TiO2 Nanotube-Enabled Glucose Biosensing: Transformative Insights from 2009 to 2024
Abstract
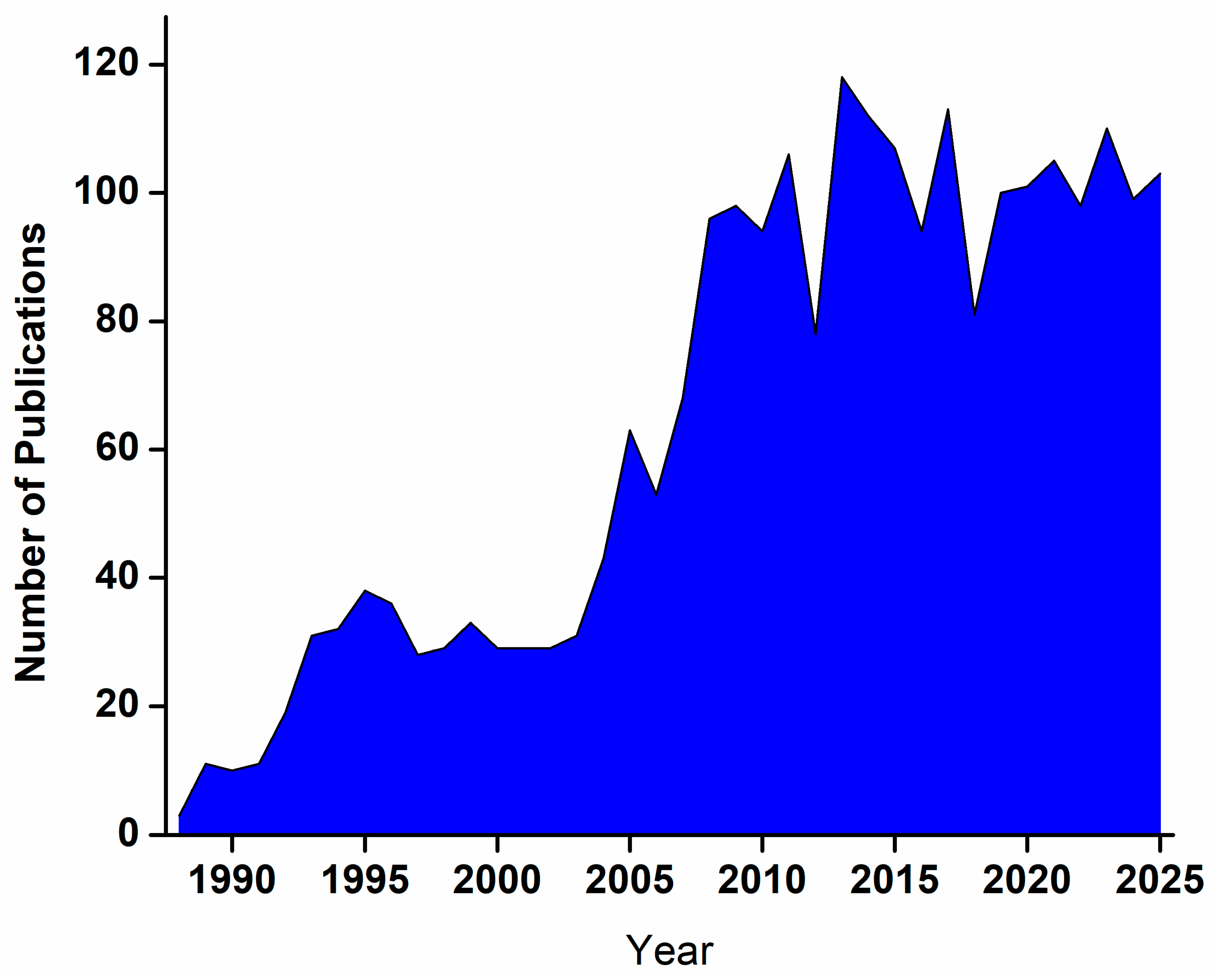
1. Introduction
2. Fundamentals of TiO2 NTAs
2.1. Synthesis
2.2. Properties
2.3. Surface Chemistry and Functionalization
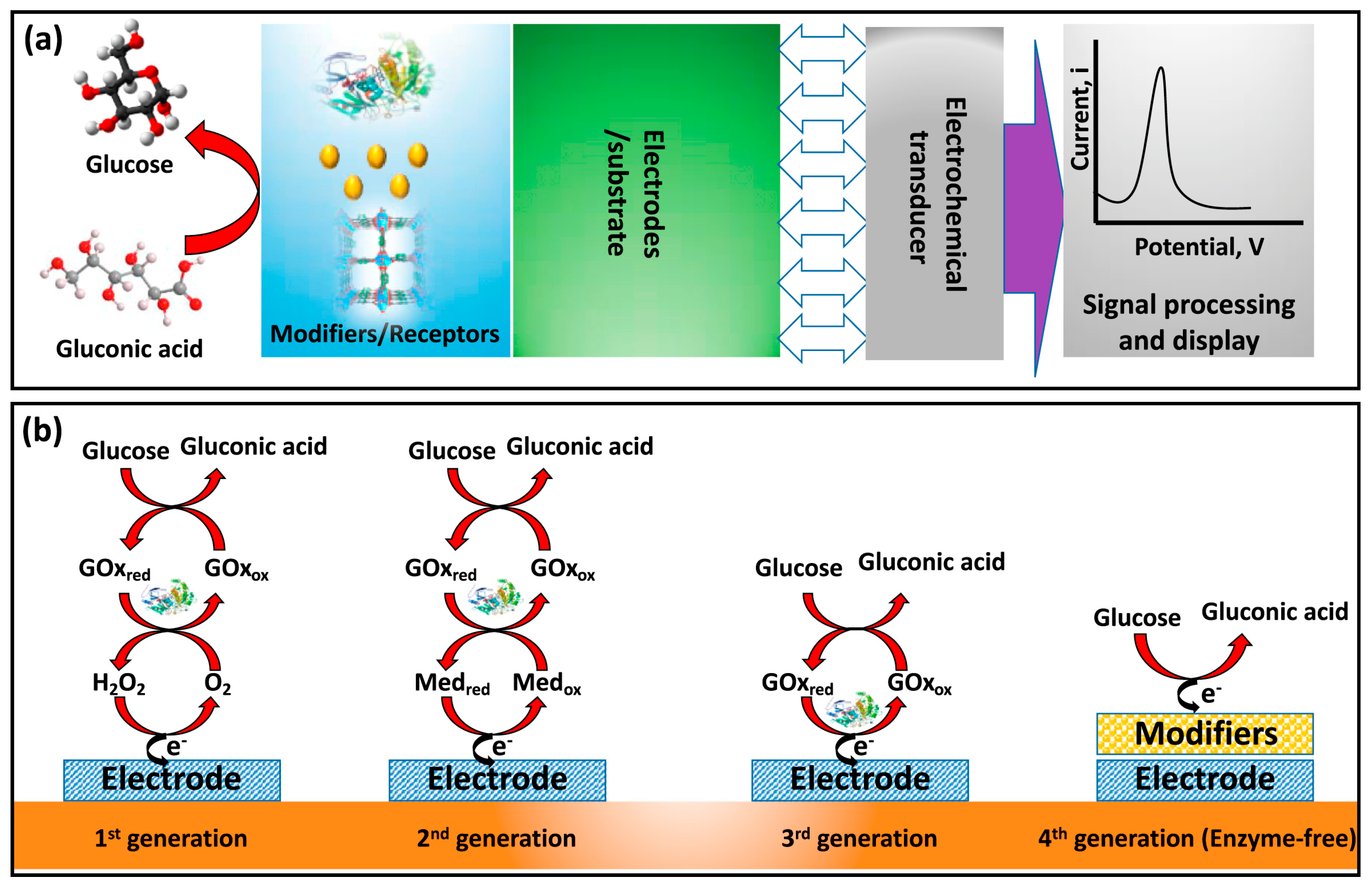
3. TiO2-Based Sensors: Types and Working Principles
4. Application of TiO2-Based Sensors in Glucose Biosensing
4.1. TiO2-Based Enzymatic Glucose Biosensors
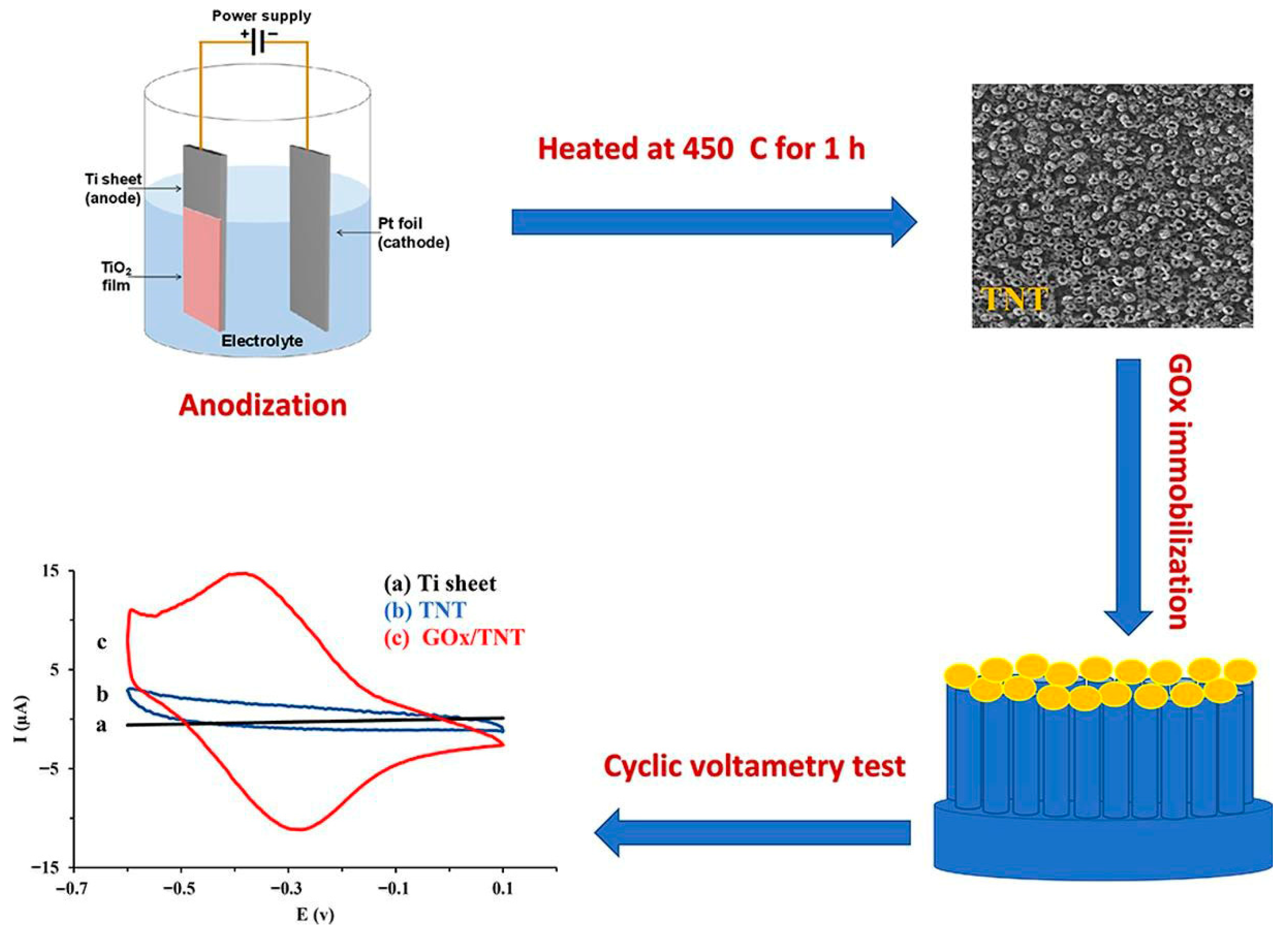
4.1.1. TiO2/GOx-Based Biosensors
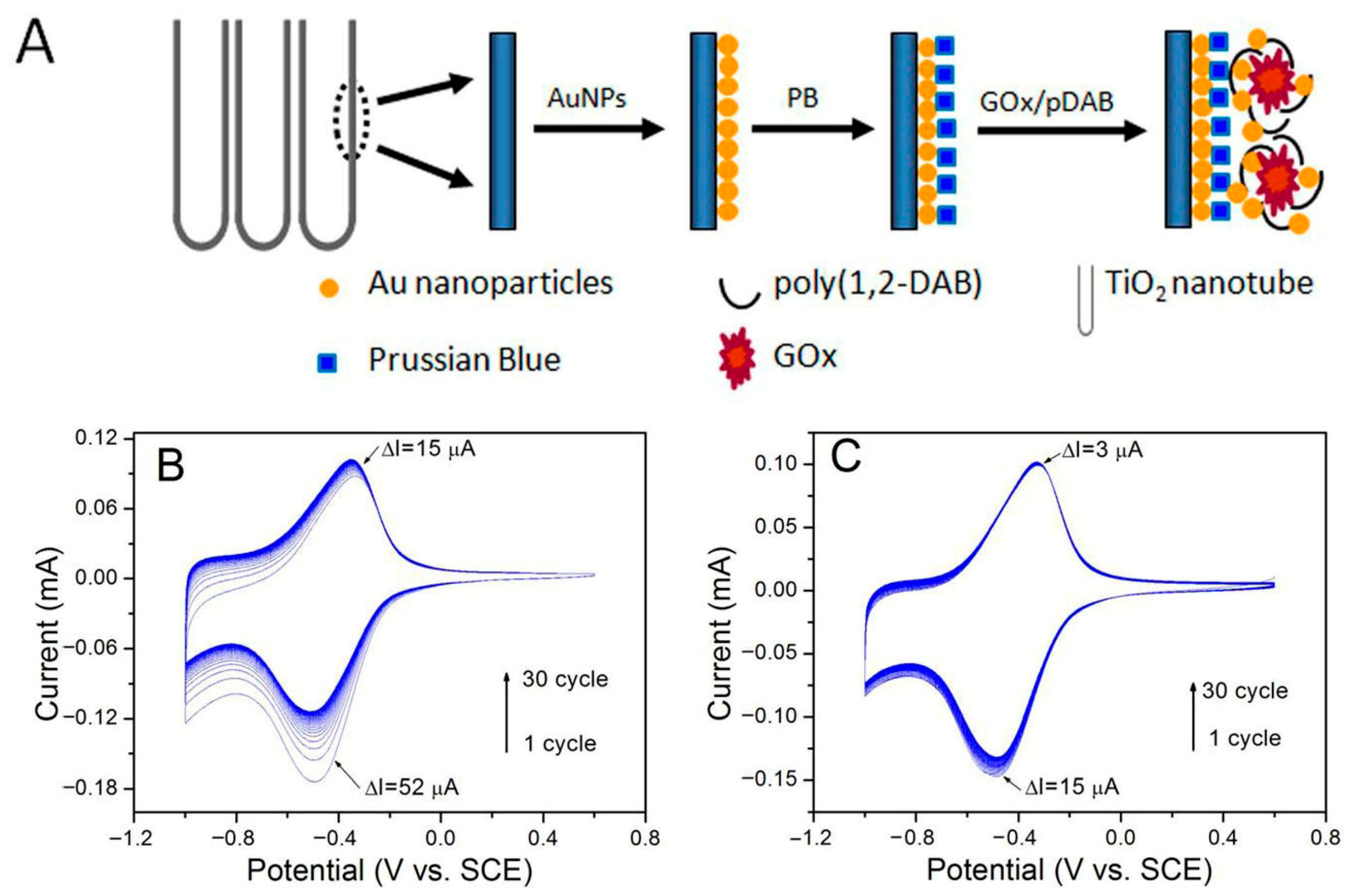
4.1.2. TiO2/Noble Metal/GOx-Based Hybrid Biosensors
4.2. TiO2-Based Non-Enzymatic Glucose Biosensors
4.2.1. TiO2/Noble Metal-Based Biosensors
4.2.2. TiO2/Transition Metal-Based Biosensors
TiO2/Nickel-Based Biosensors
TiO2/Copper-Based Biosensors
TiO2/Transition Metals Hybrid-Based Biosensors
4.3. TiO2-Based Photoelectrochemical Glucose Biosensors
4.3.1. TiO2/Noble Metal-Based Biosensors
4.3.2. TiO2/Metal Oxide-Based Biosensors
4.3.3. TiO2/Semiconductor-Based Biosensors
4.3.4. TiO2/Graphene-Based Biosensors
4.4. TiO2-Based Other Novel Glucose Biosensors
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef]
- Eckel, R.H.; Bornfeldt, K.E.; Goldberg, I.J. Cardiovascular Disease in Diabetes, beyond Glucose. Cell Metab. 2021, 33, 1519–1545. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef]
- Eid, S.A.; Rumora, A.E.; Beirowski, B.; Bennett, D.L.; Hur, J.; Savelieff, M.G.; Feldman, E.L. New Perspectives in Diabetic Neuropathy. Neuron 2023, 111, 2623–2641. [Google Scholar] [CrossRef] [PubMed]
- Jamshidnejad-Tosaramandani, T.; Kashanian, S.; Omidfar, K.; Schiöth, H.B. The Role of Nanomaterials in the Wearable Electrochemical Glucose Biosensors for Diabetes Management. Biosensors 2025, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Pikulin, S.; Yehezkel, I.; Moskovitch, R. Enhanced Blood Glucose Levels Prediction with a Smartwatch. PLoS ONE 2024, 19, e0307136. [Google Scholar] [CrossRef]
- Bamgboje, D.; Christoulakis, I.; Smanis, I.; Chavan, G.; Shah, R.; Malekzadeh, M.; Violaris, I.; Giannakeas, N.; Tsipouras, M.; Kalafatakis, K.; et al. Continuous Non-Invasive Glucose Monitoring via Contact Lenses: Current Approaches and Future Perspectives. Biosensors 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Yoon, H.; Zahed, M.A.; Park, C.; Kim, D.; Park, J.Y. Multifunctional Hybrid Skin Patch for Wearable Smart Healthcare Applications. Biosens. Bioelectron. 2022, 196, 113685. [Google Scholar] [CrossRef]
- Khosravi, S.; Soltanian, S.; Servati, A.; Khademhosseini, A.; Zhu, Y.; Servati, P. Screen-Printed Textile-Based Electrochemical Biosensor for Noninvasive Monitoring of Glucose in Sweat. Biosensors 2023, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Liao, Q.; Xie, S.; Song, Y.; Qin, L. Prospects and Challenges of Flexible Stretchable Electrodes for Electronics. Coatings 2022, 12, 558. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Wen, X.; Liu, L.; Wei, Q.; Zhang, P.; Zhang, J.-Y.; Zhao, D.; Lan, K. Facile Synthesis of Mesoporous TiO2 Architectures with Tunable Configurations and Nanometer Precision. Nat. Protoc. 2025, 1–23. [Google Scholar] [CrossRef]
- Tong, S.; Sun, X.; Wu, A.; Guo, S.; Zhang, H. Improved Biocompatibility of TiO2 Nanotubes via Co-Precipitation Loading with Hydroxyapatite and Gentamicin. Coatings 2021, 11, 1191. [Google Scholar] [CrossRef]
- Zakir, O.; Ait-Karra, A.; Idouhli, R.; Khadiri, M.; Dikici, B.; Aityoub, A.; Abouelfida, A.; Outzourhit, A. A Review on TiO2 Nanotubes: Synthesis Strategies, Modifications, and Applications. J. Solid State Electrochem. 2023, 27, 2289–2307. [Google Scholar] [CrossRef]
- Patra, S.; Das, P.; Chatterjee, S. Tuning the Optoelectronic and Surface Properties of TiO2 Nanotubes. Mater. Chem. Phys. 2023, 308, 128308. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Sanattalab, E.; Kanarya, D.; Ebrahimi, A.; Didarian, R.; Doğan Güzel, F.; Yıldırım Tirgil, N. Cutting-Edge Applications of Titanium Dioxide in Biosensors. Electroanalysis 2025, 37, e70049. [Google Scholar] [CrossRef]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef]
