Prediction Model and Experimental Verification of Surface Roughness of Single Crystal Diamond Chemical Mechanical Polishing Based on Archimedes Optimization Algorithm
Abstract
1. Introduction
2. Preliminary Analysis of Process Parameters on Polishing Effect
3. Single Factor Experiments
4. Results and Discussion
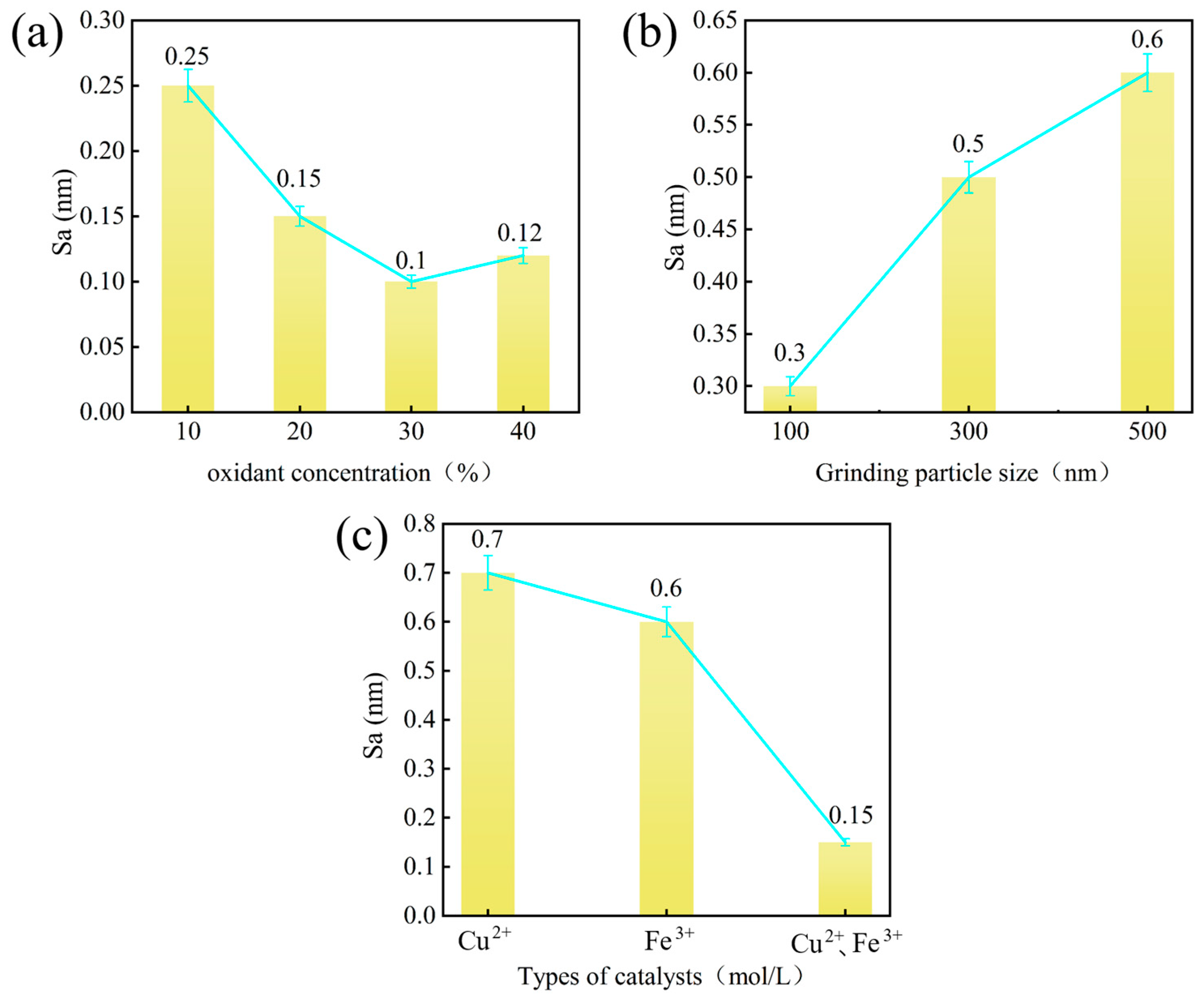
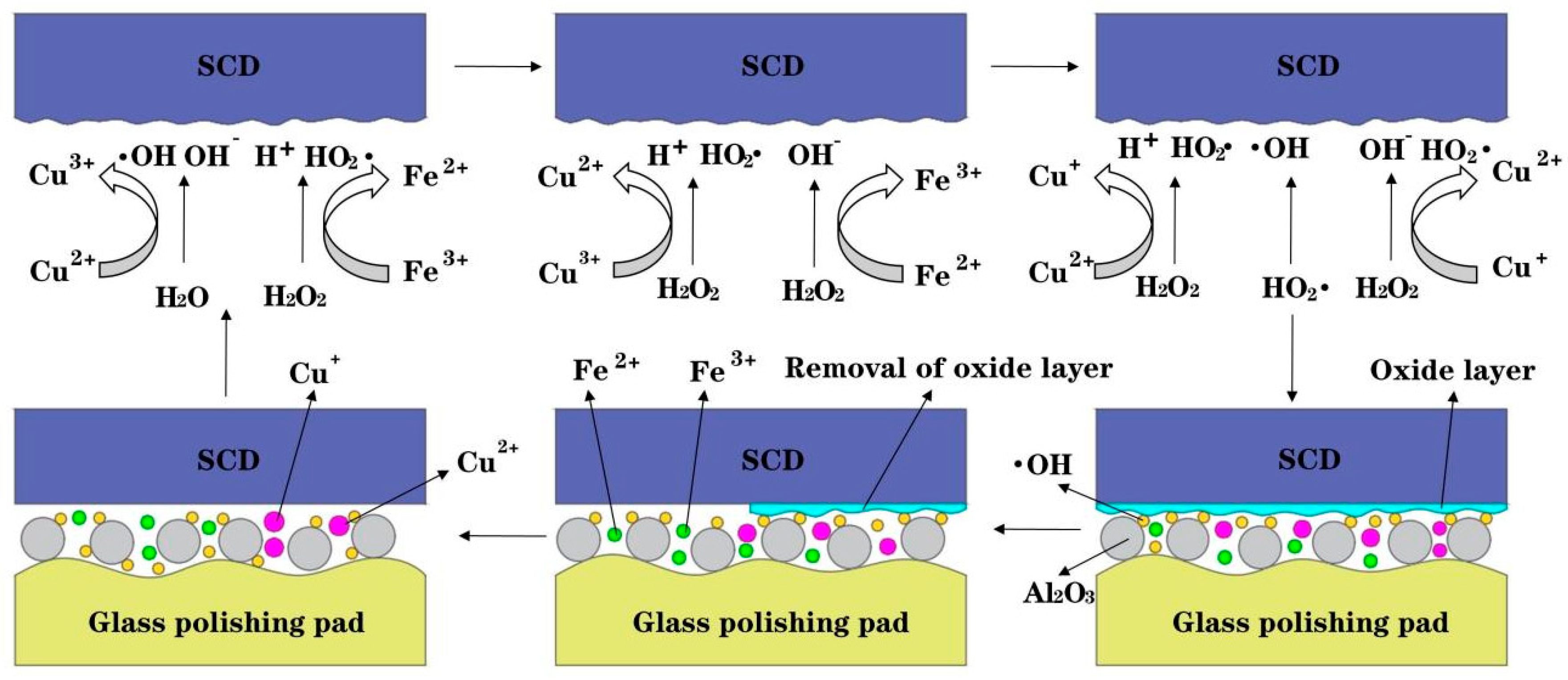
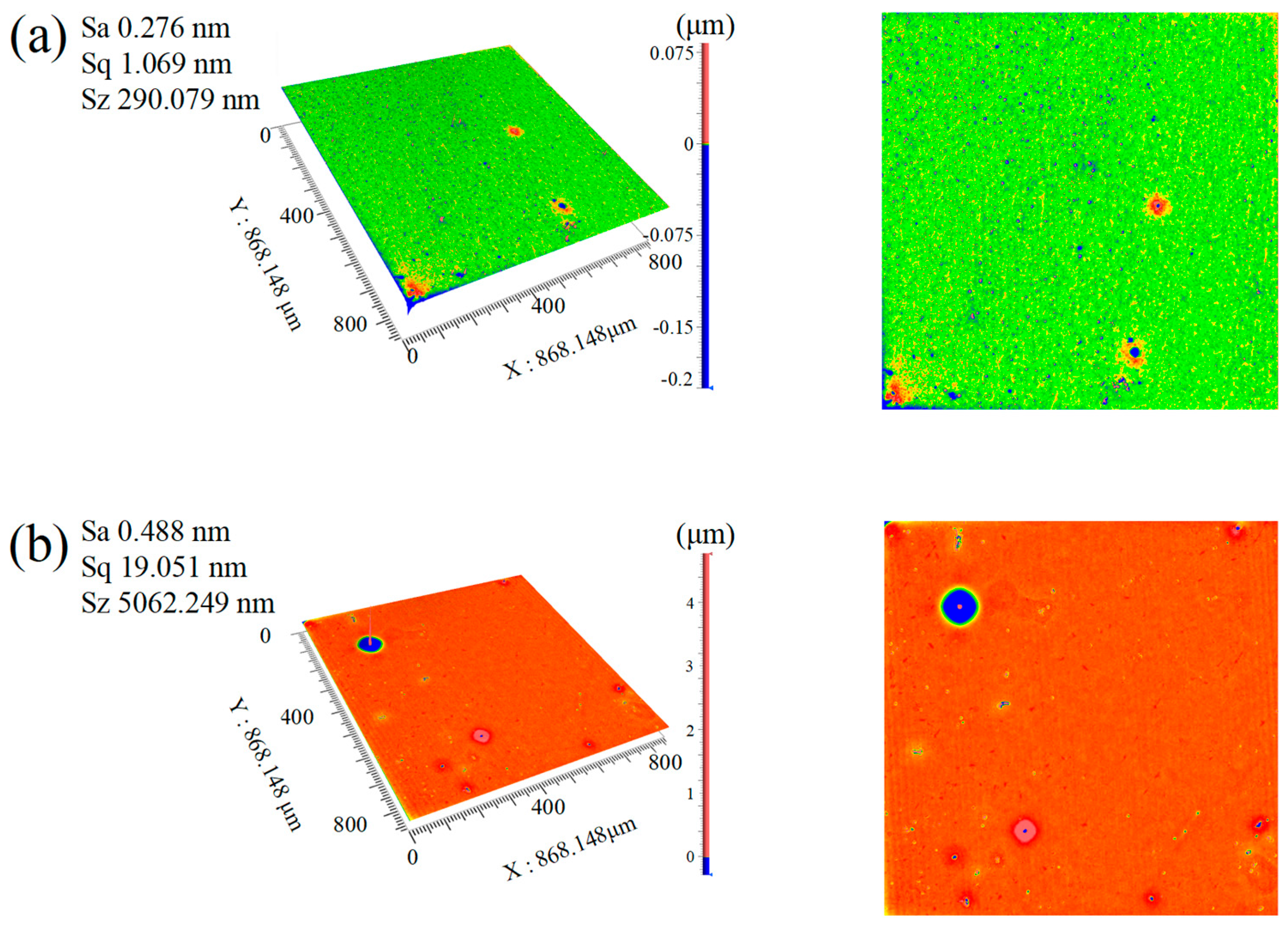
4.1. The Influence of Catalyst Types on Surface Roughness and Surface Quality
4.2. Effect of Oxidant Concentration on Surface Roughness
4.3. The Influence of Abrasive Particle Size on Surface Quality
