Application of Droplet-Array Sandwiching Technology to Click Reactions for High-Throughput Screening
Abstract
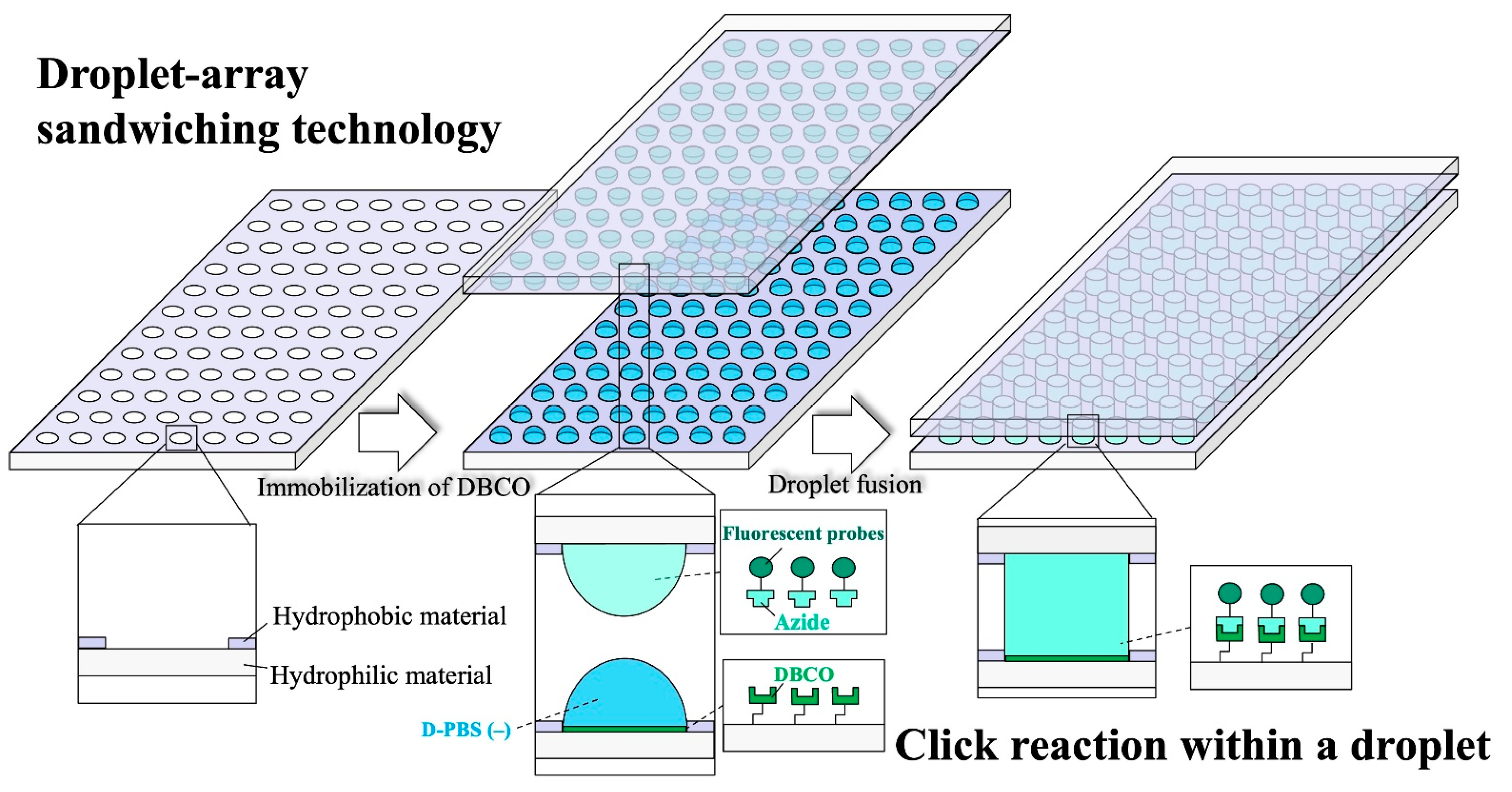
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of WP Substrates
2.3. Cleaning and Silanization
2.4. PEG Modification
2.5. DBCO Immobilization
2.6. DBCO–Azide Click Reaction
2.6.1. Manual Method
2.6.2. DAST Method (Gradient Generation and Batch Dispensing)
2.6.3. Post-Reaction Washing
2.7. Fluorescence Imaging and Analysis
2.7.1. Whole-Substrate Imaging (LuminoGraph I)
2.7.2. Per-Pattern Microscopy (Inverted Microscope)
3. Results and Discussion
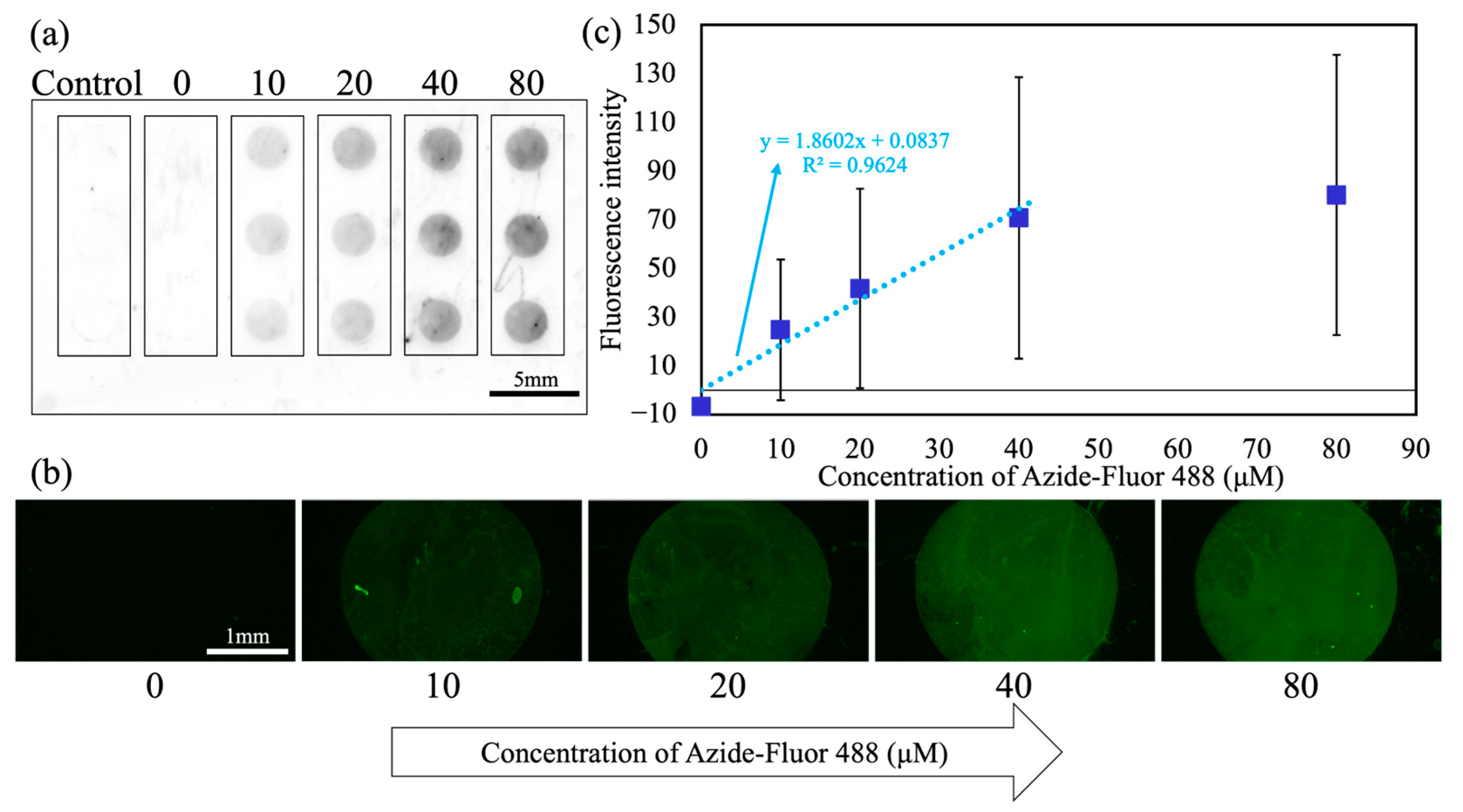
3.1. Evaluation of DBCO Immobilization on WP
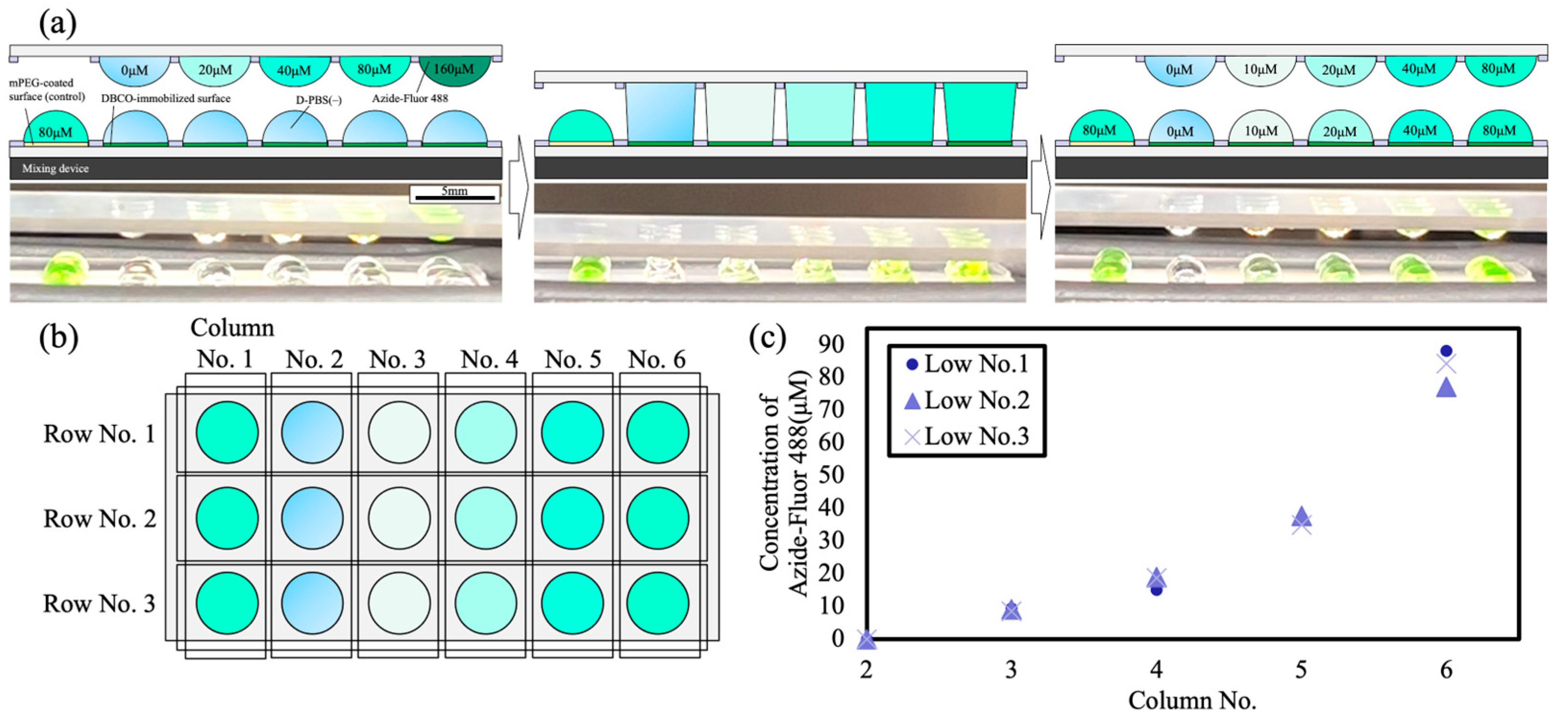
3.2. Concentration Gradient Generation by DAST
3.3. Click Reactions Executed by DAST
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Adaptation of High-Throughput Screening in Drug Discovery—Toxicological Screening Tests. Int. J. Mol. Sci. 2011, 13, 427–452. [Google Scholar] [CrossRef]
- Bleicher, K.H.; Böhm, H.-J.; Müller, K.; Alanine, A.I. Hit and Lead Generation: Beyond High-Throughput Screening. Nat. Rev. Drug Discov. 2003, 2, 369–378. [Google Scholar] [CrossRef]
- Du, G.; Fang, Q.; den Toonder, J.M. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef]
- Yang, L.; Pijuan-Galito, S.; Rho, H.S.; Vasilevich, A.S.; Eren, A.D.; Ge, L.; Habibović, P.; Alexander, M.R.; de Boer, J.; Carlier, A.; et al. High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology. Chem. Rev. 2021, 121, 4561–4677. [Google Scholar] [CrossRef]
- Lorenz, D.A.; Garner, A.L. A Click Chemistry-Based microRNA Maturation Assay Optimized for High-Throughput Screening. Chem. Commun. 2016, 52, 8267–8270. [Google Scholar] [CrossRef]
