Harnessing Microfluidics for the Effective and Precise Synthesis of Advanced Materials
Abstract
1. Introduction
2. Fundamental Microfluidic Platforms for Synthesis
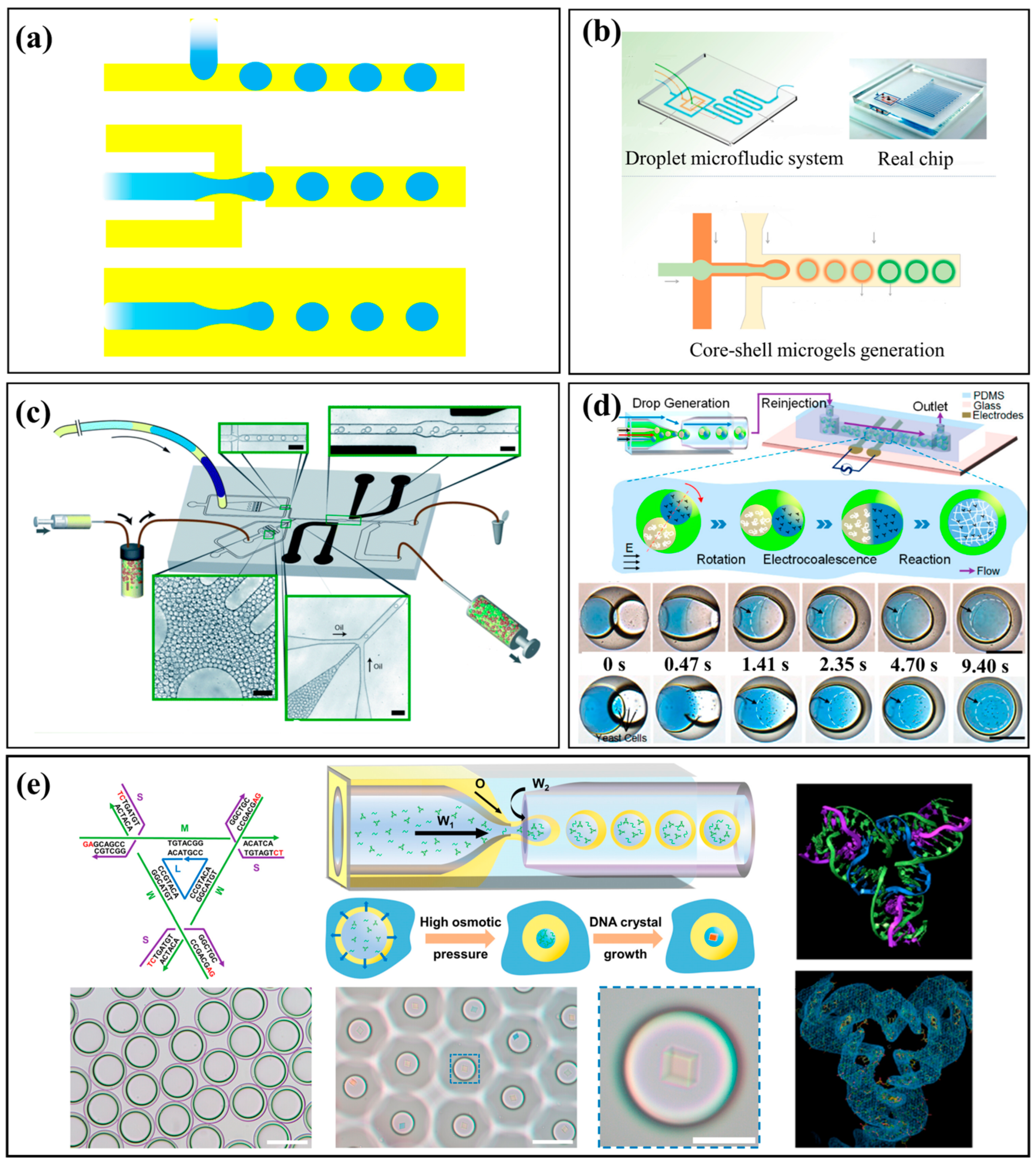
2.1. Droplet-Based Microfluidics: Monodisperse Microreactors
2.2. Continuous-Flow Microfluidics: High-Throughput Synthesis Streams
2.3. Microfluidic Synthesis Enabled by External Fields
3. Microfluidic Synthesis of Advanced Materials
3.1. Polymeric and Hydrogel Microparticles
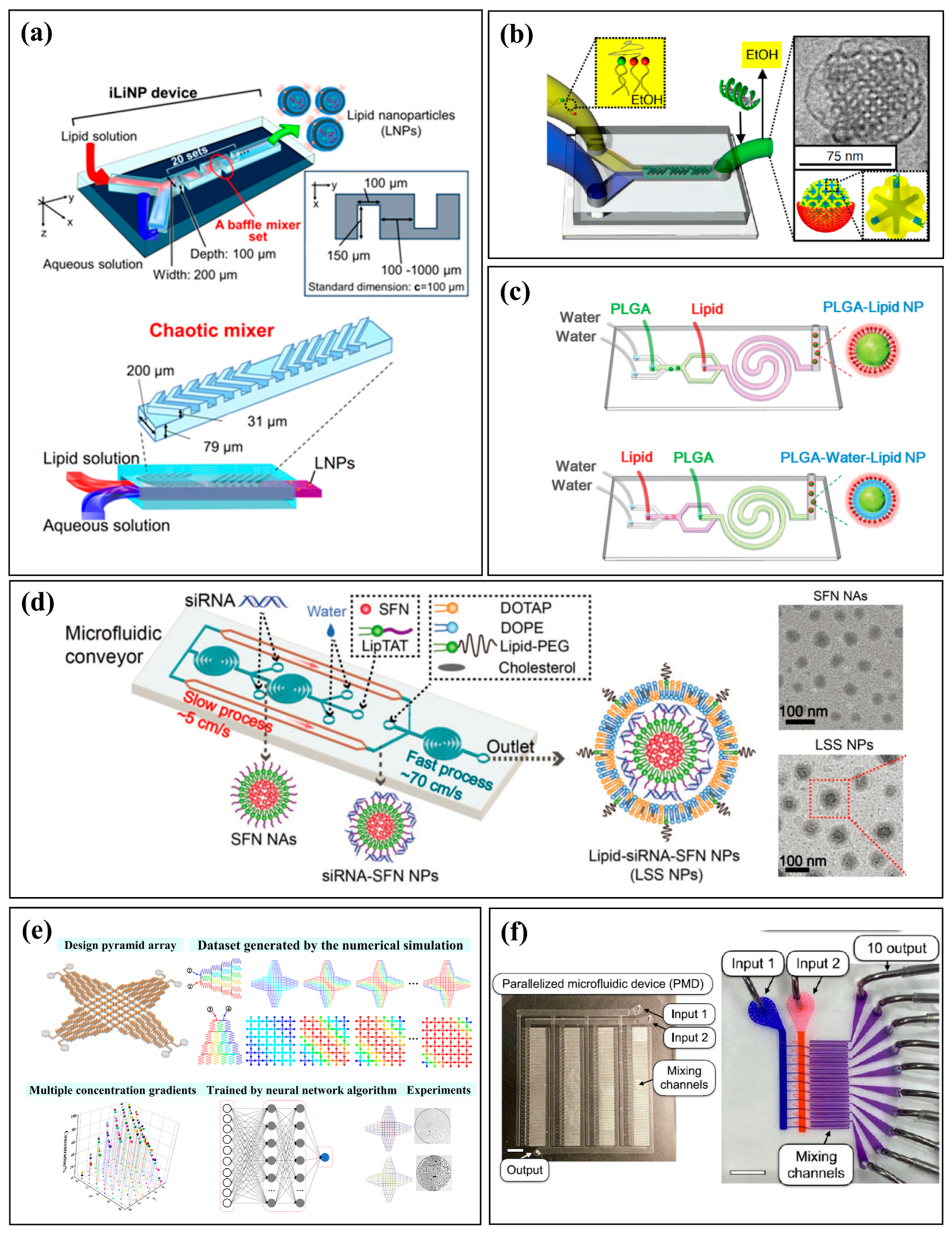
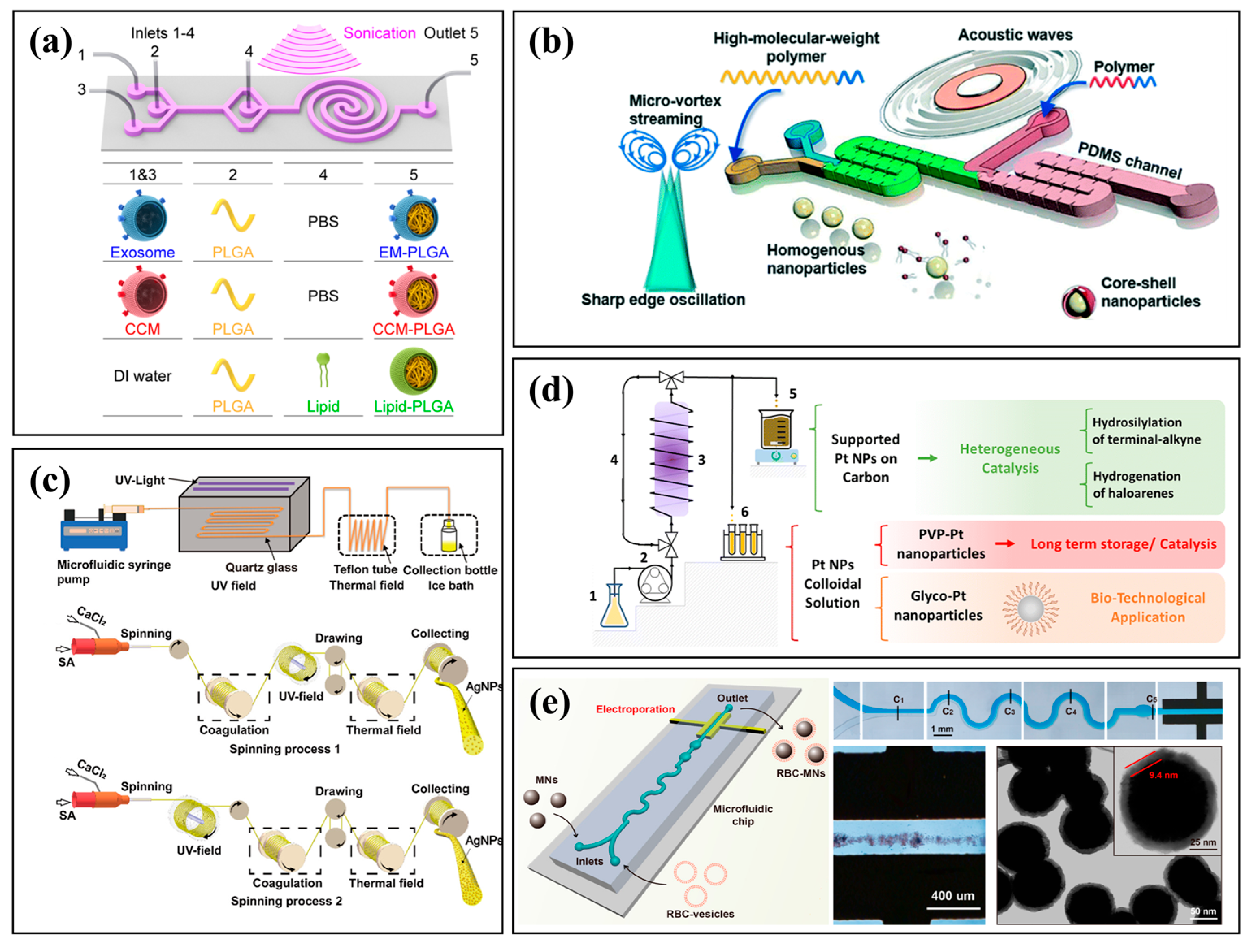
3.2. Nanoparticles: Mastering Nucleation and Growth
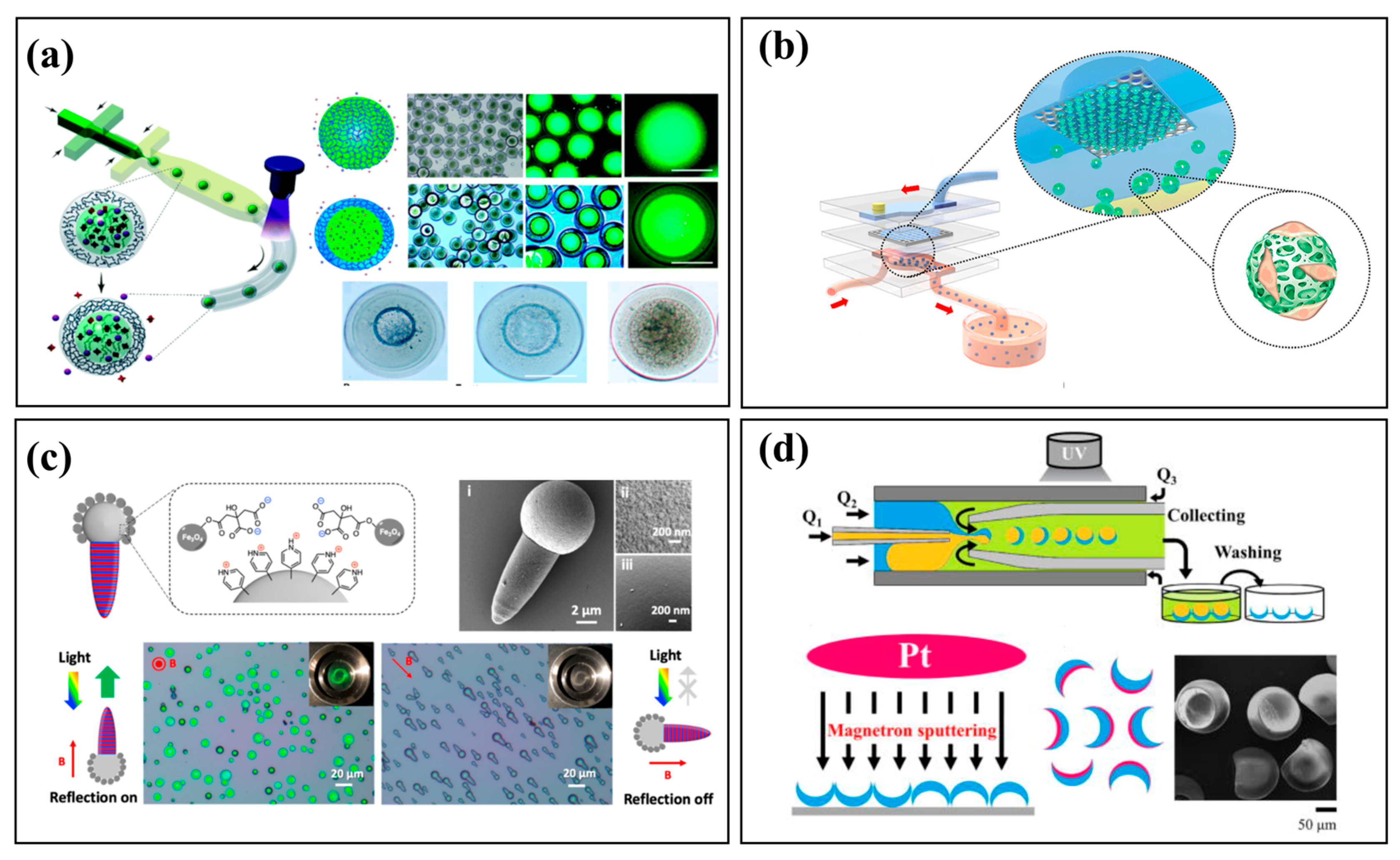
3.3. Functionalized Materials and Hierarchical Structures
4. On-Chip Manipulation and Integration
4.1. Post-Synthesis Sorting and Purification
4.2. Towards Integrated “Synthesis-to-Analysis” Platforms
5. Challenges and Future Outlook
5.1. Overcoming Current Hurdles
5.2. The Future of Microfluidic Synthesis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Xu, S.; Miao, Y.; Huang, K.; Wang, B.; Ding, B.; Zhang, Z.; Zhao, Z.; Zhang, X.; Shi, X. Curvature-mediated rapid extravasation and penetration of nanoparticles against interstitial fluid pressure for improved drug delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e2319880121. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, H.; Cai, R. Microfluidics for Nanomedicine Delivery. ACS Biomater. Sci. Eng. 2025, 11, 774–783. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.; Xiao, F.; Li, X.; Li, J.a.; Su, Z.; Jiang, X. Microfluidics-enabled Serial Assembly of Lipid-siRNA-sorafenib Nanoparticles for Synergetic Hepatocellular Carcinoma Therapy. Adv. Mater. 2023, 35, 2209672. [Google Scholar] [CrossRef]
- Creamer, A.; Fiego, A.L.; Agliano, A.; Prados-Martin, L.; Høgset, H.; Najer, A.; Richards, D.A.; Wojciechowski, J.P.; Foote, J.E.; Kim, N. Modular synthesis of semiconducting graft copolymers to achieve “clickable” fluorescent nanoparticles with long circulation and specific cancer targeting. Adv. Mater. 2024, 36, 2300413. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Becerril, L.M.; Vanzan, M.; Corni, S.; Cattelan, M.; Granozzi, G.; Frasconi, M.; Rajak, P.; Banerjee, P.; Ciancio, R. The interaction of amines with gold nanoparticles. Adv. Mater. 2024, 36, 2211624. [Google Scholar] [CrossRef]
- Xie, B.; Fu, Y.; Wang, Z.; Li, Y.; Zhu, Q.; Zhang, L.; Yang, W.; Kuhn, A. One-Pot Single-Step Approach for the Controlled Synthesis of Multifunctional Microparticles. Adv. Mater. 2025, 37, 2506777. [Google Scholar] [CrossRef] [PubMed]
- Islas, P.; Platnich, C.M.; Gidi, Y.; Karimi, R.; Ginot, L.; Saliba, D.; Luo, X.; Cosa, G.; Sleiman, H.F. Automated Synthesis of DNA Nanostructures. Adv. Mater. 2024, 36, 2403477. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Huynh, C.; Huang, X.; Desai, T.A. Bioinspired nanotopographical design of drug delivery systems. Nat. Rev. Bioeng. 2023, 1, 139–152. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kapate, N.; Clegg, J.R.; Mitragotri, S. Non-spherical micro-and nanoparticles for drug delivery: Progress over 15 years. Adv. Drug Deliv. Rev. 2021, 177, 113807. [Google Scholar] [CrossRef]
- Li, H.-J.; Du, J.-Z.; Du, X.-J.; Xu, C.-F.; Sun, C.-Y.; Wang, H.-X.; Cao, Z.-T.; Yang, X.-Z.; Zhu, Y.-H.; Nie, S. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2016, 113, 4164–4169. [Google Scholar] [CrossRef]
- Khoeini, D.; Scott, T.F.; Neild, A. Microfluidic enhancement of self-assembly systems. Lab Chip 2021, 21, 1661–1675. [Google Scholar] [CrossRef]
- Del Giudice, F.; D’Avino, G.; Maffettone, P.L. Microfluidic formation of crystal-like structures. Lab Chip 2021, 21, 2069–2094. [Google Scholar] [CrossRef] [PubMed]
- Pramoda, G.; Singh, M.; Gupta, P.K.; Shukla, R. Synergy of Microfluidics and Nanomaterials: A Revolutionary Approach for Cancer Management. ACS Appl. Bio Mater. 2025, 8, 2716–2734. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Pouryosef Miandoab, M.; Şaşmaz, M. Microfluidic synthesis, control, and sensing of magnetic nanoparticles: A review. Micromachines 2021, 12, 768. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, B.; Zeng, S.; Liu, L.; Yong, K.-T.; Ma, H.; Tang, Y. Microfluidic chip enabled one-step synthesis of biofunctionalized CuInS 2/ZnS quantum dots. Lab Chip 2020, 20, 3001–3010. [Google Scholar] [CrossRef]
- Kim, J.-W.; Han, S.H.; Choi, Y.H.; Hamonangan, W.M.; Oh, Y.; Kim, S.-H. Recent advances in the microfluidic production of functional microcapsules by multiple-emulsion templating. Lab Chip 2022, 22, 2259–2291. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hormes, J.; Kumar, C.S. Microfluidic synthesis of nanomaterials. Small 2008, 4, 698–711. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. Janus and ternary particles generated by microfluidic synthesis: Design, synthesis, and self-assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.-H.; Xiang, Z.; Wang, M.; Lee, C. A convection-driven long-range linear gradient generator with dynamic control. Lab Chip 2015, 15, 1445–1450. [Google Scholar] [CrossRef]
