Design and Analysis of a Magnetic Anchored and Cable-Driven Surgical Forceps for Minimally Invasive Surgery
Abstract
1. Introduction
2. Design and Manufacturing
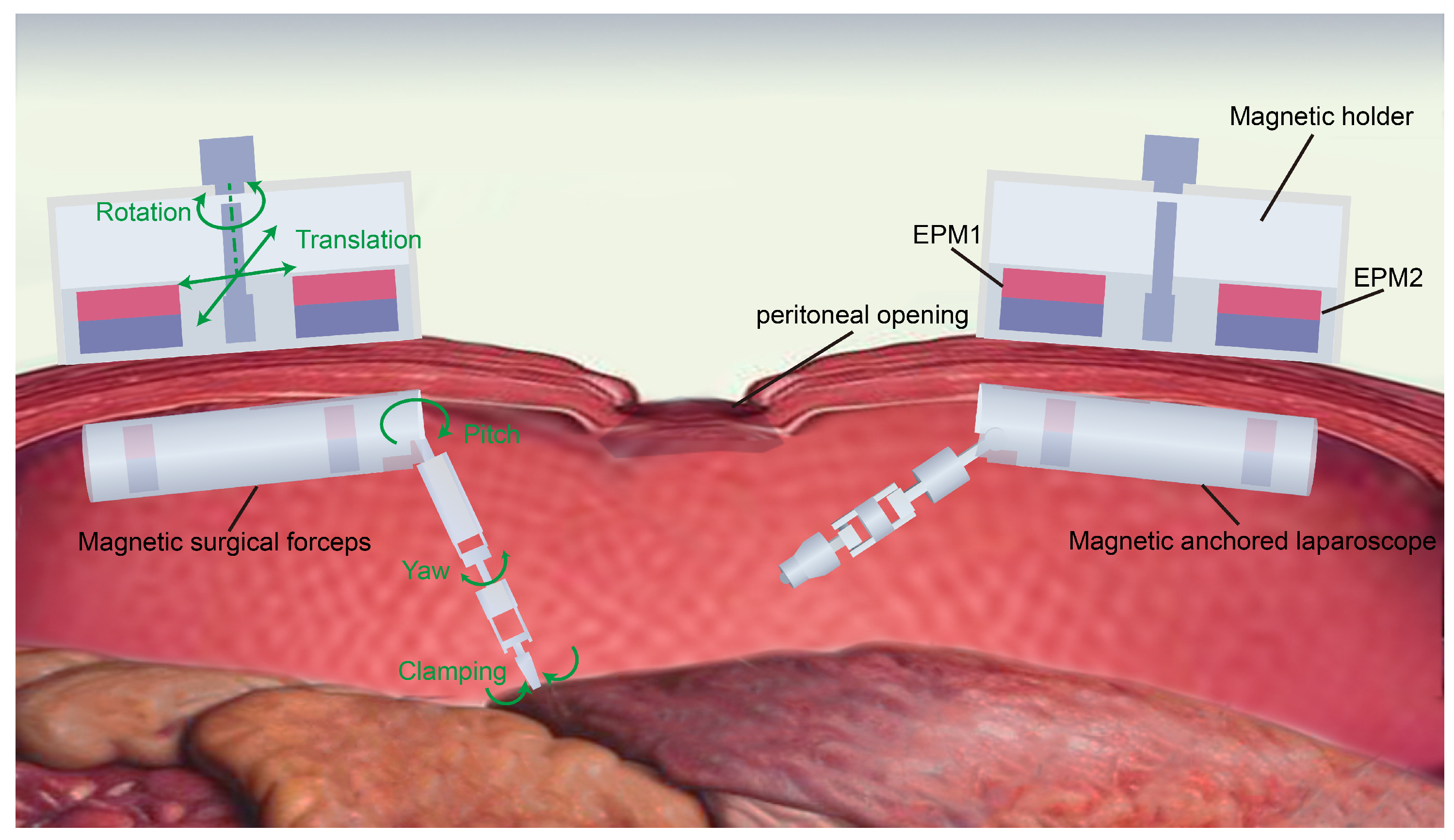
2.1. System Overview
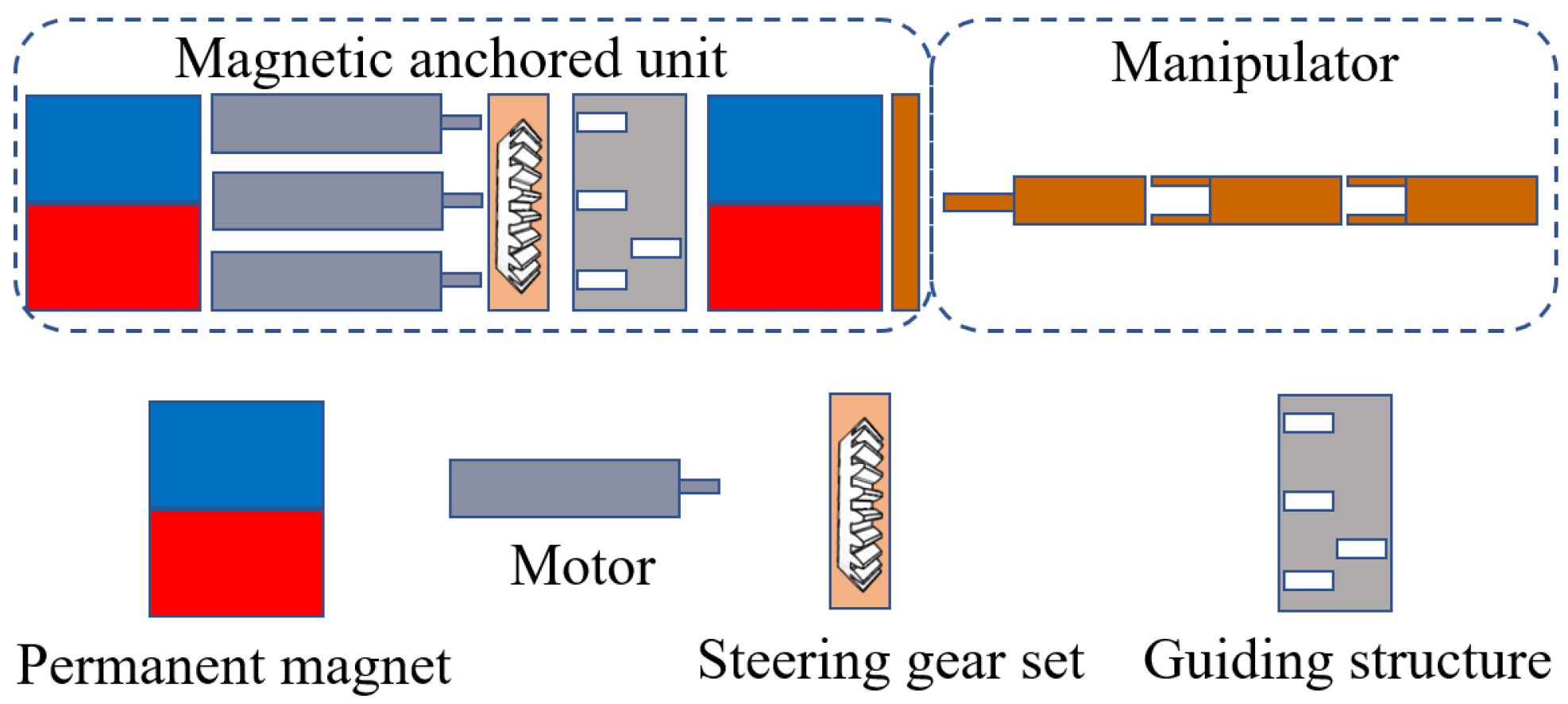
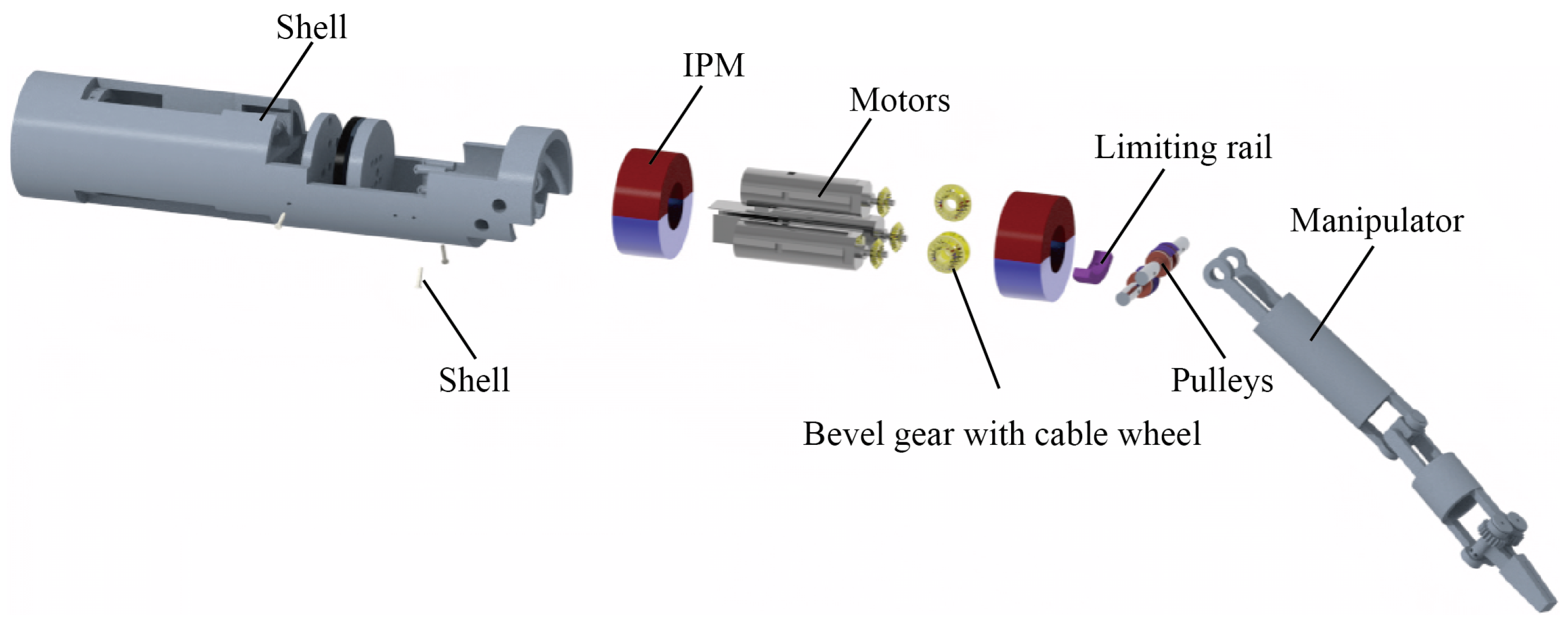
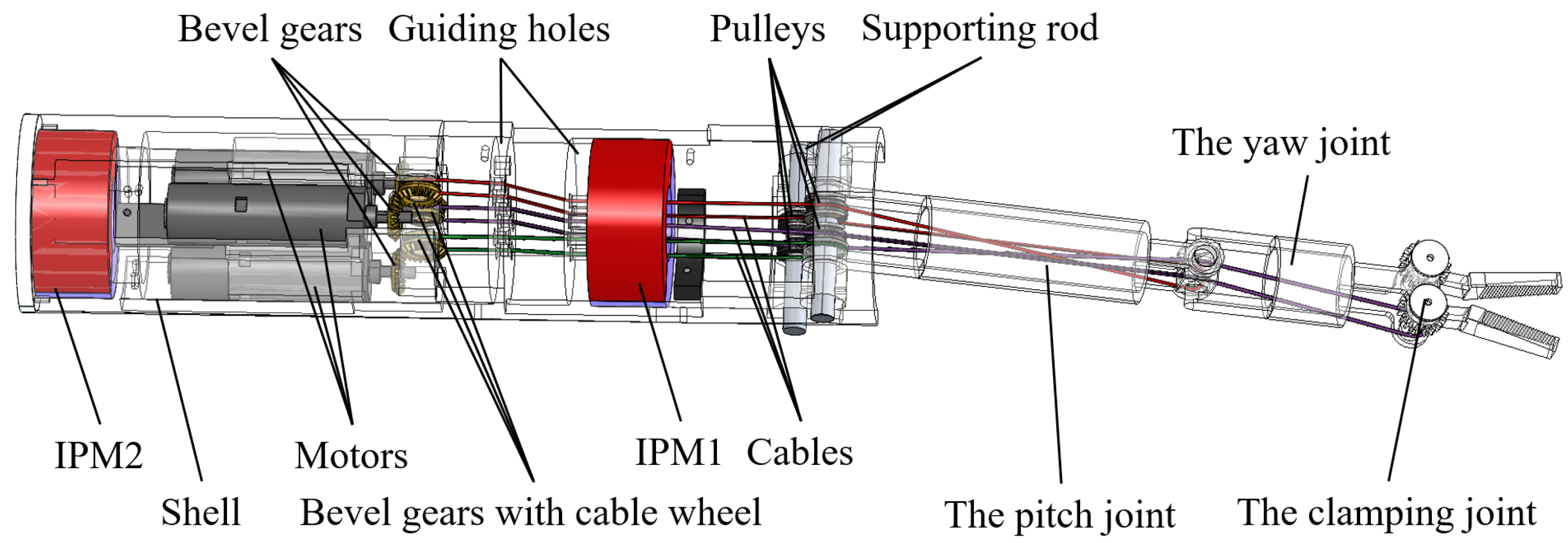
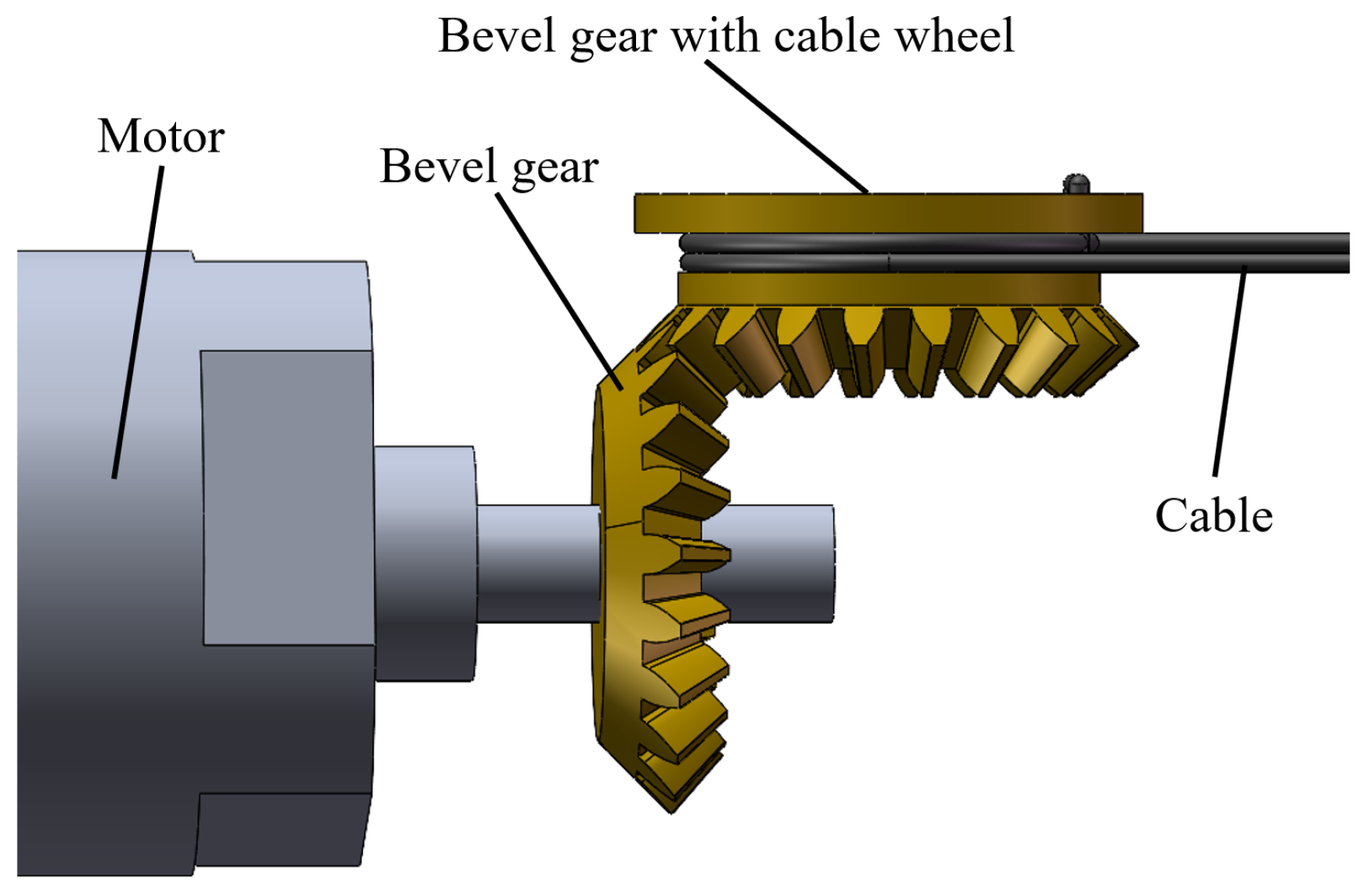
2.2. Structure of the Magnetic Anchored and Cable-Driven Surgical Forceps
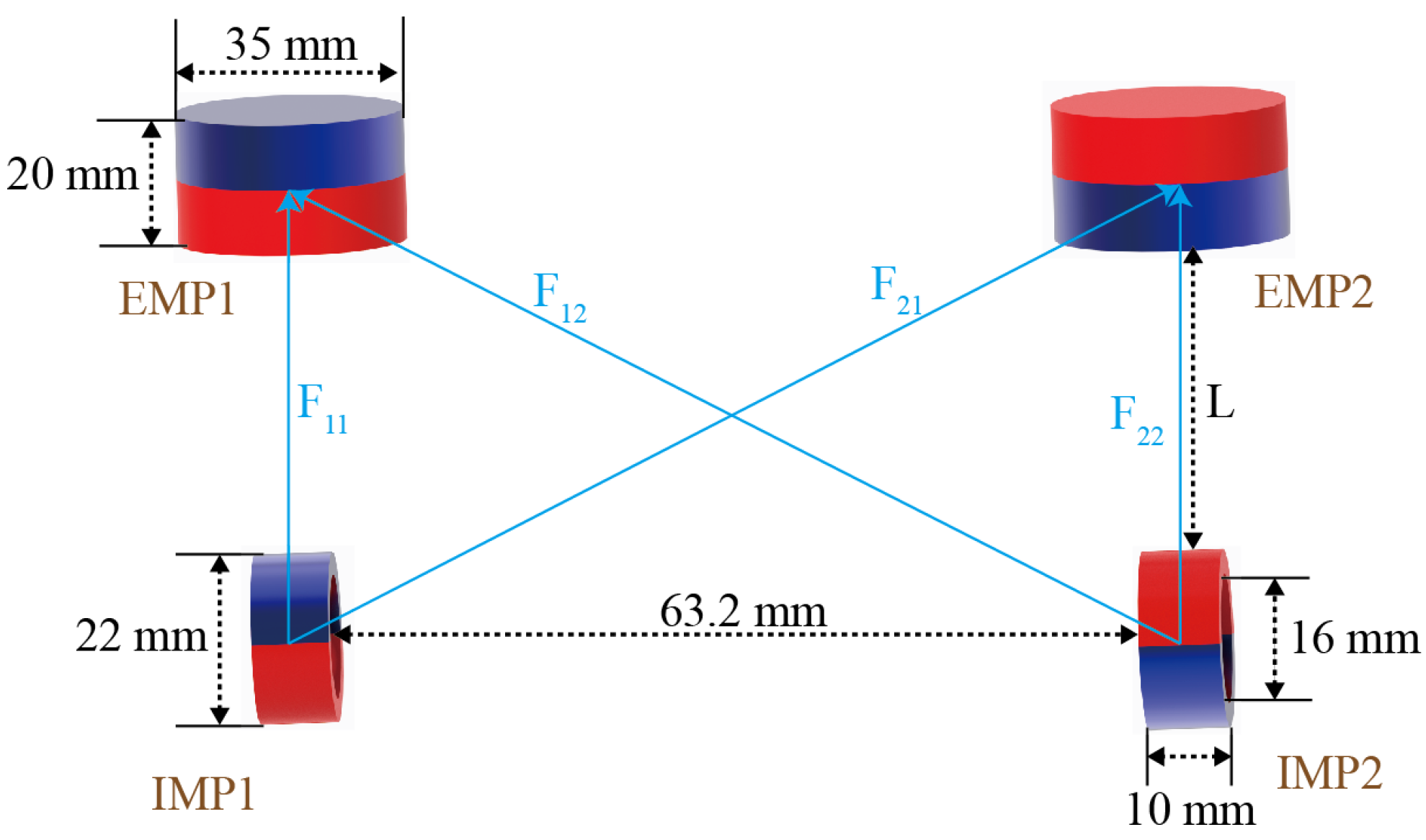
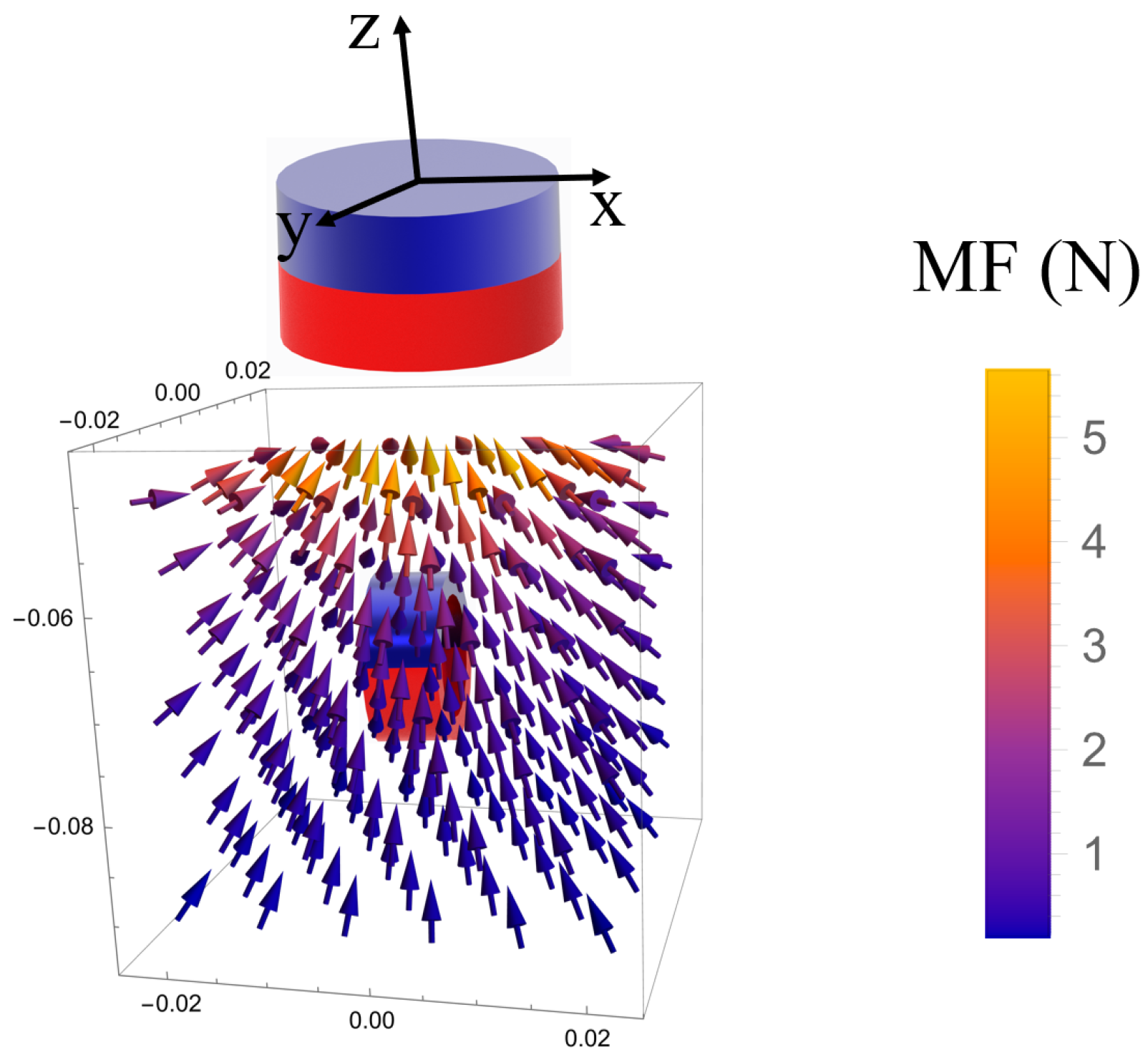
2.3. Magnet Design
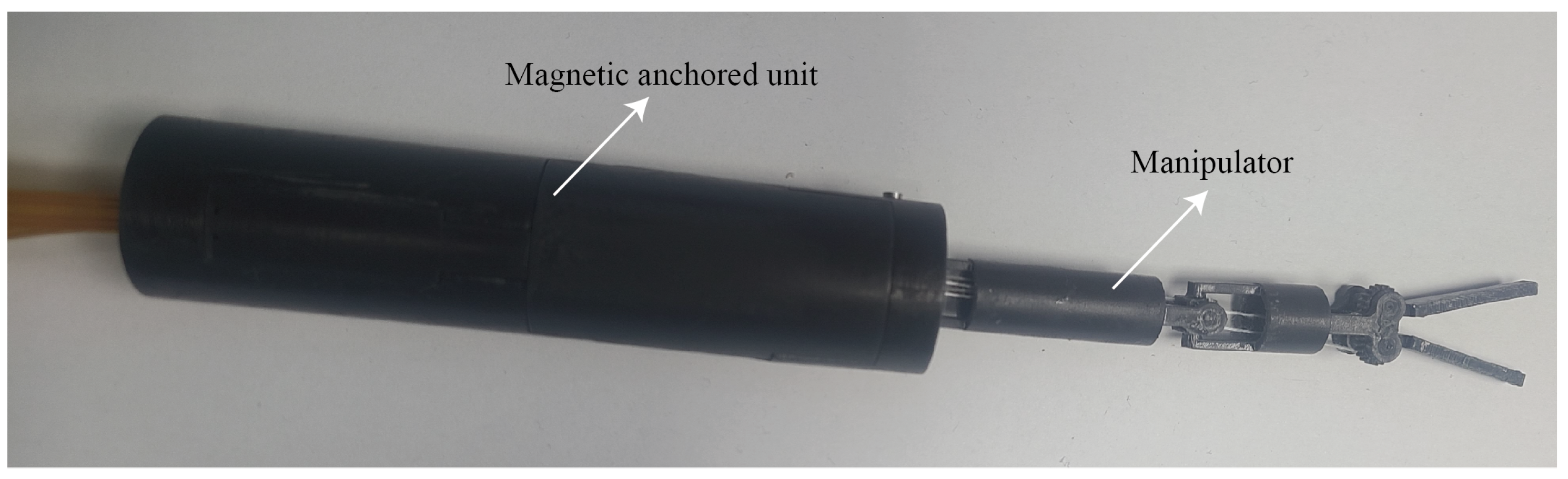
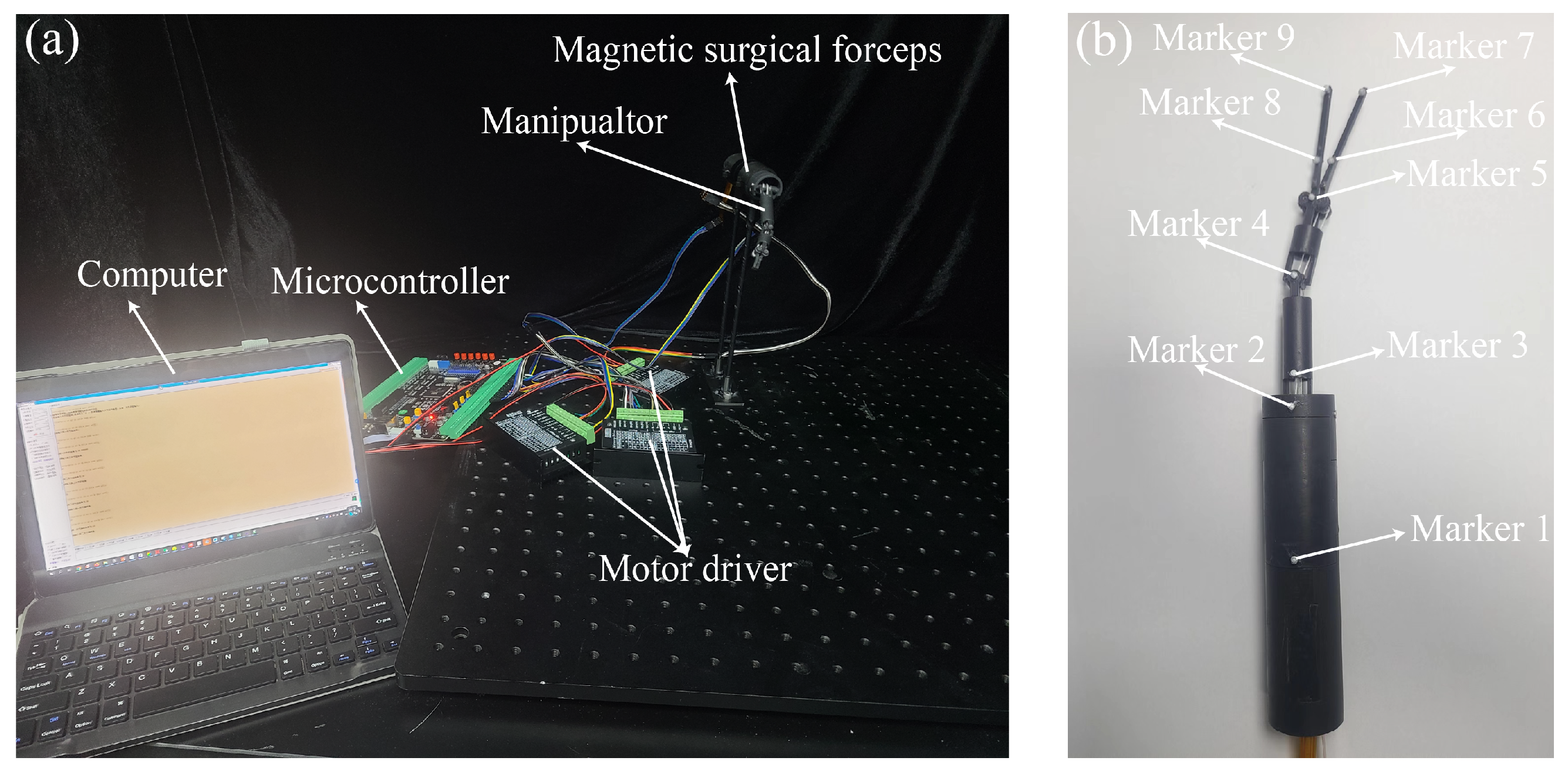
2.4. Prototype
3. Control Strategy of the Manipulator
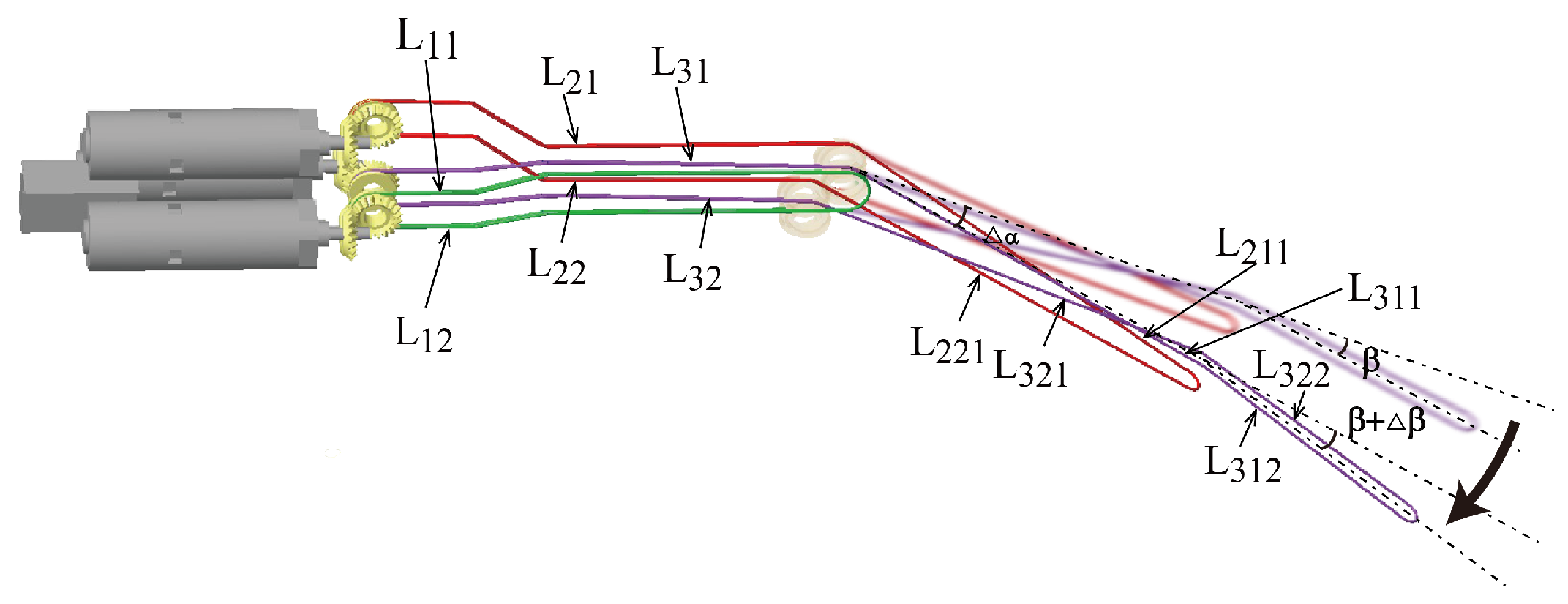
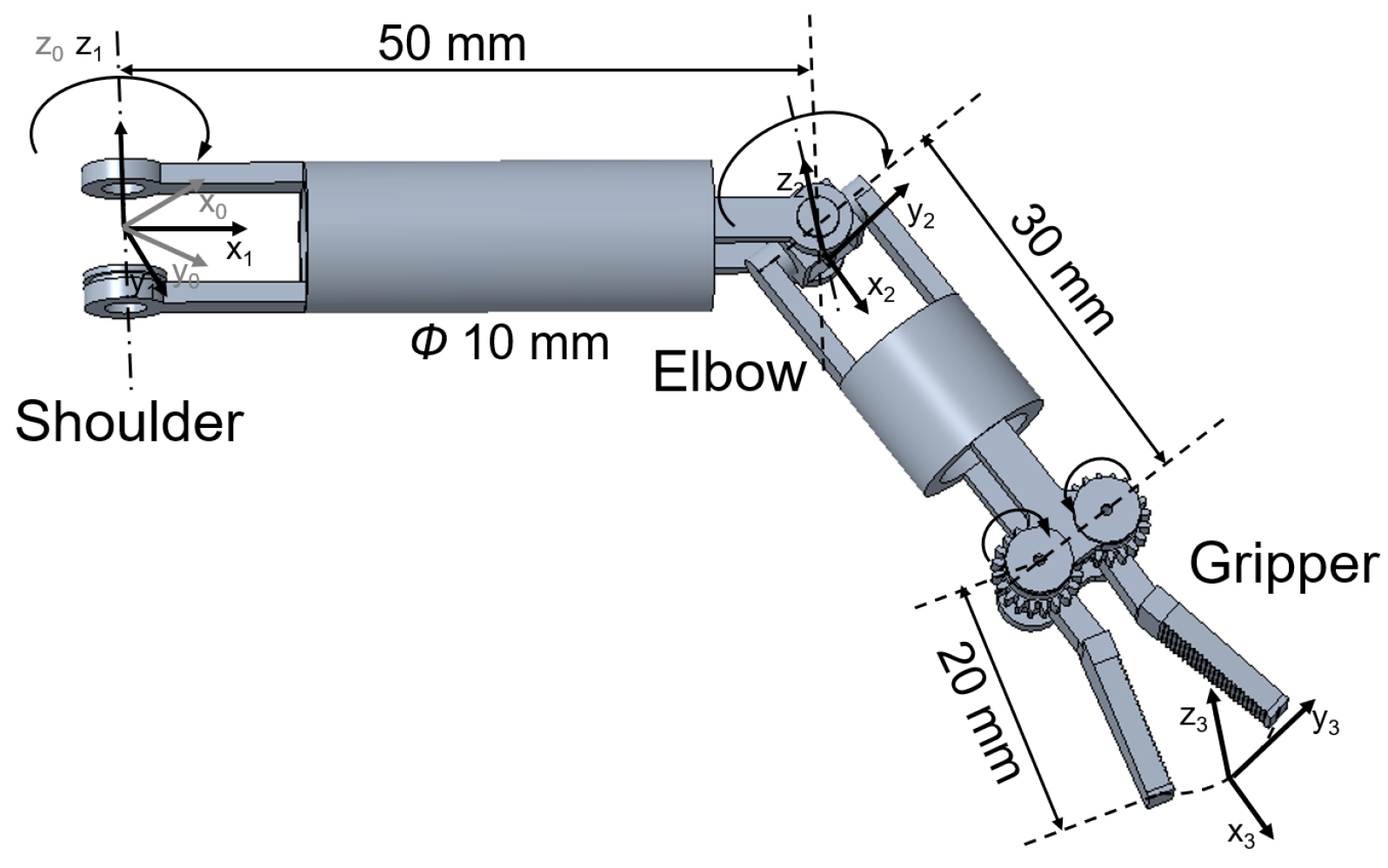
3.1. Motion of the Manipulator
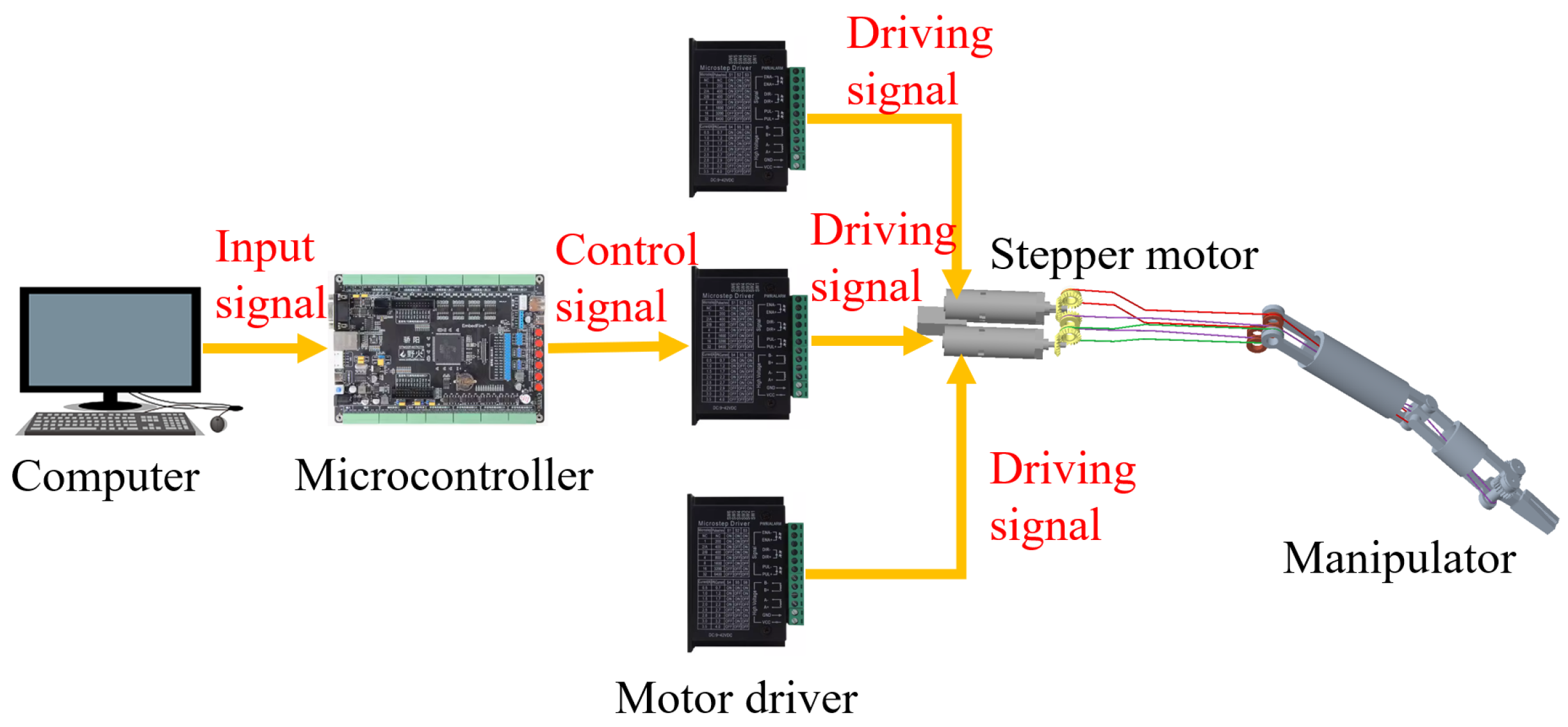
3.2. Control System of the Manipulator
4. Experimental Verification
4.1. Magnetic Attractive Force
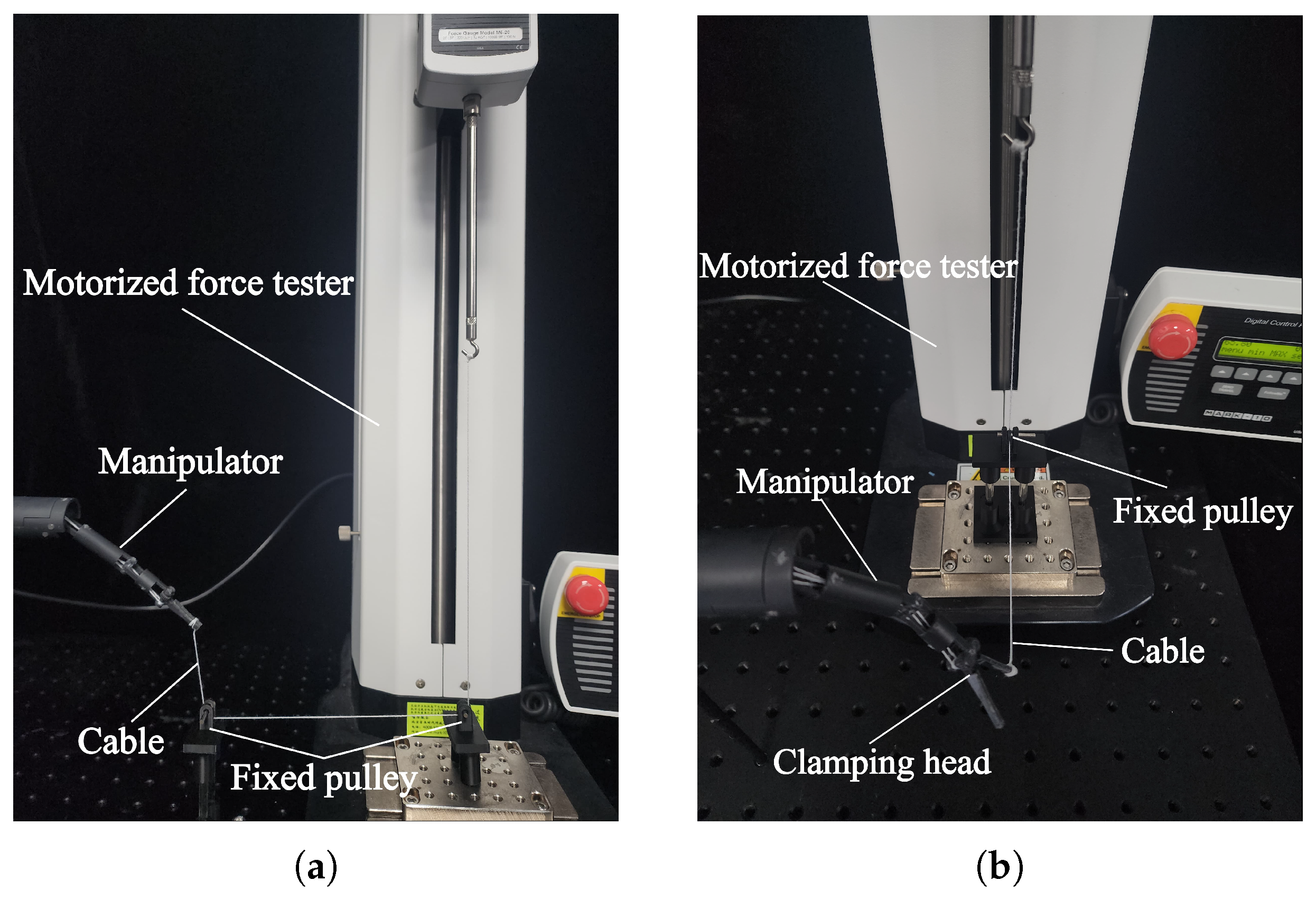
4.2. Load Capacity of the Manipulator
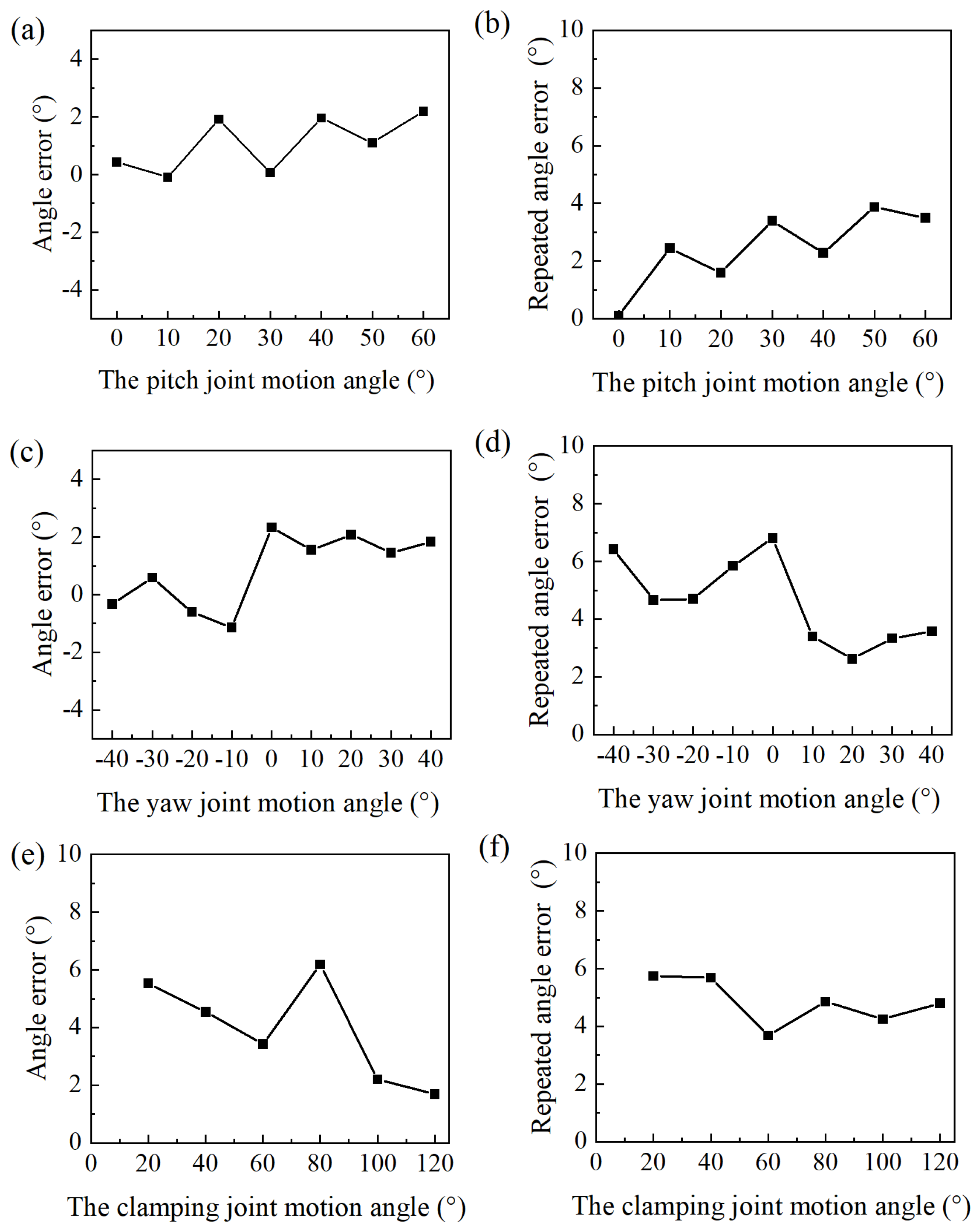
4.3. Control of the Manipulator
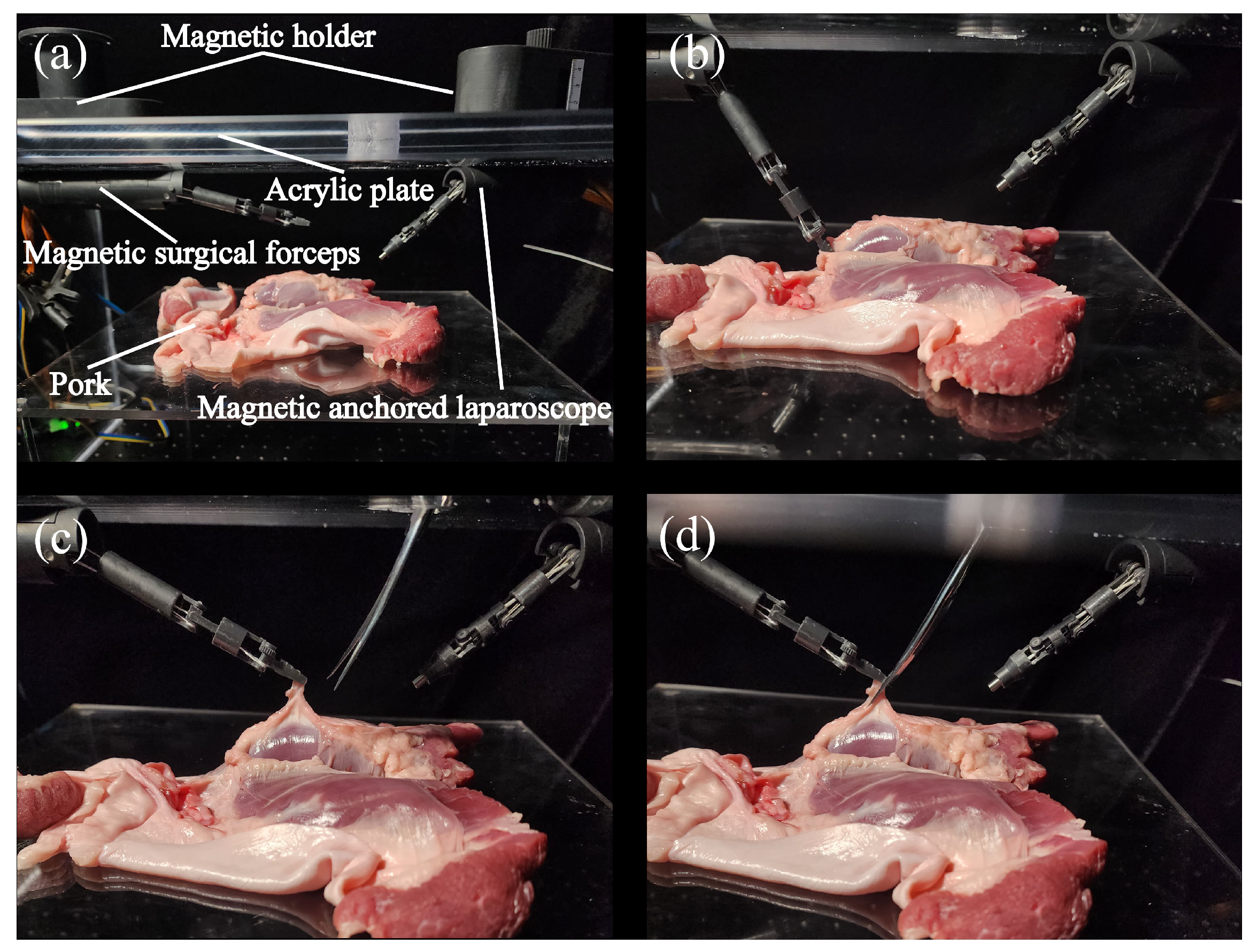
4.4. Ex Vivo Experiment
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Feng, B.; Zheng, M.H.; Xu, K. Surgical robots for SPL and NOTES: A review. Minim. Invasive Ther. Allied Technol. 2015, 24, 8–17. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wu, Z.; Yang, B.; Luo, Q.; Xu, K. Review of surgical robotic systems for keyhole and endoscopic procedures: State of the art and perspectives. Front. Med. 2020, 14, 382–403. [Google Scholar] [CrossRef]
