Advances in Impedimetric Biosensors: Current Applications and Future Directions
Abstract
1. Introduction
2. Components of Impedimetric Biosensors
2.1. Working Electrode
2.1.1. Metal and Metal Oxide Electrodes
2.1.2. Carbon-Based Materials and Advanced Nanomaterials
2.2. Biorecognition Elements
2.3. Detection Methods
2.4. Performance Limitations and Mitigation Techniques
3. Classification of Impedimetric Biosensors
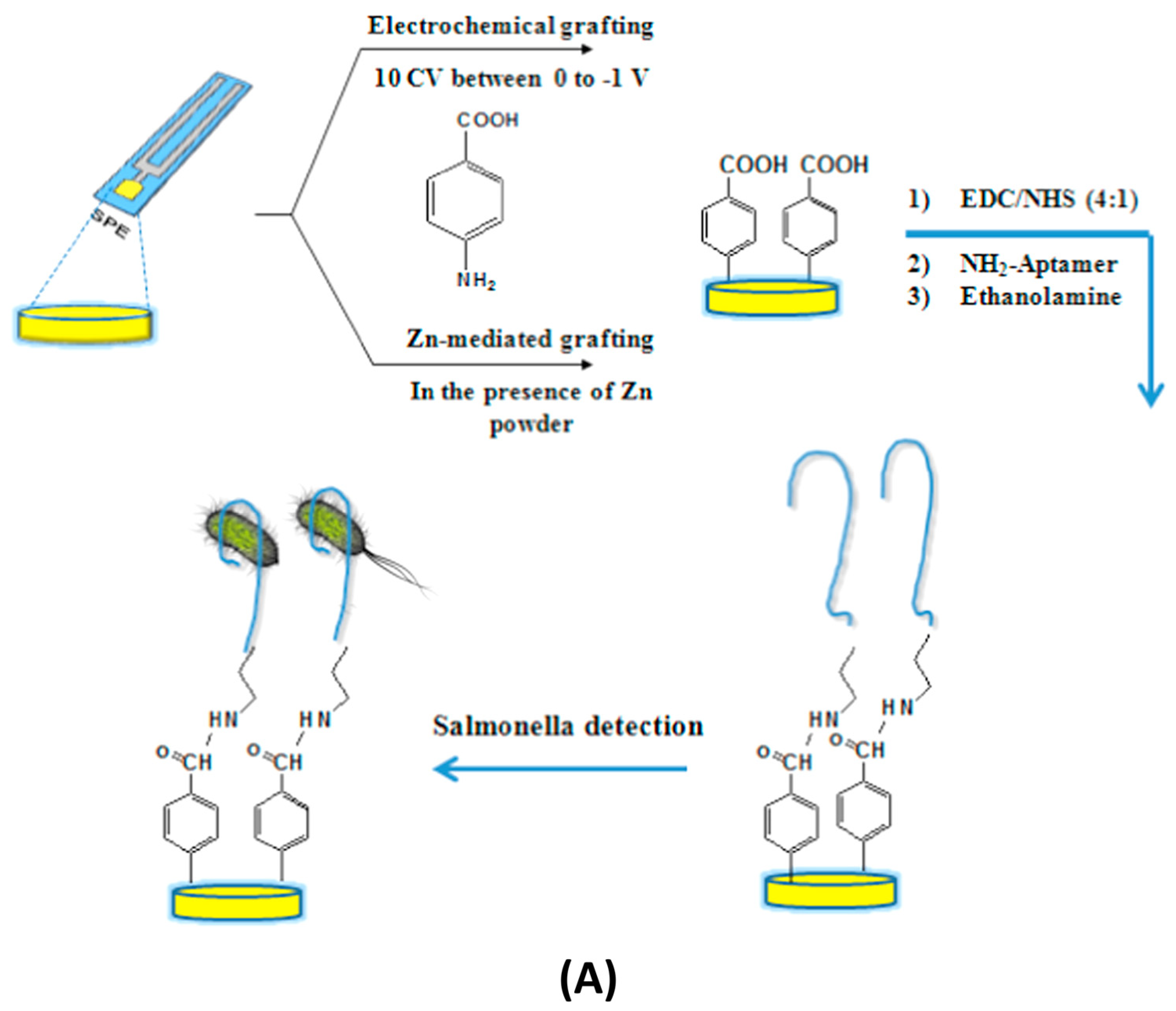
3.1. Label-Free Impedimetric Biosensors
3.1.1. Recognition Elements
3.1.2. Sensing Modalities
Interdigitated Electrodes
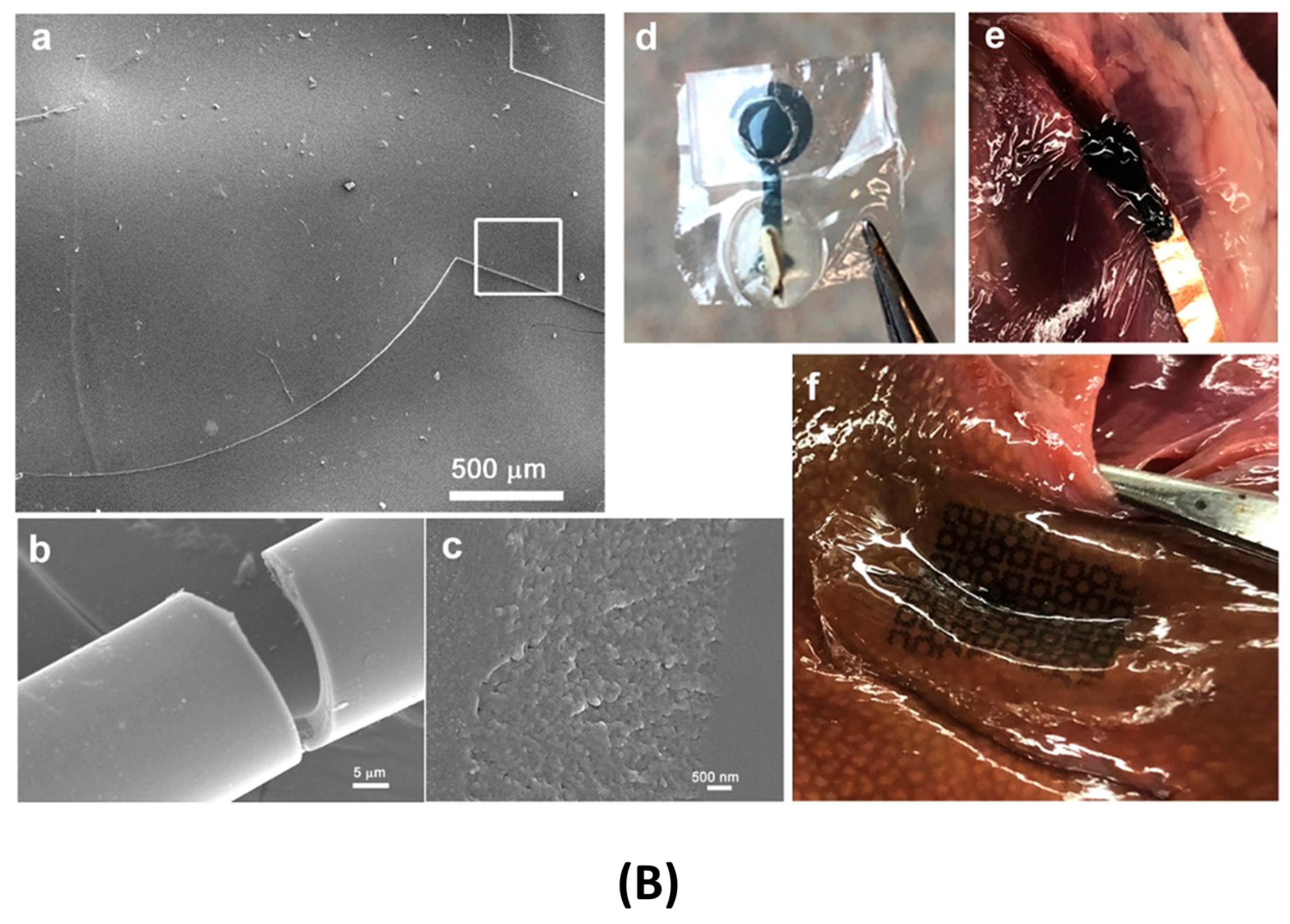
Flexible and Wearable Platforms
Microfluidic Integration
3.2. Label-Based Impedimetric Biosensors
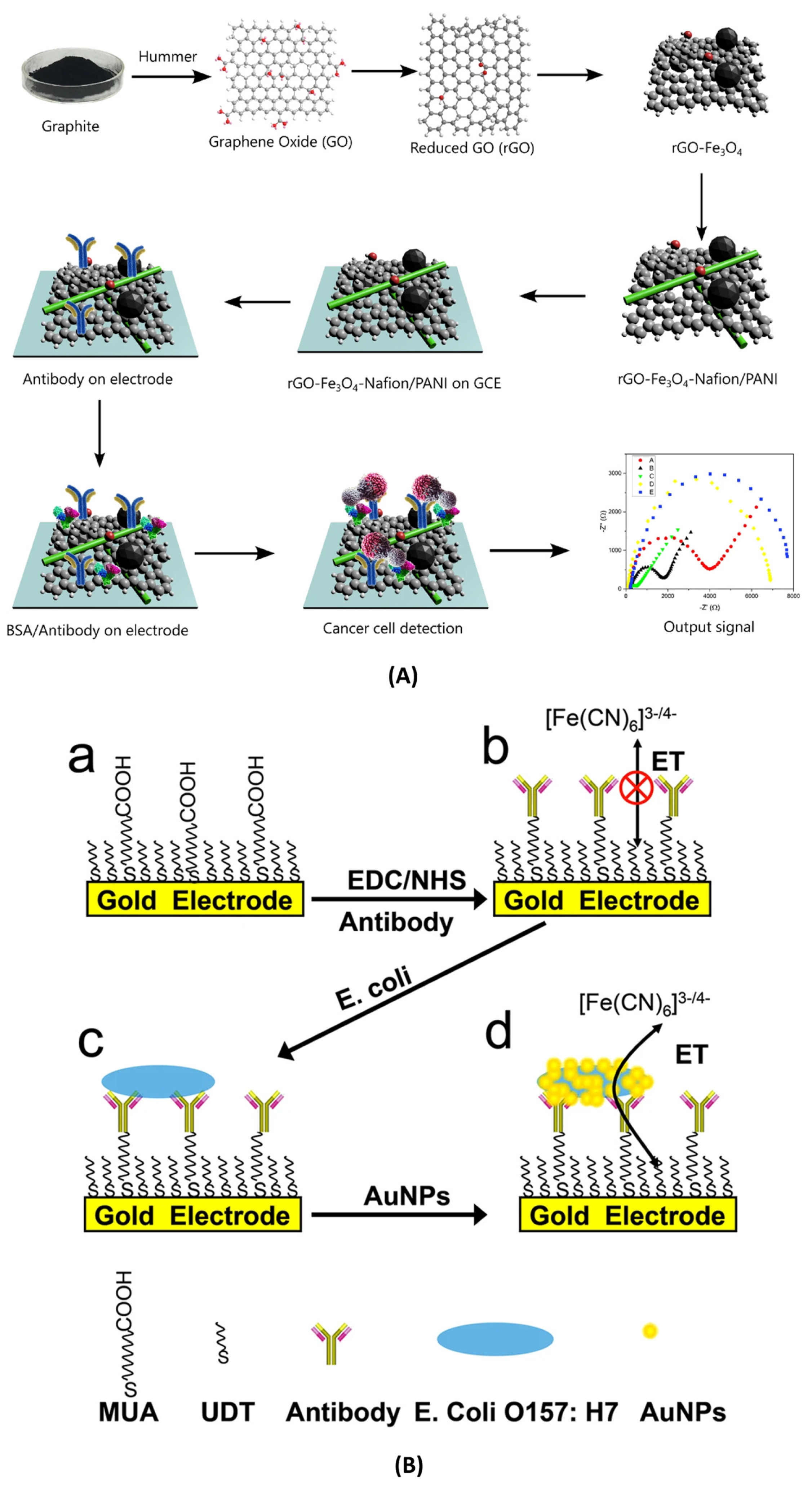
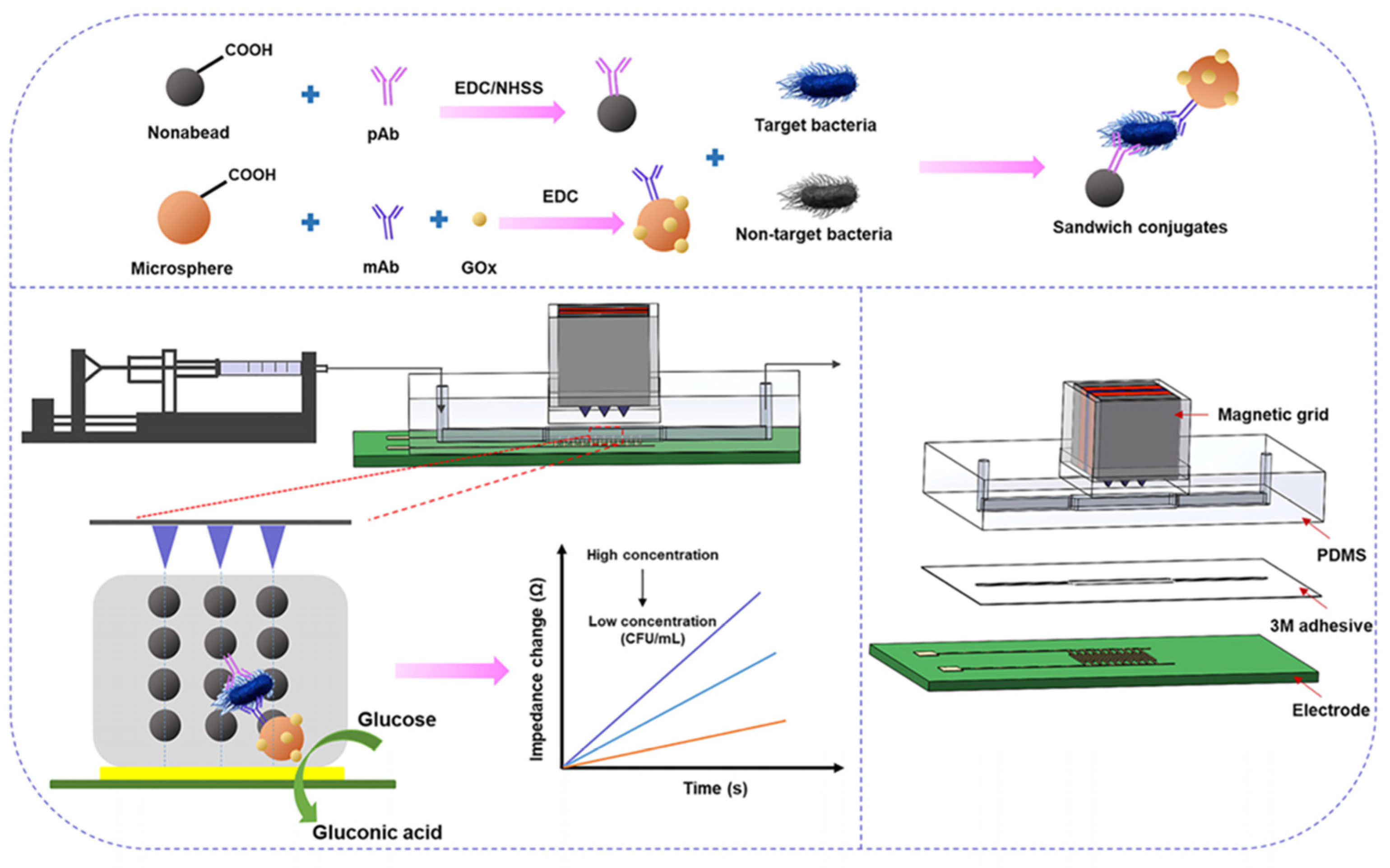
3.2.1. Recognition Elements
3.2.2. Sensing Modalities
Nanostructured Electrodes with Labels
Microfluidic Systems
4. Current Applications of Impedimetric Biosensors
4.1. Medical and Clinical Diagnostics with PoC Devices for Rapid Testing
4.1.1. Cancer Biomarker Detection
4.1.2. Diabetes Biomarkers Detection
4.1.3. Cardiac Disease Biomarker Detection
4.2. Impedimetric Biosensors for Environmental Monitoring
4.3. Impedimetric Biosensors for Food Safety and Quality Control
4.4. Impedimetric Biosensors for Agricultural Applications
5. Potential for Integration with Emerging Technologies
5.1. Artificial Intelligence and Machine Learning
5.2. Use in Wearable Devices
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mehrotra, P. Biosensors and their applications–A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Sheikhzadeh, E.; Chamsaz, M.; Turner, A.; Jager, E.; Beni, V. Label-free impedimetric biosensor for Salmonella typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016, 80, 194–200. [Google Scholar] [CrossRef]
- Bagheryan, Z.; Raoof, J.-B.; Golabi, M.; Turner, A.P.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Gao, X.; Lin, Q.; Suo, Z.; Wu, D.; Wu, X.; Chen, Q. A Label-Free and Antibody-Free Molecularly Imprinted Polymer-Based Impedimetric Sensor for NSCLC-Cells-Derived Exosomes Detection. Biosensors 2023, 13, 647. [Google Scholar] [CrossRef]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.-S.; Hahn, Y.-B. Recent advances in nanowires-based field-effect transistors for biological sensor applications. Biosens. Bioelectron. 2018, 100, 312–325. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.T.; Souto, D.E.; Barragan, J.T.; Giarola, J.d.F.; de Moraes, A.C.; Kubota, L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, P.; Kuddushi, M.; Joshi, G.; Khatua, S.; Dhanka, M.; Chauhan, D.S. Advancements in nanosensors for an early detection of cancer. In Nanosensors in Healthcare Diagnostics; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez, D.I.S.; Janicijevic, Z.; Palestina Romero, B.; Oliveros Mata, E.S.; Laube, M.; Feldmann, A.; Kegler, A.; Drewitz, L.; Fowley, C.; Pietzsch, J.; et al. Impedimetric nanobiosensor for the detection of SARS-CoV-2 antigens and antibodies. ACS Sens. 2023, 8, 576–586. [Google Scholar] [CrossRef]
- Dhull, N.; Kaur, G.; Jain, P.; Mishra, P.; Singh, D.; Ganju, L.; Gupta, V.; Tomar, M. Label-free amperometric biosensor for Escherichia coli O157: H7 detection. Appl. Surf. Sci. 2019, 495, 143548. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Khodaie, A.; Rafighirani, Y.; Heidarzadeh, H.; Javidan, J. Design and numerical evaluation of a high sensitivity plasmonic biosensor based on MISM nanoring for versatile virus detection. Sci. Rep. 2025, 15, 21484. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702. [Google Scholar] [CrossRef]
