Comparison of Environmentally Friendly Cleaning Agents and Organic Solvent Cleaning Processes in the Fabrication of Flexible Nine-in-One Microsensors and Their Application in Hydrogen/Vanadium Redox Flow Batteries
Abstract
1. Introduction
2. Research Method
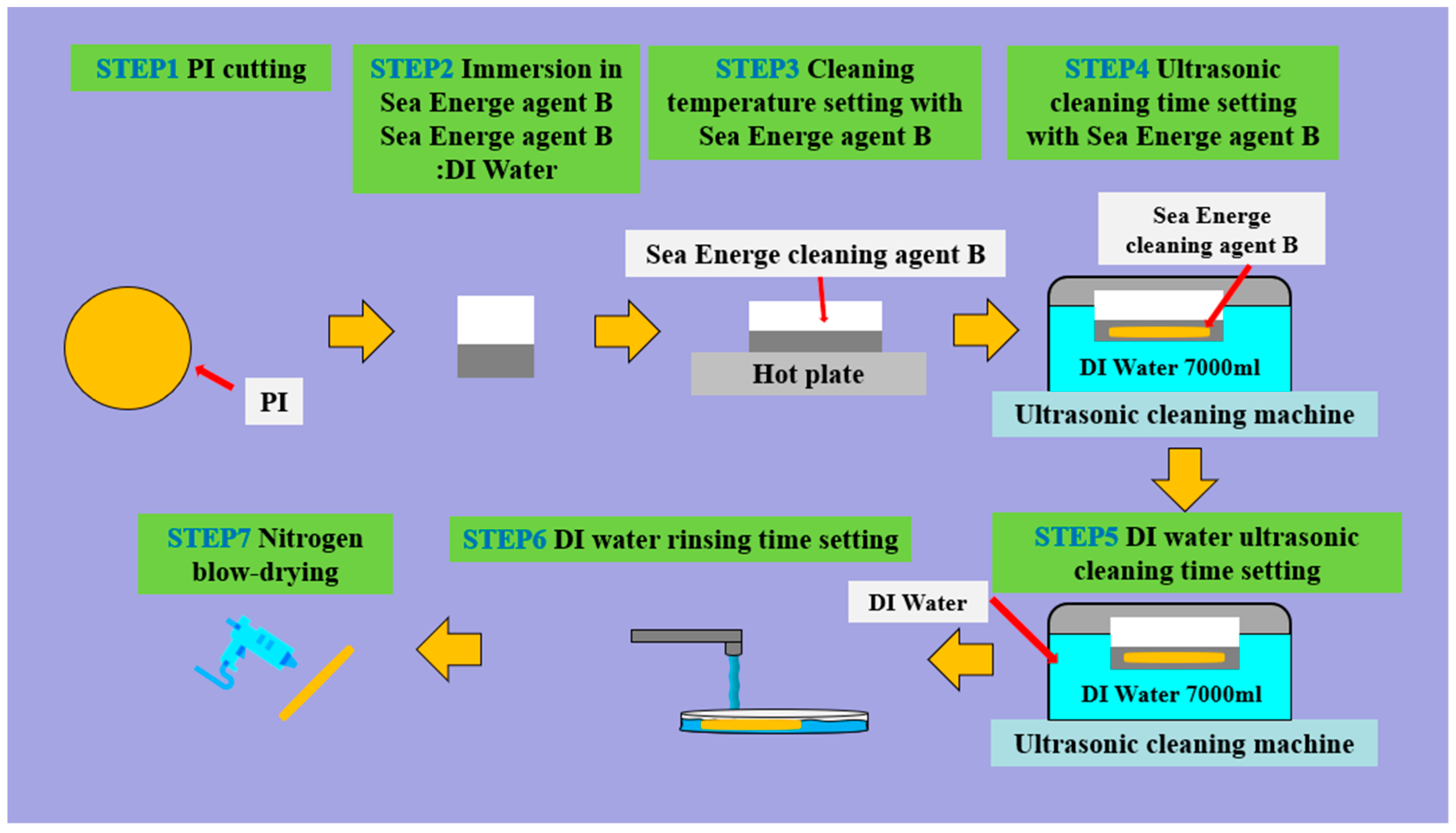
PI Film Cleaning
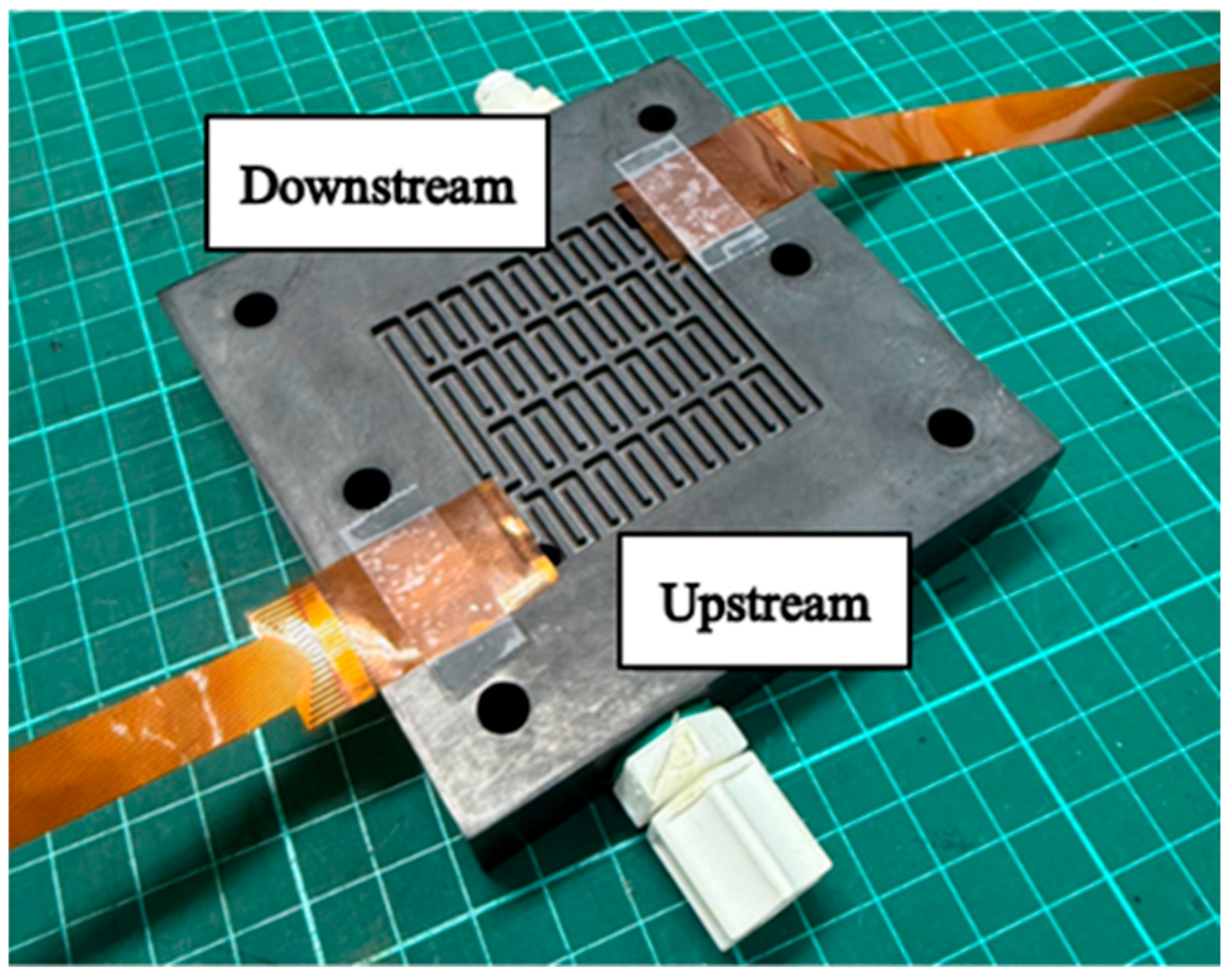
3. Flexible Nine-in-One Microsensor Embedded in HVRFBs for Real-Time Microscopic Monitoring
4. Correction of Flexible Nine-in-One Microsensor
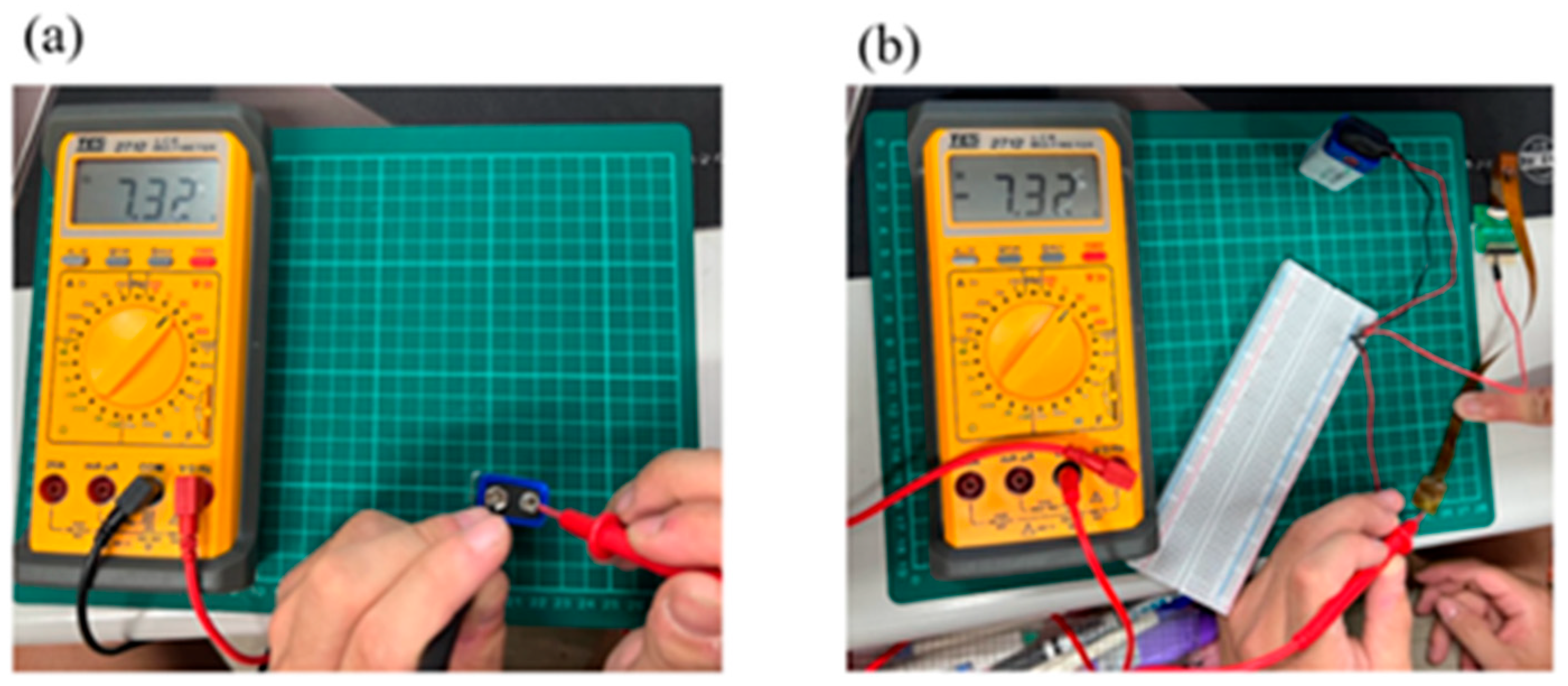
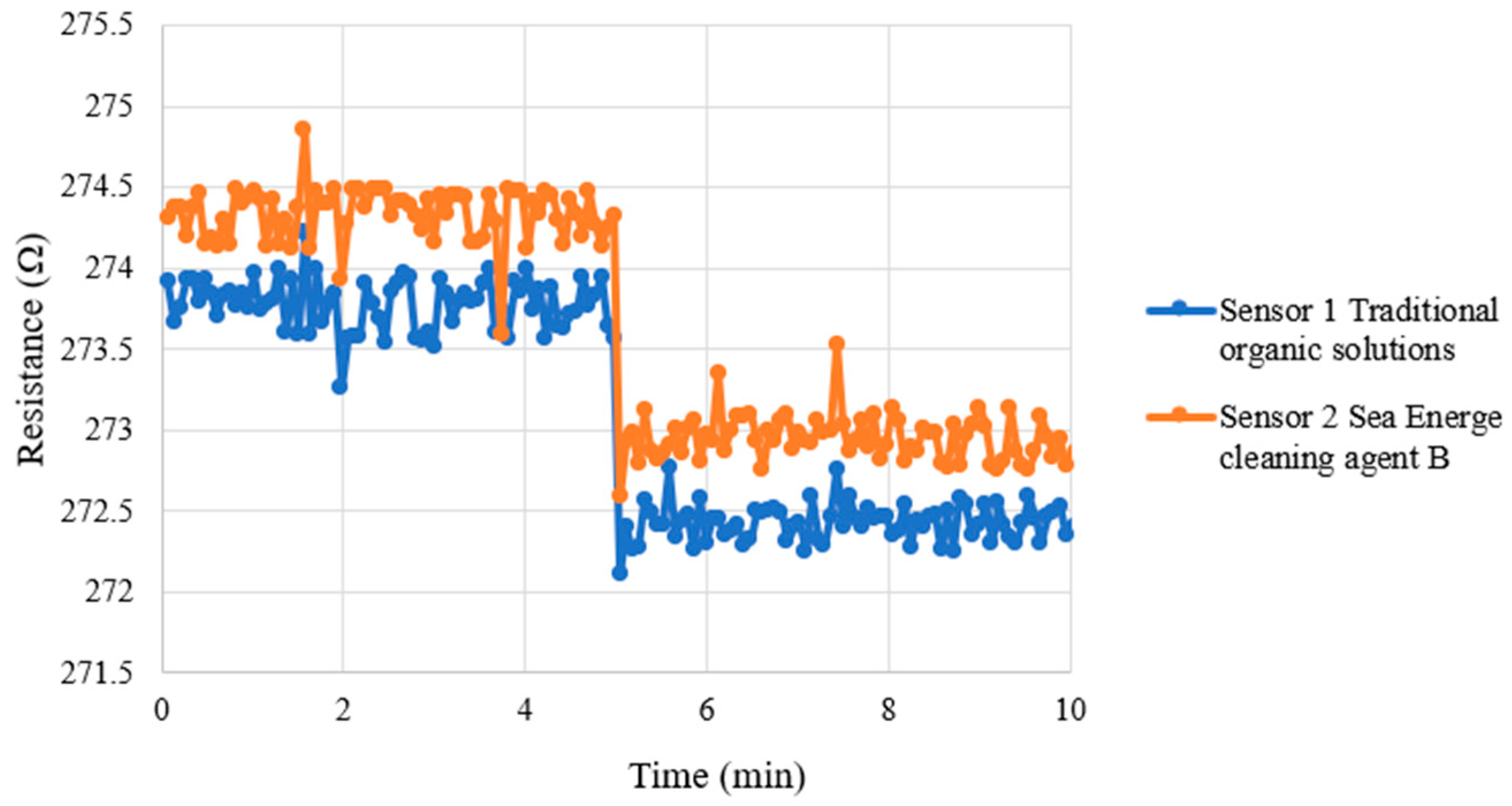
4.1. Correction Test for Micro Voltage, Current, and Conductivity Sensors
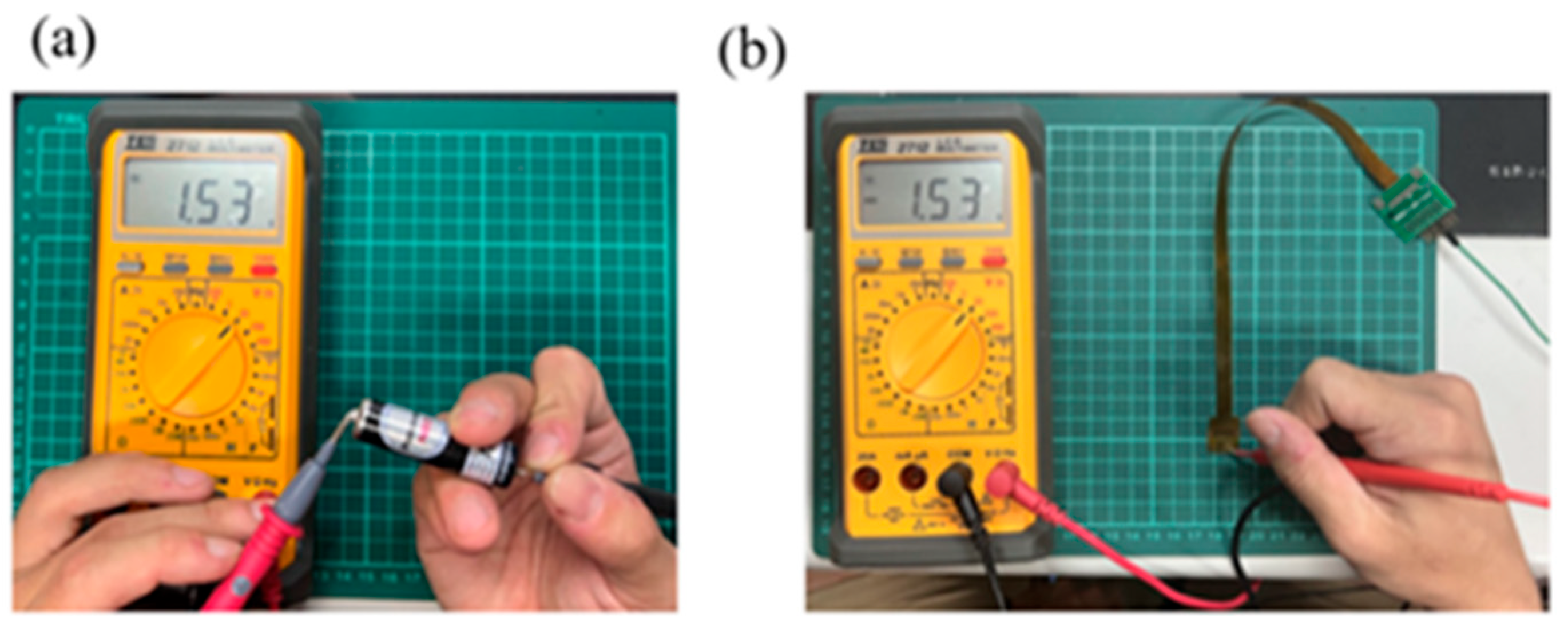
4.2. Correction Test for Micro-Temperature Sensor
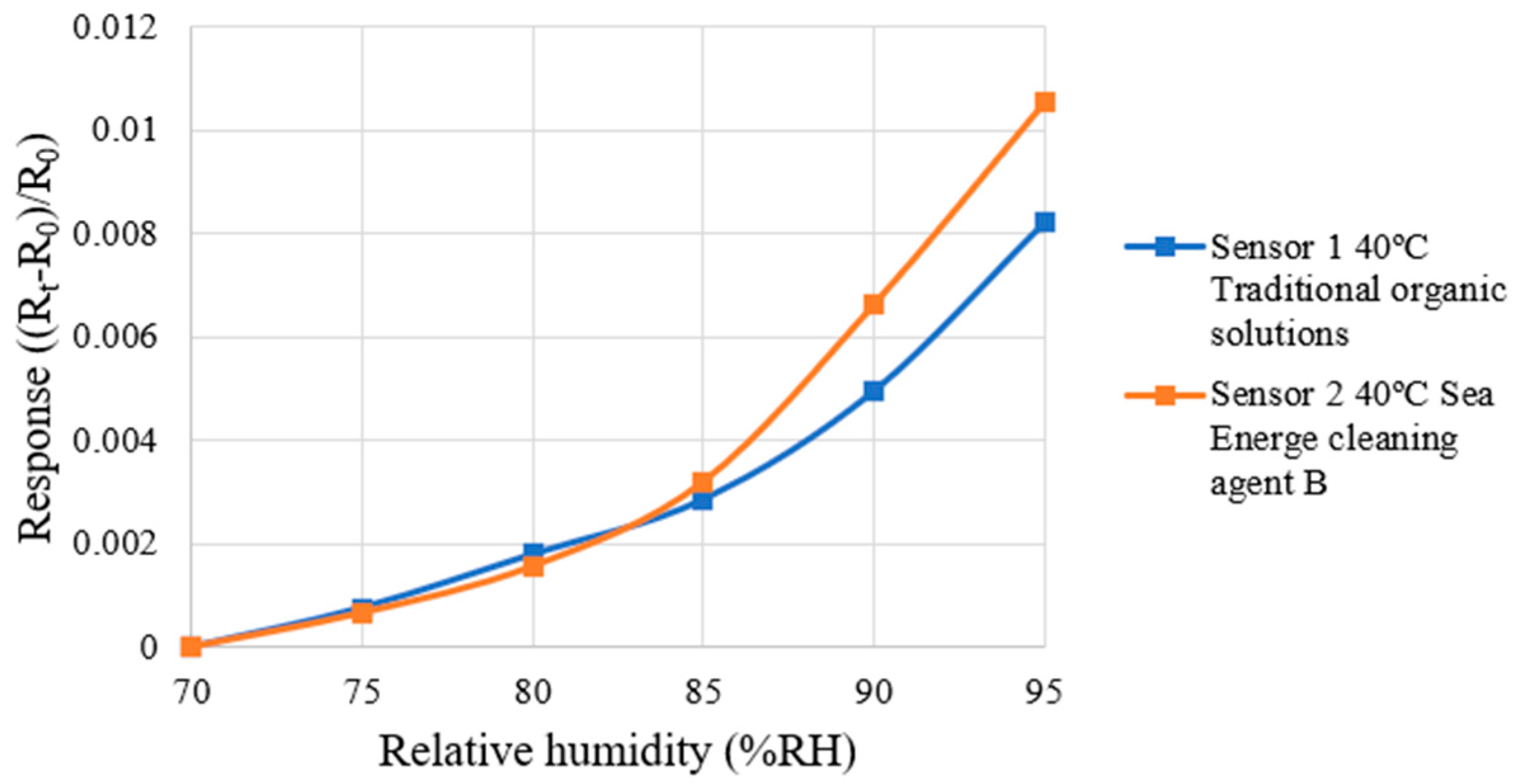
4.3. Correction Test for Micro-Humidity Sensor
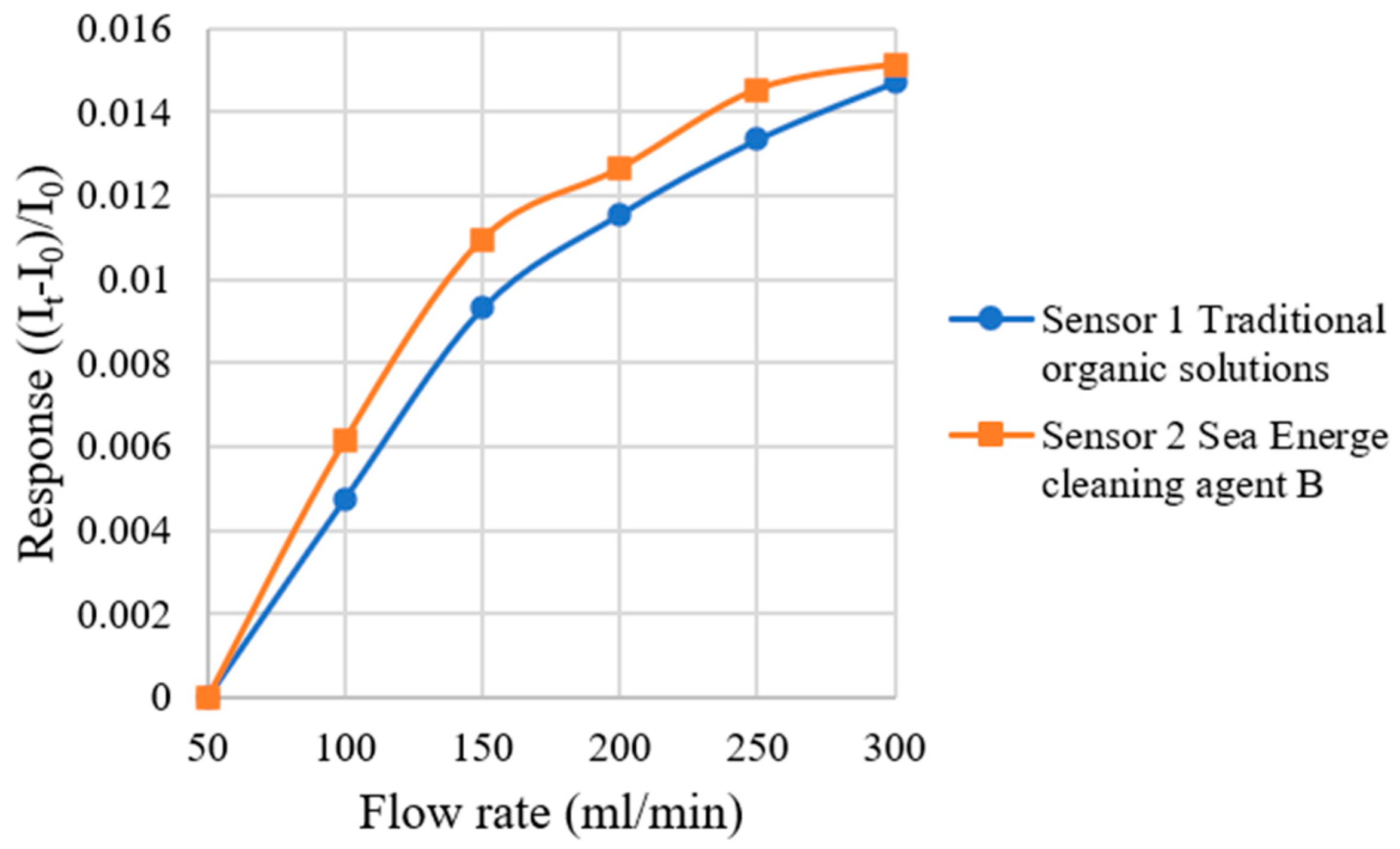
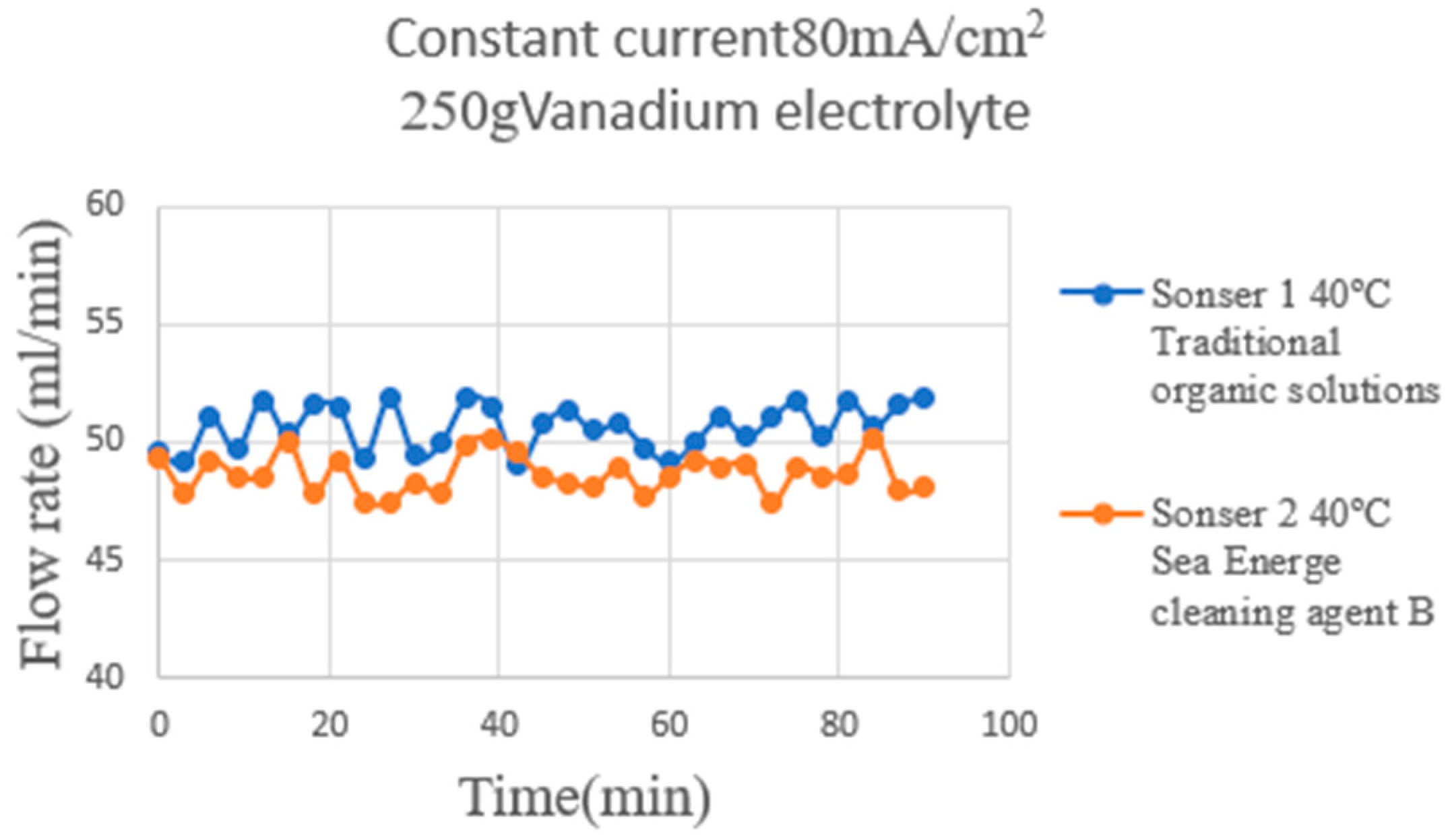
4.4. Correction Test for Micro-Flow Sensor
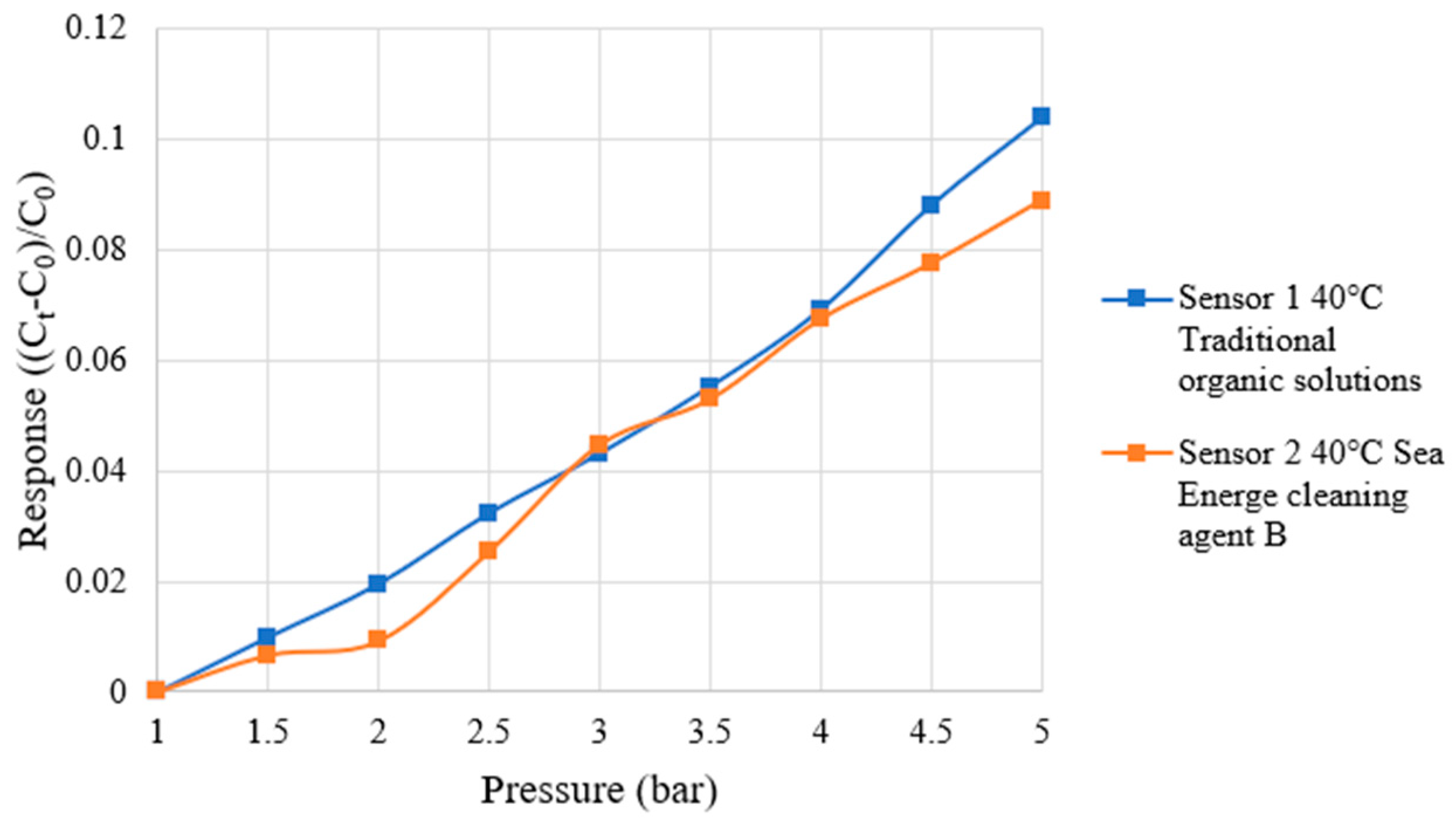
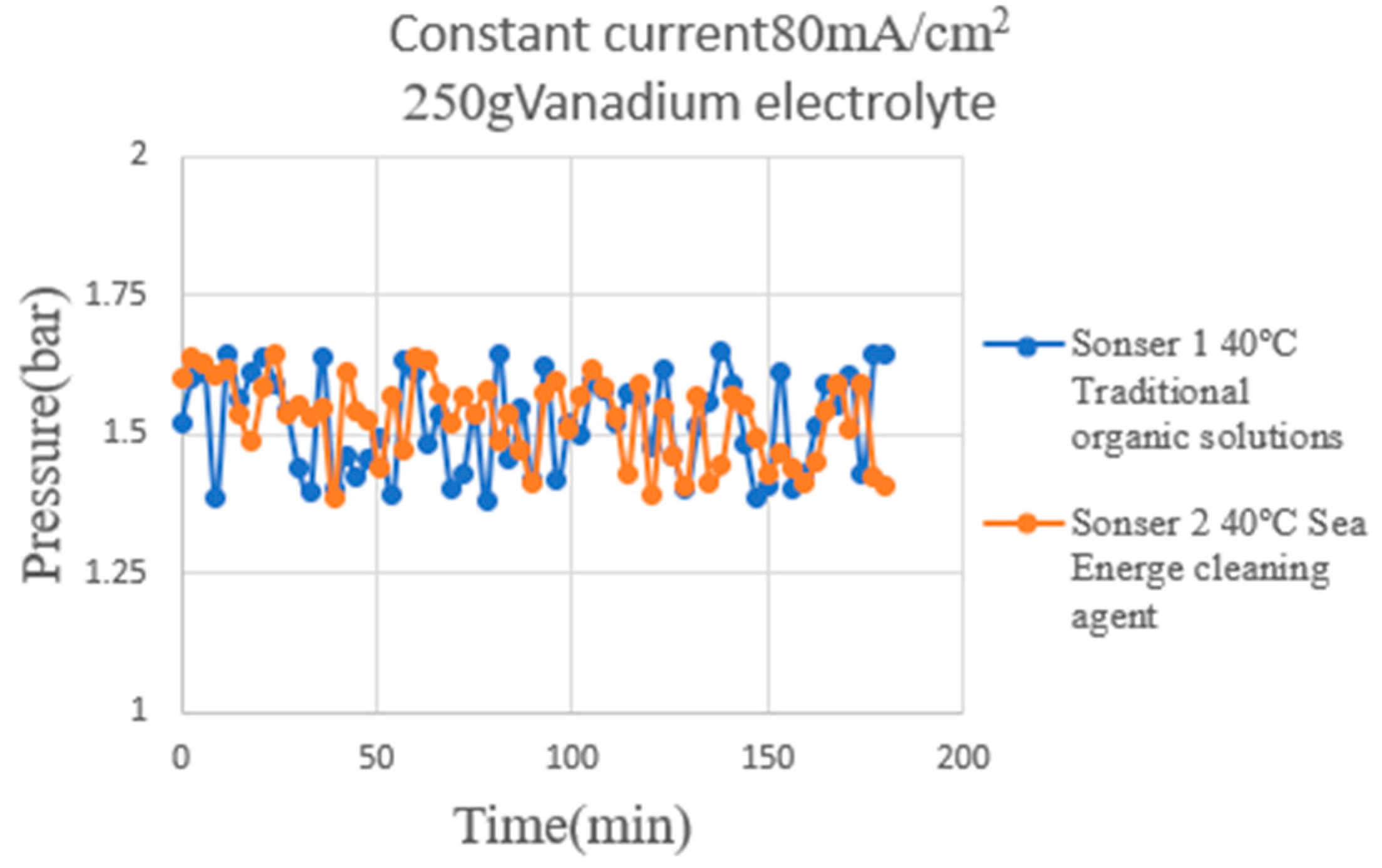
4.5. Correction Test for Micro-Pressure Sensor
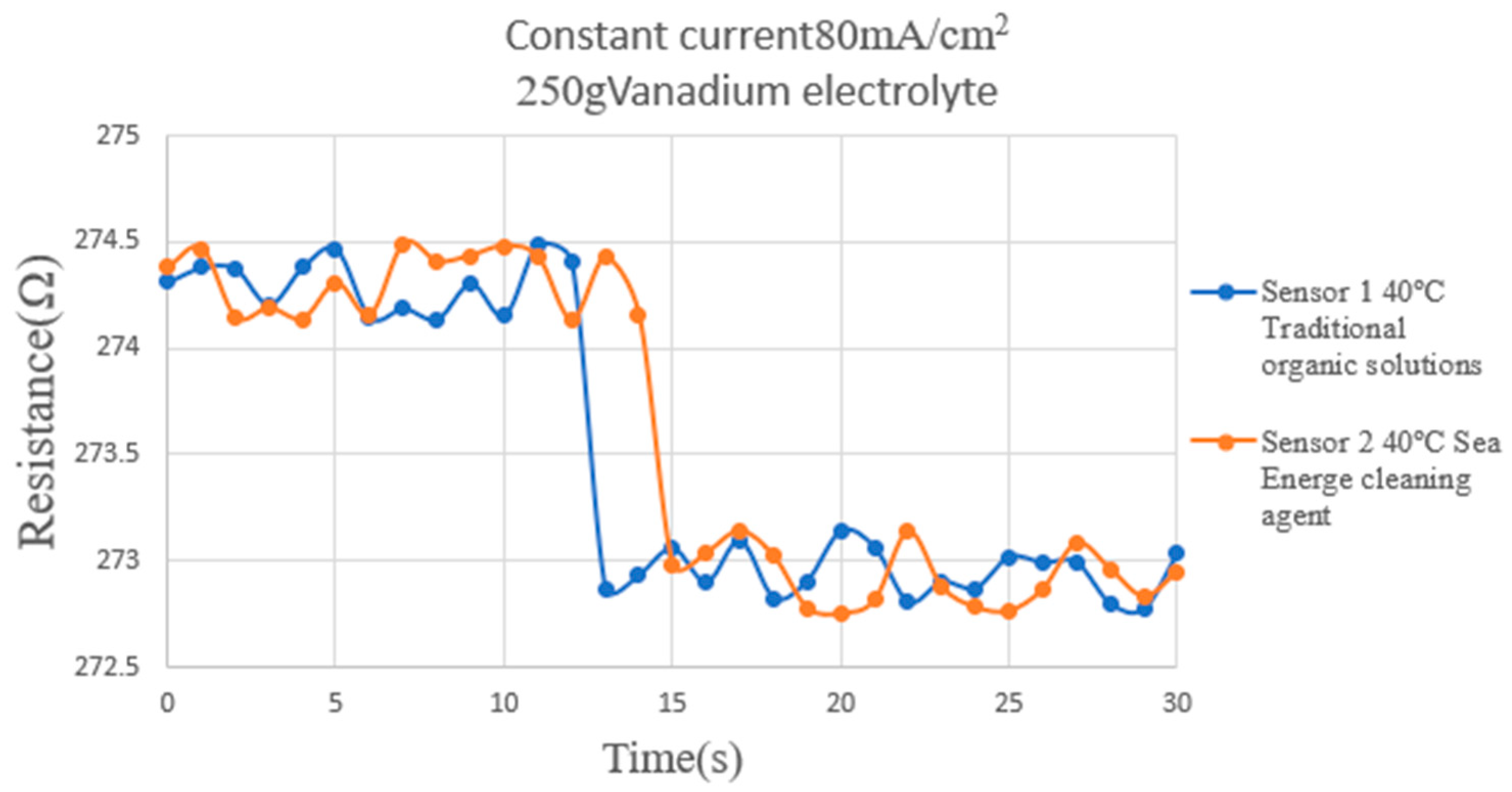
4.6. Correction Test for Micro Hydrogen Sensor
