A CMOS Hybrid System for Non-Invasive Hemoglobin and Oxygen Saturation Monitoring with Super Wavelength Infrared Light Emitting Diodes
Abstract
1. Introduction
2. Related Previous Works
3. Circuit Description
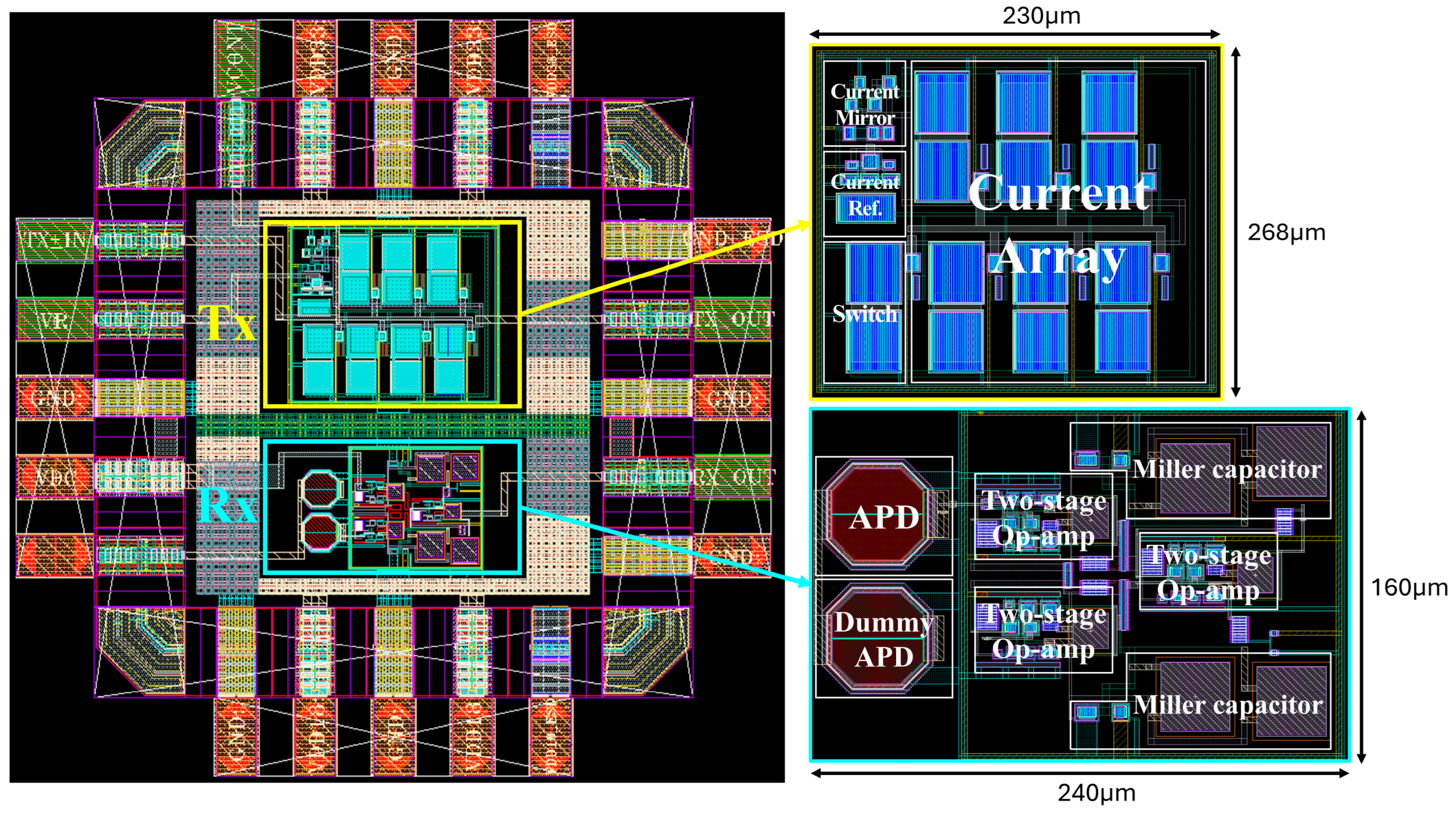
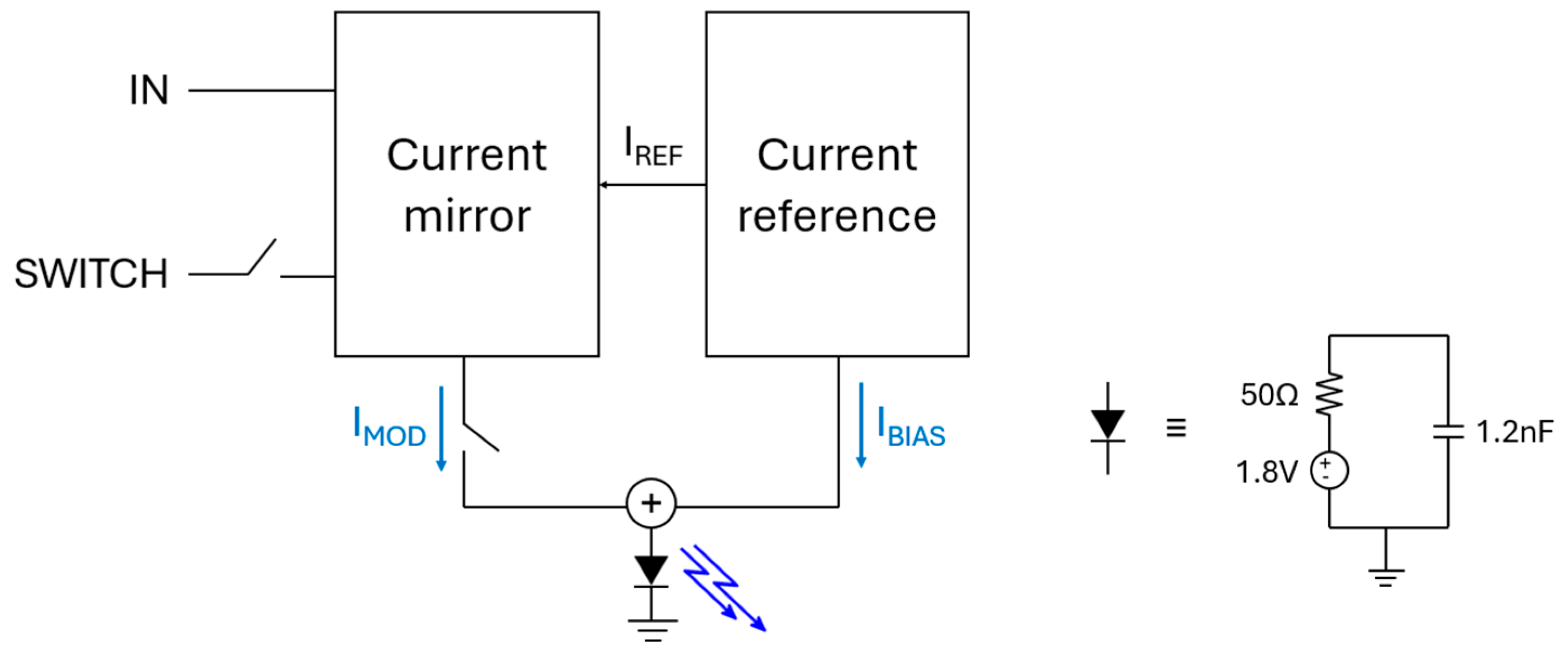
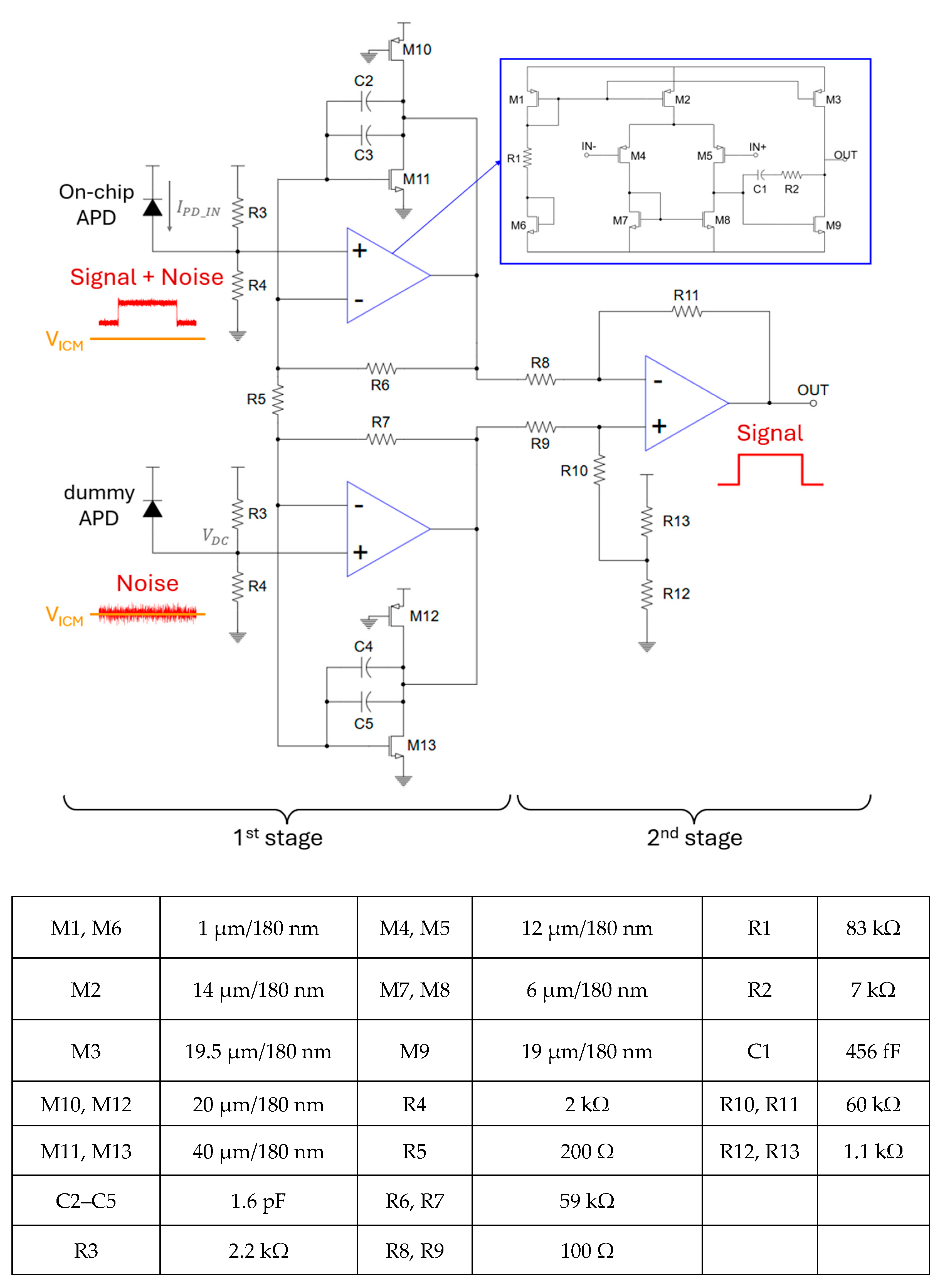
3.1. Analog Circuit Design
3.2. Digital Circuit
3.2.1. The 8th Order IIR Digital Band-Pass Filter (BPF)
3.2.2. The 60-Tap FIR Digital Low-Pass Filter (LPF)
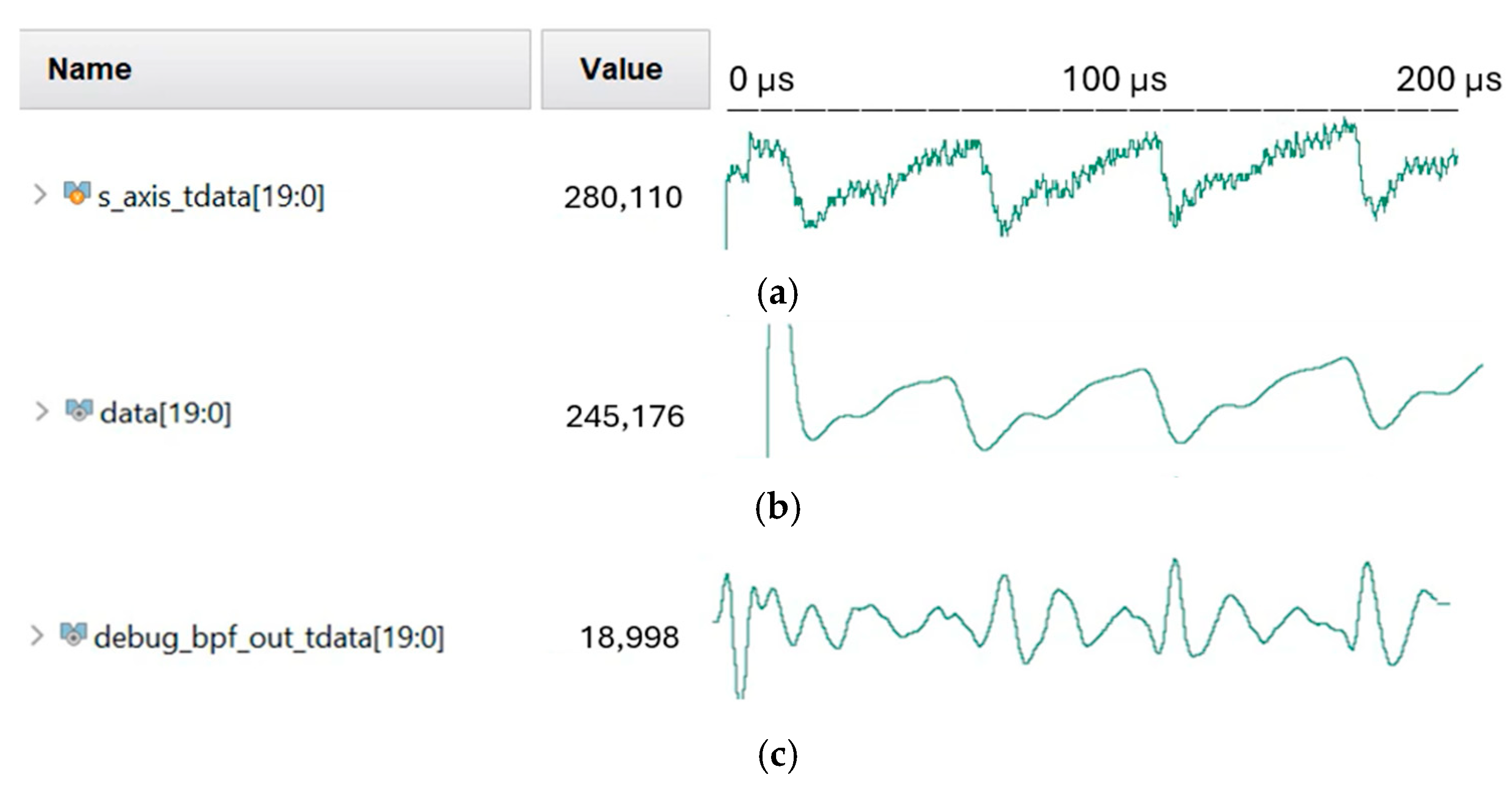
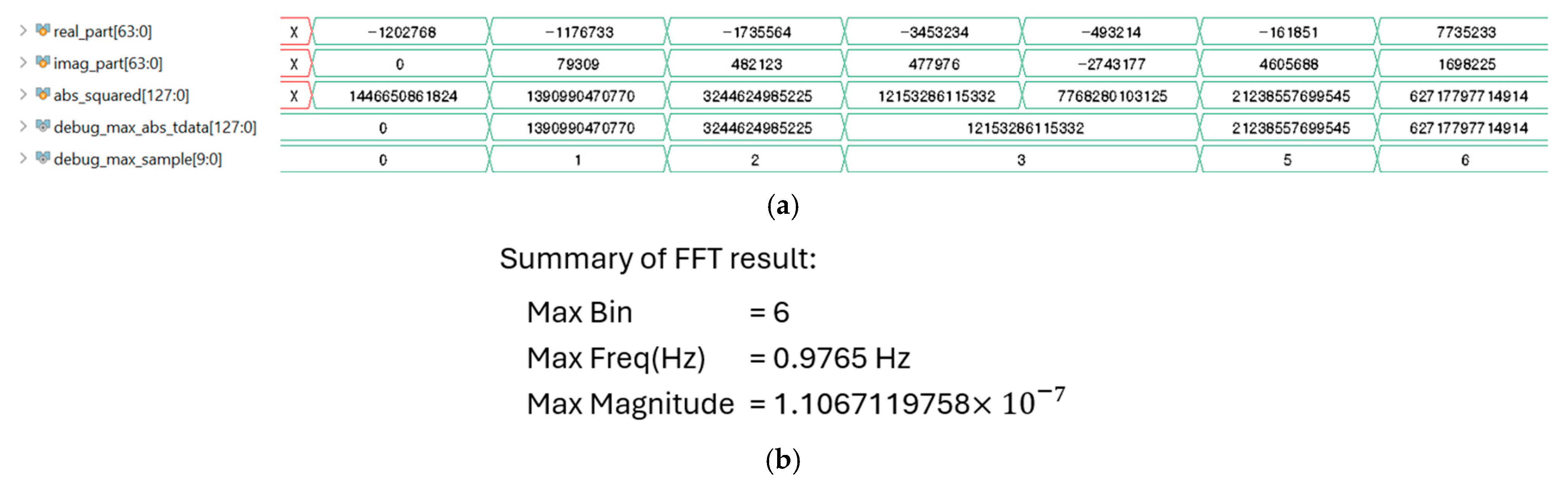
3.2.3. The 1024-Point Fast Fourier Transform (FFT)
3.2.4. Data Reader
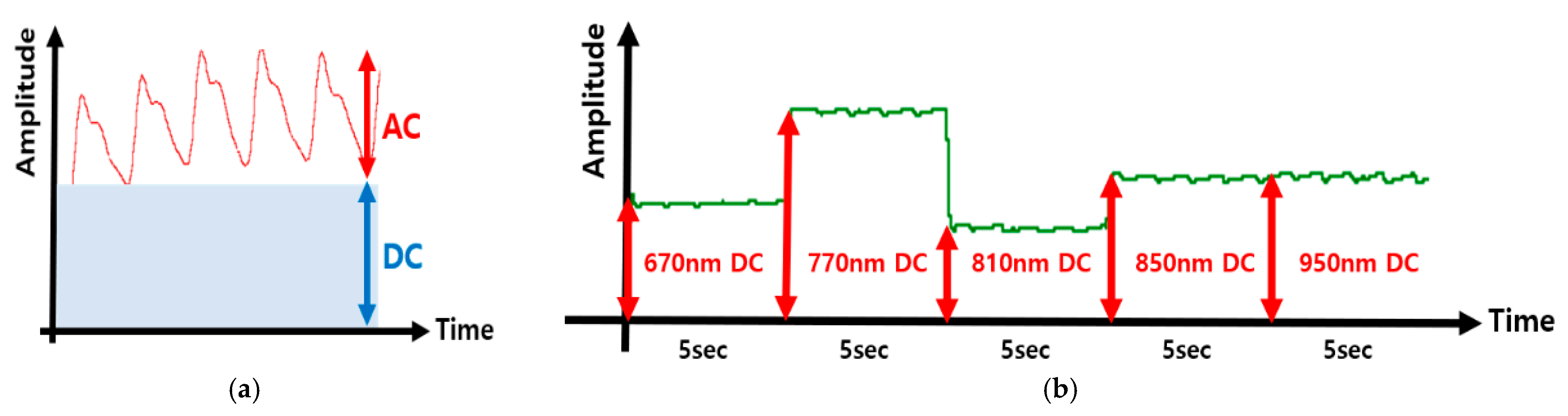
3.2.5. AC (Peak-to-Peak) Amplitude Extraction
3.2.6. DC (Chunk Average) Level Estimation
3.2.7. Calculation (Modified Beer–Lambert Law)
3.2.8. Calibration
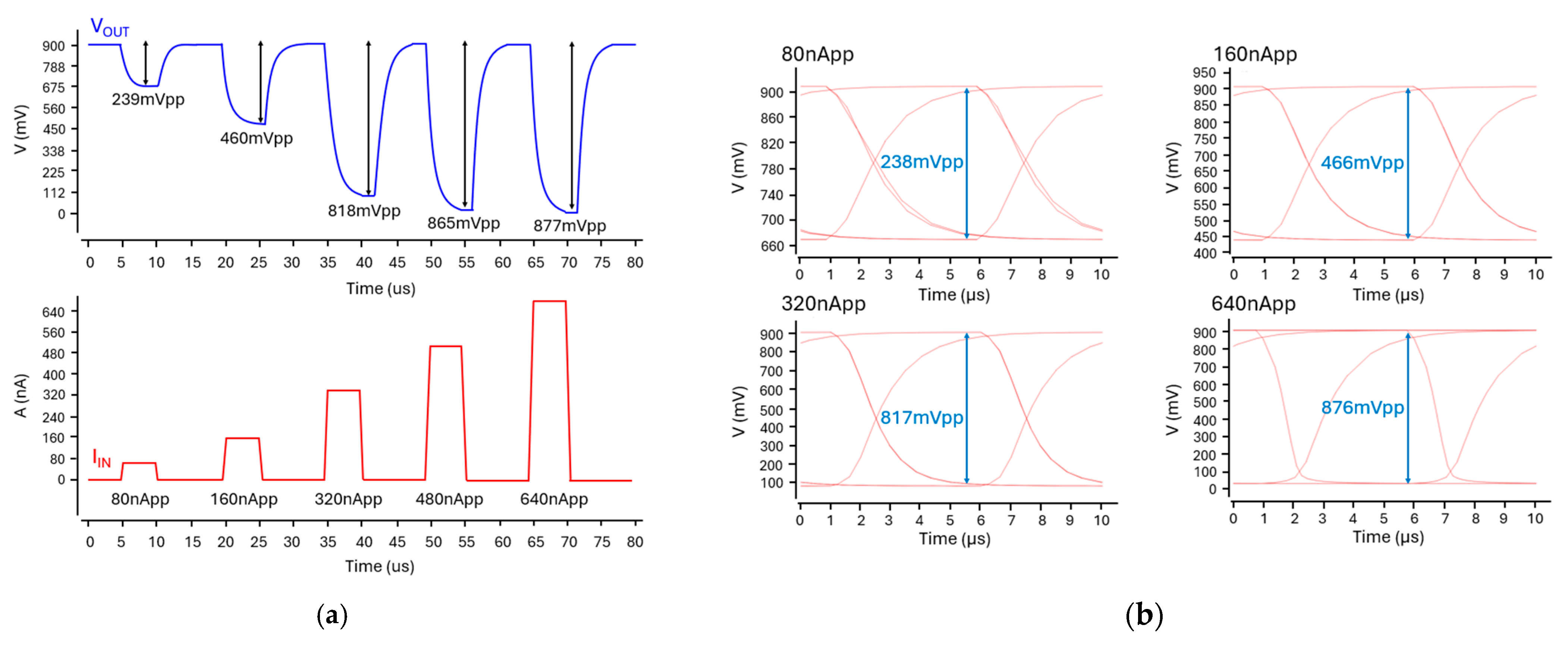
3.3. Simulation Results
4. Discussions
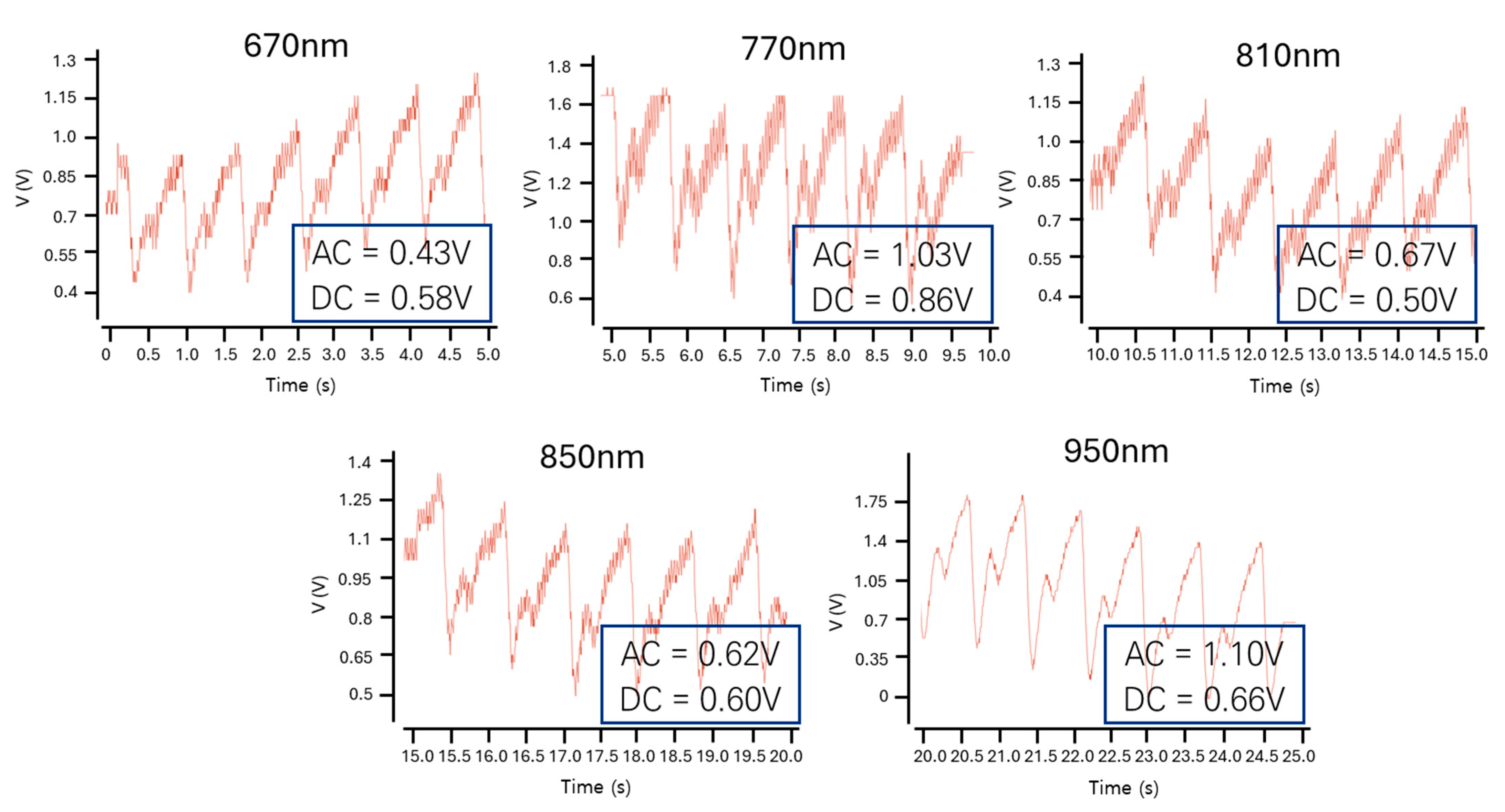
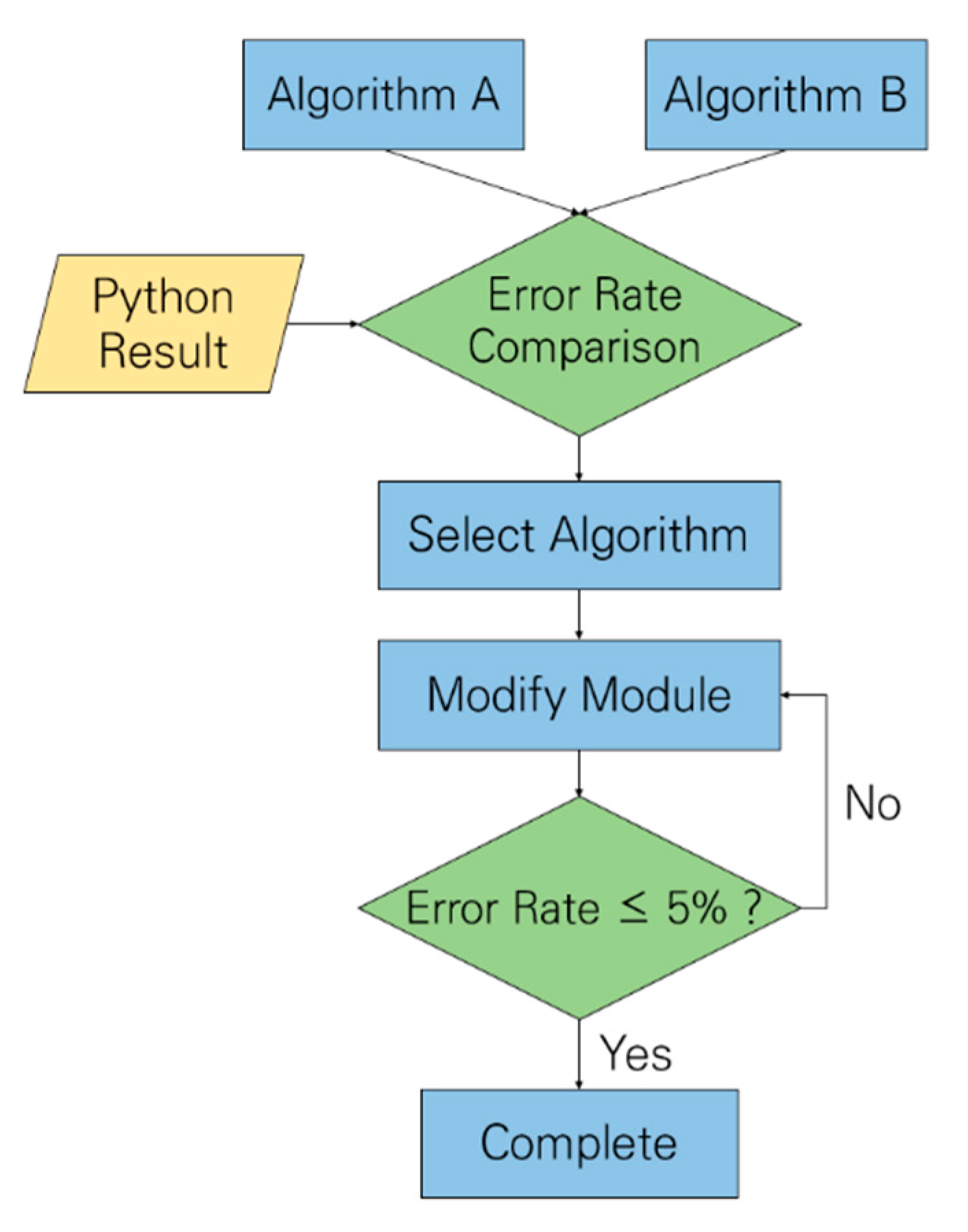
4.1. Algorithm Verification
4.2. Analog Circuit Verification Using Virtuoso