- Lovrić, M.; Osteryoung, J. Theory of differential normal pulse voltammetry. Electrochim. Acta 1982, 27, 963–968. [Google Scholar] [CrossRef]
- de Faria, R.A.D.; Heneine, L.G.D.; Matencio, T.; Messaddeq, Y. Faradaic and non-faradaic electrochemical impedance spectroscopy as transduction techniques for sensing applications. Int. J. Biosens. Bioelectron. 2019, 5, 29–31. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Uygun, H.D.E. A short footnote: Circuit design for faradaic impedimetric sensors and biosensors. Sens. Actuators B Chem. 2014, 202, 448–453. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Shanehsaz, M.; Ghaemmaghami, M. Non-Faradaic electrochemical impedance spectroscopy as a reliable and facile method: Determination of the potassium ion concentration using a guanine rich aptasensor. Mater. Sci. Eng. C 2015, 52, 151–154. [Google Scholar] [CrossRef]
- Ibau, C.; Arshad, M.M.; Gopinath, S.C.; M.N, M.N.; Fathil, M.; Shamsuddin, S.A. Immunosensing prostate-specific antigen: Faradaic vs non-Faradaic electrochemical impedance spectroscopy analysis on interdigitated microelectrode device. Int. J. Biol. Macromol. 2020, 162, 1924–1936. [Google Scholar] [CrossRef]
- Abdelrasoul, G.N.; Anwar, A.; MacKay, S.; Tamura, M.; Shah, M.A.; Khasa, D.P.; Montgomery, R.R.; Ko, A.I.; Chen, J. DNA aptamer-based non-faradaic impedance biosensor for detecting E. coli. Anal. Chim. Acta 2020, 1107, 135–144. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 1239–1257. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Xie, Q.-Z.; Lin, M.-W.; Hsu, W.-E.; Lin, C.-T. Advancements of nanoscale structures and materials in impedimetric biosensing technologies. ECS J. Solid State Sci. Technol. 2020, 9, 115027. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Štukovnik, Z.; Fuchs-Godec, R.; Bren, U. Nanomaterials and their recent applications in impedimetric biosensing. Biosensors 2023, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Alrebaish, A.S.; Alnami, L.O.; Alshraim, J.M.; Alnghemshi, R.A.; Aljammaz, A.A.; Altinawi, A.; Alhuthali, K.K.; Alfadul, H.; Assaifan, A.K. Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability. Micromachines 2025, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Rong, Y.; Vanegas, D.C.; Claussen, J.; McLamore, E.S.; Gomes, C. Impedance biosensor for the rapid detection of Listeria spp. based on aptamer functionalized Pt-interdigitated microelectrodes array. In Proceedings of the Smart Biomedical and Physiological Sensor Technology XIII, Baltimore, MD, USA, 17–21 April 2016; SPIE: Bellingham, WA, USA, 2016; pp. 77–84. [Google Scholar]
- Zarei, S.S.; Soleimanian-Zad, S.; Ensafi, A.A. An impedimetric aptasensor for Shigella dysenteriae using a gold nanoparticle-modified glassy carbon electrode. Microchim. Acta 2018, 185, 538. [Google Scholar] [CrossRef]
- Hosseine, M.; Naghib, S.M.; Khodadadi, A. Label-free electrochemical biosensor based on green-synthesized reduced graphene oxide/Fe3O4/nafion/polyaniline for ultrasensitive detection of SKBR3 cell line of HER2 breast cancer biomarker. Sci. Rep. 2024, 14, 11928. [Google Scholar] [CrossRef] [PubMed]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsyst. Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.M.; Kabiri, S.; Fotouhi, L.; Dorraji, P.S.; Omidfar, K. Impedimetric Paper-Based Enzymatic Biosensor Using Electrospun Cellulose Acetate Nanofiber and Reduced Graphene Oxide for Detection of Glucose from Whole Blood. IEEE Sens. J. 2021, 21, 9210–9217. [Google Scholar] [CrossRef]