- Leong, F.; Garbin, N.; Natali, C.D.; Mohammadi, A.; Thiruchelvam, D.; Oetomo, D.; Valdastri, P. Magnetic Surgical Instruments for Robotic Abdominal Surgery. IEEE Rev. Biomed. Eng. 2016, 9, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Gotohda, T.; Tamakawa, K.; Ueda, H.; Kakizoe, T. Magnetic anchor for more effective endoscopic mucosal resection. Jpn. J. Clin. Oncol. 2004, 34, 118–123. [Google Scholar] [CrossRef]
- Haidegger, T.; Speidel, S.; Stoyanov, D.; Satava, R.M. Robot-assisted minimally invasive surgery—Surgical robotics in the data age. Proc. IEEE 2022, 110, 835–846. [Google Scholar] [CrossRef]
- Cepolina, F.; Razzoli, R.P. An introductory review of robotically assisted surgical systems. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2409. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Cheng, T.; Li, Y.; Huang, Y.; Ng, C.S.H.; Chiu, P.W.Y.; Li, Z. Design, Analysis, and Kinematic Framework of a Long-Range Magnetic Anchored and Guided Endoscope. IEEE/ASME Trans. Mechatron. 2024, 29, 4228–4239. [Google Scholar] [CrossRef]
- Liu, H.; Li, N.; Li, S.; Mancini, G.J.; Tan, J. Design and Evaluation for a Soft Intra-abdominal Wireless Laparoscope. IEEE Trans. Med. Robot. Bionics 2024, 6, 940–950. [Google Scholar] [CrossRef]
- Cheng, T.; Li, Z. Magnetic actuated endoscope systems for surgical applications. In Magnetic Sensors and Actuators in Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 81–101. [Google Scholar]
- Garbin, N.; Slawinski, P.R.; Aiello, G.; Karraz, C.; Valdastri, P. Laparoscopic camera based on an orthogonal magnet arrangement. IEEE Robot. Autom. Lett. 2016, 1, 924–929. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, J.; Gai, J.; Zhang, Z.; Wang, H.; Zhang, Y.; Ren, Y.; Lyu, Y.; Yan, X. Magnetic anchor technique assisted laparoscopic cholecystectomy in swine. Sci. Rep. 2023, 13, 4864. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, M.; Shi, A.; Lin, Y.; Guo, K.; Geng, Z.; Zhang, D.; Ma, F.; Lyu, Y.; Yan, X. Magnetic anchor technique in laparoscopic cholecystectomy: A single-center, prospective, randomized controlled trial. Surg. Endosc. 2023, 37, 1005–1012. [Google Scholar] [CrossRef]
- Matsuzaki, I.; Hattori, M.; Yamauchi, H.; Goto, N.; Iwata, Y.; Yokoi, T.; Tsunemi, M.; Kobayashi, M.; Yamamura, T.; Miyahara, R. Magnetic anchor-guided endoscopic submucosal dissection for colorectal tumors (with video). Surg. Endosc. 2020, 34, 1012–1018. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, H.W.; Lee, S.Y.; Han, D.H.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Jeong, B.C. Laparoendoscopic single-site simple nephrectomy using a magnetic anchoring system in a porcine model. Investig. Clin. Urol. 2016, 57, 208–214. [Google Scholar] [CrossRef]
- Romero-Velez, G.; Robles, I.; Jiménez, J.; Cabrera, C.; Luengas, R.; Portenier, D.; Kroh, M. Robotic Magnetic Surgery: Results From the First Prospective Clinical Trial. Ann. Surg. Open 2022, 3, e225. [Google Scholar] [CrossRef] [PubMed]
- Rivas, H.; Robles, I.; Riquelme, F.; Vivanco, M.; Jiménez, J.; Marinkovic, B.; Uribe, M. Magnetic surgery: Results from first prospective clinical trial in 50 patients. Ann. Surg. 2018, 267, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Samsonas, D.; Leong, F.; Tan, Y.; Thiruchelvam, D.; Valdastri, P.; Oetomo, D. Modeling and control of local electromagnetic actuation for robotic-assisted surgical devices. IEEE/ASME Trans. Mechatron. 2017, 22, 2449–2460. [Google Scholar] [CrossRef]
