.; Ren, X.; Wei, Q. Advanced dual-mode microfluidic sensing platform based on enhanced activities of Au@ Pt nanozyme via photothermal effect for heart fatty acid binding protein detection. Sens. Actuators B Chem. 2025, 447, 138805. [Google Scholar] [CrossRef]
- Zeng, Y.; Feng, W.; Yang, X.; Zou, X. Microfluidic photoelectrochemical immunosensor based on BiOCl@au/CdS heterojunction for the trace detection of liver cancer marker. Bioelectrochemistry 2025, 166, 109047. [Google Scholar] [CrossRef]
- Du, Y.; Geng, H.; Chen, J.; Sun, X.; Wu, T.; Ma, H.; Gao, Z.; Wei, Q. Cascade Catalytic Iron Phosphate Nanozyme-Driven Signal Amplification in S-Scheme AgBr/La-BiOBr-OV for Sensitive Dual-Channel Microfluidic PEC Detection of CA15-3 and CA125. Anal. Chem. 2025, 97, 16611–16618. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Sun, M.; Feng, B.; Shen, H.; Zhu, J.; Chen, X.; Yu, S. A filter-electrochemical microfluidic chip for multiple surface protein analysis of exosomes to detect and classify breast cancer. Biosens. Bioelectron. 2023, 239, 115590. [Google Scholar] [CrossRef]
- Alaridhee, Z.; Alqaraguly, M.; Formanova, S.; Kuryazov, R.; Mahdi, M.; Taher, W.; Alwan, M.; Jabir, M.; Zankanah, F.; Majdi, H.; et al. Recent advances in microfluidic-based photoelectrochemical (PEC) sensing platforms for biomedical applications. Microchim. Acta 2025, 192, 297. [Google Scholar] [CrossRef]


| Photoactive Material | Analyte | Linear Range | Detection Limit |
|---|---|---|---|
| TiO2/CdS:Mn | DNA | 0.0005–50 pm | 27 am |
| ZnO flower-rod architectures | DNA | 0.00001–100 nm | 3.7 fm |
| CdTe QDs/ZnO NSs | DNA | 0.01–10 pm | 0.93 fm |
| Bi2S3 NRs | miRNA | 1–5000 fm | 0.35 fm |
| TiO2-CdS:Mn and Au NPs | miRNA | 1.0–10,000 fm | 0.5 fm |
| Au-TiO2 | prion protein | 200–2000 fm | 50.9 fm |
| phosphorylated g-C3N4 NPs | PKA | 0.05–50 U/mL | 0.077 U/mL |
| RGO-BiFeO3 | PSA | 0.001–100 ng/mL | 0.31 pg/mL |
| BiVO4-RGO | PSA | 10–80 ng/mL | 3.0 pg/mL |
| Au-BiVO4 | PSA | 10–100 ng/mL | 4.0 pg/mL |
| CdS-PAMAM film | SMMC-7721 cells | 5.0 × 103–1.0 × 107 cells/mL | 5.0 × 103 cells/mL |
| graphene-CdS film | HeLa cells | 1.0 × 102–5.0 × 106 cells/mL | 100 cells/mL |
| CdSe QDs/TiO2 | o-aminophenol | 0.4–27 μm | 80 nm |
| Strategies | Advantage | Disadvantage | Performance |
|---|---|---|---|
| heterojunctions construction | enhancing carrier separation efficiency | band matching is challenging to control with precision | a 7.0-fold higher photocurrent of BiFeO3/g-C3N4 versus BiFeO3 [73] |
| LSPR | extending the light absorption range | high cost and reproducibility related to particle size | a 3.5-fold higher current of Ag2S/AuNPs versus Ag2S [74] |
| donors/acceptors | enhancing carrier separation efficiency | performance depends on donor/acceptor concentration and diffusion efficiency | The electron donor 1,4-diazabicyclo[2.2.2]octane increased the current by 23.2-fold [75] |
| defect construction | enhancing light absorption and carrier migration efficiency | defect concentration is difficult to control | selenium doping in In2S3 resulted in a fourfold increase in the anodic photocurrent [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Wang, Y.; Zhang, Y. Review on Research Progress of Photoelectrochemical Biosensors. Micromachines 2025, 16, 1293. https://doi.org/10.3390/mi16111293
Zeng Y, Wang Y, Zhang Y. Review on Research Progress of Photoelectrochemical Biosensors. Micromachines. 2025; 16(11):1293. https://doi.org/10.3390/mi16111293
Chicago/Turabian StyleZeng, Yu, Yuheng Wang, and Yaqing Zhang. 2025. "Review on Research Progress of Photoelectrochemical Biosensors" Micromachines 16, no. 11: 1293. https://doi.org/10.3390/mi16111293
APA StyleZeng, Y., Wang, Y., & Zhang, Y. (2025). Review on Research Progress of Photoelectrochemical Biosensors. Micromachines, 16(11), 1293. https://doi.org/10.3390/mi16111293