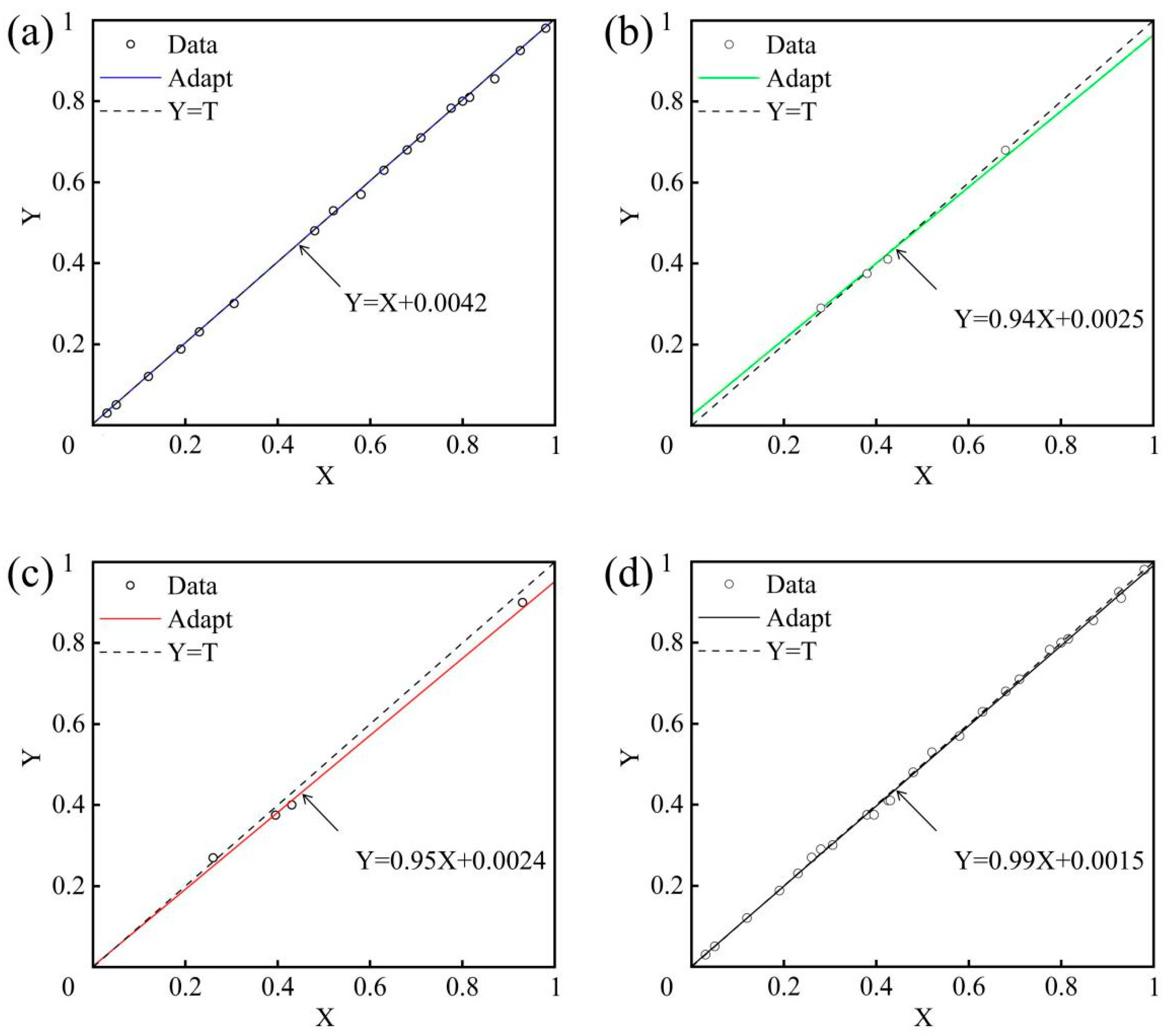
5. Diamond CMP Surface Roughness Prediction Model Based on AOA
5.1. Algorithm Introduction
5.2. Roughness Prediction Model Based on AOA
5.3. Testing and Analysis
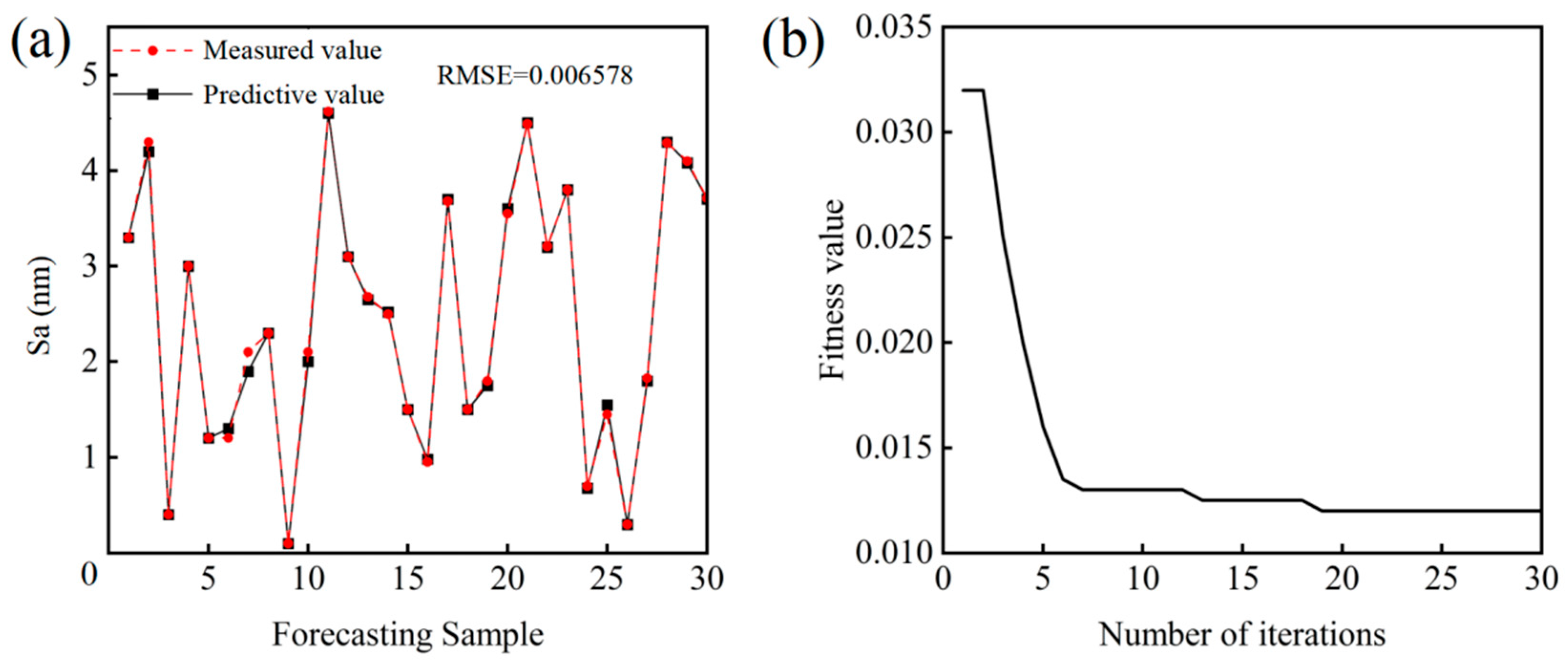
5.4. Experimental Result Verification
6. Conclusions
- (1)
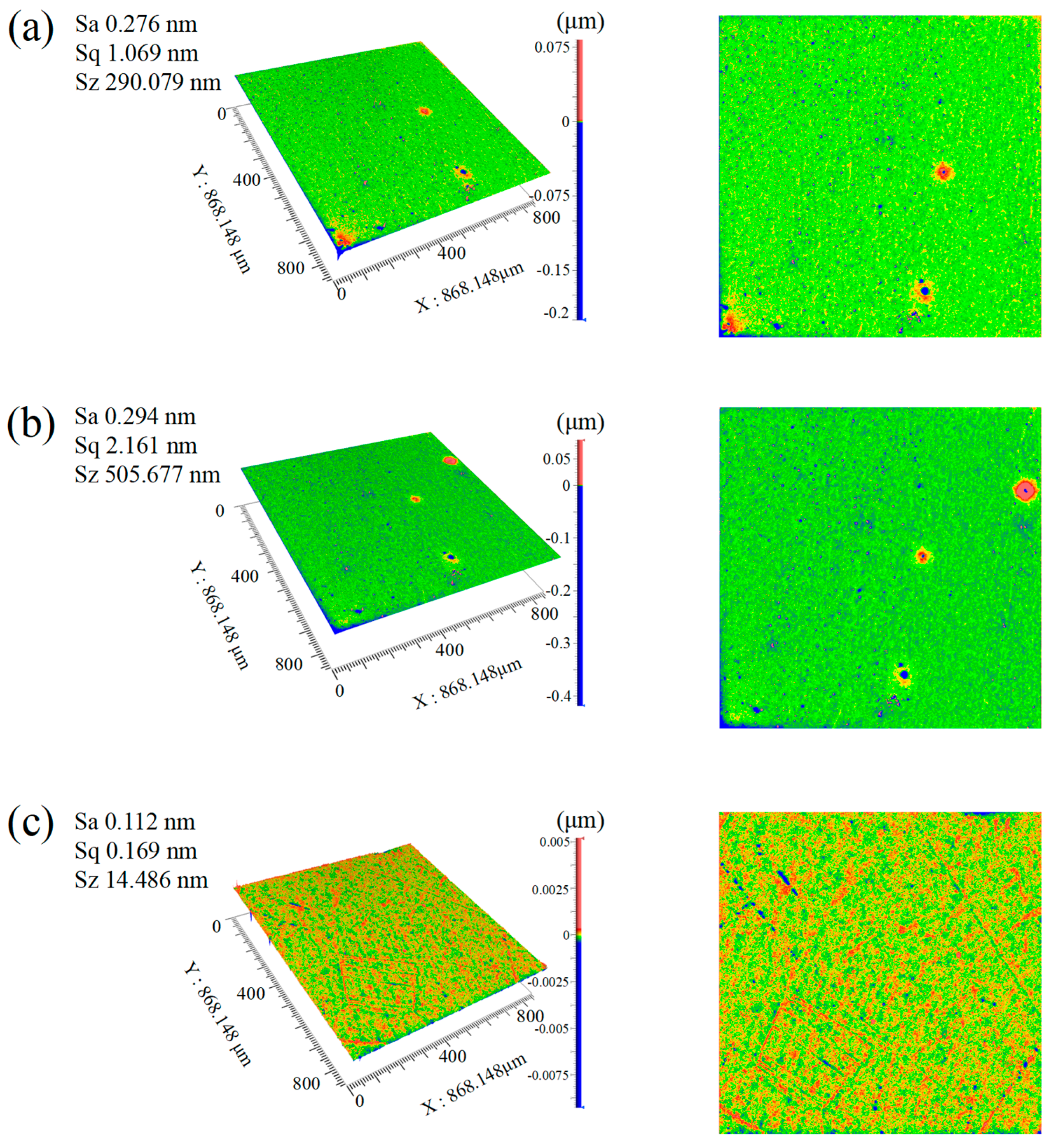
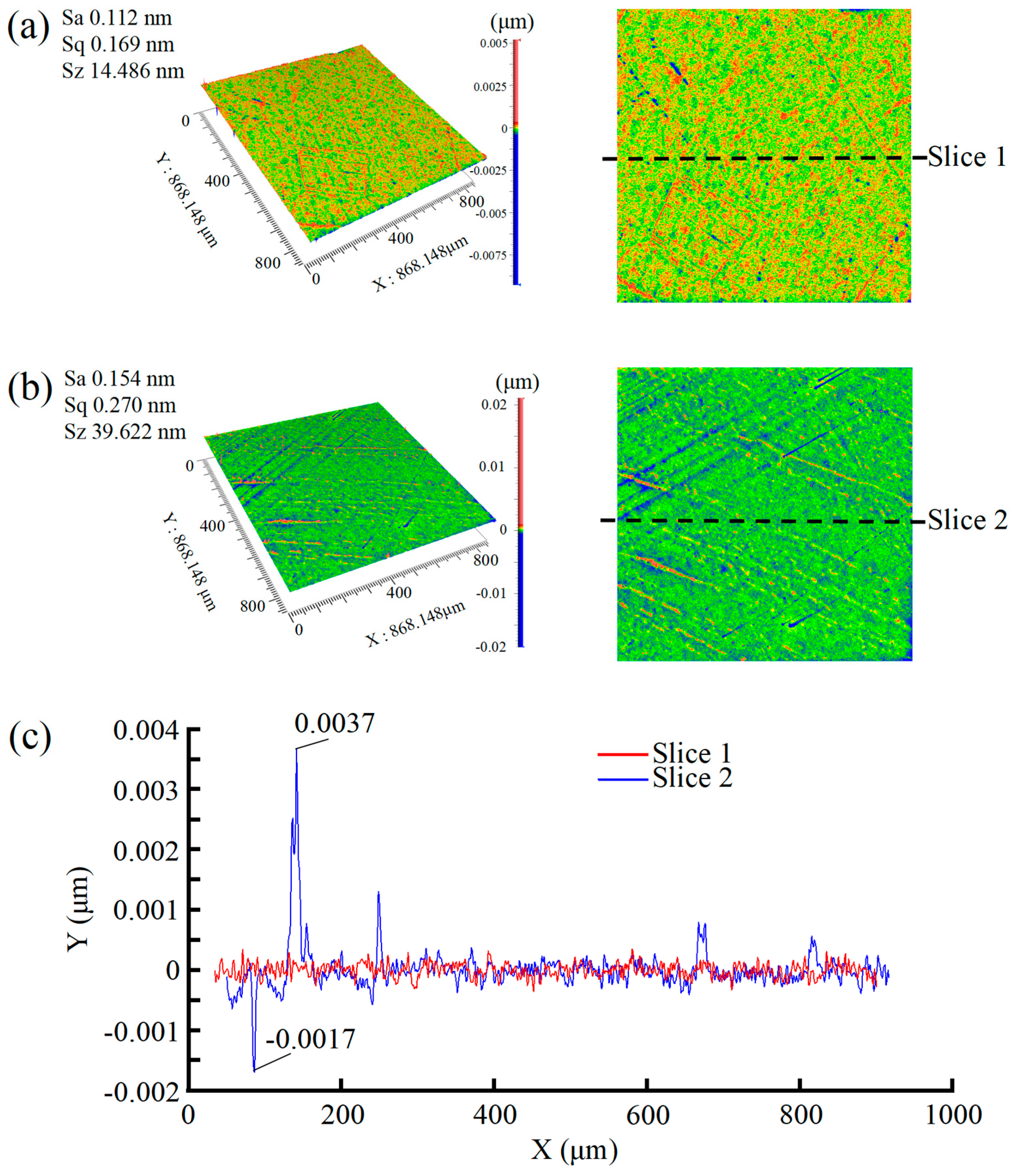
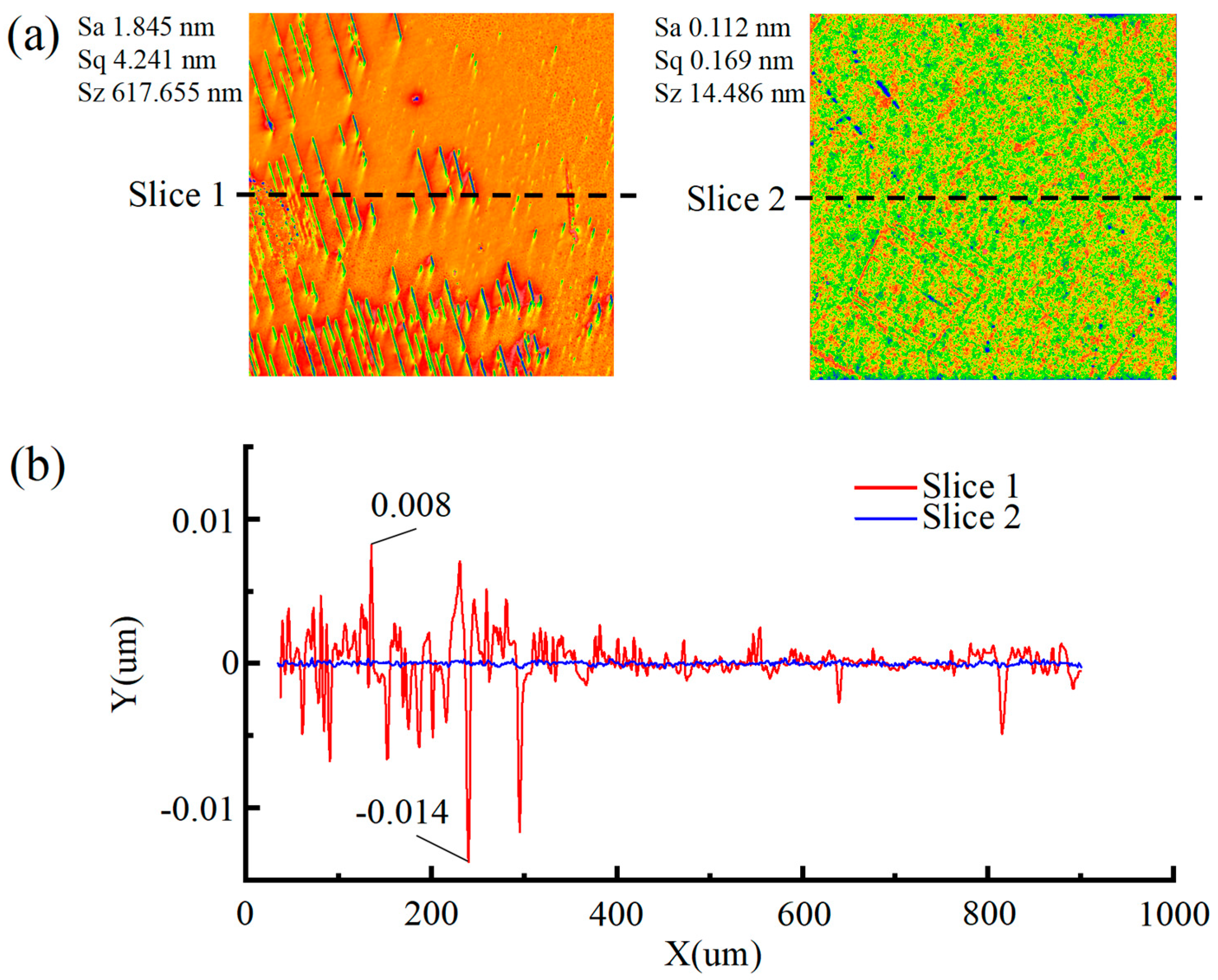
- The synergistic effect of dual catalysts significantly outperforms single catalysts. In the case of Cu2+/Fe3+ dual catalysts (SL8), their co-catalysis in the Fenton polishing solution exhibits a synergistic effect: Fe3+ activates H2O2 to generate ·OH radicals, while Cu2+ acts as an intermediate to facilitate redox cycling. This collaboration results in a final Sa of 0.112 nm, which is over 60% lower than the surface roughness values obtained with a single Fe3+ catalyst (SL6 group, Sa = 0.276 nm) and a single Cu2+ catalyst (SL8 group, Sa = 0.294 nm), highlighting the superior efficiency of dual-catalyst systems in promoting material removal and improving surface quality.
- (2)
- The concentration of oxidants significantly influences surface roughness. Under consistent polishing conditions, increasing the oxidant concentration from 15% (Group SL7) to 30% (Group SL5) reduces the Sa from 0.488 nm to 0.276 nm, representing a 43.4% decrease. This result demonstrates a direct correlation between higher oxidant concentrations and enhanced oxidation reactivity, which accelerates chemical etching of the diamond surface and effectively diminishes surface roughness, highlighting the critical role of oxidant dosage in optimizing material removal efficiency and surface quality.
- (3)
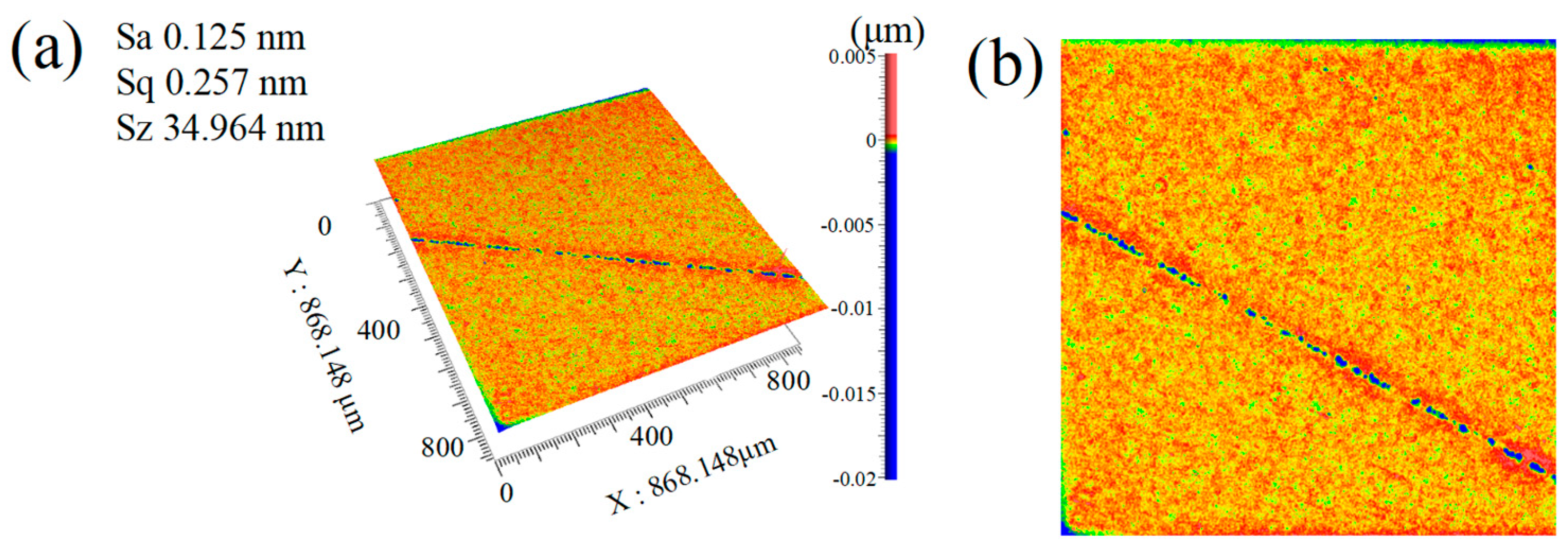
- The particle size of abrasive particles significantly influences the surface quality of SCD. Under identical polishing conditions, reducing the abrasive particle size from 500 nm (Group SL7) to 100 nm (Group SL8) optimizes the Sa from 0.154 nm to 0.112 nm. Although no order-of-magnitude change occurs in Sa, the surface quality exhibits fundamental differences: the surface of Group SL7 features periodic scratch-like networks and convex peaks with a scratch depth of 2 nm and peak height of 4 nm, whereas the scratch depth and peak height on the surface of Group SL8 workpieces are reduced by an order of magnitude, demonstrating that smaller abrasive particle sizes significantly minimize mechanical damage and enable a transition from macro-scale scratching to nanoscale surface finishing.