- Garner, A.L. Cat-ELCCA: Catalyzing Drug Discovery Through Click Chemistry. Chem. Commun. 2018, 54, 6531–6539. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.M.; Sekula-Neuner, S.; Trouillet, V.; Liu, H.-Y.; Kumar, R.; Powell, A.K.; Hirtz, M. Evaluation of Click Chemistry Microarrays for Immunosensing of Alpha-Fetoprotein (AFP). Beilstein J. Nanotechnol. 2019, 10, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Huang, Z.; am Ende, C.W.; Seneviratne, U. Protocol for Clickable Photoaffinity Labeling and Quantitative Chemical Proteomics. STAR Protoc. 2021, 2, 100593. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Kamuju, V.; Zhou, J.; Mathaes, R. Piston-Driven Automated Liquid Handlers. SLAS Technol. 2024, 29, 100128. [Google Scholar] [CrossRef]
- Michael, S.; Auld, D.; Klumpp, C.; Jadhav, A.; Zheng, W.; Thorne, N.; Austin, C.P.; Inglese, J.; Simeonov, A. A Robotic Platform for Quantitative High-Throughput Screening. Assay Drug Dev. Technol. 2008, 6, 637–657. [Google Scholar] [CrossRef]
- Bhusal, A.; Yogeshwaran, S.; Goodarzi Hosseinabadi, H.; Cecen, B.; Miri, A.K. Microfluidics for High Throughput Screening of Biological Agents and Therapeutics. Biomed. Mater. Devices 2025, 3, 93–107. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and Applications of Microfluidics in Biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- De Los Cobos, O.; Fousseret, B.; Lejeune, M.; Rossignol, F.; Dutreilh-Colas, M.; Carrion, C.; Boissière, C.; Ribot, F.; Sanchez, C.; Cattoën, X.; et al. Tunable Multifunctional Mesoporous Silica Microdots Arrays by Combination of Inkjet Printing, EISA, and Click Chemistry. Chem. Mater. 2012, 24, 4337–4342. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gourain, V.; Benz, M.; Scheiger, J.M.; Levkin, P.A.; Popova, A.A. Droplet Microarrays for Cell Culture: Effect of Surface Properties and Nanoliter Culture Volume on Global Transcriptomic Landscape. Mater. Today Bio 2021, 11, 100112. [Google Scholar] [CrossRef]