- Isik, E.; Tasyurek, L.B.; Isik, I.; Kilinc, N. Synthesis and Analysis of TiO2 Nanotubes by Electrochemical Anodization and Machine Learning Method for Hydrogen Sensors. Microelectron. Eng. 2022, 262, 111834. [Google Scholar] [CrossRef]
- Puga, M.L.; Venturini, J.; ten Caten, C.S.; Bergmann, C.P. Influencing Parameters in the Electrochemical Anodization of TiO2 Nanotubes: Systematic Review and Meta-Analysis. Ceram. Int. 2022, 48, 19513–19526. [Google Scholar] [CrossRef]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef]
- Saker, R.; Shammout, H.; Regdon, G.; Sovány, T. An Overview of Hydrothermally Synthesized Titanate Nanotubes: The Factors Affecting Preparation and Their Promising Pharmaceutical Applications. Pharmaceutics 2024, 16, 635. [Google Scholar] [CrossRef]
- Alkanad, K.; Hezam, A.; Al-Zaqri, N.; Bajiri, M.A.; Alnaggar, G.; Drmosh, Q.A.; Almukhlifi, H.A.; Neratur Krishnappagowda, L. One-Step Hydrothermal Synthesis of Anatase TiO2 Nanotubes for Efficient Photocatalytic CO2 Reduction. ACS Omega 2022, 7, 38686–38699. [Google Scholar] [CrossRef]
- Cossuet, T.; Rapenne, L.; Renou, G.; Appert, E.; Consonni, V. Template-Assisted Growth of Open-Ended TiO2 Nanotubes with Hexagonal Shape Using Atomic Layer Deposition. Cryst. Growth Des. 2021, 21, 125–132. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Hamza, A.M.; Al-Deyab, S.S.; Qurashi, A.; Kim, H.Y. Titanium-Based Polymeric Electrospun Nanofiber Mats as a Novel Organic Semiconductor. Mater. Sci. Eng. B 2012, 177, 34–42. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.A.; Nazir, A.; Arshad, S.N.; Qadir, M.B.; Khaliq, Z.; Khan, Z.S.; Satti, A.N.; Mushtaq, B.; Shahzad, A. Triaxial Electrospun Mixed-Phased TiO2 Nanofiber-in-Nanotube Structure with Enhanced Photocatalytic Activity. Microporous Mesoporous Mater. 2021, 320, 111104. [Google Scholar] [CrossRef]
- Etafa Tasisa, Y.; Kumar Sarma, T.; Krishnaraj, R.; Sarma, S. Band Gap Engineering of Titanium Dioxide (TiO2) Nanoparticles Prepared via Green Route and Its Visible Light Driven for Environmental Remediation. Results Chem. 2024, 11, 101850. [Google Scholar] [CrossRef]
- Alrashedi, W.; Kochkar, H.; Berhault, G.; Younas, M.; Ben Ali, A.; Alomair, N.A.; Hamdi, R.; Abubshait, S.A.; Alagha, O.; Gondal, M.F.; et al. Enhancement of the Photocatalytic Response of Cu-Doped TiO2 Nanotubes Induced by the Addition of Strontium. J. Photochem. Photobiol. A Chem. 2022, 428, 113858. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Guan, R.; Zhang, Y.; Zhao, Z.; Zhai, H.; Sun, Z. Vacancy-Enabled Mesoporous TiO2 Modulated by Nickel Doping with Enhanced Photocatalytic Nitrogen Fixation Performance. ACS Sustain. Chem. Eng. 2020, 8, 18258–18265. [Google Scholar] [CrossRef]
- Siliavka, E.S.; Rudakova, A.V.; Bakiev, T.V.; Murashkina, A.A.; Murzin, P.D.; Kataeva, G.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the Heterovalent Sc3+ and Nb5+ Doping on Photoelectrochemical Behavior of Anatase TiO2. Catalysts 2024, 14, 76. [Google Scholar] [CrossRef]
- Sultana, S.; Darowska, I.; Pisarek, M.; Sulka, G.D.; Syrek, K. Designing TiO2 Nanotubular Arrays with Au-CoOx Core–Shell Nanoparticles for Enhanced Photoelectrochemical Methanol and Lignin Oxidation. ACS Appl. Mater. Interfaces 2024, 16, 49262–49274. [Google Scholar] [CrossRef]
- Liang, X.; Yu, S.; Meng, B.; Wang, X.; Yang, C.; Shi, C.; Ding, J. Advanced TiO2-Based Photoelectrocatalysis: Material Modifications, Charge Dynamics, and Environmental–Energy Applications. Catalysts 2025, 15, 542. [Google Scholar] [CrossRef]
- Meng, M.; Zhou, H.; Yang, J.; Wang, L.; Yuan, H.; Hao, Y.; Gan, Z. Exploiting the Bragg Mirror Effect of TiO2 Nanotube Photonic Crystals for Promoting Photoelectrochemical Water Splitting. Nanomaterials 2024, 14, 1695. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J. Recent Advances in the Synthesis of Defective TiO2 Nanofibers and Their Applications in Energy and Catalysis. Chem. Eng. J. 2023, 472, 144831. [Google Scholar] [CrossRef]