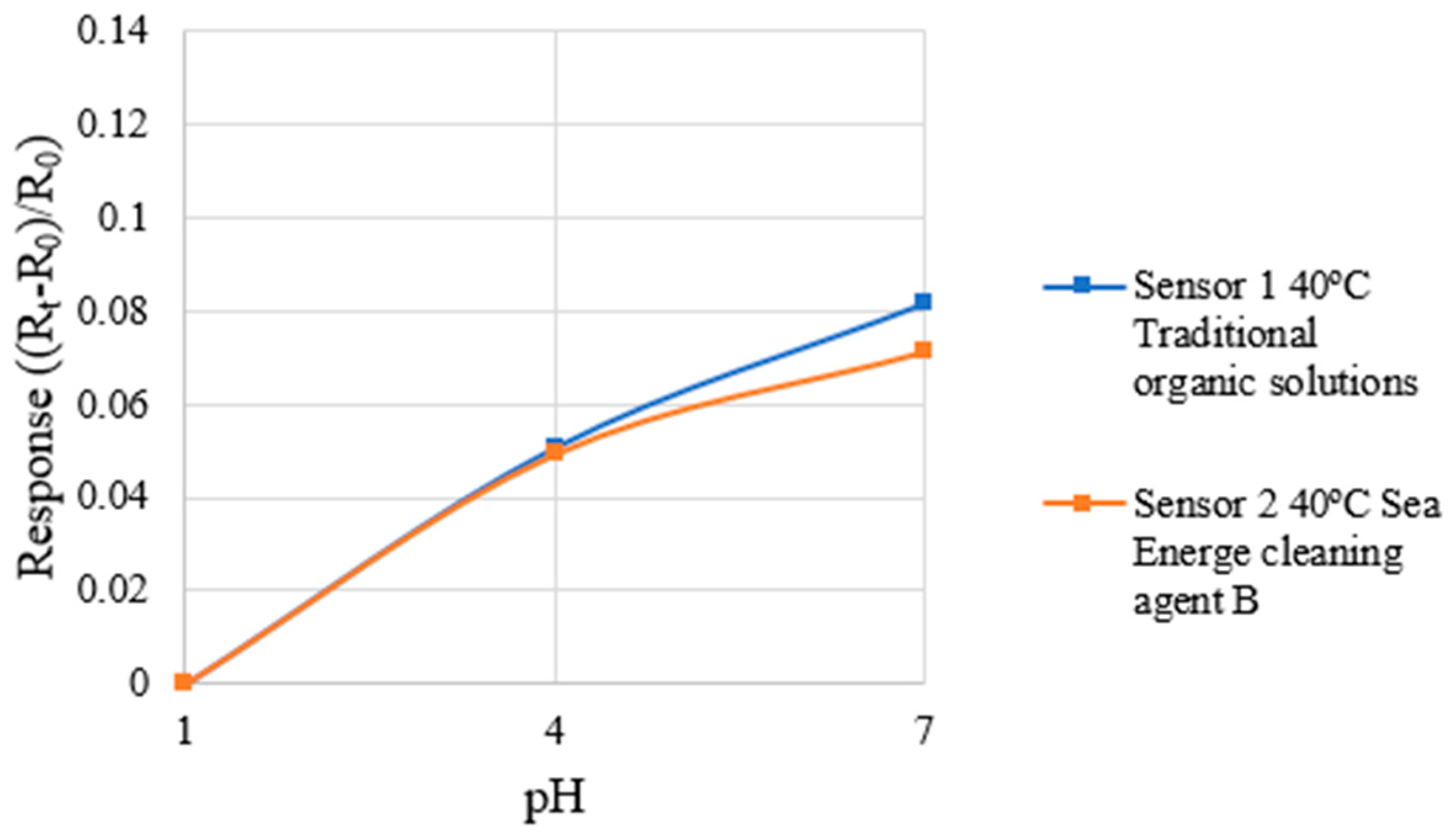
4.7. Correction Test for Micro pH Sensor
5. Flexible Nine-in-One Microsensor Embedded in an HVRFB
5.1. Local Flow Distribution at Hydrogen End of HVRFB Embedded with Flexible Nine-in-One Microsensor
5.2. Local Pressure Distribution of an HVRFB Embedded with Flexible Nine-in-One Microsensor
5.3. Local Hydrogen Distribution of an HVRFB Embedded with Flexible Nine-in-One Microsensor
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Copernicus Climate Change Service (C3S); World Meteorological Organization (WMO). European State of the Climate 2024; Copernicus Climate Change Service: Bonn, Germany, 2025; 102p. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2023; ISBN 978-92-9169-164-7. [Google Scholar]
- WMO. United in Science 2024; WMO: Geneva, Switzerland, 2024. [Google Scholar]
- Climate Change Administration, Ministry of Environment. Climate Change Response Act; Climate Change Administration, Ministry of Environment: Taipei, Taiwan, 2023.
- Jia, Q.; Zhang, T.; Zhu, Z.; Cai, R.; Song, K.; Yan, F.; Qayyum, A. Harnessing hydrogen energy storage for renewable energy stability in China: A path to carbon neutrality. Int. J. Hydrogen Energy 2025, 118, 93–101. [Google Scholar] [CrossRef]