4.3. Verification Using Programming Languages
4.4. Verilog HDL Simulation Verification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—Past and present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Sharma, C.; Kumar, S.; Bhargava, A.; Chowdhury, S.R. Field programmable gate array based embedded system for non-invasive estimation of hemoglobin in blood using photoplethysmography. Int. J. Smart Sens. Intell. Syst. 2013, 6, 1267–1282. [Google Scholar] [CrossRef]
- Jhuma, F.A.; Harada, K.; Misran, M.A.B.; Mo, H.-W.; Fujimoto, H.; Hattori, R. A Hybrid Photoplethysmography (PPG) Sensor System Design for Heart Rate Monitoring. Sensors 2024, 24, 7634. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Yoo, H. Spectroscopic optical coherence tomography: A review of concepts and biomedical applications. Appl. Spectrosc. Rev. 2017, 53, 91–111. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles and limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Banik, P.P.; Hossain, S.; Kwon, T.-H.; Kim, H.; Kim, K.-D. Development of a Wearable Reflection-Type Pulse Oximeter System to Acquire Clean PPG Signals and Measure Pulse Rate and SpO2 with and without Finger Motion. Electronics 2020, 9, 1905. [Google Scholar] [CrossRef]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef] [PubMed]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Padma, T.; Jahnavi, P. Non-Invasive Haemoglobin Estimation through Embedded Technology on Mobile Application. Int. J. Appl. Eng. Res. 2018, 13, 7853–7856. [Google Scholar]
- Jeon, K.H.; Kwon, J.M.; Kim, K.H.; Kim, M.J.; Lee, S.H.; Baek, S.D.; Jeung, S.M.; Park, J.S.; Choi, R.K.; Oh, B.H. Deep-learning-based artificial intelligence algorithm for detecting anemia using electrocardiogram. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.3446. [Google Scholar] [CrossRef]
- Timm, U.; Mcgrath, D.; Lewis, E.; Kraitl, J.; Ewald, H. Sensor system for non-invasive optical hemoglobin determination. In Proceedings of the SENSORS, 2009 IEEE, Christchurch, New Zealand, 25 October 2009; pp. 1975–1978. [Google Scholar] [CrossRef]