- Lia, F.; Farrugia, C.; Buccheri, M.A.; Rappazzo, G.; Zammit, E.; Rizzo, A.; Grech, M.; Refalo, P.; Abela, S. Effect of the Surface Morphology of TiO2 Nanotubes on Photocatalytic Efficacy Using Electron-Transfer-Based Assays and Antimicrobial Tests. Appl. Sci. 2020, 10, 5243. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, A.; Song, Z.; Bao, J.; Xue, J.; Wei, Y.; Li, S.; Lv, L.; Ding, J.; Cai, M.; et al. Enhancement and Stabilization of Isolated Hydroxyl Groups via the Construction of Coordinatively Unsaturated Sites on Surface and Subsurface of Hydrogenated TiO2 Nanotube Arrays for Photocatalytic Complete Mineralization of Toluene. J. Environ. Chem. Eng. 2021, 9, 105080. [Google Scholar] [CrossRef]
- Cui, H.; Yao, C.; Cang, Y.; Liu, W.; Zhang, Z.; Miao, Y.; Xin, Y. Oxygen Vacancy-Regulated TiO2 Nanotube Photoelectrochemical Sensor for Highly Sensitive and Selective Detection of Tetracycline Hydrochloride. Sens. Actuators B Chem. 2022, 359, 131564. [Google Scholar] [CrossRef]
- Sebek, M.; Peppel, T.; Lund, H.; Medic, I.; Springer, A.; Mazierski, P.; Zaleska-Medynska, A.; Strunk, J.; Steinfeldt, N. Thermal Annealing of Ordered TiO2 Nanotube Arrays with Water Vapor-Assisted Crystallization under a Continuous Gas Flow for Superior Photocatalytic Performance. Chem. Eng. J. 2021, 425, 130619. [Google Scholar] [CrossRef]
- Sabri, B.A.; Satgunam, M.; Manap, A.; Gnanasagaran, C.L.; Ramachandran, K. Physical and Chemical Investigation of TiO2 Nanotubes Treated with Isopropyl Triisostearoyl Titanate (KR-TTS). J. Nanomater. 2023, 2023, 7961857. [Google Scholar] [CrossRef]
- Rezaei, M.; Nezamzadeh-Ejhieh, A.; Massah, A.R. A Comprehensive Review on the Boosted Effects of Anion Vacancy in the Heterogeneous Photocatalytic Degradation, Part II: Focus on Oxygen Vacancy. ACS Omega 2024, 9, 6093–6127. [Google Scholar] [CrossRef]
- Shen, X.; Hu, J.; Lu, X.; Liu, A.; Lu, Z.; Xie, J.; Cao, Y. Interesting Molecular Adsorption Strategy Induced Surface Charge Redistribution of Commercial TiO2: Boosting 9 Times Photocatalytic Hydrogen Production Performance. Appl. Surf. Sci. 2022, 596, 153622. [Google Scholar] [CrossRef]
- Pandey, B.; Rani, S.; Roy, S.C. A Scalable Approach for Functionalization of TiO2 Nanotube Arrays with G-C3N4 for Enhanced Photo-Electrochemical Performance. J. Alloys Compd. 2020, 846, 155881. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wu, Y.; Yan, F.; Qin, S.; Yang, J.; Li, J.; Cui, Z. Fabrication of polymer@TiO2 NPs Hybrid Membrane Based on Covalent Bonding and Coordination and Its Mechanism of Enhancing Photocatalytic Performance. J. Alloys Compd. 2022, 910, 164887. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification Strategies of TiO2 Based Photocatalysts for Enhanced Visible Light Activity and Energy Storage Ability: A Review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Liu, W.; Yao, C.; Cui, H.; Cang, Y.; Zhang, Z.; Miao, Y.; Xin, Y. A Nano-Enzymatic Photoelectrochemical L-Cysteine Biosensor Based on Bi2MoO6 Modified Honeycomb TiO2 Nanotube Arrays Composite. Microchem. J. 2022, 175, 107200. [Google Scholar] [CrossRef]
- Liang, L.; Yin, J.; Bao, J.; Cong, L.; Huang, W.; Lin, H.; Shi, Z. Preparation of Au Nanoparticles Modified TiO2 Nanotube Array Sensor and Its Application as Chemical Oxygen Demand Sensor. Chin. Chem. Lett. 2019, 30, 167–170. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent Advances of Electrochemical and Optical Enzyme-Free Glucose Sensors Operating at Physiological Conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Sinha, S.K. Nanostructured Cu2O Deposited on TiO2 Nanotube Arrays for Ultra-Sensitive Non-Enzymatic Cholesterol Electrochemical Biosensor. Mater. Today Commun. 2023, 37, 107171. [Google Scholar] [CrossRef]
- Wang, K.; Amani Hamedani, H. Glucose Oxidation Performance of Zinc Nano-Hexagons Decorated on TiO2 Nanotube Arrays. Nanomanufacturing 2024, 4, 187–201. [Google Scholar] [CrossRef]
- Alaridhee, Z.A.I.; Alqaraguly, M.B.; Formanova, S.; Kuryazov, R.; Mahdi, M.S.; Taher, W.M.; Alwan, M.; Jabir, M.S.; Zankanah, F.H.; Majdi, H.; et al. Recent Advances in Microfluidic-Based Photoelectrochemical (PEC) Sensing Platforms for Biomedical Applications. Microchim. Acta 2025, 192, 297. [Google Scholar] [CrossRef]