- Dertinger, S.K.; Chiu, D.T.; Jeon, N.L.; Whitesides, G.M. Generation of gradients having complex shapes using microfluidic networks. Anal. Chem. 2001, 73, 1240–1246. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Wang, S.; Zhang, L.; Jiang, X. Microfluidics-assembled nanovesicles for nucleic acid delivery. Acc. Chem. Res. 2025, 58, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, Z.; Liu, Y.; Li, S.; Zhu, J.; Chen, P.; Liu, B.-F. Multichannel synchronous hydrodynamic gating coupling with concentration gradient generator for high-throughput probing dynamic signaling of single cells. Anal. Chem. 2020, 92, 12062–12070. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.S.; Santiago, G.T.-d.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Du, G.; Hu, C. A surfactant-free droplet based microfluidic technique for the fabrication of polymeric microspheres. Mater. Today Commun. 2022, 33, 104389. [Google Scholar] [CrossRef]
- Zheng, F.; Tian, R.; Lu, H.; Liang, X.; Shafiq, M.; Uchida, S.; Chen, H.; Ma, M. Droplet Microfluidics Powered Hydrogel Microparticles for Stem Cell-Mediated Biomedical Applications. Small 2024, 20, 2401400. [Google Scholar]
- Yang, Z.; Jin, S.; Zhang, C.; Ren, J.; Jing, W.; Wei, X. Microfluidics-assisted synthesis of hydrogel microparticles with acoustic-magnetic control. Chem. Eng. Sci. 2023, 281, 119082. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Gu, P.; Huang, C.; Chen, S.; Hu, C.; Lee, E.; Xu, J.; Zhu, J. Electric, magnetic, and shear field-directed assembly of inorganic nanoparticles. Nanoscale 2023, 15, 2018–2035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Moragues, T.; Arguijo, D.; Beneyton, T.; Modavi, C.; Simutis, K.; Abate, A.R.; Baret, J.-C.; deMello, A.J.; Densmore, D.; Griffiths, A.D. Droplet-based microfluidics. Nat. Rev. Methods Primers 2023, 3, 32. [Google Scholar] [CrossRef]
- Dai, B.; Long, Y.; Wu, J.; Huang, S.; Zhao, Y.; Zheng, L.; Tao, C.; Guo, S.; Lin, F.; Fu, Y. Generation of flow and droplets with an ultra-long-range linear concentration gradient. Lab Chip 2021, 21, 4390–4400. [Google Scholar] [CrossRef]
- Seeto, W.J.; Tian, Y.; Pradhan, S.; Minond, D.; Lipke, E.A. Droplet microfluidics-based fabrication of monodisperse poly (ethylene glycol)-fibrinogen breast cancer microspheres for automated drug screening applications. ACS Biomater. Sci. Eng. 2022, 8, 3831–3841. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Tian, F.; Cai, L.; Liu, C.; Sun, J. Microfluidic technologies for nanoparticle formation. Lab Chip 2022, 22, 512–529. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Adv. Drug Deliv. Rev. 2022, 184, 114197. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Nan, L.; Zhang, H.; Weitz, D.A.; Shum, H.C. Development and future of droplet microfluidics. Lab Chip 2024, 24, 1135–1153. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Liu, H.; Su, W.; Chen, W.; Qin, J. One-step generation of core–shell gelatin methacrylate (GelMA) microgels using a droplet microfluidic system. Adv. Mater. Technol. 2019, 4, 1800632. [Google Scholar] [CrossRef]
- Theberge, A.B.; Mayot, E.; El Harrak, A.; Kleinschmidt, F.; Huck, W.T.; Griffiths, A.D. Microfluidic platform for combinatorial synthesis in picolitre droplets. Lab Chip 2012, 12, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ren, Y.; Jia, Y.; Deng, X.; Liu, W.; Feng, X.; Jiang, H. Continuously electrotriggered core coalescence of double-emulsion drops for microreactions. ACS Appl. Mater. Interfaces 2017, 9, 12282–12289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, P.; Woloszyn, K.; Zhang, Y.; Hu, H.; Hou, L.; Li, X.; Liu, J.; Jiang, W.; Wang, L. Precision Self-assembly of 3D DNA Crystals Using Microfluidics. J. Am. Chem. Soc. 2025, 147, 11915–11924. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2014, 27, 1402–1407. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP device: Fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics synthesis of gene silencing cubosomes. ACS Nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, Q.; Li, X.; Hu, G. Generation of Multiple Concentration Gradients Using a Two-Dimensional Pyramid Array. Anal. Chem. 2024, 96, 856–865. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.-G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA lipid nanoparticle production using a parallelized microfluidic device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Han, X.; Mukalel, A.J.; El-Mayta, R.; Thatte, A.S.; Wu, J.; Padilla, M.S.; Alameh, M.-G.; Srikumar, N.; Lee, D. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc. Natl. Acad. Sci. USA 2023, 120, e2303567120. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Ma, Y.; Sun, J. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, P.-H.; Zhang, H.; Rich, J.; Bachman, H.; Ye, J.; Zhang, W.; Chen, C.; Xie, Z.; Tian, Z.; et al. Fabrication of tunable, high-molecular-weight polymeric nanoparticles via ultrafast acoustofluidic micromixing. Lab Chip 2021, 21, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lan, X.; Yin, Y.; Wang, X.; Chen, X.; Zhou, J.; Chen, K.; Zhang, X. Green Synthesis and Durable Antibacterial AgNP-Loaded Alginate Fibers Enabled by Microfluidic Technology Coupled with Ultraviolet/Thermal Fields. ACS Appl. Polym. Mater. 2025, 7, 5259–5270. [Google Scholar] [CrossRef]
- Marelli, M.; Schmidt, P.P.; Nguyen, X.T.; Pitzalis, E.; Poggini, L.; Ragona, L.; Pagano, K.; Aronica, L.A.; Polito, L.; Evangelisti, C. Photo-induced microfluidic production of ultrasmall platinum nanoparticles. Nanoscale 2024, 16, 19669–19674. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- El Itawi, H.; Fadlallah, S.; Allais, F.; Perré, P. Green assessment of polymer microparticles production processes: A critical review. Green Chem. 2022, 24, 4237–4269. [Google Scholar] [CrossRef]
