BPEI-Based N-Doped Carbon Dots with Sensitive and Selective Cu2+ Ion-Sensing Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
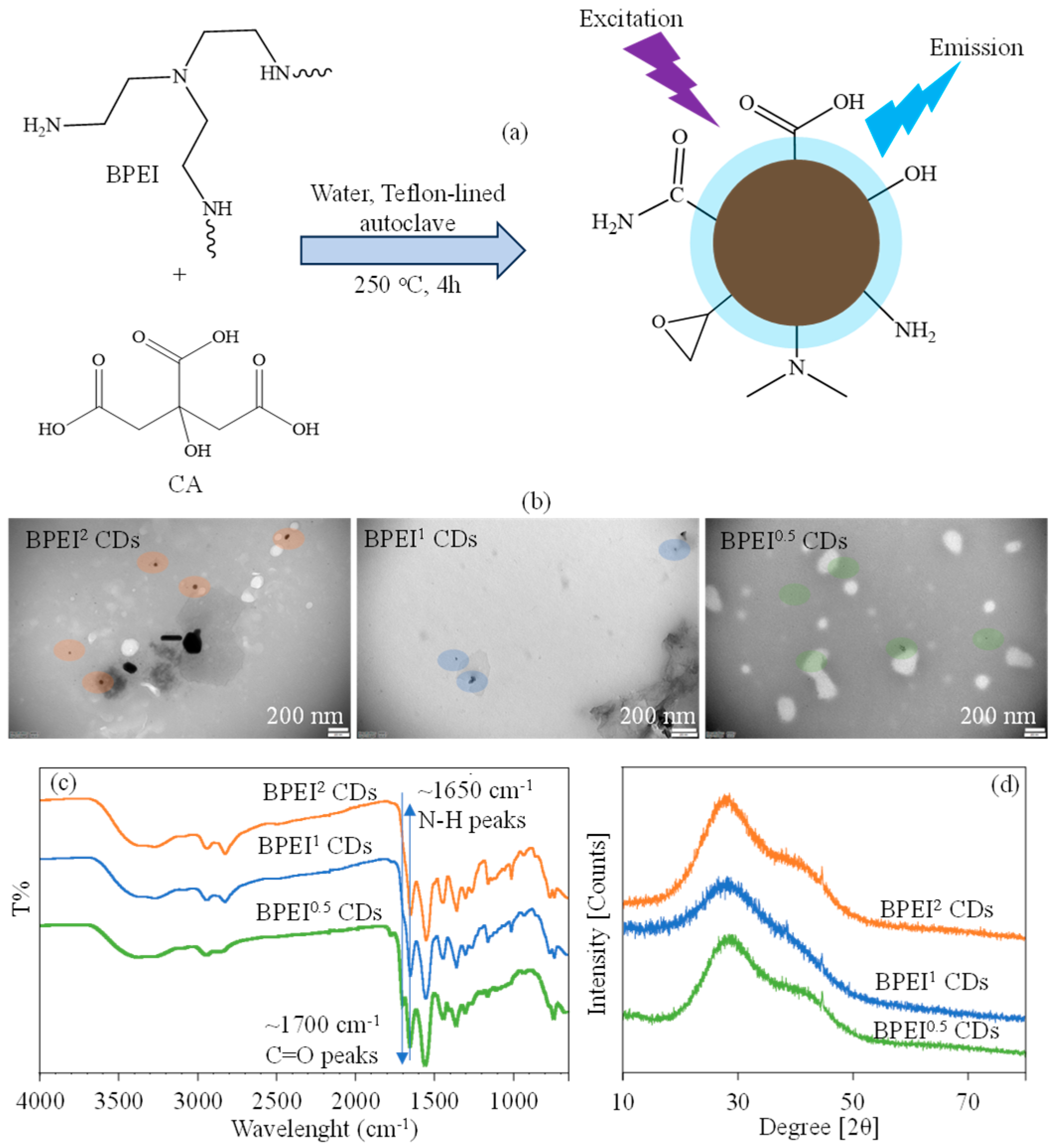
2.2. Synthesis and Characterization of BPEI CDs
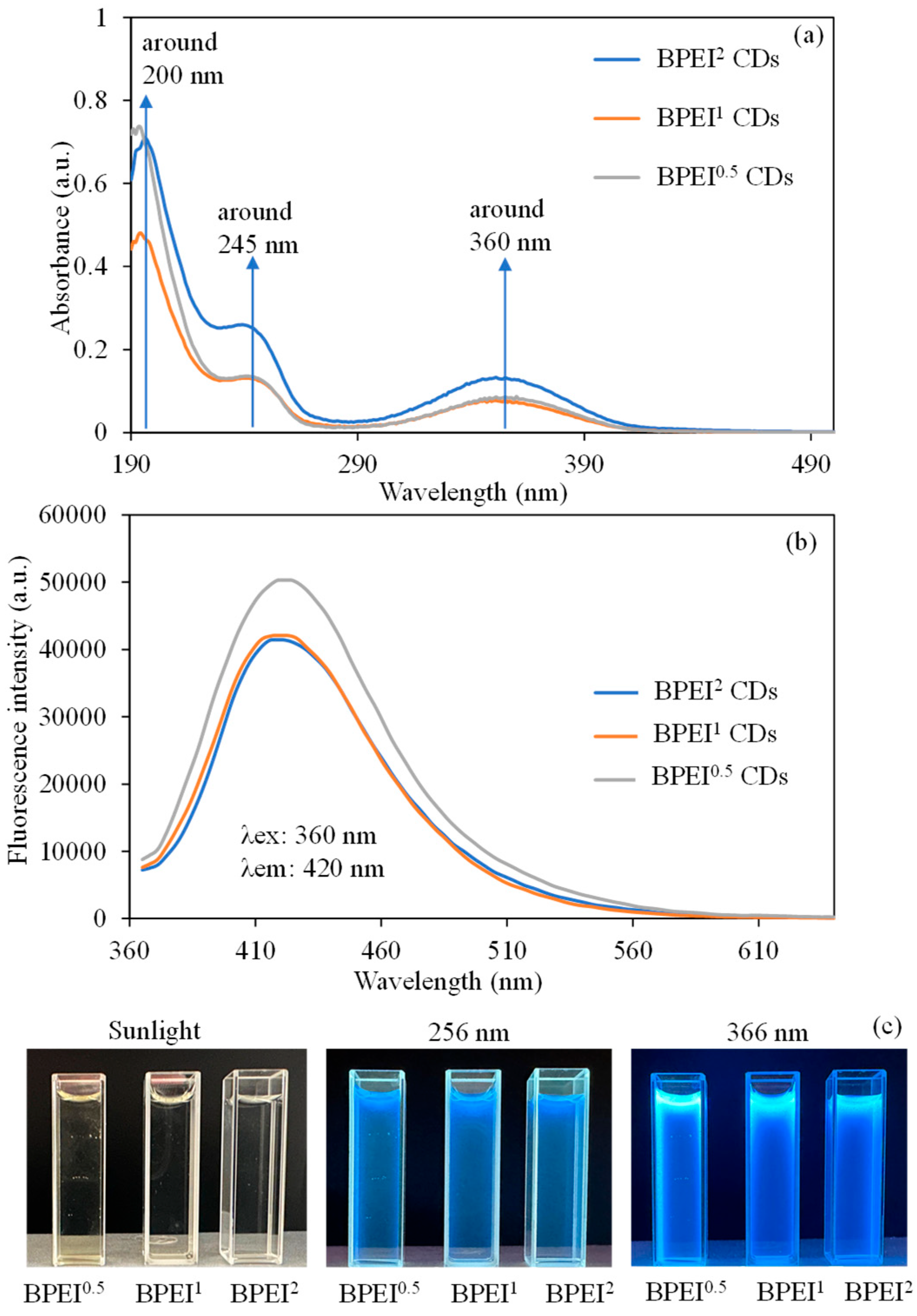
2.3. Optical Properties of BPEI CDs
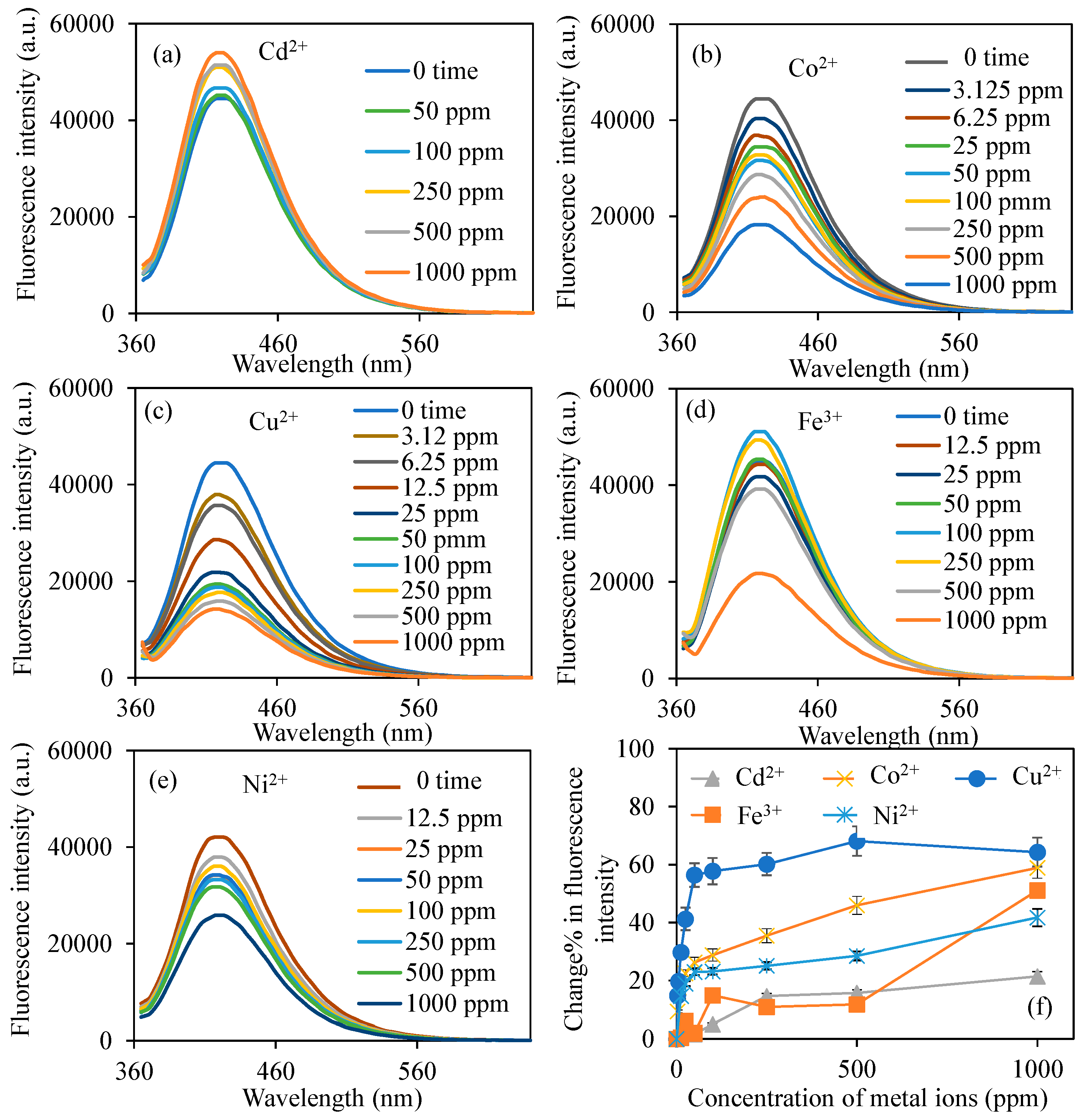
2.4. Quenching Properties of BPEI CDs
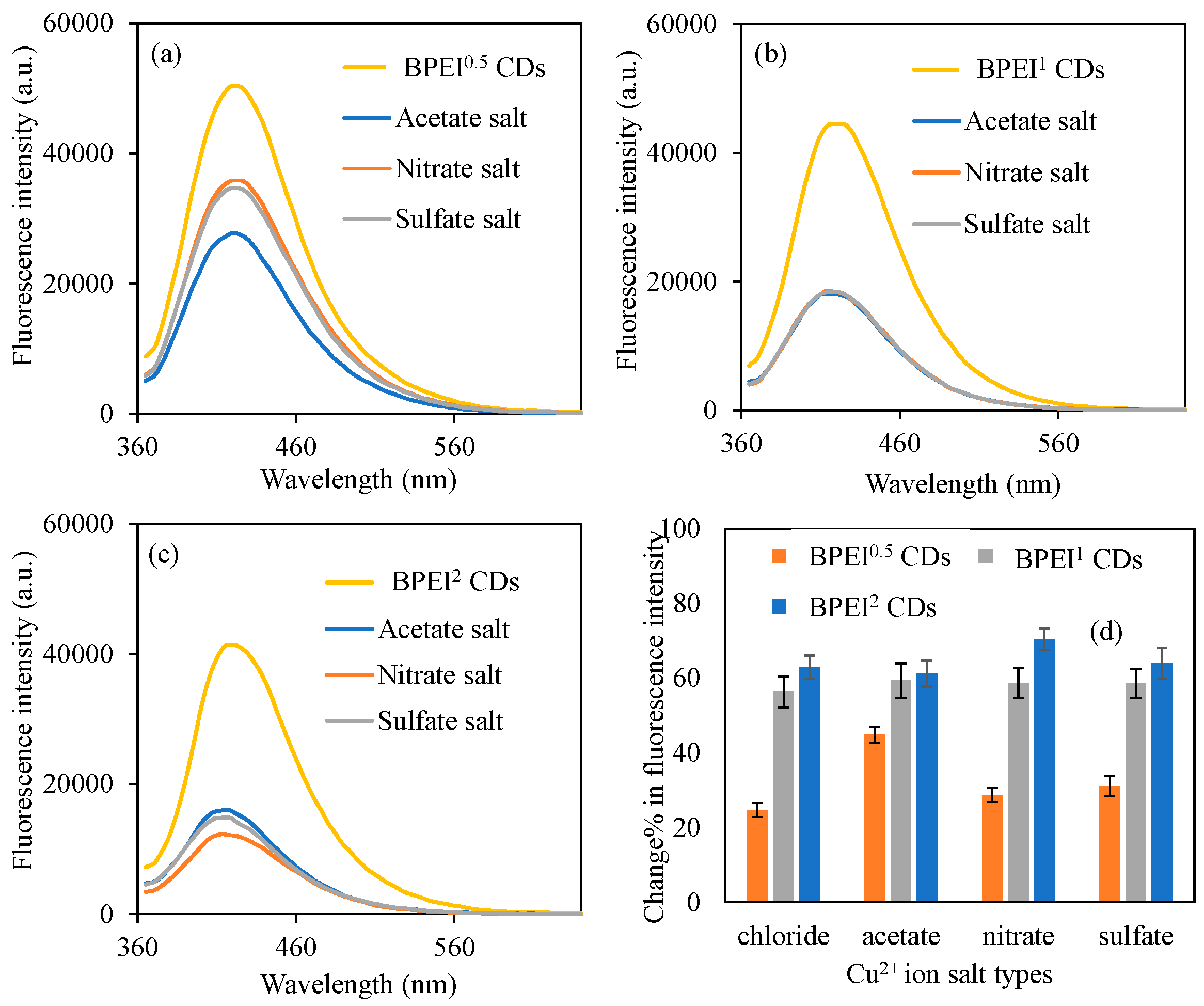
2.5. Selectivity of BPEI CDs to Cu2+ Ions
2.6. Quenching Ability of BPEI CDs in the Presence of Cu2+ Ions in Real Waters
3. Results and Discussion
3.1. Characterization and Optical Properties of BPEI CDs
3.2. Sensor Applications of BPEI CDs to Metal Ions
3.3. Sensor Applications of BPEI CDs to Cu2+ Ions
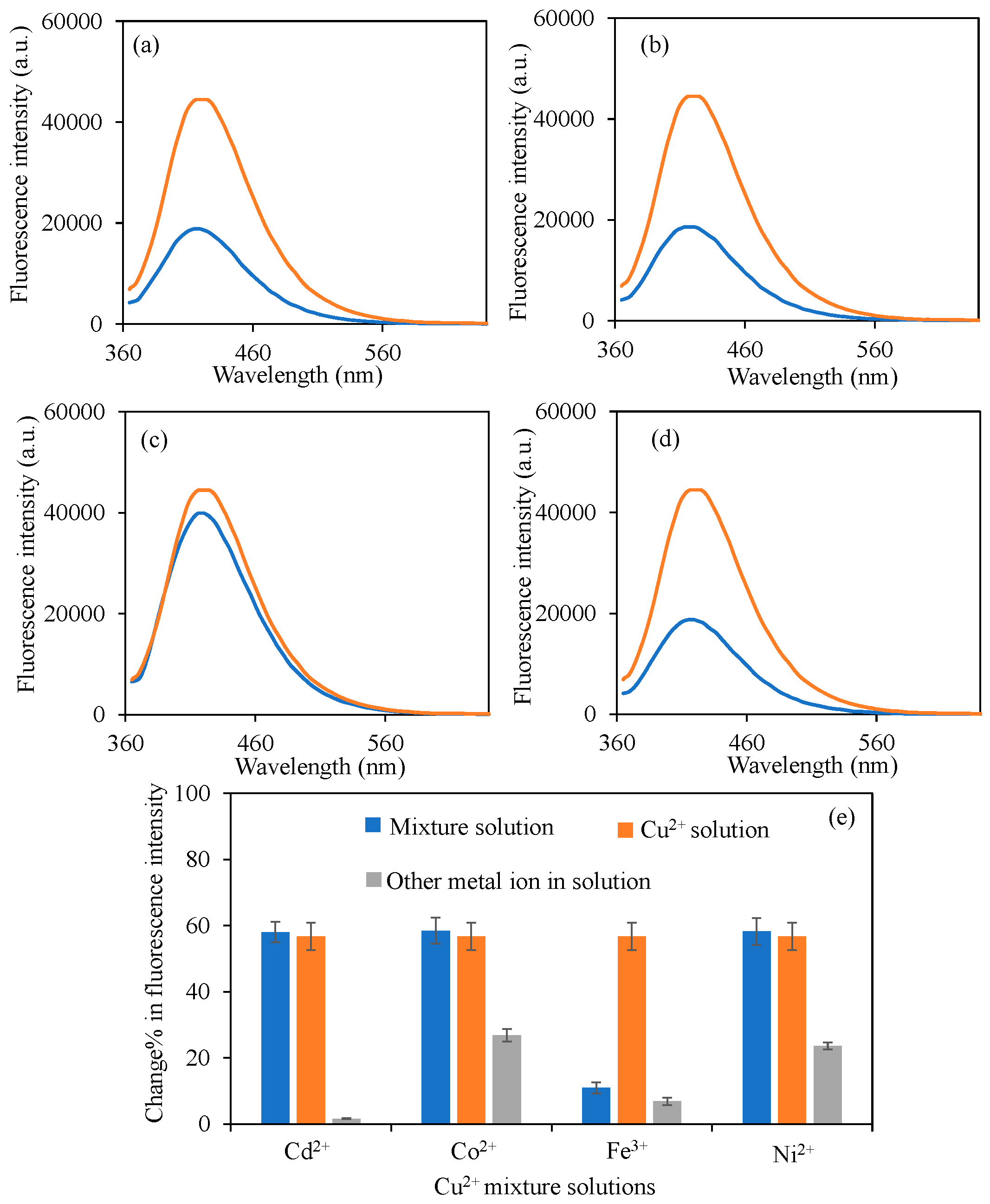
3.4. Selectivity of BPEI CDs Sensors to Cu2+ Ions
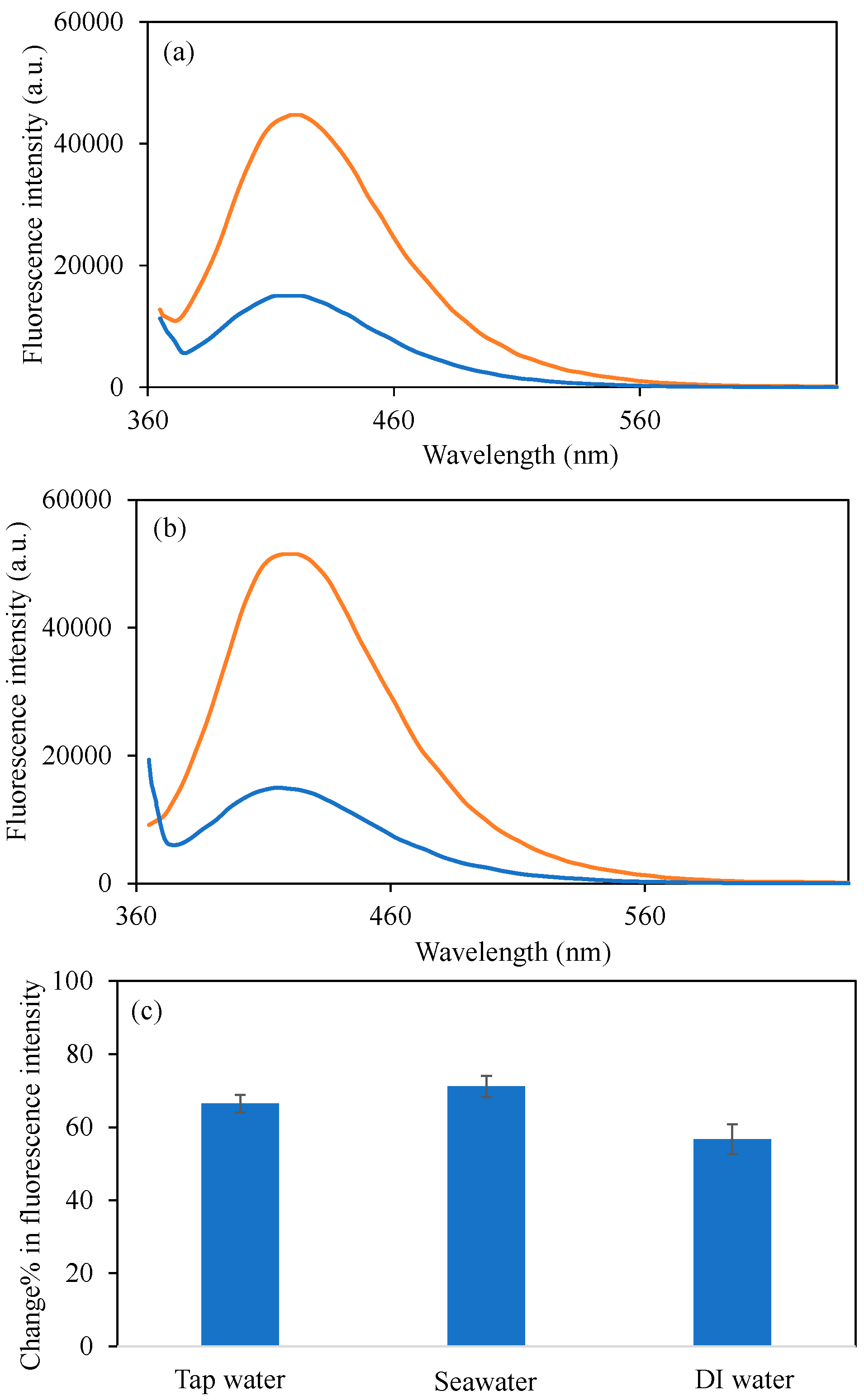
3.5. Cu2+ Ion Detections in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, W.; Zou, W.; Jiang, S.; Zhang, J.; Ge, Y.; Lu, G.; Bahnemann, D.W.; Pan, J.H. Fluorescent Carbon Quantum Dots with Controllable Physicochemical Properties Fantastic for Emerging Applications: A Review. Carbon Neutralization 2024, 3, 245–284. [Google Scholar] [CrossRef]