- Yuan, R.; Yan, B.; Lai, C.; Wang, X.; Cao, Y.; Tu, J.; Li, Y.; Wu, Q. Carbon Dot-Modified Branched TiO2 Photoelectrochemical Glucose Sensors with Visible Light Response. ACS Omega 2023, 8, 22099–22107. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Wang, Y.; Du, H.; Xia, C.; Tian, F. Glucose Biosensor Based on Glucose Oxidase Immobilized on Unhybridized Titanium Dioxide Nanotube Arrays. Microchim. Acta 2014, 181, 381–387. [Google Scholar] [CrossRef]
- Hu, Z.; Rong, J.; Zhan, Z.; Yu, X. Glucose Biosensor Based on Glucose Oxidase Immobilized on Multi-Vacancy TiO2 Nanotube Arrays. Int. J. Electrochem. Sci. 2019, 14, 9661–9670. [Google Scholar] [CrossRef]
- Akhbari Varkani, R.; Rafiee-Pour, H.-A.; Noormohammadi, M. One Step Immobilization of Glucose Oxidase on TiO2 Nanotubes towards Glucose Biosensing. Microchem. J. 2021, 170, 106712. [Google Scholar] [CrossRef]
- Benvenuto, P.; Kafi, A.K.M.; Chen, A. High Performance Glucose Biosensor Based on the Immobilization of Glucose Oxidase onto Modified Titania Nanotube Arrays. J. Electroanal. Chem. 2009, 627, 76–81. [Google Scholar] [CrossRef]
- Feng, C.; Xu, G.; Liu, H.; Lv, J.; Zheng, Z.; Wu, Y. Glucose Biosensors Based on Ag Nanoparticles Modified TiO2 Nanotube Arrays. J. Solid State Electrochem. 2014, 18, 163–171. [Google Scholar] [CrossRef]
- Gao, Z.-D.; Qu, Y.; Li, T.; Shrestha, N.K.; Song, Y.-Y. Development of Amperometric Glucose Biosensor Based on Prussian Blue Functionlized TiO2 Nanotube Arrays. Sci. Rep. 2014, 4, 6891. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, D.; Luo, S.; Cai, Q. An Amperometric Glucose Biosensor Fabricated with Pt Nanoparticle-Decorated Carbon Nanotubes/TiO2 Nanotube Arrays Composite. Sens. Actuators B Chem. 2009, 137, 134–138. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Gao, Z.; Lee, K.; Schmuki, P. A Self-Cleaning Nonenzymatic Glucose Detection System Based on Titania Nanotube Arrays Modified with Platinum Nanoparticles. Electrochem. Commun. 2011, 13, 1217–1220. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Zhang, X.; Meng, W.; Yuan, C.; Guo, M. Nonenzymatic Glucose Sensor Based on Ag&Pt Hollow Nanoparticles Supported on TiO2 Nanotubes. Mater. Sci. Eng. C 2017, 80, 174–179. [Google Scholar] [CrossRef]
- Yu, S.; Peng, X.; Cao, G.; Zhou, M.; Qiao, L.; Yao, J.; He, H. Ni Nanoparticles Decorated Titania Nanotube Arrays as Efficient Nonenzymatic Glucose Sensor. Electrochim. Acta 2012, 76, 512–517. [Google Scholar] [CrossRef]
- Huo, K.; Li, Y.; Chen, R.; Gao, B.; Peng, C.; Zhang, W.; Hu, L.; Zhang, X.; Chu, P.K. Recyclable Non-Enzymatic Glucose Sensor Based on Ni/NiTiO3/TiO2 Nanotube Arrays. ChemPlusChem 2015, 80, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ren, X.; Li, Y.; Yu, Z. Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation. Molecules 2022, 27, 5815. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Su, F.; Liu, C.; Li, J.; Liu, R.; Xiao, Y.; Li, Y.; Liu, X.; Cai, Q. A New Method for Fabricating a CuO/TiO2 Nanotube Arrays Electrode and Its Application as a Sensitive Nonenzymatic Glucose Sensor. Talanta 2011, 86, 157–163. [Google Scholar] [CrossRef]
- Stanley, J.; Sree, R.J.; Ramachandran, T.; Babu, T.G.S.; Nair, B.G. Vertically Aligned TiO2 Nanotube Arrays Decorated with CuO Mesoclusters for the Nonenzymatic Sensing of Glucose. J. Nanosci. Nanotechnol. 2017, 17, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Gousiya, B.G.; Garlapally, R.; Rao, B. A Study on Cu Decorated Anodic TiO2 Nanotubes for Non-Enzymatic Glucose Sensing and Photoelectrochemical Water Splitting Application. J. Electrochem. Soc. 2024, 171, 107517. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y. Nickel/Copper Nanoparticles Modified TiO2 Nanotubes for Non-Enzymatic Glucose Biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Suneesh, P.V.; Sara Vargis, V.; Ramachandran, T.; Nair, B.G.; Satheesh Babu, T.G. Co–Cu Alloy Nanoparticles Decorated TiO2 Nanotube Arrays for Highly Sensitive and Selective Nonenzymatic Sensing of Glucose. Sens. Actuators B Chem. 2015, 215, 337–344. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Zhang, G.; Hou, K.; Pan, H.; Du, M. Self-Assembly of Palladium Nanoparticles on Functional TiO2 Nanotubes for a Nonenzymatic Glucose Sensor. Mater. Sci. Eng. C 2016, 62, 323–328. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Ooi, P.C.; Nazeer, A.A.; Al-Hajji, L.A.; Jubu, P.R.; Dee, C.F.; Ahmadipour, M.; Hamzah, A.A. CuO/Cu/rGO Nanocomposite Anodic Titania Nanotubes for Boosted Non-Enzymatic Glucose Biosensors. New J. Chem. 2023, 47, 7890–7902. [Google Scholar] [CrossRef]