- Taylor-Williams, M.; Spicer, G.; Bale, G.; Bohndiek, S.E. Noninvasive hemoglobin sensing and imaging: Optical tools for disease diagnosis. J. Biomed. Opt. 2022, 27, 080901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, R.; Liu, H.; Wang, T.; Cai, L.; Chen, Z.; Heng, B. A Non-Invasive Hemoglobin Detection Device Based on Multispectral Photoplethysmography. Biosensors 2024, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Parab, J.; Sequeira, M.; Naik, G. Improving hemoglobin estimation accuracy through standardizing of light-emitting diode power. Int. J. Electr. Comput. Eng. (IJECE) 2022, 12, 219–228. [Google Scholar] [CrossRef]
- Pinto, C.; Parab, J.; Naik, G. Non-invasive hemoglobin measurement using embedded platform. Sens. Bio-Sens. Res. 2020, 29, 100370. [Google Scholar] [CrossRef]
- Noiri, E.; Kobayashi, N.; Takamura, Y.; Iijima, T.; Takagi, T.; Doi, K.; Nakao, A.; Yamamoto, T.; Takeda, S.; Fujita, T. Pulse total-hemoglobinometer provides accurate noninvasive monitoring. Crit. Care Med. 2005, 33, 2831–2835. [Google Scholar] [CrossRef] [PubMed]
- Marktech Optoelectronics. SWIR Multichip Emitter & Detector Datasheet (MTMD142345PDT38); Marktech Optoelectronics: Latham, NY, USA, 2023. [Google Scholar]