- Sahana, S.; Gautam, A.; Singh, R.; Chandel, S. A Recent Update on Development, Synthesis Methods, Properties and Application of Natural Products Derived Carbon Dots. Nat. Prod. Bioprospect. 2023, 13, 51. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Dabash, H.; Ponnamma, D.; Abbas, M.K.G. Carbon Dots as Versatile Nanomaterials in Sensing and Imaging: Efficiency and Beyond. Heliyon 2024, 10, e31634. [Google Scholar] [CrossRef]
- Xu, J.; Huang, B.-B.; Lai, C.-M.; Lu, Y.-S.; Shao, J.-W. Advancements in the Synthesis of Carbon Dots and Their Application in Biomedicine. J. Photochem. Photobiol. B Biol. 2024, 255, 112920. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Ren, L.; Li, S.; Feng, M.; Zhang, Q.; Qi, R.; Qin, Z.; Zhang, J.; Jiang, L. The Enhanced Photoluminescence Properties of Carbon Dots Derived from Glucose: The Effect of Natural Oxidation. Nanomaterials 2024, 14, 970. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.-T.; Zhan, L.; Chen, B.-B. Recent Progress of Carbon Dots in Fluorescence Sensing. Inorganics 2025, 13, 256. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Gao, K.; Sun, S.; Zhang, B. Recent Advancements in the Development of Graphene-Based Materials for Catalytic Applications. ChemCatChem 2024, 16, e202400696. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A.G. Heteroatom Doped High Porosity Carbon Nanomaterials as Electrodes for Energy Storage in Electrochemical Capacitors: A Review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Munusamy, S.; Mandlimath, T.R.; Swetha, P.; Al-Sehemi, A.G.; Pannipara, M.; Koppala, S.; Shanmugam, P.; Boonyuen, S.; Pothu, R.; Boddula, R. Nitrogen-Doped Carbon Dots: Recent Developments in Its Fluorescent Sensor Applications. Environ. Res. 2023, 231, 116046. [Google Scholar] [CrossRef] [PubMed]
- Basha, D.B.; Ahmed, S.; Ahmed, A.; Gondal, M.A. Recent Advances on Nitrogen Doped Porous Carbon Micro-Supercapacitors: New Directions for Wearable Electronics. J. Energy Storage 2023, 60, 106581. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Baragau, I.-A.; Gromicova, R.; Nicolaev, A.; Thomson, S.A.J.; Rennie, A.; Power, N.P.; Sajjad, M.T.; Kellici, S. Investigating the Effect of N-Doping on Carbon Quantum Dots Structure, Optical Properties and Metal Ion Screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, H.; Won, S.; Kim, H.; Kwon, W. Biocompatible Nitrogen-Doped Carbon Dots: Synthesis, Characterization, and Application. J. Mater. Chem. B 2020, 8, 8935–8951. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Li, J.; Xie, Z.; Sun, Z. Tailoring Color Emissions from N-Doped Graphene Quantum Dots for Bioimaging Applications. Light Sci. Appl. 2015, 4, e364. [Google Scholar] [CrossRef]