- Košelová, Z.; Fohlerová, Z. Enzyme-Based Impedimetric Biosensor dotted with gold nanoparticles. In Proceedings II of the 30st Conference Student EEICT 2024: Selected Papers; Brno University of Technology, Faculty of Electrical Engineering and Communication: Brno, Czech Republic, 2024; pp. 202–206. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Anwar, A.; MacKay, S.; Abdelrasoul, G.N.; Lin, D.; Yan, Z.; Nguyen, A.H.; McDermott, M.T.; Shah, M.A.; Yang, J.; et al. Immuno-impedimetric Biosensor for Onsite Monitoring of Ascospores and Forecasting of Sclerotinia Stem Rot of Canola. Sci. Rep. 2018, 8, 12396. [Google Scholar] [CrossRef]
- Jaradat, H.; Al-Hamry, A.; Ibbini, M.; Fourati, N.; Kanoun, O. Novel sensitive electrochemical immunosensor development for the selective detection of HopQ H. pylori bacteria biomarker. Biosensors 2023, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, G.B.; Ratnam, K.V.; Janardhan, S.; Shiprath, K.; Manjunatha, H.; Ramesha, M.; Prasad, N.V.K.; Ramesh, S.; Babu, T.A. Metal and metal oxide based advanced ceramics for electrochemical biosensors-A short review. Front. Mater. 2021, 8, 682025. [Google Scholar] [CrossRef]
- Akter, R.; Jeong, B.; Lee, Y.-M.; Choi, J.-S.; Rahman, M.A. Femtomolar detection of cardiac troponin I using a novel label-free and reagent-free dendrimer enhanced impedimetric immunosensor. Biosens. Bioelectron. 2017, 91, 637–643. [Google Scholar] [CrossRef]
- Rabti, A.; Zayani, R.; Meftah, M.; Salhi, I.; Raouafi, N. Impedimetric DNA E-biosensor for multiplexed sensing of Escherichia coli and its virulent f17 strains. Microchim. Acta 2020, 187, 635. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Mehrban, M.; Madrakian, T.; Afkhami, A.; Jalal, N.R. Fabrication of impedimetric sensor based on metallic nanoparticle for the determination of mesna anticancer drug. Sci. Rep. 2023, 13, 11381. [Google Scholar] [CrossRef] [PubMed]
- Elnagar, N.; Elgiddawy, N.; El Rouby, W.M.A.; Farghali, A.A.; Korri-Youssoufi, H. Impedimetric Detection of Cancer Markers Based on Nanofiber Copolymers. Biosensors 2024, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Facure, M.H.; Gahramanova, G.; Zhang, D.; Zhang, T.; Shuck, C.E.; Mercante, L.A.; Correa, D.S.; Gogotsi, Y. All-MXene electronic tongue for neurotransmitters detection. Biosens. Bioelectron. 2024, 262, 116526. [Google Scholar] [CrossRef]
- Sadri, N.; Mazloum-Ardakani, M.; Asadpour, F.; Joseph, Y.; Rahimi, P. An Enzyme-Free Impedimetric Sensor Based on Flower-like NiO/Carbon Microspheres for L-Glutamic Acid Assay. Biosensors 2024, 14, 543. [Google Scholar] [CrossRef]
- Hadian, M.; Rabbani, M.; Shariati, L.; Ghasemi, F.; Presley, J.F.; Sanati, A. MXene Nanoconfinement of SAM-Modified Molecularly Imprinted Electrochemical Biosensor for Point-of-Care Monitoring of Carcinoembryonic Antigen. ACS Sens. 2024, 10, 857–867. [Google Scholar] [CrossRef]
- Ong, V.; Soleimani, A.; Amirghasemi, F.; Nejad, S.K.; Abdelmonem, M.; Razaviyayn, M.; Hosseinzadeh, P.; Comai, L.; Mousavi, M.P.S. Impedimetric sensing: An emerging tool for combating the COVID-19 pandemic. Biosensors 2023, 13, 204. [Google Scholar] [CrossRef]
- Patel, T.; Huang, J.; Krukiewicz, K. Multifunctional organic monolayer-based coatings for implantable biosensors and bioelectronic devices: Review and perspectives. Biosens. Bioelectron. X 2023, 14, 100349. [Google Scholar] [CrossRef]