- Feng, W.; Ueda, E.; Levkin, P.A. Droplet Microarrays: From Surface Patterning to High-Throughput Applications. Adv. Mater. 2018, 30, 1706111. [Google Scholar] [CrossRef]
- Maeda, H.; Kobayashi, T.; Konishi, S. Patterning of Wettability Using the Photocatalytic Decomposition of Hydrophobic Self-Assembled Monolayer on the TiO2 Pattern. Jpn. J. Appl. Phys. 2017, 56, 06GN09. [Google Scholar] [CrossRef]
- Hirtz, M.; Greiner, A.M.; Landmann, T.; Bastmeyer, M.; Fuchs, H. Click-Chemistry Based Multi-Component Microarrays by Quill-Like Pens. Adv. Mater. Interfaces 2014, 1, 1300129. [Google Scholar] [CrossRef]
- Durand, J.-O.; Cattoen, X. Multi-Functional Silica Microdot Arrays by Inkjet Printing for Biosensor Applications. In Proceedings of the 2012 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2012, Santa Clara, CA, USA, 18–21 June 2012; Volume 2. [Google Scholar]
- Dadfar, S.M.M.; Sekula-Neuner, S.; Bog, U.; Trouillet, V.; Hirtz, M. Site-Specific Surface Functionalization via Microchannel Cantilever Spotting (µCS): Comparison between Azide–Alkyne and Thiol–Alkyne Click Chemistry Reactions. Small 2018, 14, 1800131. [Google Scholar] [CrossRef]
- Dupuis, A.; Léopoldès, J.; Bucknall, D.G.; Yeomans, J.M. Control of Drop Positioning Using Chemical Patterning. Appl. Phys. Lett. 2005, 87, 024103. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Vorobii, M.; Sauter, E.; Koenig, M.; Kumar, R.; Rodriguez-Emmenegger, C.; Hirtz, M. Evaluation of Dibenzocyclooctyne and Bicyclononyne Click Reaction on Azido-Functionalized Antifouling Polymer Brushes via Microspotting. Adv. Mater. Interfaces 2022, 9, 2102325. [Google Scholar] [CrossRef]
- Kumar, R.; Llewellyn, S.; Vasantham, S.K.; Nie, K.; Sekula-Neuner, S.; Vijayaraghavan, A.; Hirtz, M. Protein Spot Arrays on Graphene Oxide Coatings for Efficient Single-Cell Capture. Sci. Rep. 2022, 12, 3895. [Google Scholar] [CrossRef]