- King, S. Luminous Intensity of an LED as a Function of Input Power. ISB J. Phys. 2008, 2, 1–4. Available online: https://isjos.org/ISBJOP/JoPv2i2-3LED.pdf (accessed on 2 April 2025).
- Puranen, A. Effects of LED Wavelength, Intensity and Skin Tone on the Performance of Optical Beat-to-Beat Heart Rate Monitoring. Master’s Thesis, Tampere University, Tampere, Finland, 25 May 2021. [Google Scholar]
- Slade, G. The Fast Fourier Transform in Hardware: A Tutorial Based on an FPGA Implementation; ResearchGate: Berlin, Germany, 2013. [Google Scholar]
- Richards, M.A. On hardware implementation of the split-radix FFT. IEEE Trans. Acoust. Speech Signal Process. 1988, 36, 1575–1581. [Google Scholar] [CrossRef]







| Term | Definition | Normal Range |
|---|---|---|
| Total Hemoglobin (tHb) | Concentration of all hemoglobin in the blood (oxygenated hemoglobin (HbO2) + deoxygenated hemoglobin (Hb)) | Female: 12–16 g/dL Male: 14–18 g/dL |
| Blood Oxygen Saturation (SpO2) | Percentage of hemoglobin molecules bound with oxygen relative to the total hemoglobin SpO2 = (HbO2/tHb) × 100 | 95–100% |