- Gezahagne, H.F.; Brightbill, E.L.; Jin, D.S.; Krishnathas, S.; Brown, B.; Mooney, M.H.; O’rIordan, A.; Creedon, N.; Robinson, C.; Vogel, E.M. Suppression of Impedimetric Baseline Drift for Stable Biosensing. ECS Sens. Plus 2022, 1, 031605. [Google Scholar] [CrossRef]
- Dong, J.; Li, G.; Xia, L. Microfluidic magnetic spatial confinement strategy for the enrichment and ultrasensitive detection of MCF-7 and Escherichia coli O157: H7. Anal. Chem. 2022, 94, 16901–16909. [Google Scholar] [CrossRef]
- Xu, M.; Yadavalli, V.K. Flexible Biosensors for the Impedimetric Detection of Protein Targets Using Silk-Conductive Polymer Biocomposites. ACS Sens. 2019, 4, 1040–1047. [Google Scholar] [CrossRef]
- Cho, H.; Shim, S.; Cho, W.W.; Cho, S.; Baek, H.; Lee, S.-M.; Shin, D.-S. Electrochemical Impedance-Based Biosensors for the Label-Free Detection of the Nucleocapsid Protein from SARS-CoV-2. ACS Sens. 2022, 7, 1676–1684. [Google Scholar] [CrossRef]
- Fusco, G.; Gallo, F.; Tortolini, C.; Bollella, P.; Ietto, F.; De Mico, A.; D’annibale, A.; Antiochia, R.; Favero, G.; Mazzei, F. AuNPs-functionalized PANABA-MWCNTs nanocomposite-based impedimetric immunosensor for 2,4-dichlorophenoxy acetic acid detection. Biosens. Bioelectron. 2017, 93, 52–56. [Google Scholar] [CrossRef]
- Lai, W.-A.; Lin, C.-H.; Yang, Y.-S.; Lu, M.S.-C. Ultrasensitive and label-free detection of pathogenic avian influenza DNA by using CMOS impedimetric sensors. Biosens. Bioelectron. 2012, 35, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Mehennaoui, S.; Poorahong, S.; Jimenez, G.C.; Siaj, M. Selection of high affinity aptamer-ligand for dexamethasone and its electrochemical biosensor. Sci. Rep. 2019, 9, 6600. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Khajehsharifi, H.; Hajihosseini, S. Detection of oxytetracycline using an electrochemical label-free aptamer-based biosensor. Biosensors 2022, 12, 468. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Alqahtani, F.A.; Alnamlah, S.; Almutairi, R.; Alkhammash, H.I. Detection and Real-Time Monitoring of LDL-Cholesterol by Redox-Free Impedimetric Biosensors. BioChip J. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Lee, H.-B.; Meeseepong, M.; Trung, T.Q.; Kim, B.-Y.; Lee, N.-E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef]
- Farooq, A.; Hayat, F.; Zafar, S.; Butt, N.Z. Thin flexible lab-on-a-film for impedimetric sensing in biomedical applications. Sci. Rep. 2022, 12, 1066. [Google Scholar] [CrossRef]
- Zhang, P.; Williams, D.E.; Stephens, L.; Helps, R.; Pushparajah, I.P.S.; Travas-Sejdic, J.; Wood, M. Microfluidic Biosensors for the Detection of Motile Plant Zoospores. Biosensors 2025, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, C.; Qi, H.; Gao, Q.; Zhang, C. Electrochemical lectin-based biosensor array for detection and discrimination of carcinoembryonic antigen using dual amplification of gold nanoparticles and horseradish peroxidase. Sens. Actuators B Chem. 2016, 235, 575–582. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-free biosensors for laboratory-based diagnostics of infections: Current achievements and new trends. Biosensors 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- Wan, J.; Ai, J.; Zhang, Y.; Geng, X.; Gao, Q.; Cheng, Z. Signal-off impedimetric immunosensor for the detection of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 19806. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, L.; Jin, N.; Yuan, J.; Li, Y.; Lin, J. Magnetic nanobead chain-assisted real-time impedance monitoring using PCB interdigitated electrode for Salmonella detection. iScience 2023, 26, 108245. [Google Scholar] [CrossRef]