- Jayabal, R. Hydrogen energy storage in maritime operations: A pathway to decarbonization and sustainability. Int. J. Hydrogen Energy 2025, 109, 1133–1144. [Google Scholar] [CrossRef]
- Chein, H.M.; Chen, T.M.; Arpornwichanop, A.; Chen, Y.S. Emission Characteristics of Volatile Organic Compounds from Semiconductor Manufacturing. J. Air Waste Manag. Assoc. 2003, 53, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, X.; Wang, G.; Shao, X.; Li, Z.; Nie, L.; Li, G.; Arpornwichanop, A.; Chen, Y.S. Sector-based volatile organic compounds emission characteristics from the electronics manufacturing industry in China. Atmos. Pollut. Res. 2021, 316, 1–12. [Google Scholar] [CrossRef]
- Supekar, S.D.; Skerlos, S.J.; Chen, Y.S. Supercritical Carbon Dioxide in Microelectronics Manufacturing: Marginal Cradle-to-grave Emissions. Procedia CIRP 2014, 15, 461–466. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Devi, N.; Lin, Y.I.; Hu, Y.H.; Ku, H.H.; Arpornwichanop, A.; Chen, Y.S. Study on the effect of electrode configuration on the performance of a hydrogen/vanadium redox flow battery. Renew. Energy 2022, 190, 658–663. [Google Scholar] [CrossRef]