- Kumar, B.; Sinha, S.K. Growth of WO3 Nanostructure Deposited on TiO2 Nanotube Arrays for Electrochemical Enzyme-Free Glucose Sensor. J. Food Compos. Anal. 2024, 135, 106666. [Google Scholar] [CrossRef]
- Liu, W.; Duan, W.; Jia, L.; Wang, S.; Guo, Y.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials 2022, 12, 2058. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, W.; Yan, B.; Wu, B.; Ma, J.; Wang, X.; Qiao, B.; Tu, J.; Pei, H.; Chen, D.; et al. Gold and Platinum Nanoparticle-Functionalized TiO2 Nanotubes for Photoelectrochemical Glucose Sensing. ACS Omega 2022, 7, 2474–2483. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Yin, Z.; Wang, X.; Li, Z.; Wang, X. Highly Sensitive Photoelectrochemical Detection of Glucose Based on BiOBr/TiO2 Nanotube Array p-n Heterojunction Nanocomposites. Sens. Actuators B Chem. 2020, 312, 127978. [Google Scholar] [CrossRef]
- Ke, S.; Qin, L.; Zhang, R.; Zhu, W.; Lu, W.; Ma, L.; Wu, S.; Li, X. Towards Self-Driven and Enzyme-Free Sweat Glucose Photoelectrochemical Sensing via Decorating CuO Nanoparticles on TiO2 Hierarchical Nanotubes. Surf. Interfaces 2023, 40, 103102. [Google Scholar] [CrossRef]
- Yang, R.-F.; Zhang, S.-S.; Shi, D.-J.; Dong, J.-X.; Li, Y.-L.; Li, J.-X.; Guo, C.; Yue, Z.; Li, G.; Huang, W.-P.; et al. Glucose Detection via Photoelectrochemical Sensitivity of 3D CuO-TiO2 Heterojunction Nanotubes/Ti Combined with Chemometric Tools. Microchem. J. 2024, 199, 110017. [Google Scholar] [CrossRef]
- Liu, P.; Huo, X.; Tang, Y.; Xu, J.; Liu, X.; Wong, D.K.Y. A TiO2 Nanosheet-g-C3N4 Composite Photoelectrochemical Enzyme Biosensor Excitable by Visible Irradiation. Anal. Chim. Acta 2017, 984, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.O.; Tasviri, M.; Mohaghegh, N. A CdxZn1−xS/TiO2 Nanotube Array Electrode for a Highly Sensitive and Selective Nonenzymatic Photoelectrochemical Glucose Sensor. New J. Chem. 2022, 46, 9880–9888. [Google Scholar] [CrossRef]
- Li, Y.-L.; Tian, J.; Shi, D.-J.; Dong, J.-X.; Yue, Z.; Li, G.; Huang, W.-P.; Zhang, S.-M.; Zhu, B.-L. CdSe/TiO2NTs Heterojunction-Based Nonenzymatic Photoelectrochemical Sensor for Glucose Detection. Langmuir 2023, 39, 14935–14944. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, W.; Zhang, N.; Lai, X.; Peng, J.; Cao, Y.; Tu, J. TiO2 Nanotubes Modified with Polydopamine and Graphene Quantum Dots as a Photochemical Biosensor for the Ultrasensitive Detection of Glucose. J. Mater. Sci. 2020, 55, 6105–6117. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Yang, Y.; Liu, K.; Du, W. Highly Selective and Sensitive Glucose Sensors Based on Organic Electrochemical Transistors Using TiO2 Nanotube Arrays-Based Gate Electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
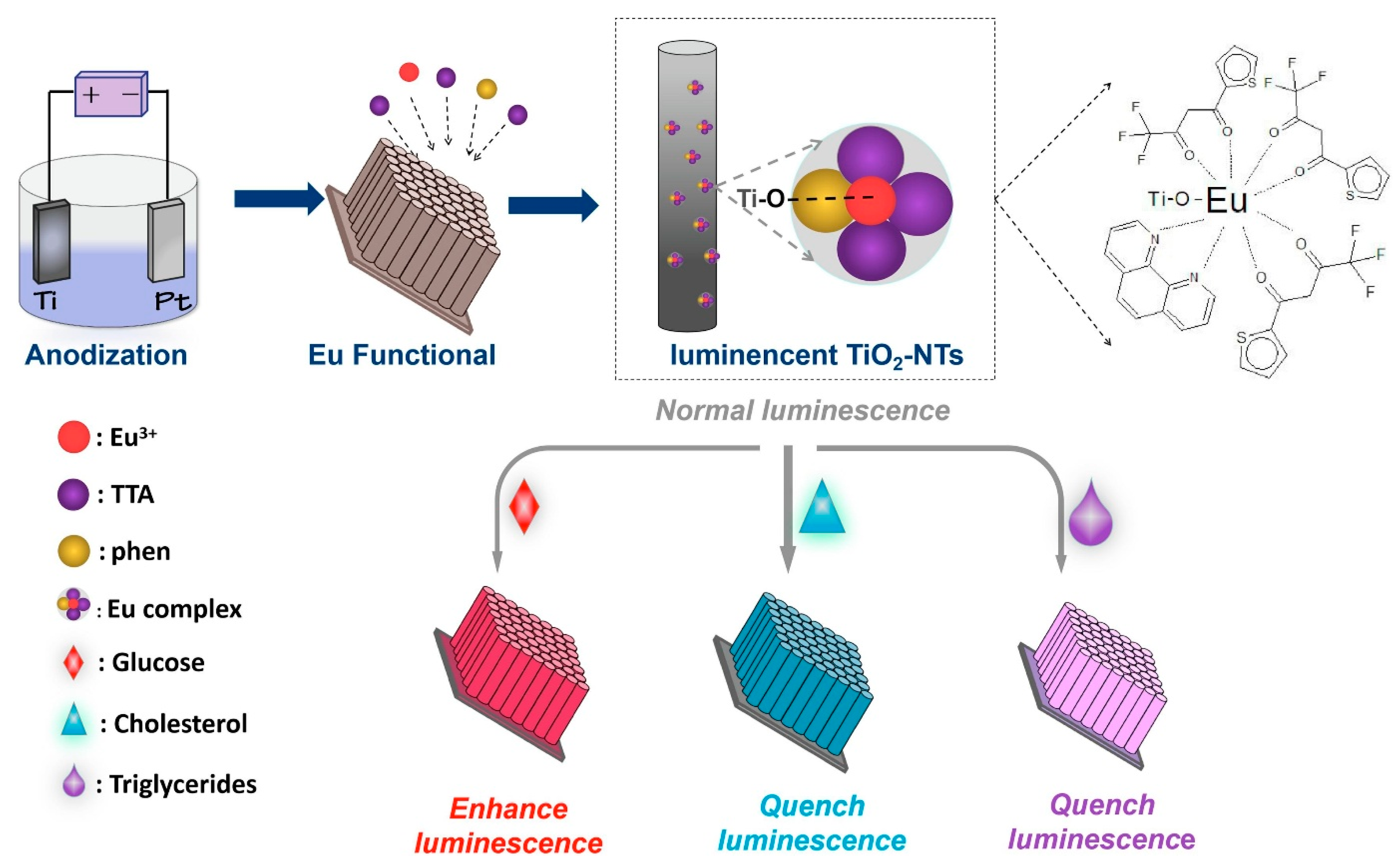
- Su, B.; Yang, W.; Wang, Y.; Huang, L.; Popat, K.C.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Europium-Functionalized Luminescent Titania Nanotube Arrays: Utilizing Interactions with Glucose, Cholesterol and Triglycerides for Rapid Detection Application. Mater. Sci. Eng. C 2020, 114, 111054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tian, D.; Yang, Y.; Cui, H.; Li, Y.; Ren, S.; Han, T.; Gao, Z. Machine Learning Assisted Biosensing Technology: An Emerging Powerful Tool for Improving the Intelligence of Food Safety Detection. Curr. Res. Food Sci. 2024, 8, 100679. [Google Scholar] [CrossRef] [PubMed]
- Nashruddin, S.N.A.B.M.; Salleh, F.H.M.; Yunus, R.M.; Zaman, H.B. Artificial Intelligence−powered Electrochemical Sensor: Recent Advances, Challenges, and Prospects. Heliyon 2024, 10, e37964. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Suh, J. 4-Roll-to-Roll Printing. In Smart and Connected Wearable Electronics; Yeo, W.-H., Kim, Y.-S., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2024; pp. 123–152. ISBN 978-0-323-99147-6. [Google Scholar]