- Asrami, P.N.; Mozaffari, S.A.; Tehrani, M.S.; Azar, P.A. A novel impedimetric glucose biosensor based on immobilized glucose oxidase on a CuO-Chitosan nanobiocomposite modified FTO electrode. Int. J. Biol. Macromol. 2018, 118, 649–660. [Google Scholar] [CrossRef]
- Wang, H.; Ohnuki, H.; Endo, H.; Izumi, M. Impedimetric and amperometric bifunctional glucose biosensor based on hybrid organic–inorganic thin films. Bioelectrochemistry 2015, 101, 1–7. [Google Scholar] [CrossRef]
- Santos, A.; Davis, J.J.; Bueno, P.R. Fundamentals and applications of impedimetric and redox capacitive biosensors. J. Anal. Bioanal. Tech. S 2014, 7, 016. [Google Scholar] [CrossRef]
- Khare, R.; Verma, S.; Singh, P.; Pal, S.; Shrivastava, R. Blueprint for impedance-based electrochemical biosensors as bioengineered tools in the field of nano-diagnostics. Curr. Res. Biotechnol. 2022, 4, 564–578. [Google Scholar] [CrossRef]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef]
- Kim, S.; Song, H.; Ahn, H.; Kim, T.; Jung, J.; Cho, S.K.; Shin, D.-M.; Choi, J.-R.; Hwang, Y.-H.; Kim, K. A review of advanced impedance biosensors with microfluidic chips for single-cell analysis. Biosensors 2021, 11, 412. [Google Scholar] [CrossRef]
- Mathur, A.; Nayak, H.C.; Rajput, S.; Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Kumar, R. An enzymatic multiplexed impedimetric sensor based on α-MnO2/GQD nano-composite for the detection of diabetes and diabetic foot ulcer using micro-fluidic platform. Chemosensors 2021, 9, 339. [Google Scholar] [CrossRef]
- Mondal, D.; Paul, D.; Mukherji, S. Impedance spectroscopy-based detection of cardiac biomarkers on polyaniline coated filter paper. IEEE Sens. J. 2017, 17, 5021–5029. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Ghodsi, E.; Abdollahi, S.; Nadri, S. Porous graphene oxide nanostructure as an excellent scaffold for label-free electrochemical biosensor: Detection of cardiac troponin I. Mater. Sci. Eng. C 2016, 69, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.-H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Gavrilaș, S.; Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.-D. Recent trends in biosensors for environmental quality monitoring. Sensors 2022, 22, 1513. [Google Scholar] [CrossRef]
- Panagopoulou, C.; Skotadis, E.; Aslanidis, E.; Tzourmana, G.; Rapesi, A.; Tsioustas, C.; Kainourgiaki, M.; Kleitsiotis, G.; Tsekenis, G.; Tsoukalas, D. Non-faradaic impedimetric detection of heavy metal ions via a hybrid nanoparticle-DNAzyme biosensor. Biosensors 2024, 14, 321. [Google Scholar] [CrossRef]
- Pichetsurnthorn, P.; Vattipalli, K.; Prasad, S. Nanoporous impedemetric biosensor for detection of trace atrazine from water samples. Biosens. Bioelectron. 2012, 32, 155–162. [Google Scholar] [CrossRef]
- Kivirand, K.; Min, M.; Rinken, T. Challenges and Applications of Impedance-Based Biosensors. In Biosensors for Environmental Monitoring; BoD: Norderstedt, Germany, 2019; p. 53. [Google Scholar]
- Zhang, W.; Han, C.; Jia, B.; Saint, C.; Nadagouda, M.; Falaras, P.; Sygellou, L.; Vogiazi, V.; Dionysiou, D.D. A 3D graphene-based biosensor as an early microcystin-LR screening tool in sources of drinking water supply. Electrochim. Acta 2017, 236, 319–327. [Google Scholar] [CrossRef]
- Malvano, F.; Pilloton, R.; Albanese, D. Label-free impedimetric biosensors for the control of food safety—A review. Int. J. Environ. Anal. Chem. 2020, 100, 468–491. [Google Scholar] [CrossRef]