- Dendukuri, D.; Pregibon, D.C.; Collins, J.; Hatton, T.A.; Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006, 5, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X.; You, S.; Cai, B.; Liu, H.; Zhang, L.; Rao, L.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Ultraviolet-assisted microfluidic generation of ferroelectric composite particles. Biomicrofluidics 2016, 10, 024106. [Google Scholar] [CrossRef]
- Feng, Z.-Y.; Liu, T.-T.; Sang, Z.-T.; Lin, Z.-S.; Su, X.; Sun, X.-T.; Yang, H.-Z.; Wang, T.; Guo, S. Microfluidic preparation of Janus microparticles with temperature and pH triggered degradation properties. Front. Bioeng. Biotechnol. 2021, 9, 756758. [Google Scholar] [CrossRef]
- Hoang, P.H. Fast synthesis of an inorganic–organic block copolymer in a droplet-based microreactor. RSC Adv. 2014, 4, 8283–8288. [Google Scholar] [CrossRef]
- Li, W.; Pham, H.H.; Nie, Z.; MacDonald, B.; Güenther, A.; Kumacheva, E. Multi-step microfluidic polymerization reactions conducted in droplets: The internal trigger approach. J. Am. Chem. Soc. 2008, 130, 9935–9941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Q.; Zhou, A.; Wang, Y.; Zhang, J.; Xiong, R.; Lenders, V.; Manshian, B.B.; Hua, D.; Soenen, S.J. Core-shell microparticles: From rational engineering to diverse applications. Adv. Colloid Interface Sci. 2022, 299, 102568. [Google Scholar] [CrossRef] [PubMed]
- Kashani, S.Y.; Afzalian, A.; Shirinichi, F.; Moraveji, M.K. Microfluidics for core–shell drug carrier particles–a review. RSC Adv. 2021, 11, 229–249. [Google Scholar] [CrossRef]
- Dinh, N.-D.; Kukumberg, M.; Nguyen, A.-T.; Keramati, H.; Guo, S.; Phan, D.-T.; Ja’Afar, N.B.; Birgersson, E.; Leo, H.L.; Huang, R.Y.-J. Functional reservoir microcapsules generated via microfluidic fabrication for long-term cardiovascular therapeutics. Lab Chip 2020, 20, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xu, X.; Li, G.; Yang, X.; Du, F.; Tan, W.; Wang, J.; Dong, S.; Luo, J.; Wang, X. High-Throughput Microfluidic Production of Droplets and Hydrogel Microspheres through Monolithically Integrated Microchannel Plates. Anal. Chem. 2023, 95, 13586–13595. [Google Scholar] [CrossRef]
- He, Q.; Vijayamohanan, H.; Li, J.; Swager, T.M. Multifunctional photonic Janus particles. J. Am. Chem. Soc. 2022, 144, 5661–5667. [Google Scholar] [CrossRef]
- Wang, D.; Guan, D.; Su, J.; Zheng, X.; Hu, G. Distinct dynamics of self-propelled bowl-shaped micromotors caused by shape effect: Concave vs. convex. Phys. Fluids 2021, 33, 122004. [Google Scholar] [CrossRef]
- Jin, S.; Wei, X.; Ren, J.; Jiang, Z.; Abell, C.; Yu, Z. Construction of core–shell microcapsules via focused surface acoustic wave microfluidics. Lab Chip 2020, 20, 3104–3108. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Van Nguyen, H.; Van Nguyen, H.; Phan, V.M.; Park, B.J.; Seo, T.S. Serially diluting centrifugal microfluidics for high-throughput gold nanoparticle synthesis using an automated and portable workstation. Chem. Eng. J. 2023, 452, 139044. [Google Scholar] [CrossRef]
- Okatenko, V.; Boulanger, C.; Chen, A.N.; Kumar, K.; Schouwink, P.; Loiudice, A.; Buonsanti, R. Voltage-Driven Chemical Reactions Enable the Synthesis of Tunable Liquid Ga–Metal Nanoparticles. J. Am. Chem. Soc. 2023, 145, 25401–25410. [Google Scholar] [CrossRef]
- Carvalho, B.G.; Ceccato, B.T.; Michelon, M.; Han, S.W.; de La Torre, L.G. Advanced microfluidic technologies for lipid nano-microsystems from synthesis to biological application. Pharmaceutics 2022, 14, 141. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, L.; Liu, C.; Li, X.; Hu, G.; Sun, J.; Jiang, X. Microfluidic based high throughput synthesis of lipid-polymer hybrid nanoparticles with tunable diameters. Biomicrofluidics 2015, 9, 052604. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, C.P.; Gispert, I.; Chui, S.Y.; Seddon, J.M.; Elani, Y. Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release. Nat. Chem. 2024, 16, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Firmino, P.C.; Vianna, S.S.; da Costa, O.M.; Malfatti-Gasperini, A.A.; Gobbi, A.L.; Lima, R.S.; de la Torre, L.G. 3D micromixer for nanoliposome synthesis: A promising advance in high mass productivity. Lab Chip 2021, 21, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Bandara, S.R.; Tan, Z.; Leal, C. Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc. Natl. Acad. Sci. USA 2023, 120, e2301067120. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Huang, H.; Chang, A.; Peng, H.; Liu, J.; Yao, A.; Ruan, Y.; Zhang, P.; Wang, T.; Qu, C.; Yin, X. Preparation and anti-tumor effect in hepatocellular carcinoma treatment of AS1411 aptamer-targeted polyphyllin II-loaded PLGA nanoparticles. J. Sci. Adv. Mater. 2024, 9, 100755. [Google Scholar] [CrossRef]
- RS, P.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Dual drug delivery of curcumin and niclosamide using PLGA nanoparticles for improved therapeutic effect on breast cancer cells. J. Polym. Res. 2020, 27, 133. [Google Scholar] [CrossRef]