- (4)
- Based on experimental results, the AOA was employed to establish a diamond CMP surface roughness prediction model incorporating three variables, including catalyst type, oxidant concentration and abrasive particle size. The model demonstrates remarkable predictive accuracy, with the root mean square error between predicted and actual values controlled at approximately 0.06, a correlation coefficient reaching 0.998, and relative errors in model validation maintained within 3%, highlighting its high reliability for optimizing polishing process parameters and predicting surface roughness in SCD applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Lu, J.; Xiong, Q.; Wiong, X.; Yan, X. Investigation on influence of polishing disc materials in UV-catalytic polishing of single crystal diamond. Diam. Relat. Mater. 2024, 141, 110678. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, H.; Xiao, J.; Zhang, J.; Chen, X.; Zhu, J.; Chen, X.; Zhu, J.; Xu, J.; Y, K. Insights into the atomic-scale removal mechanism of single crystal diamond in plasma-assisted polishing with quartz glass. Tribol. Int. 2024, 194, 109507. [Google Scholar] [CrossRef]
- Watanabe, J.; Touge, M.; Sakamoto, T. Ultraviolet-irradiated precision polishing of diamond and its related materials. Diam. Relat. Mater. 2013, 39, 14–19. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Huang, Y.; Zhu, X.; Fu, W.; Li, C.; Miao, J. A diamond made microchannel heat sink for high-density heat flux dissipation. Appl. Therm. Eng. 2019, 158, 113804. [Google Scholar] [CrossRef]
- Xiao, C.; Hsia, F.; Sutton-Cook, A.; Webar, B.; Franklin, S. Polishing of polycrystalline diamond using synergies between chemical and mechanical inputs: A review of mechanisms and processes. Carbon 2022, 196, 29–48. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, J.; Yan, Q.; Cai, W.; Huang, W. Experimental study of chemical mechanical polishing of polycrystalline diamond based on photo-Fenton reaction. Mater. Sci. Semicond. Process. 2025, 186, 109072. [Google Scholar] [CrossRef]
- Liu, N.; Lei, L.; Lu, H.; Jiang, H.; Zhang, Y.; Xiao, J.; Zhang, J.; Chen, X.; Xu, J. C–C bond rupture initiated graphitization achieves highly efficient diamond polishing. Int. J. Mech. Sci. 2025, 287, 109958. [Google Scholar] [CrossRef]
- Bi, G.; Li, Y.; Lai, M.; Fang, F. Mechanism of polishing lutetium oxide single crystals with polyhedral diamond abrasive grains based on molecular dynamics simulation. Appl. Surf. Sci. 2023, 616, 156549. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, G.; Guan, S.; Mao, Y.; Yang, Z.; Long, X.; Zhao, L.; Qi, Q.; Tan, Z. A novel low-rotation-speed dynamic friction polishing of diamond. Diam. Relat. Mater. 2023, 136, 109932. [Google Scholar] [CrossRef]
- He, Y.; Zhou, G.; Tang, M.; Fan, L.; Gao, X.; Sun, J. Investigation on mechanism of mechanical activation and chemical reactions in CMP of diamond assisted by hydroxyl free radicals. Appl. Surf. Sci. 2025, 681, 161527. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Li, M.; Jin, Z.; Guo, D. An insight into polishing slurry for high quality and efficiency polishing of diamond. Tribol. Int. 2022, 174, 107789. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Cai, W.; Hu, D.; Yan, Q. Optimisation of chemically assisted mechanical polishing process parameters for polycrystalline diamond based on photo-Fenton reaction. Diam. Relat. Mater. 2024, 150, 111750. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Meng, F.; Liu, D.; Wu, B.; Li, X.; Xie, W. A novel slurry for chemical mechanical polishing of single crystal diamond. Appl. Surf. Sci. 2021, 564, 150431. [Google Scholar] [CrossRef]
- Liu, H.; Zong, W.; Cheng, X. Behaviors of carbon atoms induced by friction in mechanical polishing of diamond. Comput. Mater. Sci. 2021, 186, 110069. [Google Scholar] [CrossRef]
- Liu, N.; Sugimoto, K.; Yoshitaka, N.; Yamada, H.; Sun, R.; Arima, K.; Yamamura, K. Highly efficient finishing of large-sized single crystal diamond substrates by combining nanosecond pulsed laser trimming and plasma-assisted polishing. Ceram. Int. 2023, 49 Pt B, 19109–19123. [Google Scholar] [CrossRef]
- Sammer, T.; Shi, X.; Khan, M.Z.; Khan, M.; Matkovic, A.; Teichert, C.; Raith, J. Influence of broad ion beam polishing on the surface roughness of hydrated cement paste and its implications on microstructural analysis. Cem. Concr. Res. 2024, 182, 107555. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Mokuno, Y.; Shikata, S. Characterizations of etch pits formed on single crystal diamond surface using oxygen/hydrogen plasma surface treatment. Diam. Relat. Mater. 2016, 63, 43–46. [Google Scholar] [CrossRef]
- Kubota, A.; Nagae, S.; Motoyama, S. High-precision mechanical polishing method for diamond substrate using micron-sized diamond abrasive grains. Diam. Relat. Mater. 2020, 101, 107644. [Google Scholar] [CrossRef]