- Chai, C.; Oh, S.-W. Electrochemical impedimetric biosensors for food safety. Food Sci. Biotechnol. 2020, 29, 879–887. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance sensing platform for detection of the food pathogen listeria monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef]
- Abdelhamied, N.; Abdelrahman, F.; El-Shibiny, A.; Hassan, R.Y.A. Bacteriophage-based nano-biosensors for the fast impedimetric determination of pathogens in food samples. Sci. Rep. 2023, 13, 3498. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A review on the use of impedimetric sensors for the inspection of food quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef]
- Patel, R.; Vinchurkar, M.; Patkar, R.; Pranjale, G.; Baghini, M.S. Impedance based biosensor for agricultural pathogen detection. In Proceedings of the 2021 IEEE 21st International Conference on Nanotechnology (NANO), Montreal, QC, Canada, 28–30 July 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 385–388. [Google Scholar]
- Mondal, R.; Dam, P.; Chakraborty, J.; Paret, M.L.; Katı, A.; Altuntas, S.; Sarkar, R.; Ghorai, S.; Gangopadhyay, D.; Mandal, A.K.; et al. Potential of nanobiosensor in sustainable agriculture: The state-of-art. Heliyon 2022, 8, e12207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, J.; Yang, J.; MacKay, S.; Shoute, L.C. Method and device for detecting a component in a sample. U.S. Patent 18/081,894, 13 April 2023. [Google Scholar]
- Bukhamsin, A.; Kosel, J.; Blilou, I.; Salama, K.N. Accelerating adoption of species-agnostic plant sensors for precision farming. Nat. Rev. Electr. Eng. 2025, 2, 58–70. [Google Scholar] [CrossRef]
- Kwekha-Rashid, A.S.; Abduljabbar, H.N.; Alhayani, B. Coronavirus disease (COVID-19) cases analysis using machine-learning applications. Appl. Nanosci. 2023, 13, 2013–2025. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Jiang, Y.; Guo, M.; Yang, Y.; Yang, Y.; Yu, H. Electrochemical Impedance Spectroscopic Detection of E.coli with Machine Learning. J. Electrochem. Soc. 2020, 167, 047508. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, X.; Gao, X.; Wang, J.; Zhao, N.; Sha, J. Equivalent circuit model recognition of electrochemical impedance spectroscopy via machine learning. J. Electroanal. Chem. 2019, 855, 113627. [Google Scholar] [CrossRef]
- Tayyab, M.; Lin, Z.; Mahmoodi, S.R.; Javanmard, M. Automated Electrical Detection of Proteins for Oral Squamous Cell Carcinoma in an Integrated Microfluidic Chip Using Multi-Frequency Impedance Cytometry and Machine Learning. Sensors 2025, 25, 1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nat. Commun. 2020, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, W.; Suarez, J.E.D.D.; Athavan, H.; Wang, Y.; Yeung, C.; Lin, S.; Sankararaman, S.; Milla, C.; Emaminejad, S. Autonomous wearable sweat rate monitoring based on digitized microbubble detection. Lab A Chip 2022, 22, 4267–4275. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of machine learning assisted biosensors in point-of-care-testing for clinical decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef]
- Ramola, A.; Shakya, A.K.; Bergman, A. Finite Element Method-Based Modeling of a Novel Square Photonic Crystal Fiber Surface Plasmon Resonance Sensor with a Au–TiO2 Interface and the Relevance of Artificial Intelligence Techniques in Sensor Optimization. Photonics 2025, 12, 565. [Google Scholar] [CrossRef]