- Lin, T.Z.; Chen, K.T.; Chen, Y.H. Renewable energy certificate mechanism and markets in Taiwan: The evolution and characteristics. Energy Policy 2025, 205, 114689–114698. [Google Scholar] [CrossRef]
- Taheri, A.H.; Ardehali, M.M. Optimal design and management of renewable energy system for charging infrastructure of hydrogen fuel cell and battery electric vehicles. Energy Convers. Manag. 2025, 326, 119415–119434. [Google Scholar] [CrossRef]
- Wang, Z. Assessing the economic feasibility of hydrogen generation from renewable energy sources. Int. J. Hydrogen Energy 2025, 152, 149940–149982. [Google Scholar] [CrossRef]
- Liu, P.; Brika, S.K.; Vagif, K.M.; Yusupov, U.; Jeang, Z. A hybrid decision-making framework for renewable-hydrogen energy storage integration: Enhancing economic and energy security. Int. J. Hydrogen Energy 2025, 144, 482–495. [Google Scholar] [CrossRef]
- Badawi, N.M.; Agrawal, N.; Luqman, M.; Ramesh, S.; Ramesh, K.; Khan, M.; Adil, S.F. Developments and challenges in batteries, and hydrogen as a future fuel, and storage and carrier devices. Int. J. Hydrogen Energy 2025, 105, 1242–1260. [Google Scholar] [CrossRef]