- Doronin, M.A.; Polyakov, S.N.; Kravchuk, K.S.; Molchanov, S.P.; Lomov, A.A.; Troschiev, S.Y.; Terentiev, S.A. Limits of single crystal diamond surface mechanical polishing. Diam. Relat. Mater. 2018, 87, 149–155. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, J.; Wu, L.; Yan, Q. Study on the material removal characteristics of single crystal diamond polished by infrared picosecond laser. Int. J. Refract. Met. Hard Mater. 2025, 132, 107275. [Google Scholar] [CrossRef]
- Zou, J.; Ye, S.; Sedov, V.; Leng, W.; Liu, P.; Pivovarov, P.; Chen, L.; Zhang, J.; Liu, J.; Li, C.; et al. Ultrafast planarization of large-scale polycrystalline diamond by microsecond laser processing: Investigation of polishing mechanisms and impact on the material. Appl. Surf. Sci. 2025, 713, 164282. [Google Scholar] [CrossRef]
- Mi, S.; Toros, A.; Graziosi, T.; Quack, N. Non-contact polishing of single crystal diamond by ion beam etching. Diam. Relat. Mater. 2019, 92, 248–252. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Chen, L.; Ma, C.; Zheng, X.; Shi, J. Orientation-dependent effects in single-crystal diamond during focused gallium ion beam processing. Diam. Relat. Mater. 2025, 158, 112631. [Google Scholar] [CrossRef]
- Guo, X.G.; Yuan, S.; Wang, X.L.; Jin, Z.J.; Kang, R.K. Atomistic mechanisms of chemical mechanical polishing of diamond (100) in aqueous H2O2/pure H2O: Molecular dynamics simulations using reactive force field (ReaxFF). Comput. Mater. Sci. 2019, 157, 99–106. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Huang, J.; Gou, Y.; Jin, Z.; Kang, R.; Guo, D. Insight into the mechanism of low friction and wear during the chemical mechanical polishing process of diamond: A reactive molecular dynamics simulation. Tribol. Int. 2020, 148, 106308. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Tsai, H.Y.; Wu, C.H.; Liu, P.Y.; Hsieh, C.H.; Chang, Y.Y. An oxidation enhanced mechanical polishing technique for CVD diamond films. Diam. Relat. Mater. 2005, 14, 622–625. [Google Scholar] [CrossRef]
- Tokuda, N.; Takeuchi, D.; Ri, S.; Umezawa, H.; Yamaba, K.; Okushi, H.; Yamasaki, S. Flattening of oxidized diamond (111) surfaces with H2SO4/H2O2 solutions. Diam. Relat. Mater. 2009, 18, 213–215. [Google Scholar] [CrossRef]
- Yuan, Z.; Zheng, P.; Wen, Q. Chemical kinetics mechanism for chemical mechanical polishing diamond and its related hard-inert materials. Int. J. Adv. Manuf. Technol. 2018, 95, 1715–1727. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, Z.; Feng, Y. A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing. Micromachines 2024, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jin, Z.; Zhang, Y.; Wen, Q. Chemical Mechanical Polishing Slurries for Chemically Vapor-Deposited Diamond Films. J. Manuf. Sci. Eng. 2013, 135, 041006. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Jin, Z.J.; Xue, H.M.; Shi, S.J. Oxidant for Chemical Mechanical Polishing of Single Crystal Diamond. Adv. Mater. Res. 2014, 1027, 80–83. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Lu, M.; Jin, Z.; Kang, R.; Guo, D. Diamond nanoscale surface processing and tribochemical wear mechanism. Diam. Relat. Mater. 2019, 94, 8–13. [Google Scholar] [CrossRef]
- Liao, L.; Luo, S.; Chang, X.; Li, S.; Shudin, D. Study on the mechanism of chemical mechanical polishing on high-quality surface of single crystal diamond. J. Manuf. Process. 2023, 105, 386–398. [Google Scholar] [CrossRef]
- Shi, D.; Zhou, W.; Zhao, T. Polishing of diamond, SiC, GaN based on the oxidation modification of hydroxyl radical: Status, challenges and strategies. Mater. Sci. Semicond. Process. 2023, 166, 107737. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Lin, M.; Chen, Z. Self-Fenton Cu-Mn catalysts for efficient ciprofloxacin removal: In-situ H2O2 generation and activation. J. Environ. Manag. 2025, 384, 125569. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y. Regulating pore size of Co-P and Fe-Co-P mesoporous nanosheet array for coupling hydrogen evolution with Fenton reaction. Sep. Purif. Technol. 2025, 365, 132569. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, Q.; Mo, C.; Ma, X.; Zhang, W.; Fan, Z.; Wang, Y.; Fan, G.; Zhang, L. Construction of Fe-Mn-La/Ce@biochar electrodes 3D/HEF systems for efficient electro-Fenton degradation of coking wastewater: Performance and mechanism insights. J. Environ. Manag. 2025, 386, 125779. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, J.; Zeng, J.; Wang, X.; Yan, Q. Experimental study on single crystal diamond CMP based on Fenton reaction and analysis of oxidation mechanism. Mater. Sci. Semicond. Process. 2024, 182, 108739. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, A.; Ma, X. Copper-incorporated dendritic mesoporous silica nanospheres and enhanced chemical mechanical polishing (CMP) performance via Cu2+/H2O2 heterogeneous Fenton-like system. Appl. Surf. Sci. 2022, 601, 154262. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Gong, C.; Liu, X.; Wang, K.; He, W.; Li, C.; Li, Z.; Li, L. Study of Fe/Cu composite Fenton-like catalyst in the treatment of antibiotic wastewater: Preparation, application, degradation pathways and working mechanism. J. Environ. Chem. Eng. 2025, 13, 115926. [Google Scholar] [CrossRef]