- Popova, A.A.; Schillo, S.M.; Demir, K.; Ueda, E.; Nesterov-Mueller, A.; Levkin, P.A. Droplet-Array (DA) Sandwich Chip: A Versatile Platform for High-Throughput Cell Screening Based on Superhydrophobic–Superhydrophilic Micropatterning. Adv. Mater. 2015, 27, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Ohya, C.; Yamada, T. Selective Control of the Contact and Transport Between Droplet Pairs by Electrowetting-on-Dielectric for Droplet-Array Sandwiching Technology. Sci. Rep. 2021, 11, 12355. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.A.; Demir, K.; Hartanto, T.G.; Schmitt, E.; Levkin, P.A. Droplet-Microarray on Superhydrophobic–Superhydrophilic Patterns for High-Throughput Live Cell Screenings. Rsc Adv. 2016, 6, 38263–38276. [Google Scholar] [CrossRef]
- Wu, J.; Wheeldon, I.; Guo, Y.; Lu, T.; Du, Y.; Wang, B.; He, J.; Hu, Y.; Khademhosseini, A. A Sandwiched Microarray Platform for Benchtop Cell-Based High Throughput Screening. Biomaterials 2011, 32, 841–848. [Google Scholar] [CrossRef]
- Miyata, Y.; Bono, S.; Konishi, S. Homogenization of Solute Concentration in Vertically Coalescent Droplets Using Acoustic Wave. In Proceedings of the 2025 23rd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 29 June–3 July 2025; pp. 1264–1267. [Google Scholar]
- Bono, S.; Kinugasa, H.; Kajita, H.; Konishi, S. Resonant Oscillation of Droplets under an Alternating Electric Field to Enhance Solute Diffusion. Sci. Rep. 2024, 14, 21326. [Google Scholar] [CrossRef] [PubMed]
- Bono, S.; Sakai, K.; Konishi, S. Enhancement of Solute Diffusion in Microdroplets Using Microrotors under Rotational Magnetic Field. Sci. Rep. 2023, 13, 11169. [Google Scholar] [CrossRef]
- Bono, S.; Miyata, Y.; Konishi, S. Volume Difference in Microdroplets in Vertical Contact Control under Electrowetting-Induced Asymmetric Boundary Condition. Jpn. J. Appl. Phys. 2023, 62, 017003. [Google Scholar] [CrossRef]