- Hong, Y.Y.; Magararu, P. Techno-economic analysis of Taiwan’s new energy policy for 2025. Sustain. Energy Technol. Assess. 2021, 46, 101307–101322. [Google Scholar] [CrossRef]
- Phogat, P.; Chand, B.; Shreya; Jha, R.; Singh, S. Hydrogen and methanol fuel cells: A comprehensive analysis of challenges, advances, and future prospects in clean energy. Int. J. Hydrogen Energy 2025, 109, 465–485. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, Q.; Zhang, X.; Sun, Z.; Bao, D. Detection Method and Common Characteristics of Waste Solvent from Semiconductor Industry. Molecules 2023, 28, 5992. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chen, C.H.; Fang, L.H.; Hung, C.T.; Yang, L.J.; Yang, C.Y.; Yu, C.S. An internal real-time microscopic monitoring and diagnostic tool for improved proton battery stacks. Sens. Actuators A Phys. 2025, 139, 116608–116619. [Google Scholar] [CrossRef]
- Sammut, S. A Comprehensive Review of Plasma Cleaning Processes Used in Semiconductor Packaging. Appl. Sci. 2025, 15, 7361. [Google Scholar] [CrossRef]
- Lemaire, E.; Thuau, D.; Caillard, B.; Dufour, I. Fast-Fabrication Process for Low Environmental Impact Microsystems. J. Clean. Prod. 2015, 108, 207–216. [Google Scholar] [CrossRef]