- Jaggessar, A.; Senevirathne, S.W.M.A.I.; Velic, A.; Yarlagadda, P.K.D.V. Antibacterial Activity of 3D versus 2D TiO2 Nanostructured Surfaces to Investigate Curvature and Orientation Effects. Curr. Opin. Biomed. Eng. 2022, 23, 100404. [Google Scholar] [CrossRef]


| Biosensor Configuration | Biosensor Type | Target | Medium Used | Primary Detection Method | Linear Range | Sensitivity | Detection Limit | Response Time | Stability | Anti-Interference Performance | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2/GOx | Electrochemical (Enzymatic) | Glucose | PBS | Amperometry | 0.05 to 0.65 mM | 199.6 μA mM−1cm−2 | 3.8 μM | NA | 86% (after 6 days) | Minimal response to ascorbic acid, sucrose, L-cysteine, L-histidine, and L-glycine | Wang et al. [53] |
| TiO2/GOx | Electrochemical (Enzymatic) | Glucose | PBS | Amperometry | 0.05 to 3.2 mM | 8.5 μAmM−1cm−2 | 3.2 μM | <10 s | 93.5% (after 40 days) | Minimal response to ascorbic acid, uric acid, and dopamine, KCL | Hu et al. [54] |
| TiO2/GOx | Electrochemical (Enzymatic) | Glucose | PBS | Voltammetry | 0.03 to 1.0 mM | 56.60 µA mM−1 cm−2 | 8.5 µM | 10 s | 90% (after 20 days) | Minimal response to ascorbic acid, acetaminophen, and uric acid | Akhbari Varkani et al. [55] |
| Ti/TiO2/Au/PB/GOx | Electrochemical (Enzymatic) | Glucose | PBS | Amperometry | 0.015 to 4.00 mM | 36 μA mM−1 | 5 μM | <10 s | 90% (after 21 days) | Minimal response to ascorbic acid, uric acid, acetaminophen | Benvenuto et al. [56] |
| GOx/Ag/TiO2 | Electrochemical (Enzymatic) | Glucose | PBS | Amperometry | 0.1 to 4 mM | 0.39 μA mM−1 cm−2 | 0.1 mM | 40 s | NA | Minimal response to H2O2 | Feng et al. [57] |
| GOx/Au/pDAB)-PB/AuNP/TiO2 | Electrochemical (Enzymatic) | Glucose | PBS, Serum | Amperometry | 0.01 to 0.70 mM | 248 mA M−1 cm−2 | 3.2 μM | <1 s | >90% (after 30 days) | Minimal response to ascorbic acid, uric acid, p-acetamidophenol | Gao et al. [58] |
| TiO2/CNT/Pt/GOx | Electrochemical (Enzymatic) | Glucose | PBS | Amperometry | 0.006 to 1.5 mM | 0.24 μA mM−1 cm−2 | 5.7 μM | <3 s | 82% (after 30 days) | Not specified | Pang et al. [59] |
| Pt/TiO2 | Electrochemical (Non-enzymatic) | Glucose | H2SO4 | Amperometry | NA | NA | NA | NA | Self-cleaning via UV exposure | Minimal response to ascorbic acid, uric acid, and p-acetamidophenol | Song et al. [60] |
| Ag/Pt-TiO2 | Electrochemical (Non-enzymatic) | Glucose | PBS | Voltammetry | 30 to 180 mM | 3.99 μA·cm−2·mM−1 | 22.6 μM | NA | NA | Minimal response to Cl ion | Wang et al. [61] |
| Ni-NPs/TiO2 | Electrochemical (Non-enzymatic) | Glucose | NaOH solution | Amperometry | 0.004 to 4.8 mM | 700.2 μA mM−1 cm−2 | 2 μM | <5 s | 80.3% (after 20 days) | Minimal response to ascorbic acid, uric acid | Yu et al. [62] |
| Ni/NiTiO3/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Serum | Amperometry | 0.005 to 0.5 mM | 456.4 μA mM−1 cm−2 | 0.7 μM | <5 s | NA | Minimal response to ascorbic acid, uric acid | Huo et al. [63] |
| Ni-DLC/TiO2 | Electrochemical (Non-enzymatic) | Glucose | NaOH | Amperometry | 0.99 to 22.97 mM | 1063.78 μA·mM−1·cm−2 | 0.53 μM | <5 s | 82.6% (after 30 days) | Minimal response to dopamine, ascorbic acid, uric acid, and galactose | Kang et al. [64] |
| CuO/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Serum | Amperometry | Up to 2.0 mM | 79.79 μA·mM−1·cm−2 | 1 μM | <4 s | >90% (after 30 days) | Minimal response to Cl ion, ascorbic acid, uric acid, lactose, sucrose, fructose, dopamine | Luo et al. [65] |
| CuO/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Serum | Amperometry | 0.625 to 6.25 m mol L−1; 6.87 to 12.5 m mol L−1 | 1836 μA mmol−1 L cm−2 (low range); 1416 μA mmol−1 L cm−2 (high range) | 3.4 μ mol L−1 | ≤2 s | >96% (after 30 days) | Minimal response to ascorbic acid, dopamine, galactose, uric acid, lactose | Stanley et al. [66] |
| Cu/TiO2 | Electrochemical (Non-enzymatic) | Glucose | NaOH | Amperometry | 0.5 to 7 mM | 522 μA mM−1 cm−2 | NA | 0.1 s | NA | Minimal response to ascorbic acid, NaCl, lactose, sucrose, D-fructose | Bhanu et al. [67] |