- Garbin, N.; Di Natali, C.; Buzzi, J.; De Momi, E.; Valdastri, P. Laparoscopic tissue retractor based on local magnetic actuation. J. Med. Devices 2015, 9, 011005. [Google Scholar] [CrossRef]
- Karimi, M.; Ghidary, S.S.; Shekhar, R.; Kane, T.D.; Monfaredi, R. Magnetically anchored pan-tilt stereoscopic robot with optical-inertial stabilization for minimally invasive surgery. In Proceedings of the Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 17–19 February 2019. [Google Scholar]
- Feng, H.; Dong, D.; Ma, T.; Zhuang, J.; Fu, Y.; Yi, L.; Li, L. Development of an in vivo visual robot system with a magnetic anchoring mechanism and a lens cleaning mechanism for laparoendoscopic single-site surgery (LESS). Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13. [Google Scholar] [CrossRef]
- Huda, M.N.; Yu, H.; Cang, S. Robots for minimally invasive diagnosis and intervention. Robot. Comput.-Integr. Manuf. 2016, 41, 127–144. [Google Scholar] [CrossRef]
- Tortora, G.; Salerno, M.; Ranzani, T.; Tognarelli, S.; Menciassi, A. A modular magnetic platform for natural orifice transluminal endoscopic surgery. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Platt, S.R.; Hawks, J.A.; Rentschler, M.E. Vision and Task Assistance Using Modular Wireless In Vivo Surgical Robots. IEEE Trans. Bio-Med. Eng. 2009, 56, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Simaan, N.; Yasin, R.M.; Wang, L. Medical Technologies and Challenges of Robot-Assisted Minimally Invasive Intervention and Diagnostics. Annu. Rev. Control Robot. Auton. Syst. 2018, 1, 465–490. [Google Scholar] [CrossRef]
- Hong, W.; Schmitz, A.; Bai, W.; Berthet-Rayne, P.; Xie, L.; Yang, G.Z. Design and Compensation Control of a Flexible Instrument for Endoscopic Surgery. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020. [Google Scholar]