- Qin, C.; Hu, Z.; Tang, A.; Yang, Z.; Luo, S. An efficient material removal rate prediction model for cemented carbide inserts chemical mechanical polishing. Wear 2020, 452–453, 203293. [Google Scholar] [CrossRef]
- Ji, B.; Liu, H.; Cheng, P.; Ren, X.; Pi, H.; Li, L. Phased optimization of active distribution networks incorporating distributed photovoltaic storage system: A multi-objective coati optimization algorithm. J. Energy Storage 2024, 91, 112093. [Google Scholar] [CrossRef]
- Hashim, F.A.; Hussain, K.; Houssein, E.H.; Mabrouk, M.S.; Al-Atabany, W. Archimedes optimization algorithm: A new metaheuristic algorithm for solving optimization problems. Appl. Intell. 2021, 51, 1531–1551. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-Embedded p-Type Copper(I) Oxide Nanocrystals for Broad-Spectrum Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1501865. [Google Scholar] [CrossRef]
- Huang, W.; Li, H.; Lu, J.; Hu, D.; Lin, C.; Yan, Q. Experimental study on UV photocatalytic assisted chemical mechanical polishing process for single-crystal diamond. Diam. Relat. Mater. 2025, 159, 112771. [Google Scholar] [CrossRef]
- Yang, H.; Jin, Z.; Niu, H.; Shen, Y.; Zhang, X.; Ao, M. A novel visible-light catalyzed assisted single crystal diamond chemical mechanical polishing slurry and polishing mechanism. Mater. Today Commun. 2022, 33, 104249. [Google Scholar] [CrossRef]
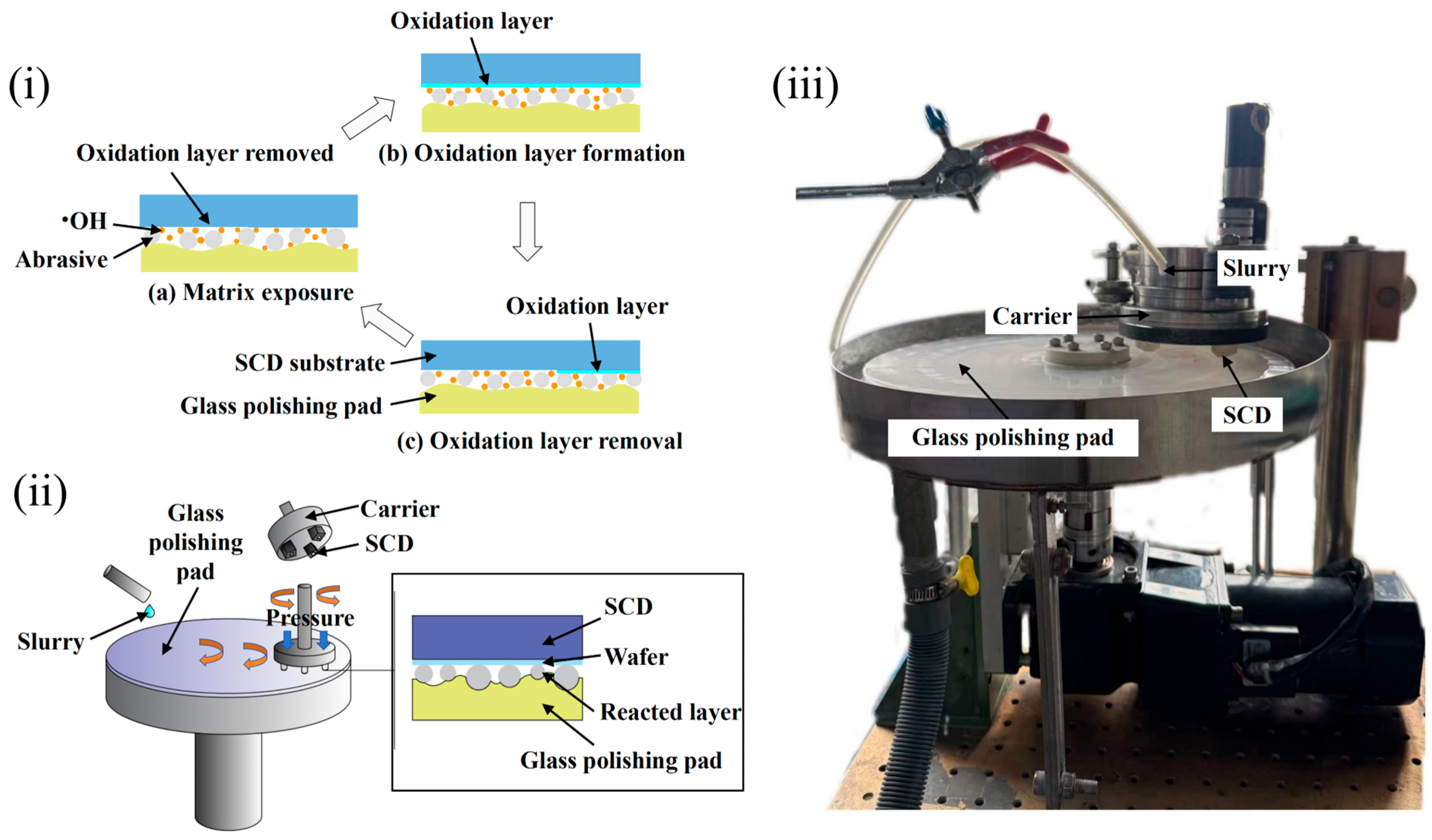
| Parameters | Types |
|---|---|
| Disc polishing speed/r/min | 60 |
| Diamond rotation speed/r/min | 20 |
| Pressure/MPa | 1.8 |
| Abrasive type | Al2O3 |
| Disc throwing type | Glass polishing pad |
| Abrasive concentration/wt.% | 5 |
| Slurry flow rate/mL/min | 10 |
| The size of SCD/mm3 | 3 × 3 × 1 |
| Group Number | H2O2 Concentration (%) | Abrasive Particle Size (nm) | Fe2(SO4)3 Concentration (mol/L) | CuSO4 Concentration (mol/L) | Final Roughness (nm) |
|---|---|---|---|---|---|
| SL1 | 30 | 500 | \ | \ | 1.3 |
| SL2 | 30 | 100 | \ | \ | 1.2 |
| SL3 | 30 | 500 | 0.2 | \ | 0.6 |
| SL4 | 30 | 500 | \ | 0.2 | 0.7 |
| SL5 | 30 | 100 | 0.2 | \ | 0.3 |
| SL6 | 30 | 100 | \ | 0.2 | 0.4 |
| SL7 | 30 | 500 | 0.2 | 0.2 | 0.15 |
| SL8 | 30 | 100 | 0.2 | 0.2 | 0.1 |
| SL9 | 15 | 500 | \ | \ | 1.8 |
| SL10 | 15 | 100 | \ | \ | 1.6 |
| SL11 | 15 | 500 | 0.2 | \ | 1.2 |
| SL12 | 15 | 500 | \ | 0.2 | 1.4 |
| SL13 | 15 | 100 | 0.2 | \ | 0.5 |
| SL14 | 15 | 100 | \ | 0.2 | 0.6 |
| SL15 | 15 | 500 | 0.2 | 0.2 | 0.3 |
| SL16 | 15 | 100 | 0.2 | 0.2 | 0.2 |
| Parameter | Type |
|---|---|
| Accavg | Average acceleration |
| Accit | The acceleration of the i-th |
| α | Density adjustment factor 1 |
| β | Density adjustment factor 2 |
| γ | Volume adjustment factor 1 |
| δ | Volume adjustment factor 2 |
| φ | Resistance adjustment factor 1 |
| ω | Resistance adjustment factor 2 |
| Xbest | Optimal surface roughness |
| ρbest | Optimal catalyst type |
| kbest | Optimal oxidant concentration |
| Vbest | Optimal abrasive particle size |
| ρ | Types of catalysts |
| V | Abrasive particle size |
| k | Oxidant concentration |
| X | Polishing quality |
| No. | Measured Value | Predictive Value | Relative Error |
|---|---|---|---|
| S1 | 1.466 | 1.502 | 2.46% |
| S2 | 3.936 | 3.853 | −2.11% |
| S3 | 0.302 | 0.308 | 1.98% |
| S4 | 0.125 | 0.128 | 2.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Guo, X.; Fan, G.; Deng, Y.; Kang, R.; Wang, X. Prediction Model and Experimental Verification of Surface Roughness of Single Crystal Diamond Chemical Mechanical Polishing Based on Archimedes Optimization Algorithm. Micromachines 2025, 16, 1121. https://doi.org/10.3390/mi16101121
Li Z, Guo X, Fan G, Deng Y, Kang R, Wang X. Prediction Model and Experimental Verification of Surface Roughness of Single Crystal Diamond Chemical Mechanical Polishing Based on Archimedes Optimization Algorithm. Micromachines. 2025; 16(10):1121. https://doi.org/10.3390/mi16101121
Chicago/Turabian StyleLi, Zhaoze, Xiaoguang Guo, Guanghui Fan, Yueming Deng, Renke Kang, and Xuefei Wang. 2025. "Prediction Model and Experimental Verification of Surface Roughness of Single Crystal Diamond Chemical Mechanical Polishing Based on Archimedes Optimization Algorithm" Micromachines 16, no. 10: 1121. https://doi.org/10.3390/mi16101121
APA StyleLi, Z., Guo, X., Fan, G., Deng, Y., Kang, R., & Wang, X. (2025). Prediction Model and Experimental Verification of Surface Roughness of Single Crystal Diamond Chemical Mechanical Polishing Based on Archimedes Optimization Algorithm. Micromachines, 16(10), 1121. https://doi.org/10.3390/mi16101121