- Xu, M.; He, G.; Li, Z.; He, F.; Gao, F.; Su, Y.; Zhang, L.; Yang, Z.; Zhang, Y. A Green Heterogeneous Synthesis of N-Doped Carbon Dots and Their Photoluminescence Applications in Solid and Aqueous States. Nanoscale 2014, 6, 10307–10315. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics 2023, 11, 262. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Hu, G.; Mo, L.; Zheng, M.; Lei, B.; Zhang, X.; Hu, C.; Zhuang, J.; Liu, Y. Surface Functional Carbon Dots: Chemical Engineering Applications beyond Optical Properties. J. Mater. Chem. C Mater. 2020, 8, 16282–16294. [Google Scholar] [CrossRef]
- Hasan, M.; Baheerathan, B.; Sutradhar, S.; Shahbandinejad, R.; Rakshit, S.; Kozinski, J.; Li, D.; Hu, Y.; Kang, K. Microwave-Assisted Synthesis of Biomass-Derived N-Doped Carbon Dots for Metal Ion Sensing. Carbon Res. 2025, 4, 49. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining Carbon Dots: Synthesis and Biomedical and Optoelectronic Applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Guo, T.; Hu, P. The Influence of Defect Engineering on the Electronic Structure of Active Centers on the Catalyst Surface. Catalysts 2025, 15, 651. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, W.; Xu, R.; Wang, P.; Zhao, H. Tuning Electronic and Pore Structures of Biochar via Nitrogen and Magnesium Doping for Superior Methylene Blue Adsorption: Synergistic Mechanisms and Kinetic Analysis. ACS Omega 2025, 10, 31679–31692. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Yukta, Y.; Mondal, J.; Kumar, R.; Pani, B.; Singh, B. Carbon Dots: Synthesis, Characterizations, and Recent Advancements in Biomedical, Optoelectronics, Sensing, and Catalysis Applications. ACS Appl. Bio Mater. 2024, 7, 2086–2127. [Google Scholar] [CrossRef]
- Sudewi, S.; Sai Sashank, P.V.; Kamaraj, R.; Zulfajri, M.; Huang, G.G. Understanding Antibiotic Detection with Fluorescence Quantum Dots: A Review. J. Fluoresc. 2024, 35, 2527–2551. [Google Scholar] [CrossRef]
- Song, Y.; Xia, X.; Xiao, Z.; Zhao, Y.; Yan, M.; Li, J.; Li, H.; Liu, X. Synthesis of N,S Co-Doped Carbon Dots for Fluorescence Turn-on Detection of Fe2+ and Al3+ in a Wide PH Range. J. Mol. Liq. 2022, 368, 120663. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-Doped Carbon Materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Antolini, E. Nitrogen-Doped Carbons by Sustainable N- and C-Containing Natural Resources as Nonprecious Catalysts and Catalyst Supports for Low Temperature Fuel Cells. Renew. Sustain. Energy Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Wu, B.; Meng, H.; Morales, D.M.; Zeng, F.; Zhu, J.; Wang, B.; Risch, M.; Xu, Z.J.; Petit, T. Nitrogen-Rich