- Lens, T.; Stryk, O.V. Investigation of safety in human-robot-interaction for a series elastic, tendon-driven robot arm. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012. [Google Scholar]
- Li, J.; Zhang, T.; Cheng, T.; Li, Y.; Ng, C.S.H.; Chiu, P.W.Y.; Li, Z. Design, Analysis, and Preliminary Validation of Magnetic Anchored and Cable Driven Endoscope for Minimally Invasive Surgery. IEEE Trans. Med. Robot. Bionics 2020, 6, 1397–1400. [Google Scholar] [CrossRef]
- Rivas-Blanco, I.; Lopez-Casado, C.; Perez-Del-Pulgar, C.J.; Garcia-Vacas, F.; Fraile, J.C.; Munoz, V.F. Smart Cable-Driven Camera Robotic Assistant. IEEE Trans. Hum.-Mach. Syst. 2017, 48, 183–196. [Google Scholar] [CrossRef]
- Liu, X.; Abdolmalaki, R.Y.; Zuo, T.; Guan, Y.; Mancini, G.J.; Tan, J. Transformable In Vivo Robotic Laparoscopic Camera with Optimized Illumination System for Single-Port Access Surgery: Initial Prototype. IEEE/ASME Trans. Mechatron. 2018, 23, 1585–1596. [Google Scholar] [CrossRef]
- Feng, H.; Zhai, Y.; Fu, Y. Development of master-slave magnetic anchoring vision robotic system for single-port laparoscopy (SPL) surgery. Ind. Robot. Int. J. 2018, 45, 458–468. [Google Scholar] [CrossRef]
- Feng, H.; Lu, Y.; Chen, D.; Ma, T.; Fu, Y. Development on a magnetic anchoring robot system based on visual servo control for laparoendoscopic single-site surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1904. [Google Scholar] [CrossRef]
- Tortora, G.; Dimitracopoulos, A.; Valdastri, P.; Menciassi, A.; Dario, P. Design of miniature modular in vivo robots for dedicated tasks in minimally invasive surgery. In Proceedings of the 2011 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Budapest, Hungary, 3–7 July 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 327–332. [Google Scholar]