- Wang, R.; Zou, L.; Yi, Z.; Zhang, Z.; Zhao, M.; Shi, S. PLGA nanoparticles loaded with curcumin produced luminescence for cell bioimaging. Int. J. Pharm. 2023, 639, 122944. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.; Manickam, D.S. Lipid nanoparticle-mediated drug delivery to the brain. Adv. Drug Deliv. Rev 2023, 197, 114861. [Google Scholar] [CrossRef]
- Patel, D.; Solanki, J.; Kher, M.M.; Azagury, A. A review: Surface engineering of lipid-based drug delivery systems. Small 2024, 20, 2401990. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zou, X.; Su, H.; Li, C. Methotrexate-loaded folic acid of solid-phase synthesis conjugated gold nanoparticles targeted treatment for rheumatoid arthritis. Eur. J. Pharm. Sci. 2021, 170, 106101. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; Ahmed, F.; Mohammed, A.M.; Alsharidah, M.; Al-Subaiyel, A.; Samman, W.A.; Alhaddad, A.A.; Al-Mijalli, S.H.; Amin, M.A.; Barakat, H. Recent advances in the pharmaceutical and biomedical applications of cyclodextrin-capped gold nanoparticles. Int. J. Nanomed. 2023, 18, 3247–3281. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, X.; Wang, F.; Yu, Q.; Chen, F.; Shen, D.; Yang, Z.; Wang, T.; Jiang, M.; Deng, T. Duplex metal co-doped carbon quantum dots-based drug delivery system with intelligent adjustable size as adjuvant for synergistic cancer therapy. Carbon 2021, 183, 789–808. [Google Scholar] [CrossRef]
- Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent breakthroughs in using quantum dots for cancer imaging and drug delivery purposes. Nanomaterials 2023, 13, 2566. [Google Scholar] [CrossRef]
- Soumya, K.; More, N.; Choppadandi, M.; Aishwarya, D.; Singh, G.; Kapusetti, G. A comprehensive review on carbon quantum dots as an effective photosensitizer and drug delivery system for cancer treatment. Biomed. Technol. 2023, 4, 11–20. [Google Scholar] [CrossRef]
- Jana, P.; Dev, A. Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer. Mater. Today Commun. 2022, 32, 104068. [Google Scholar] [CrossRef]
- Bai, X.; Tang, S.; Butterworth, S.; Tirella, A. Design of PLGA nanoparticles for sustained release of hydroxyl-FK866 by microfluidics. Biomater. Adv. 2023, 154, 213649. [Google Scholar] [CrossRef] [PubMed]
- Udepurkar, A.P.; Mampaey, L.; Clasen, C.; Cabeza, V.S.; Kuhn, S. Microfluidic synthesis of PLGA nanoparticles enabled by an ultrasonic microreactor. React. Chem. Eng. 2024, 9, 2208–2217. [Google Scholar] [CrossRef]
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. PLoS ONE 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.-Y.; Choi, I.; Ryu, T.-K.; Ryu, Y.-H.; Oh, D.-H.; Kang, H.-W.; Kang, M.-H.; Choi, S.-W. Continuous production of lipid nanoparticles by multiple-splitting in microfluidic devices with chaotic microfibrous channels. Colloids Surf. B 2023, 224, 113212. [Google Scholar] [CrossRef]
- Maeki, M.; Okada, Y.; Uno, S.; Sugiura, K.; Suzuki, Y.; Okuda, K.; Sato, Y.; Ando, M.; Yamazaki, H.; Takeuchi, M. Mass production system for RNA-loaded lipid nanoparticles using piling up microfluidic devices. Appl. Mater. Today 2023, 31, 101754. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.; Park, H.; Ahn, D.; Lee, J.M.; Choe, H.; Kim, D.; Kim, J.H.; Chon, C.H. Size-controllable and monodispersed lipid nanoparticle production with high mRNA delivery efficiency using 3D-printed Ring micromixers. ACS Appl. Mater. Interfaces 2024, 16, 46044–46052. [Google Scholar] [CrossRef]
- Lin, W.-Z.S.; Bostic, W.K.V.; Malmstadt, N. 3D-printed microfluidic device for high-throughput production of lipid nanoparticles incorporating SARS-CoV-2 spike protein mRNA. Lab Chip 2024, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vinnacombe-Willson, G.A.; Lee, J.K.; Chiang, N.; Scarabelli, L.; Yue, S.; Foley, R.; Frost, I.; Weiss, P.S.; Jonas, S.J. Exploring the bottom-up growth of anisotropic gold nanoparticles from substrate-bound seeds in microfluidic reactors. ACS Appl. Nano Mater. 2023, 6, 6454–6460. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, H.; Guo, Y.; Fang, H.; Zhu, C.; Xue, J.; Wang, W.; Luo, G.; Sun, Y. Synthesis of uniform Pickering microspheres doped with quantum dot by microfluidic technology and its application in tumor marker. Talanta 2023, 262, 124495. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, H.; Chen, Y.; Chen, X.; Zhang, W.; Wang, J.; Zhang, G.; Belotelov, V.I.; Song, Y. Magnetic field coupling microfluidic synthesis of diluted magnetic semiconductor quantum dots: The case of Co doping ZnSe quantum dots. J. Mater. Chem. C 2021, 9, 4619–4627. [Google Scholar] [CrossRef]
- Kurassova, K.; Filatov, N.; Karamysheva, S.; Bukatin, A.; Starovoytov, A.; Vartanyan, T.; Vollmer, F.; Toropov, N.A. Microfluidics-Driven Dripping Technique for Fabricating Polymer Microspheres Doped with AgInS2/ZnS Quantum Dots. ACS Omega 2024, 9, 39287–39295. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, O.; Koyuncu, A.; Hubatová-Vacková, A.; Balouch, M.; Tokárová, V. Influence of channel height on mixing efficiency and synthesis of iron oxide nanoparticles using droplet-based microfluidics. RSC Adv. 2020, 10, 15179–15189. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496. [Google Scholar] [CrossRef]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Synthesis and characterization of paclitaxel-loaded PEGylated liposomes by the microfluidics method. Mol. Pharm. 2023, 20, 6184–6196. [Google Scholar] [CrossRef]