- Maeda, H.; Ohya, C.; Matsuyoshi, T.; Kobayashi, T.; Konishi, S. Contact Fusion of Droplets Patterned on Opposing Plates for Cellular Transportation and Medium Exchange for Hanging Droplet Cell Culture. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 115–118. [Google Scholar]
- Konishi, S.; Higuchi, Y.; Tamayori, A. Cellular Calcium Oscillations in Droplets with Different Chemical Concentrations Supplied by Droplet-Array Sandwiching Technology. Sens. Actuators B Chem. 2022, 370, 132435. [Google Scholar] [CrossRef]
- Bono, S.; Konishi, S. Chirality Sensing Mechanism Susing Vertical Contact Control of Liquid Crystal Micro-Droplets. In Proceedings of the 2023 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kyoto, Japan, 25–29 June 2023; pp. 397–400. [Google Scholar]
- Agnihotri, S.N.; Das, P.K.; Tolboom, F.; Werr, G.; Palierse, E.; Persson, C.; Tenje, M. Dynamics of Non-Newtonian Agarose Gel Droplet Formation in Two-Phase Microfluidic Systems. Phys. Fluids 2025, 37, 032010. [Google Scholar] [CrossRef]
- Khater, A.; Abdelrehim, O.; Mohammadi, M.; Azarmanesh, M.; Janmaleki, M.; Salahandish, R.; Mohamad, A.; Sanati-Nezhad, A. Picoliter Agar Droplet Breakup in Microfluidics Meets Microbiology Application: Numerical and Experimental Approaches. Lab. Chip 2020, 20, 2175–2187. [Google Scholar] [CrossRef]
- Zhu, C.; Takemoto, H.; Higuchi, Y.; Yamashita, F. Programmed Immobilization of Living Cells Using Independent Click Pairs. Biochem. Biophys. Res. Commun. 2024, 699, 149556. [Google Scholar] [CrossRef]
- Miyata, Y.; Bono, S.; Konishi, S. Volume Control of Droplets in Vertical Contact-Separation Process Using Radius Difference of Solid–Liquid Interface on Substrate. J. Phys. Soc. Jpn. 2023, 92, 104802. [Google Scholar] [CrossRef]