- Dufour, T. From Basics to Frontiers: A Comprehensive Review of Plasma-Modified and Plasma-Synthesized Polymer Films. Polymers 2023, 15, 3607. [Google Scholar] [CrossRef] [PubMed]
- Hatescu, I.; Borcia, C.; Ciobanu, R.; Borcia, G. Discharge Energy Versus Exposure Time in Atmospheric-Pressure Air Plasma Surface Treatment of Polyimide and Polyamide 6 Films. Polymers 2025, 17, 1394. [Google Scholar] [CrossRef] [PubMed]




| Traditional Organic Solutions | Sea Energe Cleaning Agent | |
|---|---|---|
| Sensing area | 390 µm × 390 µm | 390 µm × 390 µm |
| Sensitivity | 0.00309 °C−1 | 0.00287 °C−1 |
| Accuracy | ±0.2 °C | ±0.2 °C |
| Sensing range | 25~60 °C | 25~60 °C |
| Traditional Organic Solutions | Sea Energe Cleaning Agent | |
|---|---|---|
| Sensing area | 390 µm × 390 µm | 390 µm × 390 µm |
| Sensitivity | 0.00307%RH−1 | 0.00370%RH−1 |
| Accuracy | ±2%RH | ±2%RH |
| Sensing range | 70–95%RH | 70–95%RH |
| Traditional Organic Solutions | Sea Energe Cleaning Agent | |
|---|---|---|
| Sensing area | 390 µm × 430 µm | 390 µm × 430 µm |
| Sensitivity (Liquid flow rate) | 0.000308 mL/min−1 | 0.000395 mL/min−1 |
| Accuracy (Liquid flow rate) | ±2%mL/min | ±2%mL/min |