- Huang, C.; Shang, X.; Zhou, X.; Zhang, Z.; Huang, X.; Lu, Y.; Wang, M.; Löffler, M.; Liao, Z.; Qi, H.; et al. Hierarchical conductive metal-organic framework films enabling efficient interfacial mass transfer. Nat. Commun. 2023, 14, 3850. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Guo, Z.; Zheng, X.; Chen, X.; Xue, Z.; Zhang, S.; Li, X.; Guan, B.; Li, X.; Hu, G.; et al. Deformable metal–organic framework nanosheets for heterogeneous catalytic reactions. J. Am. Chem. Soc. 2020, 142, 9408–9414. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Giménez-Marqués, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–organic frameworks for biological applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Q.; Li, C.; Yang, Q.; Xu, F.; Mai, Y. Block copolymer self-assembly directed synthesis of porous materials with ordered bicontinuous structures and their potential applications. Adv. Mater. 2023, 35, 2207684. [Google Scholar] [CrossRef]
- Cui, C.; Cao, Y.; Han, L. Deep-Learning-Assisted Understanding of the Self-Assembly of Miktoarm Star Block Copolymers. ACS Nano 2025, 19, 11427–11439. [Google Scholar] [CrossRef]
- Wang, J.; Le-The, H.; Wang, Z.; Li, H.; Jin, M.; van den Berg, A.; Zhou, G.; Segerink, L.I.; Shui, L.; Eijkel, J.C. Microfluidics assisted fabrication of three-tier hierarchical microparticles for constructing bioinspired surfaces. ACS Nano 2019, 13, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour Tamrin, S.; Sanati Nezhad, A.; Sen, A. Label-free isolation of exosomes using microfluidic technologies. ACS Nano 2021, 15, 17047–17079. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Cha, H.; Ouyang, L.; Mudugamuwa, A.; An, H.; Kijanka, G.; Kashaninejad, N.; Nguyen, N.-T.; Zhang, J. Recent microfluidic advances in submicron to nanoparticle manipulation and separation. Lab Chip 2023, 23, 982–1010. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Sun, J.; Hu, G. A generalized formula for inertial lift on a sphere in microchannels. Lab Chip 2016, 16, 884–892. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, H.; Di Carlo, D. Inertial flow of a dilute suspension over cavities in a microchannel. J. Fluid Mech. 2017, 811, 436–467. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef]
- Liu, C.; Hu, G.; Jiang, X.; Sun, J. Inertial focusing of spherical particles in rectangular microchannels over a wide range of Reynolds numbers. Lab Chip 2015, 15, 1168–1177. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Viscoelastic microfluidics: Progress and challenges. Microsyst. Nanoeng. 2020, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ding, B.; Xue, C.; Tian, Y.; Hu, G.; Sun, J. Sheathless focusing and separation of diverse nanoparticles in viscoelastic solutions with minimized shear thinning. Anal. Chem. 2016, 88, 12547–12553. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, J.; Tian, F.; Yang, N.; Yan, F.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 2017, 11, 6968–6976. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, Q.; Yan, S.; Tang, S.-Y.; Alici, G.; Zhang, J.; Li, W. Recent progress of particle migration in viscoelastic fluids. Lab Chip 2018, 18, 551–567. [Google Scholar] [CrossRef]
- Lu, X.; Liu, C.; Hu, G.; Xuan, X. Particle manipulations in non-Newtonian microfluidics: A review. J. Colloid Interface Sci. 2017, 500, 182–201. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Ouyang, L.; Cha, H.; Hansen, H.H.; An, H.; Nguyen, N.-T.; Zhang, J. Viscoelastic microfluidics for enhanced separation resolution of submicron particles and extracellular vesicles. Nanoscale 2024, 16, 3560–3570. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.; Tayebi, M.; Ai, Y. Submicron particle focusing and exosome sorting by wavy microchannel structures within viscoelastic fluids. Anal. Chem. 2019, 91, 4577–4584. [Google Scholar] [CrossRef]
- Bai, J.-J.; Zhang, X.; Wei, X.; Wang, Y.; Du, C.; Wang, Z.-J.; Chen, M.-L.; Wang, J.-H. Dean-flow-coupled elasto-inertial focusing accelerates exosome purification to facilitate single vesicle profiling. Anal. Chem. 2023, 95, 2523–2531. [Google Scholar] [CrossRef]
- Hochstetter, A.; Vernekar, R.; Austin, R.H.; Becker, H.; Beech, J.P.; Fedosov, D.A.; Gompper, G.; Kim, S.-C.; Smith, J.T.; Stolovitzky, G. Deterministic lateral displacement: Challenges and perspectives. ACS Nano 2020, 14, 10784–10795. [Google Scholar] [CrossRef]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.-C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V. The exosome total isolation chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X. Recent advances in direct current electrokinetic manipulation of particles for microfluidic applications. Electrophoresis 2019, 40, 2484–2513. [Google Scholar] [CrossRef]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.-Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef] [PubMed]
- Chinappi, M.; Yamaji, M.; Kawano, R.; Cecconi, F. Analytical model for particle capture in nanopores elucidates competition among electrophoresis, electroosmosis, and dielectrophoresis. ACS Nano 2020, 14, 15816–15828. [Google Scholar] [CrossRef]
- Huo, X.; Yossifon, G. Tunable electrorheological fluid microfluidic rectifier: Irreversibility of viscous flow due to spatial asymmetry induced memory effects. Phys. Rev. Lett. 2019, 123, 194502. [Google Scholar] [CrossRef]