Carbonaceous Materials for Advanced Oxygen Electrocatalysis: Synthesis, Characterization, and Activity of Nitrogen Sites. Adv. Funct. Mater. 2022, 32, 2204137. [Google Scholar] [CrossRef]
- Ejaz, A.; Jeon, S. The Individual Role of Pyrrolic, Pyridinic and Graphitic Nitrogen in the Growth Kinetics of Pd NPs on N-RGO Followed by a Comprehensive Study on ORR. Int. J. Hydrogen Energy 2018, 43, 5690–5702. [Google Scholar] [CrossRef]
- Filimon, M.N.; Caraba, I.V.; Popescu, R.; Dumitrescu, G.; Verdes, D.; Petculescu Ciochina, L.; Sinitean, A. Potential Ecological and Human Health Risks of Heavy Metals in Soils in Selected Copper Mining Areas—A Case Study: The Bor Area. Int. J. Environ. Res. Public Health 2021, 18, 1516. [Google Scholar] [CrossRef]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Environmental Copper: Its Dynamics and Human Exposure Issues. J. Toxicol. Environ. Health Part B 2001, 4, 341–394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Copper in Drinking-Water; World Health Organization: Geneva, Switzerland, 2024.
- The United States Environmental Protection Agency. Lead and Copper Rule; The United States Environmental Protection Agency: Washington, DC, USA, 2010.