- Tortora, G.; Dario, P.; Menciassi, A. Array of robots augmenting the kinematics of endocavitary surgery. IEEE/ASME Trans. Mechatron. 2014, 19, 1821–1829. [Google Scholar] [CrossRef]
- Lehman, A.C.; Wood, N.A.; Farritor, S.; Goede, M.R.; Oleynikov, D. Dexterous miniature robot for advanced minimally invasive surgery. Surg. Endosc. 2011, 25, 119–123. [Google Scholar] [CrossRef]
- Li, J.; Sun, Z.; Sun, Z.; Gao, X.; Cao, C.; Li, Y. A Magnetic Force Calculation of Permanent Magnet for Magnetic Surgical Instruments. In Proceedings of the 2023 IEEE International Conference on Robotics and Biomimetics (ROBIO), Koh Samui, Thailand, 4–9 December 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–6. [Google Scholar]
- Song, C.; Alijani, A.; Frank, T.; Hanna, G.B.; Cuschieri, A. Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg. Endosc. Other Interv. Tech. 2006, 20, 987–990. [Google Scholar] [CrossRef]
- Li, W.; Cheng, T.; Ye, M.; Ng, C.S.H.; Li, Z. Kinematic Modeling and Visual Servo Control of a Soft-Bodied Magnetic Anchored and Guided Endoscope. IEEE/ASME Trans. Mechatron. 2020, 25, 1531–1542. [Google Scholar] [CrossRef]
- Leong, F.; Mohammadi, A.; Tan, Y.; Thiruchelvam, D.; Lai, C.Y.; Valdastri, P.; Oetomo, D. Disturbance Rejection in Multi-DOF Local Magnetic Actuation for the Robotic Abdominal Surgery. IEEE Robot. Autom. Lett. 2018, 3, 1568–1575. [Google Scholar] [CrossRef]
- Scaglioni, B.; Fornarelli, N.; Garbin, N.; Menciassi, A.; Valdastri, P. Independent control of multiple degrees of freedom local magnetic actuators with magnetic cross-coupling compensation. IEEE Robot. Autom. Lett. 2018, 3, 3622–3629. [Google Scholar] [CrossRef]