- Raihan, M.K.; Baghdady, M.; Dort, H.; Bentor, J.; Xuan, X. Fluid Elasticity-Enhanced Insulator-Based Dielectrophoresis for Sheath-Free Particle Focusing in Very Dilute Polymer Solutions. Anal. Chem. 2023, 95, 16013–16020. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xuan, X. Electrophoretic slip-tuned particle migration in microchannel viscoelastic fluid flows. Phys. Rev. Fluids 2018, 3, 074202. [Google Scholar] [CrossRef]
- Serhatlioglu, M.; Isiksacan, Z.; Özkan, M.; Tuncel, D.; Elbuken, C. Electro-viscoelastic migration under simultaneously applied microfluidic pressure-driven flow and electric field. Anal. Chem. 2020, 92, 6932–6940. [Google Scholar] [CrossRef]
- Choudhary, A.; Li, D.; Renganathan, T.; Xuan, X.; Pushpavanam, S. Electrokinetically enhanced cross-stream particle migration in viscoelastic flows. J. Fluid Mech. 2020, 898, A20. [Google Scholar] [CrossRef]
- Li, D.; Xuan, X. Electro-elastic migration of particles in viscoelastic fluid flows. Phys. Fluids 2023, 35, 092013. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, S.; Chen, X.; Zhang, P.; Gu, G.; Wang, Y.; Zhang, H.; Zhang, Y.; Yang, H. Extraction of small extracellular vesicles by label-free and biocompatible on-chip magnetic separation. Lab Chip 2022, 22, 2476–2488. [Google Scholar] [CrossRef]
- Zhang, P.; Rufo, J.; Chen, C.; Xia, J.; Tian, Z.; Zhang, L.; Hao, N.; Zhong, Z.; Gu, Y.; Chakrabarty, K. Acoustoelectronic nanotweezers enable dynamic and large-scale control of nanomaterials. Nat. Commun. 2021, 12, 3844. [Google Scholar] [CrossRef]
- Läubli, N.F.; Burri, J.T.; Marquard, J.; Vogler, H.; Mosca, G.; Vertti-Quintero, N.; Shamsudhin, N.; DeMello, A.; Grossniklaus, U.; Ahmed, D. 3D mechanical characterization of single cells and small organisms using acoustic manipulation and force microscopy. Nat. Commun. 2021, 12, 2583. [Google Scholar] [CrossRef]
- Fujiwara, H.; Yamauchi, K.; Wada, T.; Ishihara, H.; Sasaki, K. Optical selection and sorting of nanoparticles according to quantum mechanical properties. Sci. Adv. 2021, 7, eabd9551. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, G.; Truong, V.G.; Esporlas, C.L.; Sanskriti, I.; Nic Chormaic, S. Evanescent field trapping and propulsion of Janus particles along optical nanofibers. Nat. Commun. 2023, 14, 1691. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Cao, X.; Mateescu, B.; van Leeuwen, D.; Aslan, M.K.; Stavrakis, S.; deMello, A.J. Oscillatory viscoelastic microfluidics for efficient focusing and separation of nanoscale species. ACS Nano 2019, 14, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-M.; Wang, H.-F.; Guo, Q.-H.; Wang, J.-W.; Li, T.-T.; Chen, K.-X.; Zhang, M.-T.; Chen, J.-B.; Shi, Q.-N.; Huang, Y. Roboticized AI-assisted microfluidic photocatalytic synthesis and screening up to 10,000 reactions per day. Nat. Commun. 2024, 15, 1–13. [Google Scholar] [CrossRef]
- Li, S.; Baker, R.W.; Lignos, I.; Yang, Z.; Stavrakis, S.; Howes, P.D.; deMello, A.J. Automated microfluidic screening of ligand interactions during the synthesis of cesium lead bromide nanocrystals. Mol. Syst. Des. Eng. 2020, 5, 1118–1130. [Google Scholar] [CrossRef]
- Li, J.; Carney, R.P.; Liu, R.; Fan, J.; Zhao, S.; Chen, Y.; Lam, K.S.; Pan, T. Microfluidic print-to-synthesis platform for efficient preparation and screening of combinatorial peptide microarrays. Anal. Chem. 2018, 90, 5833–5840. [Google Scholar] [CrossRef]
- Kaiser, M.; Jug, F.; Julou, T.; Deshpande, S.; Pfohl, T.; Silander, O.K.; Myers, G.; Van Nimwegen, E. Monitoring single-cell gene regulation under dynamically controllable conditions with integrated microfluidics and software. Nat. Commun. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Guo, J.; Ling, S.D.; Wu, X.; Liu, H.; Chen, Z.; Chen, S.; Xu, J. A nano-liter droplet-based microfluidic reactor serves as continuous large-scale production of inorganic perovskite nanocrystals. Sci. China Mater. 2022, 65, 2746–2754. [Google Scholar] [CrossRef]
- Coliaie, P.; Kelkar, M.S.; Langston, M.; Liu, C.; Nazemifard, N.; Patience, D.; Skliar, D.; Nere, N.K.; Singh, M.R. Advanced continuous-flow microfluidic device for parallel screening of crystal polymorphs, morphology, and kinetics at controlled supersaturation. Lab Chip 2021, 21, 2333–2342. [Google Scholar] [CrossRef]
- Eder, T.; Mautner, A.; Xu, Y.; Reithofer, M.R.; Bismarck, A.; Chin, J.M. Transparent PDMS surfaces with covalently attached lubricants for enhanced anti-adhesion performance. ACS Appl. Mater. Interfaces 2024, 16, 10942–10952. [Google Scholar] [CrossRef]
- Hao, L.; Fan, B. Slippery liquid-like surfaces as a promising solution for sustainable drag reduction. Nanoscale 2025, 17, 6448–6459. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, P.; Han, H.; Ohl, C.-D.; Zuo, Z.; Liu, S. Removal of surface-attached micro-and nanobubbles by ultrasonic cavitation in microfluidics. Ultrason. Sonochem. 2024, 109, 107011. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lam, C.W.E.; Donati, M.; Regulagadda, K.; Yavuz, E.; Pfeiffer, T.; Sarkiris, P.; Gogolides, E.; Milionis, A.; Poulikakos, D. Durable, ultrathin, and antifouling polymer brush coating for efficient condensation heat transfer. ACS Appl. Mater. Interfaces 2023, 16, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, F.; McAlpine, M.C. 3D printed microfluidics: Advances in strategies, integration, and applications. Lab Chip 2023, 23, 1279–1299. [Google Scholar] [CrossRef]