| Ni-Cu/TiO2 | Electrochemical (Non-enzymatic) | Glucose | NaOH | Amperometry | 10 μM to 3.2 mM | 1590.9 μA mM−1 cm−2 | 5 μM | <5 s | 98% (after 49 days) | Minimal response to uric acid, ascorbic acid | Li et al. [68] |
| Co/Cu/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Serum, NaOH | Amperometry | Up to 12 mM | 4651.0 μA mM−1 cm−2 up to 5 mM and 2581.70 μA mM−1 cm−2 from 5 mM to 12 mM. | 0.6 μM | NA | 92% (after 90 days) | Minimal response to fructose, maltose, galactose, lactose, ascorbic acid, uric acid, acetamidophenol, creatinine, urea, chloride | Suneesh et al. [69] |
| Pd NPs/PDDA/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Serum | Amperometry | 4 × 10−7 to 8 × 10−4 M | NA | 8 × 10−8 M | NA | Stable (after 14 days) | Minimal response to chloride ions, ascorbic acid, uric acid, urea | Chen et al. [70] |
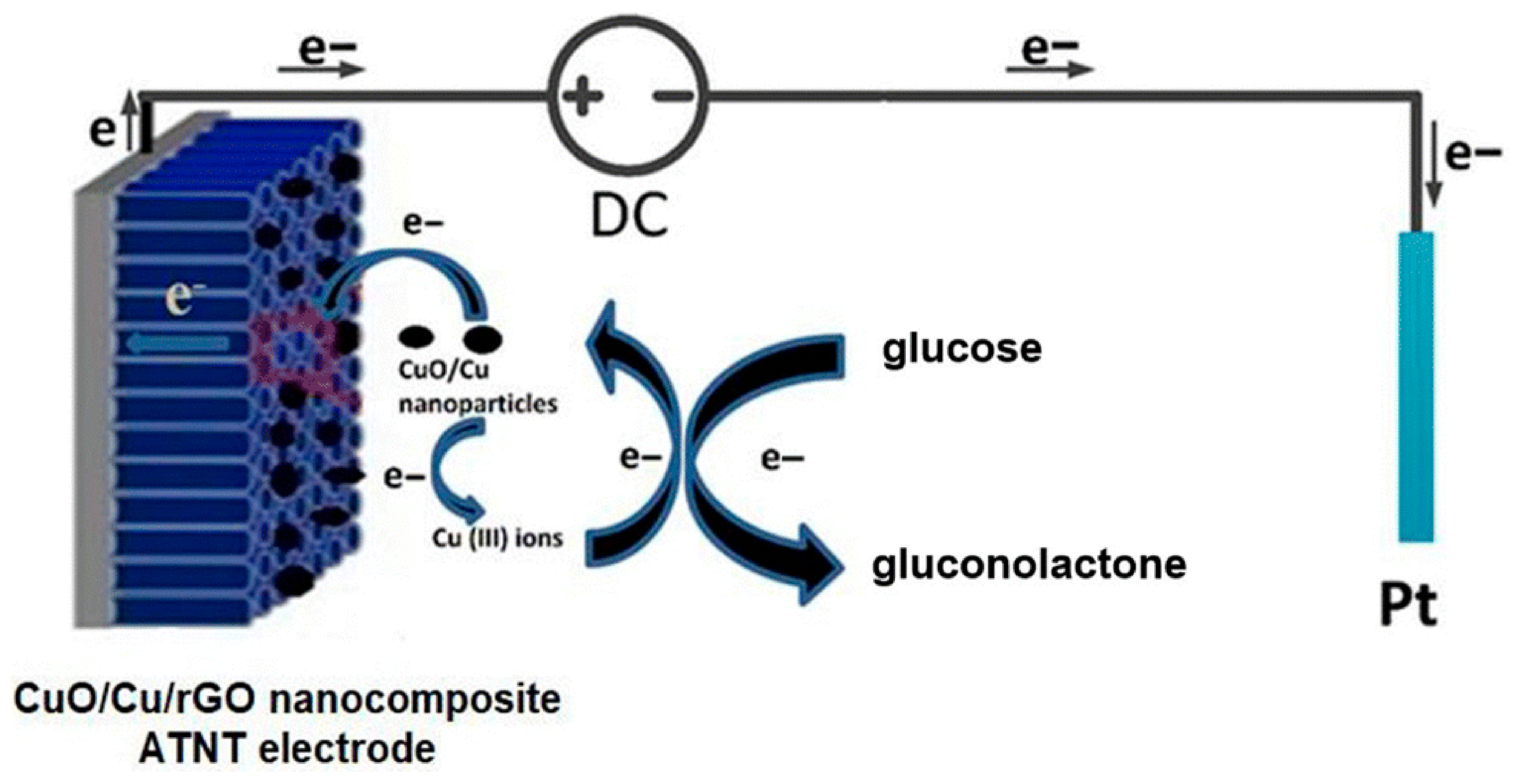
| CuO/Cu/rGO/TiO2 | Electrochemical (Non-enzymatic) | Glucose | PBS | Amperometry | 0.5 to 16 mM | 371.6 μA mM−1 cm−2 | 22.8 μM | ~5 s | 92% (after 5 days) | Minimal response to uric acid, ascorbic acid, lactose, sucrose, fructose | Chahrour et al. [71] |
| WO3/TiO2 | Electrochemical (Non-enzymatic) | Glucose | Orange juice | Amperometry | 1.0 to 6.5 mM | 1228.12 μA mM−1 cm−2 | 0.19 mM | 2 s | 97.1% (after 25 days) | Minimal response to uric acid, ascorbic acid, NaCl | Kumar & Sinha [72] |
| Au/TiO2 | Photoelectrochemical | Glucose | NaOH | Visible red light | 1 to 90 μM | 170.37 μA·mM−1·cm−2 | 1.3 μM | NA | 96% after 25 days | Minimal response to sucrose, lactose, ascorbic acid, saccharose, fructose | Liu et al. [73] |
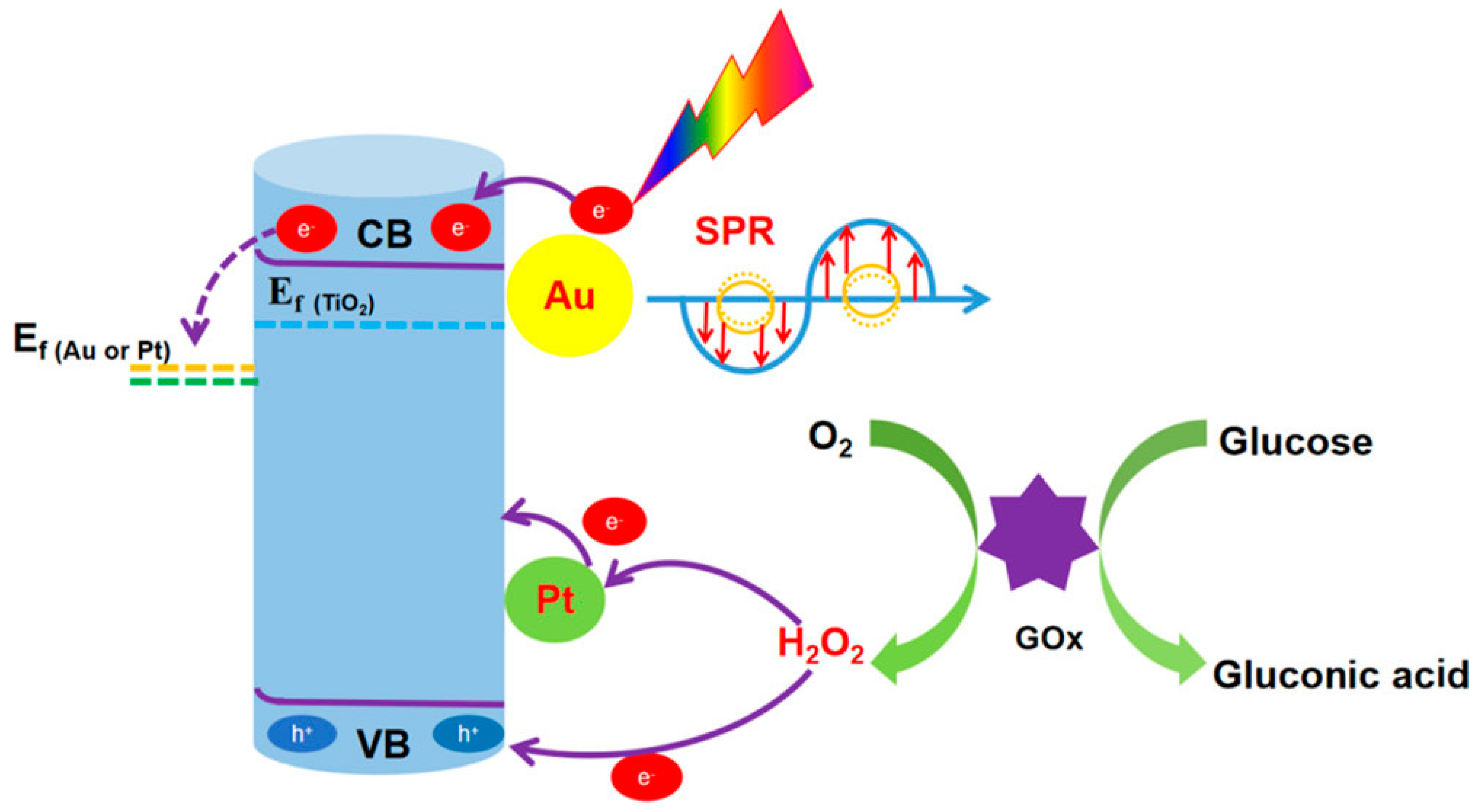
| TiO2/Au/Pt/GOx | Photoelectrochemical | Glucose | PBS | Amperometry (visible light) | 0 to 4 mM | 81.93 μA mM–1 cm–2 | 1.39 μM | NA | NA | Minimal response to NaCl, sucrose, ascorbic acid, uric acid, galactose, fructose | Yang et al. [74] |
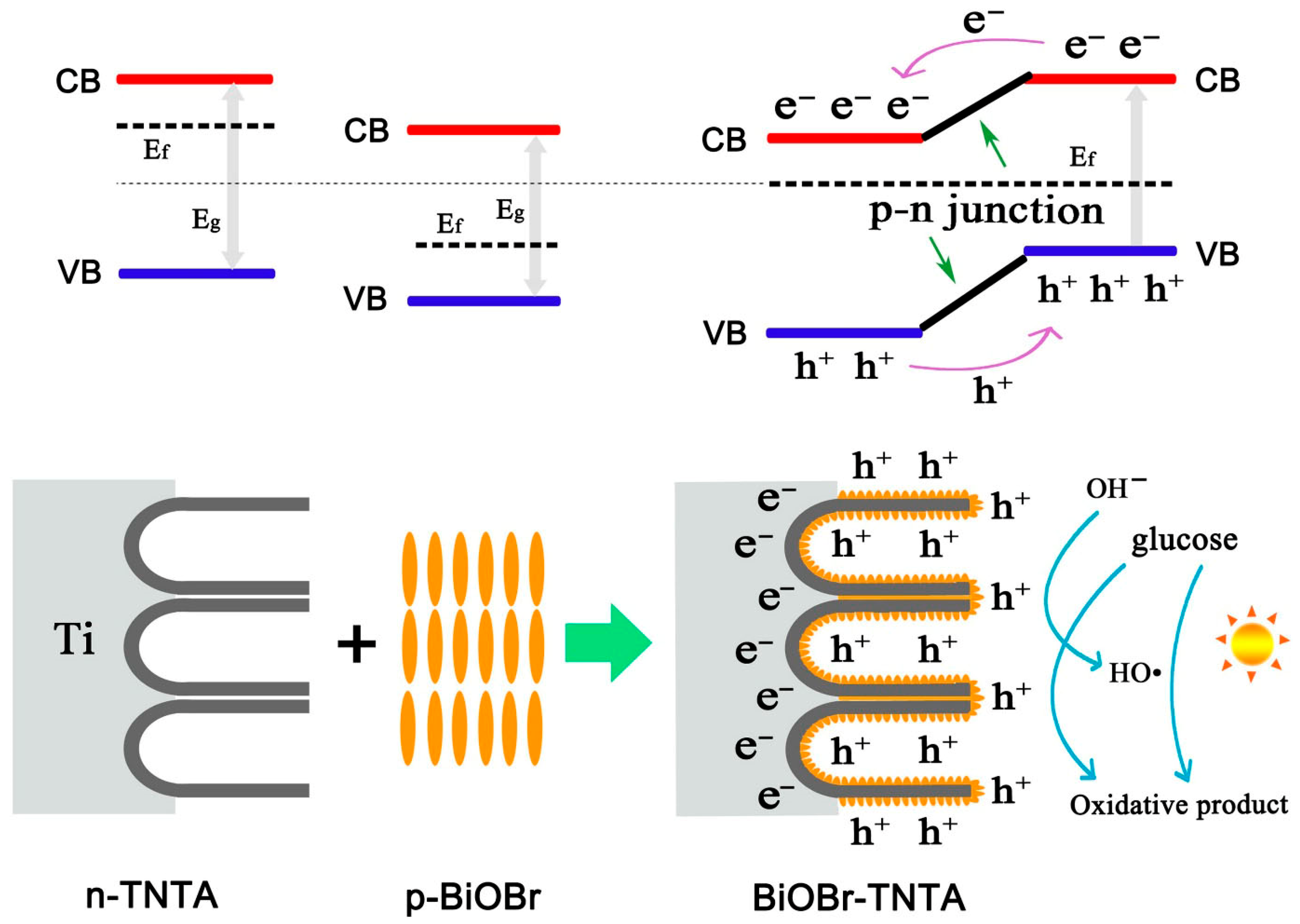
| BiOBr/TiO2 | Photoelectrochemical | Glucose | Serum, NaOH) | Amperometry (visible light) | 5 × 102 to 3 × 107 nM | NA | 10 nM | NA | >95% (after 28 days) | Minimal response to ascorbic acid, uric acid, urea, dopamine | Wu et al. [75] |
| CuO/TiO2 | Photoelectrochemical | Glucose | Human sweat | Visible light | 1 to 200 μM (sweat)/0.5 to 10 mM (blood) | 138.5 μA·mM−1·cm−2 | 0.7 μM | <1 s | NA | Minimal response to NaCl, KCl, dopamine, uric acid, lactic acid, ascorbic acid | Ke et al. [76] |
| 3D CuO/TiO2/Ti | Photoelectrochemical | Glucose | Serum | Visible light | 70 to 900 μM | 155 μA·mM−1·cm−2 | 20 μM | NA | NA | Minimal response to fructose, lactose, sucrose, dopamine, ascorbic acid, uric acid, and L-cysteine | Yang et al. [77] |
| GOx/g-C3N4-TiO2/ITO | Photoelectrochemical | Glucose | Serum | Visible light | 0.05 to 16 mM | 16.7 µA mM−1 cm−2 | 0.01 mM | NA | 90.5% (after 14 days) | Minimal response to ascorbic acid, uric acid, dopamine, fructose, lactose, and sucrose. | Liu et al. [78] |
| CdxZn1-xS/TiO2 | Photoelectrochemical | Glucose | Plasma, NaNO3 | Amperometry (UV light) | 0.014 to 214 mM | 1331.7 μA mM−1 cm−2 | 0.225 μM | NA | 82% (after 35 days) | Minimal response to ascorbic acid, uric acid, dopamine, urea, lysine, tyrosine, histidine | Esmaeili et al. [79] |
| CdSe/TiO2 | Photoelectrochemical | Glucose | Serum | Visible light | 10 to 90 μM | NA | 3.1 μM | NA | NA | Minimal response to ascorbic acid, uric acid, urea, fructose, xylose | Li et al. [80] |
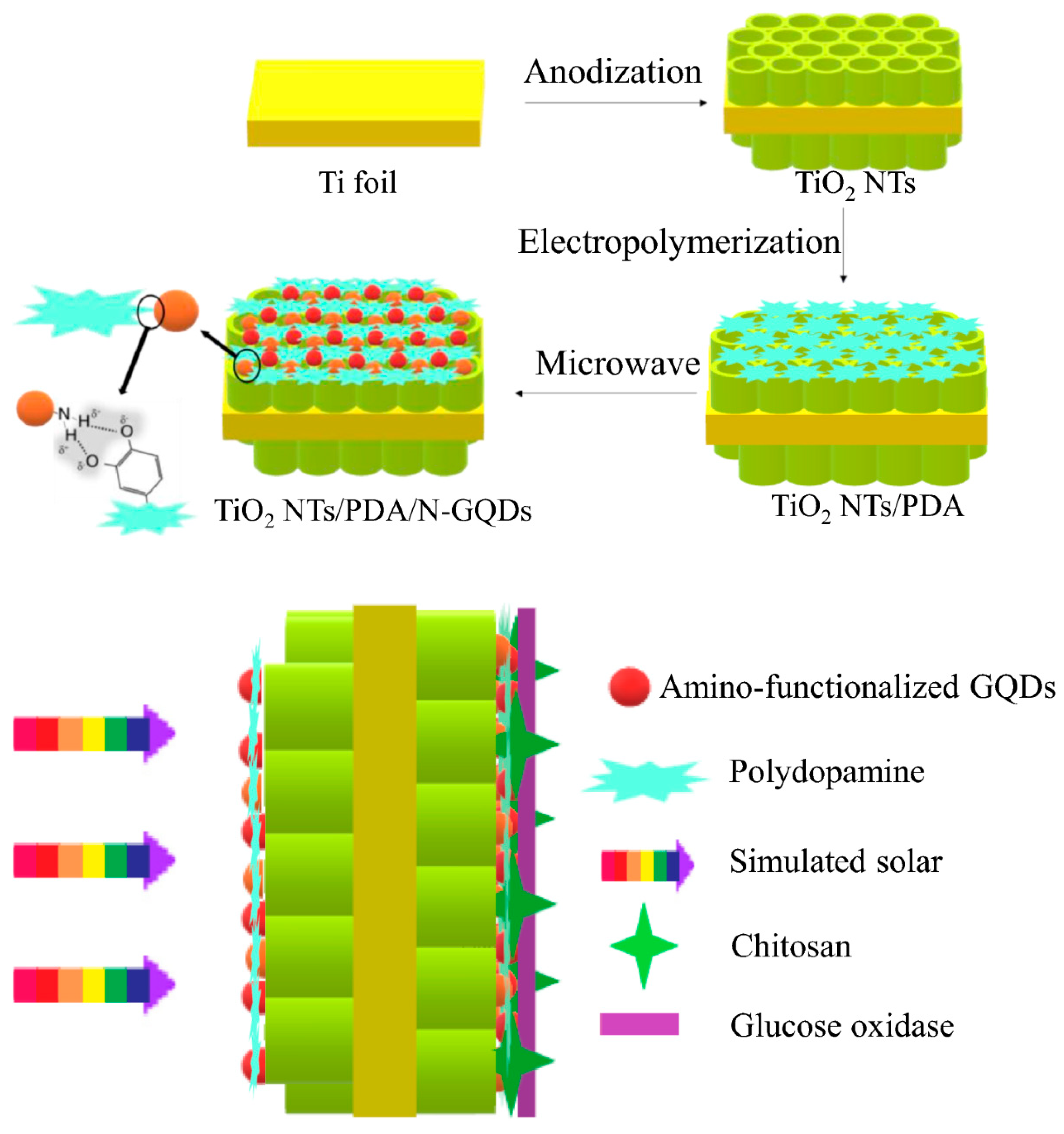
| TiO2/PDA/N-GQDs/GOx | Photoelectrochemical | Glucose | PBS | Amperometry (visible light) | Up to 11 mM | 13.6 μA mM−1 cm−2 | 0.015 mM | <1 s | 86.95% (after 30 days) | Minimal response to NaCl, sucrose, ascorbic acid, uric acid, dopamine | Yang et al. [81] |
| Nafion/GOx/Pt-NPs/TiO2 | Organic Electrochemical Transistor | Glucose | PBS, Serum | Amperometry | 100 nM to 5 mM | 0.09 NCR/1μM | 100 nM | NA | 90% (after 10 days) | Minimal response to ascorbic acid, uric acid | Liao et al. [82] |
| Eu(III) complex/TiO2 | Fluorescence (Optical) | Glucose | Orange juice | Fluorescence intensity change | 0 to 15 mmol/L | NA | 1.02 mmol/L | NA | NA | Minimal response to urea, fructose, sucrose, galactose | Su et al. [83] |