| Sensing range (Liquid flow rate) | 200~400 mL/min | 200~400 mL/min |
| Sensitivity (Gas flow rate) | 0.00388 mL/min−1 | 0.00417 mL/min−1 |
| Accuracy (Gas flow rate) | ±0.1 mL/min | ±0.1 mL/min |
| Sensing range (Gas flow rate) | 50~300 mL/min | 50~300 mL/min |
| Traditional Organic Solutions | Sea Energe Cleaning Agent | |
|---|---|---|
| Sensing area | 800 µm × 800 µm | 800 µm × 800 µm |
| Sensitivity | 0.0336 bar−1 | 0.0471 bar−1 |
| Accuracy | ±0.1 bar | ±0.1 bar |
| Sensing range | 1~5 bar | 1~5 bar |
| Traditional Organic Solutions | Sea Energe Cleaning Agent | |
|---|---|---|
| Sensing area | 600 µm × 600 µm | 600 µm × 600 µm |
| Sensitivity | 0.0122 pH−1 | 0.0111 pH−1 |
| Sensing range | pH 1~7 | pH 1~7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Jung, G.-B.; Chen, H.-C.; Chen, M.-H.; Chen, C.-H.; Lai, K.-T.; Liao, C.-K.; Chang, Y.-L.; Chang, H.-P. Comparison of Environmentally Friendly Cleaning Agents and Organic Solvent Cleaning Processes in the Fabrication of Flexible Nine-in-One Microsensors and Their Application in Hydrogen/Vanadium Redox Flow Batteries. Micromachines 2025, 16, 1219. https://doi.org/10.3390/mi16111219