- Selemani, M.A.; Cenhrang, K.; Azibere, S.; Singhateh, M.; Martin, R.S. 3D printed microfluidic devices with electrodes for electrochemical analysis. Anal. Methods 2024, 16, 6941–6953. [Google Scholar] [CrossRef]
- Kibar, G.; Sarıarslan, B.S.R.; Doganay, S.; Yıldız, G.K.; Usta, O.B.; Çetin, B. Novel 3D-printed microfluidic magnetic platform for rapid DNA isolation. Anal. Chem. 2024, 96, 1985–1992. [Google Scholar] [CrossRef]
- Weaver, E.; Mathew, E.; Caldwell, J.; Hooker, A.; Uddin, S.; Lamprou, D.A. The manufacturing of 3D-printed microfluidic chips to analyse the effect upon particle size during the synthesis of lipid nanoparticles. J. Pharm. Pharmacol. 2023, 75, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.; Palma, M.S.; Lopes, M.G.; Souza, J.; Lima, G.A.; Taranto, O.P.; Silva, J.L., Jr. Microfluidic devices and 3D printing for synthesis and screening of drugs and tissue engineering. Ind. Eng. Chem. Res. 2019, 59, 3794–3810. [Google Scholar] [CrossRef]
- Curtin, K.; Godary, T.; Li, P. Synthesis of tunable gold nanostars via 3D-printed microfluidic device with vibrating sharp-tip acoustic mixing. Microfluid. Nanofluid. 2023, 27, 77. [Google Scholar] [CrossRef]
- Apoorva, S.; Nguyen, N.-T.; Sreejith, K.R. Recent developments and future perspectives of microfluidics and smart technologies in wearable devices. Lab Chip 2024, 24, 1833–1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shah, A.; Lee, H.; Lee, C.H. Microfluidic technologies for wearable and implantable biomedical devices. Lab Chip 2025, 25, 4542–4576. [Google Scholar] [CrossRef]
- Ju, F.; Wang, Y.; Yin, B.; Zhao, M.; Zhang, Y.; Gong, Y.; Jiao, C. Microfluidic wearable devices for sports applications. Micromachines 2023, 14, 1792. [Google Scholar] [CrossRef]
- Božinović, M.; Jereb, M.; Šketa, B.; Gaber, A.; Seručnik, M.; Košmrlj, J.; Žnidaršič-Plazl, P. Development of Sustainable Biocatalytic Furfurylamine Production in a Magnetic Field-Assisted Microfluidic Reactor. ACS Sustain. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- He, Y.; Guo, S.; Chen, K.; Li, S.; Zhang, L.; Yin, S. Sustainable green production: A review of recent development on rare earths extraction and separation using microreactors. ACS Sustain. Chem. Eng. 2019, 7, 17616–17626. [Google Scholar] [CrossRef]
- Zizzari, A.; Bloise, E.; Perrone, E.; Perinelli, D.R.; Schmutz, M.; Arima, V.; Mele, G.; Carbone, L. Environmentally friendly method of assembly of cardanol and cholesterol into nanostructures using a continuous flow microfluidic device. ACS Sustain. Chem. Eng. 2022, 10, 8484–8494. [Google Scholar] [CrossRef]
- Saeed, M.M.; Carthy, E.; Dunne, N.; Kinahan, D. Advances in nanoparticle synthesis assisted by microfluidics. Lab Chip 2025, 25, 3060–3093. [Google Scholar] [CrossRef]
- Liu, L.; Bi, M.; Wang, Y.; Liu, J.; Jiang, X.; Xu, Z.; Zhang, X. Artificial intelligence-powered microfluidics for nanomedicine and materials synthesis. Nanoscale 2021, 13, 19352–19366. [Google Scholar] [CrossRef]
- Ahmadi, F.; Simchi, M.; Perry, J.M.; Frenette, S.; Benali, H.; Soucy, J.-P.; Massarweh, G.; Shih, S.C. Integrating machine learning and digital microfluidics for screening experimental conditions. Lab Chip 2023, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, G.; Wu, X.; Wu, Q.; Liu, F.; Zhang, H.; Shi, N.; Guan, Y. Advances in integration, wearable applications, and artificial intelligence of biomedical microfluidics systems. Micromachines 2023, 14, 972. [Google Scholar] [CrossRef]
- Chen, X.; Lv, H. Intelligent control of nanoparticle synthesis on microfluidic chips with machine learning. NPG Asia Mater. 2022, 14, 69. [Google Scholar] [CrossRef]
- Rizkin, B.A.; Shkolnik, A.S.; Ferraro, N.J.; Hartman, R.L. Combining automated microfluidic experimentation with machine learning for efficient polymerization design. Nat. Mach. Intell. 2020, 2, 200–209. [Google Scholar] [CrossRef]
- Jia, Y.; Liang, X.; Zhang, L.; Zhang, J.; Zafar, H.; Huang, S.; Shi, Y.; Chen, J.; Shen, Q. Machine learning-assisted microfluidic approach for broad-spectrum liposome size control. J. Pharm. Anal. 2025, 15, 101221. [Google Scholar] [CrossRef]
- Di Francesco, V.; Boso, D.P.; Moore, T.L.; Schrefler, B.A.; Decuzzi, P. Machine learning instructed microfluidic synthesis of curcumin-loaded liposomes. Biomed. Microdevices 2023, 25, 29. [Google Scholar] [CrossRef]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W.K.; Zheng, F.; Xie, J.; Tian, I.P.S.; Jayavelu, S.; Mahfoud, Z.; Bash, D. Two-step machine learning enables optimized nanoparticle synthesis. npj Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.M.; Hong, S.; Han, B.; Nam, K.T.; Jung, Y. Closed-loop optimization of nanoparticle synthesis enabled by robotics and machine learning. Matter 2023, 6, 677–690. [Google Scholar] [CrossRef]
| Type | Microfluidic Methods and Controlled Parameters | Advantages | Reference |
|---|---|---|---|
| PLGA (Polymeric) NPs | A hydrodynamic flow-focusing microfluidic method was employed to fabricate PLGA nanoparticles, with key parameters including flow rate ratio, total flow rate, and polymer/surfactant concentrations, enabling high encapsulation efficiency and sustained, pH-dependent drug release. | Z-average size of 128 ± 8 nm (PDI < 0.2), ζ-potential of −14.8 ± 5.3 mV and high encapsulation efficiency (98.6 ± 5.8%). | Bai et al. [95] |