- Vo, T.T.T.; Peng, T.-Y.; Nguyen, T.H.; Bui, T.N.H.; Wang, C.-S.; Lee, W.-J.; Chen, Y.-L.; Wu, Y.-C.; Lee, I.-T. The Crosstalk between Copper-Induced Oxidative Stress and Cuproptosis: A Novel Potential Anticancer Paradigm. Cell Commun. Signal. 2024, 22, 353. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Du, T.; Yang, D. The Impact of Copper on Bone Metabolism. J. Orthop. Transl. 2024, 47, 125–131. [Google Scholar] [CrossRef]
- Sailer, J.; Nagel, J.; Akdogan, B.; Jauch, A.T.; Engler, J.; Knolle, P.A.; Zischka, H. Deadly Excess Copper. Redox Biol. 2024, 75, 103256. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, A. Copper-Instigated Modulatory Cell Mortality Mechanisms and Progress in Oncological Treatment Investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef] [PubMed]
- de Bie, P.; van de Sluis, B.; Burstein, E.; van de Berghe, P.V.E.; Muller, P.; Berger, R.; Gitlin, J.D.; Wijmenga, C.; Klomp, L.W.J. Distinct Wilson’s Disease Mutations in ATP7B Are Associated With Enhanced Binding to COMMD1 and Reduced Stability of ATP7B. Gastroenterology 2007, 133, 1316–1326. [Google Scholar] [CrossRef]
- Ovchinnikova, E.V.; Garbuz, M.M.; Ovchinnikova, A.A.; Kumeiko, V.V. Epidemiology of Wilson’s Disease and Pathogenic Variants of the ATP7B Gene Leading to Diversified Protein Disfunctions. Int. J. Mol. Sci. 2024, 25, 2402. [Google Scholar] [CrossRef]
- Patil, M.; Sheth, K.A.; Krishnamurthy, A.C.; Devarbhavi, H. A Review and Current Perspective on Wilson Disease. J. Clin. Exp. Hepatol. 2013, 3, 321–336. [Google Scholar] [CrossRef]
- Liu, M.; Cohen, E.J.; Brewer, G.J.; Laibson, P.R. Kayser-Fleischer Ring as the Presenting Sign of Wilson Disease. Am. J. Ophthalmol. 2002, 133, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Acıpayam, C.; Altunay, A.; İlhan, N.; Atçı, N. Wilson’s Disease Presenting With Pancytopenia. Mustafa Kemal Üniversitesi Tıp Dergisi 2015, 6, 43–45. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Pan, J.; Zheng, Y.; Xu, X. Copper Homeostasis and Cuproptosis in Radiation-Induced Injury. Biomed. Pharmacother. 2024, 178, 117150. [Google Scholar] [CrossRef]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Pivariu, D.; Oros, A.N.; Tabaran, A.; Caloni, F.; Bolfa, P.; Nagy, A.-L. Chronic Copper Bilysinate Poisoning in Five Texel Sheep: A Case Report. Life 2024, 14, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Xu, K.; Wang, G.; Zhang, F. Copper Homeostasis and Neurodegenerative Diseases. Neural Regen. Res. 2025, 20, 3124–3143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, Y.; Shu, Q.; Kan, Y.-K.; Wang, Z. Cuproptosis and Copper as Potential Mechanisms and Intervention Targets in Alzheimer’s Disease. Biomed. Pharmacother. 2025, 183, 117814. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, X.; Li, J.; Xie, Z.; Wu, Q.; Chen, J.; Wang, Y.; Chen, Z.; Cao, X.; Li, T.; et al. Insights Into the Role of Copper in Neurodegenerative Diseases and the Therapeutic Potential of Natural Compounds. Curr. Neuropharmacol. 2024, 22, 1650–1671. [Google Scholar] [CrossRef]
- Twomey, P.J.; Viljoen, A.; House, I.M.; Reynolds, T.M.; Wierzbicki, A.S. Adjusting Copper Concentrations for Caeruloplasmin Levels in Routine Clinical Practice. J. Clin. Pathol. 2006, 59, 867–869. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and Characterization of Nitrogen-Doped Carbon Dots as Fluorescent Nanoprobes with Antimicrobial Properties and Skin Permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Demirci, S.; Suner, S.S.; Sahiner, M.; Akcali, A.; Guven, O.; Sahiner, N. A Comparative Study of Nitrogen Doped Carbon Dots Prepared from Linear Polyethyleneimine (L-PEI) and Branched Polyethyleneimine (B-PEI): Thermal, Optical, Biocompatibility, Sensor, Antibacterial, and Light-Induced Antibacterial Activity. J. Fluoresc. 2025. [Google Scholar] [CrossRef] [PubMed]
- Sousa, H.B.A.; Martins, C.S.M.; Prior, J.A.V. You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials 2021, 11, 611. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon Dots: Synthesis, Formation Mechanism, Fluorescence Origin and Sensing Applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, Y.; Tao, S.; Han, X.; Zhang, K.; Yang, B. Carbonized Polymer Dots: A Class of Highly Functionalized Nanoparticles with Polymeric Characteristics. Polym. Sci. Technol. 2025, 1, 436–457. [Google Scholar] [CrossRef]
- Wang, Z.; Li, F.; Zhang, L.; Qian, J.; Cao, S. Phase-Transfer-Assisted Synthesis of Cysteine-Ag Nanoparticles/Graphene Oxide Nanocomposite and Its Enhanced Performance in Antibiosis and Biosensing. Nanotechnology 2020, 31, 455603. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A Minireview on Doped Carbon Dots for Photocatalytic and Electrocatalytic Applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Hu, R.; Li, L.; Jin, W.J. Controlling Speciation of Nitrogen in Nitrogen-Doped Carbon Dots by Ferric Ion Catalysis for Enhancing Fluorescence. Carbon 2017, 111, 133–141. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Ren, W.; Cheng, H.; Tang, N.; Wu, W.; Zhong, W.; Du, Y. The Doping of Reduced Graphene Oxide with Nitrogen and Its Effect on the Quenching of the Material’s Photoluminescence. Carbon 2012, 50, 5286–5291. [Google Scholar] [CrossRef]