- Rong, Y.; Padron, A.V.; Hagerty, K.J.; Nelson, N.; Chi, S.; Keyhani, N.O.; Katz, J.; Datta, S.P.A.; Gomes, C.; McLamore, E.S. Post hoc support vector machine learning for impedimetric biosensors based on weak protein–ligand interactions. Analyst 2018, 143, 2066–2075. [Google Scholar] [CrossRef]
- Uzun, S.D. Machine learning-based prediction and interpretation of electrochemical biosensor responses: A comprehensive framework. Microchem. J. 2025, 218, 115656. [Google Scholar] [CrossRef]
- Schackart, K.E.; Yoon, J.-Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Jagannath, B.; Prasad, S. A new paradigm in sweat based wearable diagnostics biosensors using Room Temperature Ionic Liquids (RTILs). Sci. Rep. 2017, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Islam, S.; Jia, F.; Cao, Y.; Li, Y.; Cao, C. Flexible Biosensors for Food Pathogen Detection. Adv. Electron. Mater. 2024, 10, 2300898. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Yaakoubi, N.; Trivella, M.G.; Elaissari, A.; Sigaud, M.; Jaffrezic-Renault, N.; Errachid, A. A Flexible Label-Free Biosensor Sensitive and Selective to TNF-α: Application for Chronic Heart Failure. Sens. Transducers 2014, 27, 15. [Google Scholar]
- Chang, Y.-T.; Huang, J.-H.; Tu, M.-C.; Chang, P.; Yew, T.-R. Flexible direct-growth CNT biosensors. Biosens. Bioelectron. 2013, 41, 898–902. [Google Scholar] [CrossRef]
- Jafari, H.; Amiri, M.; Abdi, E.; Navid, S.L.; Bouckaert, J.; Jijie, R.; Boukherroub, R.; Szunerits, S. Entrapment of uropathogenic E. coli cells into ultra-thin sol-gel matrices on gold thin films: A low cost alternative for impedimetric bacteria sensing. Biosens. Bioelectron. 2019, 124–125, 161–166. [Google Scholar] [CrossRef]
- Arabsalmani, N.; Ghouchani, A.; Ashtiani, S.J.; Zamani, M. Exploring Bio-Impedance Sensing for Intelligent Wearable Devices. Bioengineering 2025, 12, 521. [Google Scholar] [CrossRef]
- Qiao, X.; Cai, Y.; Kong, Z.; Xu, Z.; Luo, X. A wearable electrochemical sensor based on anti-fouling and self-healing polypeptide complex hydrogels for sweat monitoring. ACS Sens. 2023, 8, 2834–2842. [Google Scholar] [CrossRef] [PubMed]

| Category /Subtype | Recognition Element | Limit of Detection (LoD) | Time to Response | Anti-Fouling Strategy | Reusability | Sample Matrix | Redox Probe Used | Advantages | Limitations | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Label-free (Faradaic) | Antibody (2,4-D) | 0.3 ppb | Not reported | PANABA/MWCNT/AuNP | No | Water, agro Samples | Yes | Selectivity, sensitivity, robust signal | Needs a redox probe, single use | [55] |
| Label-free (Non-faradaic) | DNA probe (Avian Influenza) | 1 fM | Not reported | Dielectric film, APTES/Glutaraldehyde | No | Buffer, serum | No | Real-time, reagent-free | Lower sensitivity | [56] |
| Label-free (Faradaic) | Aptamer (Salmonella) | 6 CFU/mL | 30 min | Diazonium-polymer | No | Apple juice, buffer | Yes | Real sample validated, ultra-low LoD | Needs a redox probe, single use | [5] |
| Label-free (Faradaic) | Aptamer (DXN) | 2.12 nM | Not reported | MCH | No | Milk, buffer | No | Reproducible, selective, food compatible | No active antifouling | [57] |
| Label-free (Non-faradaic) | MIP (Exosome, NSCLC) | 2.03 × 103 particles/mL | Not reported | MIP layer | Yes | Cell, serum | No | Selectivity, reuse, stability | Complex synthesis | [6] |
| Label-free (Non-faradaic) | Antibody (IL-8) | 90 pg/mL | Not reported | Au-IDE, Zimag analysis | No | Serum, buffer | No | Sensitive cytokine detection | Single use | [30] |
| Label-free (Non-faradaic) | Antibody (LDL cholesterol) | 120 pg/mL | Not reported | Not reported | No | PBS, blood | No | Wearable integration, continuous | Low stability and specificity | [59] |