| Wavelength | HbO2 Absorption | Hb Absorption | Characteristics |
|---|---|---|---|
| 670 nm (Red) | Low | High | Hb-selective absorption |
| 950 nm (NIR) | High | Low | HbO2-selective absorption |
| 810 nm | Medium | Medium | Isosbestic point |
| Parameter | Target Performance | Benchmark/Remark |
|---|---|---|
| tHb concentration | ±0.3 g/dL | Over 80% improvement vs. 3-wavelength PPG (avg. error 1.38 g/dL); comparable to blood test accuracy |
| SpO2 concentration | ±1.5% | Comparable to commercial pulse oximeters |
| Heart rate | ±0.2% | Higher precision than commercial pulse oximeters |
| Real-time and robustness | FPGA-based high-speed AC/DC extraction Motion artifact suppression via local peak filtering | Reliable under movement and noise |
| Parameters | LED Driver (Tx) | Optoelectronic Rx | ||
|---|---|---|---|---|
| CMOS Process (nm) | 180 | 180 | ||
| Operating Voltage (V) | 3.3 | 1.8 | ||
| Optical Components | Five-wavelength multichip LED emitter | TIA integrated w/on-chip APD | ||
| Signal Configuration | Single-ended | Single-ended | ||
| Topology | Common cathode | IA-based TIA | ||
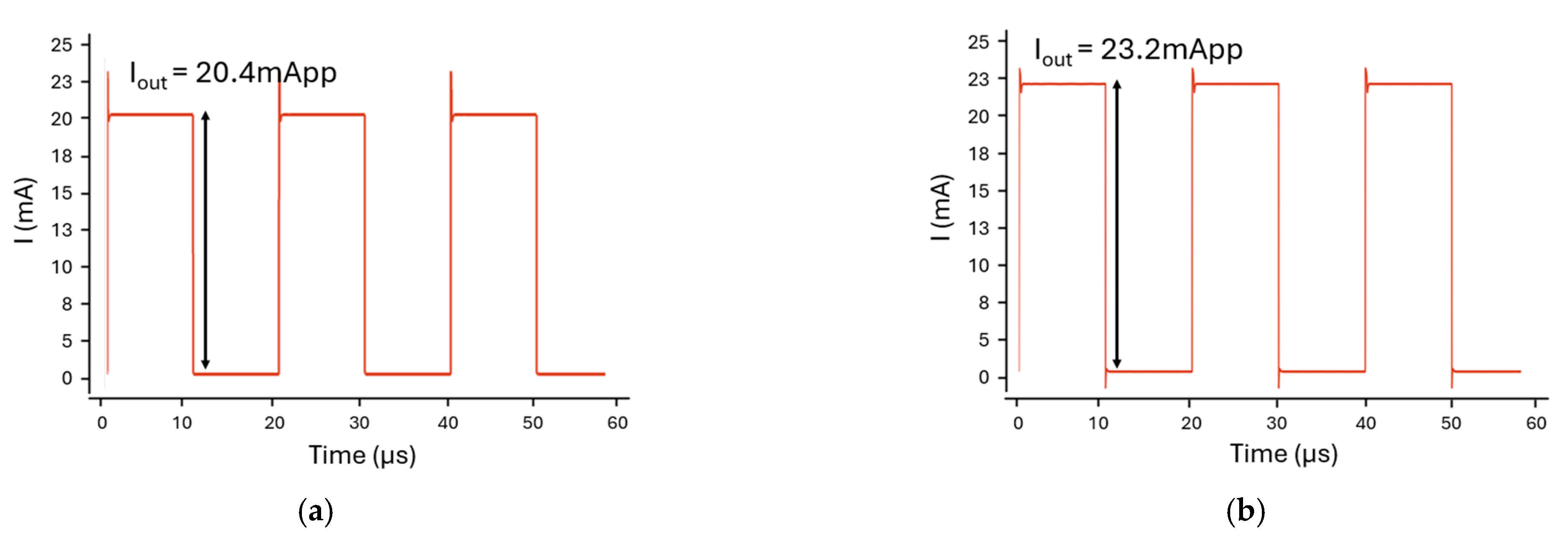
| Performance | Modulation current (mA) | 0–23.5 | TIA gain (dBΩ) | 129 |
| Bias current (mA) | 3.6 | Bandwidth (kHz) | 85 | |
| Max Power Consumption (mW) | 73.4 | 19 | ||
| Area (µm2) | 230 × 268 | 240 × 160 | ||
| Parameters | Proposed Method | Blood Test | Commercial Noninvasive Devices |
|---|---|---|---|
| tHb (g/dL) | 11.8 | 11.5 | Not provided |
| SpO2 (%) | 98 | – | 98–99 |
| Filter (Hz) | 0.5–3 | – | 0.5–5.5 |
| Heart Rate (bpm) | 58.6 | – | 60 |
| Parameter | Proposed Method | Commercial Non-Invasive Products | |
|---|---|---|---|
| SpO2 (%) | Measurement Range | 70–100 | 70–100 |
| Accuracy | Within ±1.5% | Within ±2% | |
| Heart Rate (bpm) | Measurement Range | 30–180 | 30–235 |
| Accuracy | Within ±0.2% | Within ±2% | |
| tHb (g/dL) | ±0.3 | Not provided; blood test results used as target | |