- Bono, S.; Takahashi, R.; Konishi, S. Quantitative Analysis of the Volume Difference of Microdroplets in Vertical Contact Control. Phys. Rev. Appl. 2021, 16, 054044. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Saini, G.; Parasa, M.K.; Clayton, K.N.; Fraseur, J.G.; Bolton, S.C.; Lin, K.P.; Wereley, S.T.; Kinzer-Ursem, T.L. Immobilization of Azide-Functionalized Proteins to Micro- and Nanoparticles Directly from Cell Lysate. Mikrochim. Acta 2024, 191, 46. [Google Scholar] [CrossRef]
- Hudalla, G.A.; Murphy, W.L. Using “Click” Chemistry to Prepare SAM Substrates to Study Stem Cell Adhesion. Langmuir 2009, 25, 5737–5746. [Google Scholar] [CrossRef][Green Version]
- Song, Y.; Wang, L.; Xu, T.; Zhang, G.; Zhang, X. Emerging Open-Channel Droplet Arrays for Biosensing. Natl. Sci. Rev. 2023, 10, nwad106. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyata, Y.; Nishimura, S.; Kawakami, S.; Higuchi, Y.; Konishi, S. Application of Droplet-Array Sandwiching Technology to Click Reactions for High-Throughput Screening. Micromachines 2025, 16, 1270. https://doi.org/10.3390/mi16111270
Miyata Y, Nishimura S, Kawakami S, Higuchi Y, Konishi S. Application of Droplet-Array Sandwiching Technology to Click Reactions for High-Throughput Screening. Micromachines. 2025; 16(11):1270. https://doi.org/10.3390/mi16111270
Chicago/Turabian StyleMiyata, Yoshinori, Shoma Nishimura, Sora Kawakami, Yuriko Higuchi, and Satoshi Konishi. 2025. "Application of Droplet-Array Sandwiching Technology to Click Reactions for High-Throughput Screening" Micromachines 16, no. 11: 1270. https://doi.org/10.3390/mi16111270
APA StyleMiyata, Y., Nishimura, S., Kawakami, S., Higuchi, Y., & Konishi, S. (2025). Application of Droplet-Array Sandwiching Technology to Click Reactions for High-Throughput Screening. Micromachines, 16(11), 1270. https://doi.org/10.3390/mi16111270