| An ultrasonic microreactor was used to synthesize PLGA nanoparticles by emulsion-solvent evaporation, with key parameters—ultrasonic power, PLGA concentration, and flow rate ratio—optimized to control particle size and uniformity. | PDI of 0.1–0.2, 115–150 nm | Udepurkar et al. [96] | |
| A microfluidic iLiNP device was used to precisely tune PLGA nanoparticle sizes (40–114 nm) by adjusting flow rates, enabling size-controlled sub-200 nm drug-loaded nanoparticles without changing polymer precursors. | PLGA NPs: 44–101 nm; PEG-PLGA NPs: 29–76 nm; blend NPs: 40–114 nm | Bao et al. [97] | |
| Lipid NPs (LNPs) | Chaotic microfibrous channels enable continuous lipid nanoparticle production via multiple phase-splitting, with smaller fiber diameters and higher continuous-phase flow rates yielding smaller, more uniform particles. | 89.7 ± 35.1 and 190.4 ± 66.4 nm | Ahn et al. [98] |
| A glass-based piling-up microfluidic device system was developed, enabling controlled RNA-loaded lipid nanoparticle production at high flow rates (20–50 mL/min) with particle sizes of 20–60 nm for scalable mass manufacturing. | 20 and 60 nm at a flow rate of 20–50 mL/min | Maeki et al. [99] | |
| 3D-printed ring micromixers with controllable flow rate and ring asymmetry enable high-throughput production of size-controlled, monodisperse lipid nanoparticles with efficient mRNA encapsulation. | Diameters less than 90 nm, low polydispersity (<0.2), and high mRNA encapsulation efficiency (>91%) | Hong et al. [100] | |
| A stereolithography-fabricated 3D-printed microfluidic device using omnidirectional sheath flow and a staggered herringbone mixer enables high-throughput (60 mL min−1) production of mRNA-loaded lipid nanoparticles. | Diameter less than 90 nm, with low polydispersity (2–8%) and high mRNA encapsulation efficiency (>90%). | Lin et al. [101] | |
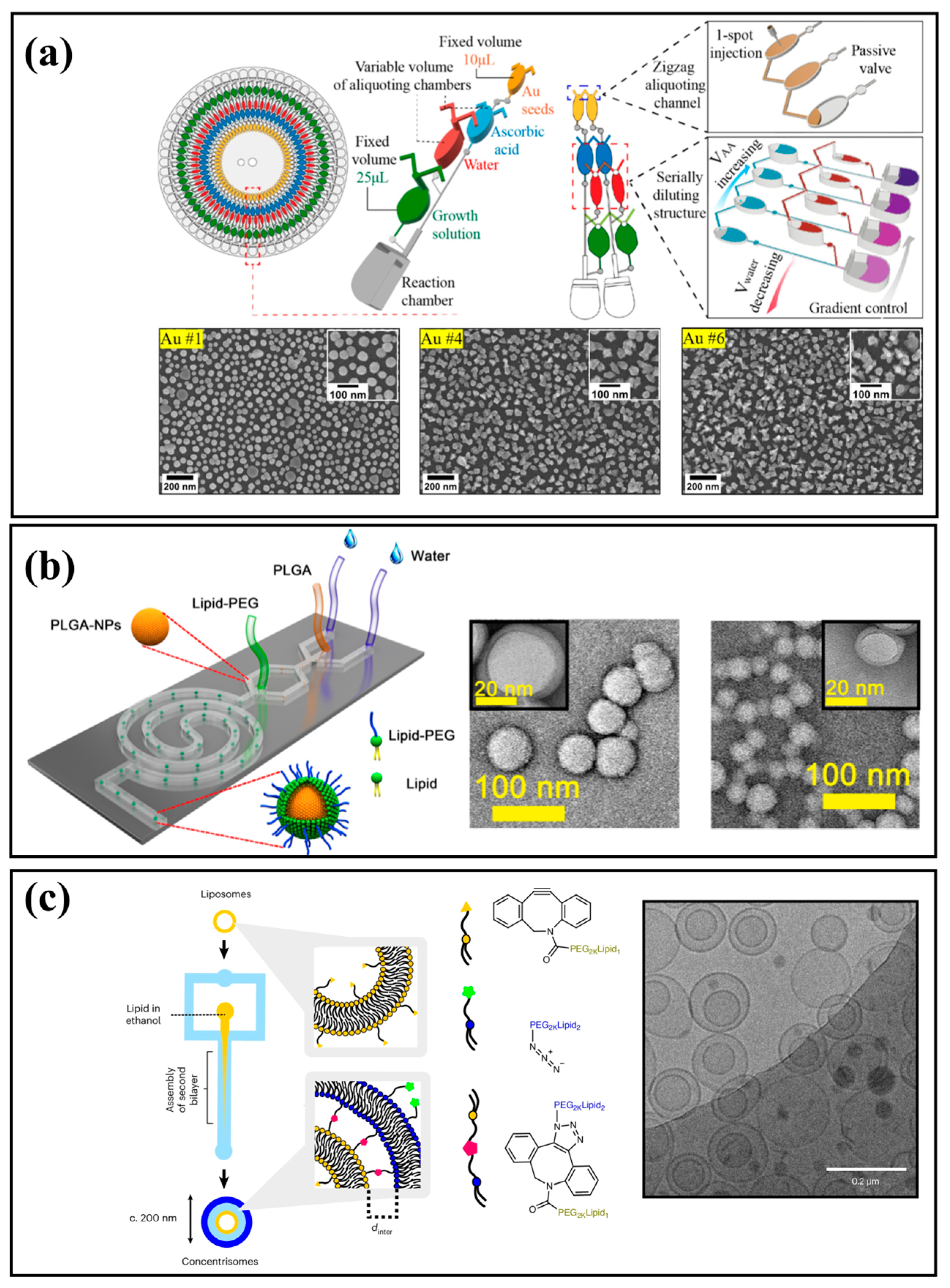
| Metallic NPs | A high-throughput centrifugal microfluidic platform integrated with a portable automated workstation enables 60 parallel gold nanoparticle syntheses. | 120.5 nm, 117.3 nm, and 114.1 nm in diameter | Nguyen et al. [74] |
| A seed-mediated in situ synthesis method was implemented in microfluidic reactors, where flow rate and channel geometry were identified as key parameters influencing gold NPs growth, morphology, and surface coverage. | Nanostar, 60 nm~100 nm | Vinnacombe-Willson et al. [102] | |
| Quantum Dots | A microfluidic Pickering emulsion method was developed to synthesize uniform magnetic/fluorescent microspheres with tunable optical barcoding, using droplet size control, silica nanoparticle stabilization, and quantum dot encapsulation for multiplex tumor marker detection. | High-throughput ultrasensitive detection, the detection limits of 0.027 ng/ mL for CEA, 1.48 KU/L for CA125 and 1.09 KU/L for CA199 | Li et al. [103] |
| A magnetic-field-coupled microfluidic method was used to synthesize Co-doped ZnSe quantum dots, where varying magnetic fields (0–100 mT) controlled doping level, particle size, and band gap, thereby tuning their magnetic and optical properties. | Co-doped ZnCoSe quantum dots (~4–6 nm) | Zhao et al. [104] | |
| A microfluidic dripping technique was employed to fabricate acrylamide polymer microspheres doped with AgInS2/ZnS quantum dots, controlling droplet formation via flow rates and channel design parameters. | Mean value of the decay time for quantum dots in solutions is 91 and 3.5 ns | Kurassova et al. [105] |
| Mixing Mechanism/Geometry | Typical Mixing Time | Key Features/Advantages |
|---|---|---|
| Hydrodynamic Flow Focusing | Good size control; narrow size distribution; smaller particles; high encapsulation efficiency for drug delivery; reproducible. | |
| Passive Micromixers (e.g., lamination, staggered herringbone) | Depending on channel design and flow rates. SHM can achieve chaotic mixing within milliseconds. | Good mixing without external fields; relatively simple devices; lower energy/ lower complexity. |
| Droplet-Based Microfluidics | Rapid solute homogenization via internal circulation (some reported a few milliseconds). | Excellent compartmentalization; reduced cross-contamination; control over individual reaction “chambers”; improved mixing via internal flows. |
| Active Micromixers (acoustic, electrical, etc.) | Some active mixers achieve mixing times faster than passive counterparts (i.e., lower ms), though exact reported values depend on device. | Mixing can be tuned; high efficiency even at higher flow rates; can reduce required channel length; may reduce residence time. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Hu, G. Harnessing Microfluidics for the Effective and Precise Synthesis of Advanced Materials. Micromachines 2025, 16, 1106. https://doi.org/10.3390/mi16101106
Qi X, Hu G. Harnessing Microfluidics for the Effective and Precise Synthesis of Advanced Materials. Micromachines. 2025; 16(10):1106. https://doi.org/10.3390/mi16101106
Chicago/Turabian StyleQi, Xinlei, and Guoqing Hu. 2025. "Harnessing Microfluidics for the Effective and Precise Synthesis of Advanced Materials" Micromachines 16, no. 10: 1106. https://doi.org/10.3390/mi16101106
APA StyleQi, X., & Hu, G. (2025). Harnessing Microfluidics for the Effective and Precise Synthesis of Advanced Materials. Micromachines, 16(10), 1106. https://doi.org/10.3390/mi16101106