- Di Natali, C.; Buzzi, J.; Garbin, N.; Beccani, M.; Valdastri, P. Closed-loop control of local magnetic actuation for robotic surgical instruments. IEEE Trans. Robot. 2015, 31, 143–156. [Google Scholar] [CrossRef]
- Tognarelli, S.; Salerno, M.; Tortora, G.; Quaglia, C.; Dario, P.; Menciassi, A. An endoluminal robotic platform for Minimally Invasive Surgery. In Proceedings of the 2012 4th IEEE RAS &EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 7–12. [Google Scholar]
- Lehman, A.C.; Dumpert, J.; Wood, N.A.; Redden, L.; Visty, A.Q.; Farritor, S.; Varnell, B.; Oleynikov, D. Natural orifice cholecystectomy using a miniature robot. Surg. Endosc. 2009, 23, 260–266. [Google Scholar] [CrossRef]
- Singh, S.; Cheung, J.L.; Sreedhar, B.; Dai Hoa, X.; Ng, H.P.; Yeung, C.K. A novel robotic platform for single-port abdominal surgery. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 320, p. 012008. [Google Scholar]
- Rodriguez-Cianca, D.; Verstraten, T.; Rodriguez-Guerrero, C.; Jimenez-Fabian, R.; Naef, M.; Vanderborght, B.; Lefeber, D. The two-degree-of-freedom cable pulley (2DCP) transmission system: An under-actuated and motion decoupled transmission for robotic applications. Mech. Mach. Theory 2020, 148, 103765. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, T.; Chen, J.; Xu, W.; Wei, H.; Song, Y.; Guan, Y. A modular cable-driven humanoid arm with anti-parallelogram mechanisms and Bowden cables. Front. Mech. Eng. 2023, 18, 6. [Google Scholar] [CrossRef]
- Liu, F.; Xu, W.; Huang, H.; Ning, Y.; Li, B. Design and Analysis of a High-Payload Manipulator Based on Cable-Driven Serial-Parallel Mechanism. J. Mech. Robot. 2019, 11, 1. [Google Scholar] [CrossRef]