| Max Power Consumption (mW) | 92.4 | <40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kang, S.; Kim, J.; Lee, J.; Park, S.; Park, S.-M. A CMOS Hybrid System for Non-Invasive Hemoglobin and Oxygen Saturation Monitoring with Super Wavelength Infrared Light Emitting Diodes. Micromachines 2025, 16, 1086. https://doi.org/10.3390/mi16101086
Park H, Kang S, Kim J, Lee J, Park S, Park S-M. A CMOS Hybrid System for Non-Invasive Hemoglobin and Oxygen Saturation Monitoring with Super Wavelength Infrared Light Emitting Diodes. Micromachines. 2025; 16(10):1086. https://doi.org/10.3390/mi16101086
Chicago/Turabian StylePark, Hyunjin, Seoyeon Kang, Jiwon Kim, Jeena Lee, Somi Park, and Sung-Min Park. 2025. "A CMOS Hybrid System for Non-Invasive Hemoglobin and Oxygen Saturation Monitoring with Super Wavelength Infrared Light Emitting Diodes" Micromachines 16, no. 10: 1086. https://doi.org/10.3390/mi16101086
APA StylePark, H., Kang, S., Kim, J., Lee, J., Park, S., & Park, S.-M. (2025). A CMOS Hybrid System for Non-Invasive Hemoglobin and Oxygen Saturation Monitoring with Super Wavelength Infrared Light Emitting Diodes. Micromachines, 16(10), 1086. https://doi.org/10.3390/mi16101086