| Feature | Enzymatic Sensors | Non-Enzymatic Sensors | Photoelectrochemical (PEC) Sensors |
|---|---|---|---|
| Sensing Principle | Uses an enzyme, such as glucose oxidase (GOx), for specific biorecognition of the target analyte. The biological event is then converted into a measurable signal by a transducer, often electrochemical. | Uses an electrode material (e.g., noble metal, metal oxide, or carbon nanomaterial) to directly catalyze the oxidation or reduction in the target analyte. | Combines light excitation and an electrical signal readout. A photoactive material converts an optical signal into a measurable electrical current or voltage. |
| Strengths | High selectivity and sensitivity: Enzymes provide excellent specificity for their target molecules, resulting in high accuracy. Rapid response: Enzymatic reactions can provide a fast response time. | High stability and long-term lifespan: Not dependent on biological components, so they are more robust against environmental factors like temperature and pH. Lower cost: Enzymes are often expensive to produce and immobilize, so non-enzymatic sensors have lower manufacturing costs. Simpler fabrication: Avoids the complex and delicate process of immobilizing enzymes. | High sensitivity: Separating the optical excitation and electrical detection minimizes background noise, leading to very high sensitivity and low detection limits. High signal-to-noise ratio: The separation of the input light signal and output electrical signal allows for reduced noise and drift. Low background signal: Inherently low background current enables sensitive detection. Miniaturization: Relies on simple light sources and electrodes, allowing for smaller, more portable devices. |
| Weaknesses | Low stability: Enzymes can denature due to changes in temperature, pH, or exposure to organic solvents, which reduces sensor lifespan. High cost: Enzymes are costly to source, purify, and immobilize. Complex immobilization: Ensuring the enzyme remains active and securely attached to the electrode is a complicated process. Oxygen dependence: Early generations of sensors were limited by the availability of oxygen, though this has been addressed in later generations. | Lower selectivity: Catalytic materials are not as specific as enzymes, leading to potential interference from other electroactive species in the sample. High working potential: Some non-enzymatic sensors require a high potential, which can increase interference. Surface fouling: The electrode surface can become blocked or “poisoned” by intermediate oxidation products, which degrades performance over time. Sensitivity can be lower: It remains a challenge to achieve sensitivity levels comparable to enzymatic sensors. | Limited material choice: Performance is highly dependent on the photoactive material used, which can have intrinsic limitations like a narrow light absorption range. Complex systems: The use of nanomaterials and heterojunctions can increase complexity, which may affect scalability. Potential for toxicity: Some photoactive materials, like early quantum dots, have toxicity concerns, though research is shifting to more biocompatible options. Developmental stage: PEC technology is still an emerging field, and widespread commercialization is not yet realized for many applications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, J.; Hussain, C.M. TiO2 Nanotube-Enabled Glucose Biosensing: Transformative Insights from 2009 to 2024. Micromachines 2025, 16, 1235. https://doi.org/10.3390/mi16111235
Sengupta J, Hussain CM. TiO2 Nanotube-Enabled Glucose Biosensing: Transformative Insights from 2009 to 2024. Micromachines. 2025; 16(11):1235. https://doi.org/10.3390/mi16111235
Chicago/Turabian StyleSengupta, Joydip, and Chaudhery Mustansar Hussain. 2025. "TiO2 Nanotube-Enabled Glucose Biosensing: Transformative Insights from 2009 to 2024" Micromachines 16, no. 11: 1235. https://doi.org/10.3390/mi16111235
APA StyleSengupta, J., & Hussain, C. M. (2025). TiO2 Nanotube-Enabled Glucose Biosensing: Transformative Insights from 2009 to 2024. Micromachines, 16(11), 1235. https://doi.org/10.3390/mi16111235