- Barman, B.K.; Okano, K.; Deguchi, K.; Ohki, S.; Hashi, K.; Goto, A.; Nagao, T. N-Dopant Site Formulation for White-Light-Emitting Carbon Dots with Tunable Chromaticity. ACS Sustain. Chem. Eng. 2022, 10, 16136–16149. [Google Scholar] [CrossRef]
- Osorio, H.M.; Castillo-Solís, F.; Barragán, S.Y.; Rodríguez-Pólit, C.; Gonzalez-Pastor, R. Graphene Quantum Dots from Natural Carbon Sources for Drug and Gene Delivery in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 10539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly Luminescent N-Doped Carbon Quantum Dots as an Effective Multifunctional Fluorescence Sensing Platform. Chem.—Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Zhang, H.; Ji, X.; Sun, W.; Yu, Y.; Liu, Y.; Huang, J.; Sarshar, Z.; Sain, M. High Quantum Yield Photoluminescent N-Doped Carbon Dots for Switch Sensing and Imaging. Talanta 2021, 222, 121663. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Muthu, A.; Elsakhawy, T.; Sheta, M.H.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials 2025, 15, 725. [Google Scholar] [CrossRef]
- Thanjavur, N.; Kim, Y.-J. Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing. Chemosensors 2025, 13, 241. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; El-Ramady, H.; Prokisch, J. Food Safety Aspects of Carbon Dots: A Review. Environ. Chem. Lett. 2025, 23, 337–360. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Vinodh, R.; Sangaraju, S.; Kishore, S.C.; Lee, Y.R. Natural Nitrogen-Doped Carbon Dots Obtained from Hydrothermal Carbonization of Chebulic Myrobalan and Their Sensing Ability toward Heavy Metal Ions. Sensors 2023, 23, 787. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-Doped Carbon Quantum Dots as Fluorometric Probes for Iron Detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Zeng, J.; Liao, L.; Lin, X.; Liu, G.; Luo, X.; Luo, M.; Wu, F. Red-Emissive Sulfur-Doped Carbon Dots for Selective and Sensitive Detection of Mercury (II) Ion and Glutathione. Int. J. Mol. Sci. 2022, 23, 9213. [Google Scholar] [CrossRef]
- Kamali, S.R.; Chen, C.-N.; Agrawal, D.C.; Wei, T.-H. Sulfur-Doped Carbon Dots Synthesis under Microwave Irradiation as Turn-off Fluorescent Sensor for Cr(III). J. Anal. Sci. Technol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Chaghaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, K.; Joseph, Y.; Rahimi, P. Nitrogen and Sulfur Co-Doped Carbon Quantum Dots Fluorescence Quenching Assay for Detection of Mercury (II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122448. [Google Scholar] [CrossRef]
- Mohandoss, S.; Ganesan, S.; Palanisamy, S.; You, S.; Velsankar, K.; Sudhahar, S.; Lo, H.-M.; Lee, Y.R. Nitrogen, Sulfur, and Phosphorus Co-Doped Carbon Dots-Based Ratiometric Chemosensor for Highly Selective Sequential Detection of Al3+ and Fe3+ Ions in Logic Gate, Cell Imaging, and Real Sample Analysis. Chemosphere 2023, 313, 137444. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Xu, H.; Wang, R.; Dong, L.; Gao, S.; Tang, B.; Fang, W.; Hou, F.; Zhong, L.; et al. Nitrogen-Doped Carbon Quantum Dots from Poly(Ethyleneimine) for Optical Dual-Mode Determination of Cu 2+ and l-Cysteine and Their Logic Gate Operation. ACS Appl. Mater. Interfaces 2020, 12, 47245–47255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, X.; Lin, Z.; Fan, Y.; Shi, G.; Zhang, S.; Zhang, M. Facile Reflux Synthesis of Polyethyleneimine-capped Fluorescent Carbon Dots for Sequential Bioassays toward Cu2+/H2S and Its Application for a Logic System. Biotechnol. Appl. Biochem. 2019, 66, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Pei, Z.; Shen, B.; Li, Y.; Wei, W.; Zhang, J.; Li, J. Correlation between Surface Structure of Carbon Dots and Selective Detection of Heavy Metal Ions. Appl. Phys. A 2024, 130, 122. [Google Scholar] [CrossRef]
- Yang, J.; Jin, X.; Cheng, Z.; Zhou, H.; Gao, L.; Jiang, D.; Jie, X.; Ma, Y.; Chen, W. Facile and Green Synthesis of Bifunctional Carbon Dots for Detection of Cu2+ and ClO− in Aqueous Solution. ACS Sustain. Chem. Eng. 2021, 9, 13206–13214. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Mao, Z.; Deng, Y.; He, J.; Mu, H.; Li, P.; Zou, W.; Zhao, Q. Synthesis of Up-Conversion Fluorescence N-Doped Carbon Dots with High Selectivity and Sensitivity for Detection of Cu2+ Ions. Crystals 2023, 13, 812. [Google Scholar] [CrossRef]
- Won, S.; Kim, J. The Detection of Fe (III) and Ascorbic Acid by Fluorescence Quenching and Recovery of Carbon Dots Prepared from Coffee Waste. Korean J. Chem. Eng. 2022, 39, 2826–2833. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S.; Torres, J.H.; Sahiner, N. BPEI-Based N-Doped Carbon Dots with Sensitive and Selective Cu2+ Ion-Sensing Ability. Micromachines 2025, 16, 1275. https://doi.org/10.3390/mi16111275
Demirci S, Torres JH, Sahiner N. BPEI-Based N-Doped Carbon Dots with Sensitive and Selective Cu2+ Ion-Sensing Ability. Micromachines. 2025; 16(11):1275. https://doi.org/10.3390/mi16111275
Chicago/Turabian StyleDemirci, Sahin, Jorge H. Torres, and Nurettin Sahiner. 2025. "BPEI-Based N-Doped Carbon Dots with Sensitive and Selective Cu2+ Ion-Sensing Ability" Micromachines 16, no. 11: 1275. https://doi.org/10.3390/mi16111275
APA StyleDemirci, S., Torres, J. H., & Sahiner, N. (2025). BPEI-Based N-Doped Carbon Dots with Sensitive and Selective Cu2+ Ion-Sensing Ability. Micromachines, 16(11), 1275. https://doi.org/10.3390/mi16111275