- Jiang, S.; Hua, D.; Wang, Y.; Ju, F.; Yin, L.; Chen, B. Design and modeling of motion-decoupling mechanism for cable-driven joints. Adv. Mech. Eng. 2018, 10, 1687814018777428. [Google Scholar] [CrossRef]



| Surgical Instrument | Magnetic Anchored Unit | Manipulator | Motor Diameter (mm) | DoF of Manipulator | ||
|---|---|---|---|---|---|---|
| Length (mm) | Diameter (mm) | Length (mm) | Diameter (mm) | |||
| Feng [31] | 110 | 20 | 6 | 3 | ||
| Tortora [33] | 186 | 17 | 95 | 12 | 4 | 4 |
| Oleynikov [34] | 80 | 26 | 53 | 26 | 6 | 4 |
| Valdastri [18] | 154 | 12.5 | No motor | 1 | ||
| This paper | 110 | 25 | 100 | 10 | 8 | 3 |
| Joint | (mm) | (mm) | ||
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0∼70 |
| 2 | 90 | 50 | 0 | −40∼40 |
| 3 | 0 | 50 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Y.; Sun, Z.; Sun, Z. Design and Analysis of a Magnetic Anchored and Cable-Driven Surgical Forceps for Minimally Invasive Surgery. Micromachines 2025, 16, 1109. https://doi.org/10.3390/mi16101109
Li J, Li Y, Sun Z, Sun Z. Design and Analysis of a Magnetic Anchored and Cable-Driven Surgical Forceps for Minimally Invasive Surgery. Micromachines. 2025; 16(10):1109. https://doi.org/10.3390/mi16101109
Chicago/Turabian StyleLi, Jingwu, Yingtian Li, Zhongqing Sun, and Zhijun Sun. 2025. "Design and Analysis of a Magnetic Anchored and Cable-Driven Surgical Forceps for Minimally Invasive Surgery" Micromachines 16, no. 10: 1109. https://doi.org/10.3390/mi16101109
APA StyleLi, J., Li, Y., Sun, Z., & Sun, Z. (2025). Design and Analysis of a Magnetic Anchored and Cable-Driven Surgical Forceps for Minimally Invasive Surgery. Micromachines, 16(10), 1109. https://doi.org/10.3390/mi16101109