| Label-free (Non-faradaic) | Spike protein (SARS-CoV-2) | 0.4 BAU/mL | <1 h | Ionic strength tuning | No | Serum | No | PoC/serology, label-free | Ionic dependence | [34] |
| Label-free (Non-faradaic) | Nucleocapsid (SARS-CoV-2) | 0.1 ng/mL | 60 min | ToAD 96-IDE array | No | Serum | No | High throughput, multiplexed | Multiplexing complexity | [54] |
| Label-free (Non-faradaic) | Cortisol antibody | pM range | Not reported | Stretchable microfluidic, AuNS | Yes | Sweat | No | Reusable, non-invasive, robust | Fabrication cost | [60] |
| Label-free (Non-faradaic) | VEGF antibody | 1.03 pg/mL | Not reported | Biodegradable silk polymer | Yes | Buffer serum, simulated urine | No | Biocompatibility, eco-friendly | single use | [53] |
| Label-free (Non-faradaic) | Chemotactic capture (zoospore) | Not reported | Not reported | Microfluidics | Yes | Plant water, field sample | No | Field detection, high selectivity | Low throughput | [62] |
| Label-based (Faradaic) | Lectin (CEA/Bacteria) | 0.01–0.05 ng/mL | Not reported | Lectin array multiplexed MEA | No | Blood | Yes | Multiplexing, robust signal | Probe/addition needed | [63] |
| Label-based (Faradaic) | Antibody-AuNP (E. coli) | 100 cfu/mL | Not reported | AuNP-modified SAM | No | Buffer, water | Yes | Signal-off, nanoparticle amplification | Nanoparticle requirement | [66] |
| Label-based (Faradaic) | GOx enzyme (Glucose) | 27 μM | <10 s | CuO-chitosan nanocomposite | No | Serum | Yes | Fast, enzyme-amplified, robust | Enzyme stability | [68] |
| Label-based (Faradaic) | Magnetic nanobead/ALP (Salmonella) | 50 cfu/mL | 3 min | Magnetic separation, microfluidic | No | Spiked chicken supernatant | Yes | Fast, sensitive, multiplexing | Bead-label integration complexity | [67] |
| Domain | Target(s) | Recognition Element/Platform | Format | References |
|---|---|---|---|---|
| Medical/Clinical | PSA, cTnI, IL-6, VEGF, Myoglobin, MPO, CK-MB | Antibodies, aptamers, nanocomposites | Label-free (mostly Faradaic) | [75,76,77] |
| Glucose, Tyrosine | Enzymes (GOx), nanocomposites | Label-based Faradaic | [68,69] | |
| Exosomes | MIP-based GCE | Label-free Faradaic | [6] | |
| Environmental Monitoring | Herbicides (2,4-D), heavy metals, pesticides, toxins | Antibodies, aptamers, nanostructures | Both | [78,79,80,81,82] |
| Food Safety | Salmonella, E. coli, and antibiotic residues | Aptamers, antibodies, phages | Both | [83,84,85,86,87] |
| Agriculture | Airborne ascospores, plant pathogens | IDEs, DNA probes | Label-free non-faradaic | [88,89,90,91] |
| Wearables/Flexible | Cortisol, VEGF, cytokines | Stretchable Au electrodes, silk-sericin inks | Label-free non-faradaic | [53,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Arqam, M.; Fraiwan, A. Advances in Impedimetric Biosensors: Current Applications and Future Directions. Micromachines 2025, 16, 1244. https://doi.org/10.3390/mi16111244
Verma A, Arqam M, Fraiwan A. Advances in Impedimetric Biosensors: Current Applications and Future Directions. Micromachines. 2025; 16(11):1244. https://doi.org/10.3390/mi16111244
Chicago/Turabian StyleVerma, Ashmit, Mohammad Arqam, and Arwa Fraiwan. 2025. "Advances in Impedimetric Biosensors: Current Applications and Future Directions" Micromachines 16, no. 11: 1244. https://doi.org/10.3390/mi16111244
APA StyleVerma, A., Arqam, M., & Fraiwan, A. (2025). Advances in Impedimetric Biosensors: Current Applications and Future Directions. Micromachines, 16(11), 1244. https://doi.org/10.3390/mi16111244