Lee C-Y, Jung G-B, Chen H-C, Chen M-H, Chen C-H, Lai K-T, Liao C-K, Chang Y-L, Chang H-P. Comparison of Environmentally Friendly Cleaning Agents and Organic Solvent Cleaning Processes in the Fabrication of Flexible Nine-in-One Microsensors and Their Application in Hydrogen/Vanadium Redox Flow Batteries. Micromachines. 2025; 16(11):1219. https://doi.org/10.3390/mi16111219
Chicago/Turabian StyleLee, Chi-Yuan, Guo-Bin Jung, Huan-Chu Chen, Mau-Hsiung Chen, Chia-Hung Chen, Kuan-Ting Lai, Cheng-Kai Liao, Yung-Lin Chang, and Hao-Peng Chang. 2025. "Comparison of Environmentally Friendly Cleaning Agents and Organic Solvent Cleaning Processes in the Fabrication of Flexible Nine-in-One Microsensors and Their Application in Hydrogen/Vanadium Redox Flow Batteries" Micromachines 16, no. 11: 1219. https://doi.org/10.3390/mi16111219
APA StyleLee, C.-Y., Jung, G.-B., Chen, H.-C., Chen, M.-H., Chen, C.-H., Lai, K.-T., Liao, C.-K., Chang, Y.-L., & Chang, H.-P. (2025). Comparison of Environmentally Friendly Cleaning Agents and Organic Solvent Cleaning Processes in the Fabrication of Flexible Nine-in-One Microsensors and Their Application in Hydrogen/Vanadium Redox Flow Batteries. Micromachines, 16(11), 1219. https://doi.org/10.3390/mi16111219








