Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation
Abstract
1. Introduction
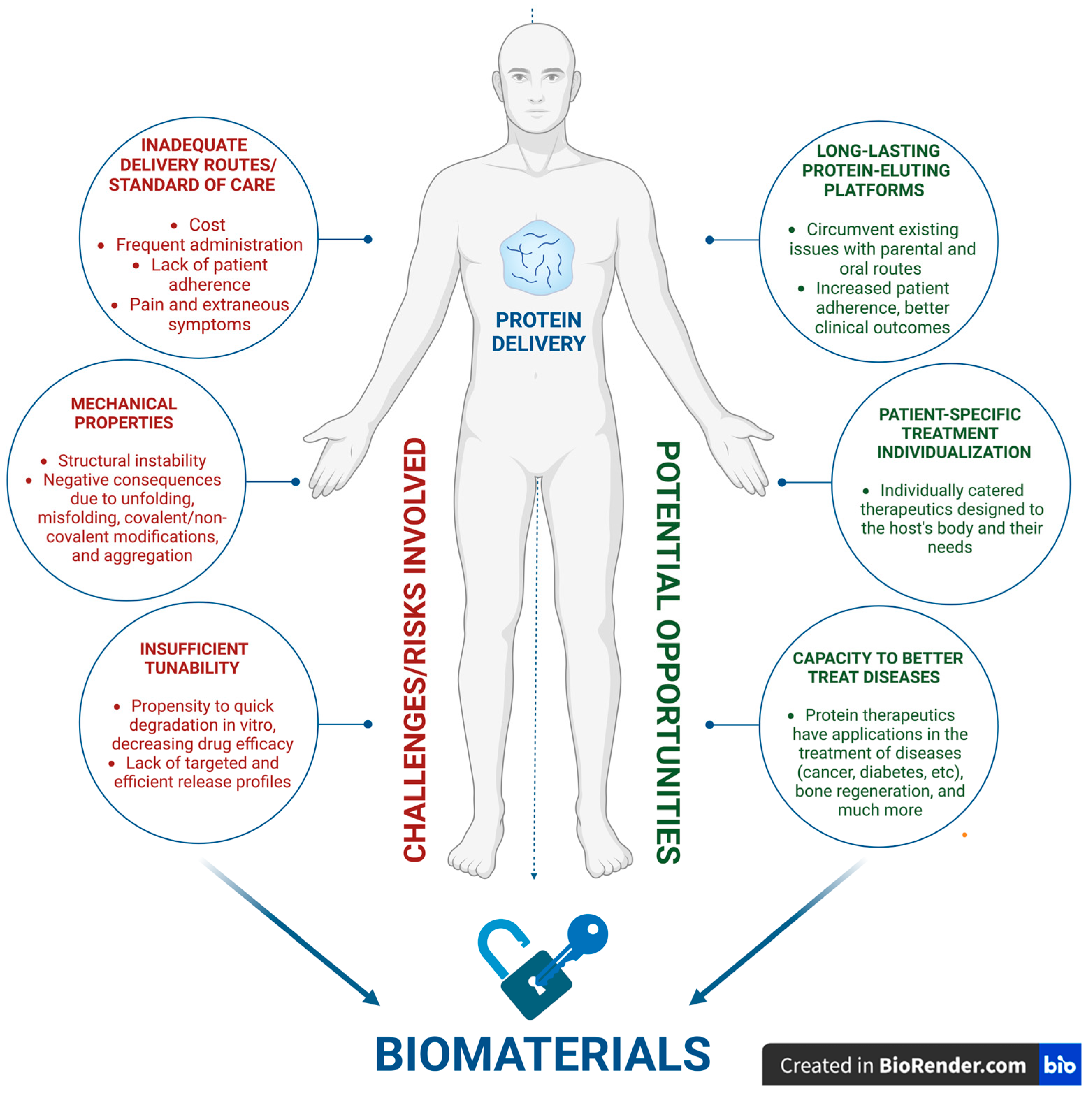
2. Opportunities and Challenges
2.1. Viable Biomaterial Platforms for Therapeutic Protein Delivery
2.1.1. Hydrogels
2.1.2. Scaffold Systems
2.1.3. Nanogels
2.1.4. Polymeric Nanoparticles
2.2. Efficient and Targeted Delivery: The Core Challenge
2.3. Addressing Immunogenicity and Biocompatibility: Safety, Toxicity, and Tolerability
2.4. Stability Issues
2.5. Production Scale-Up and Navigating Regulatory Challenges
2.6. Advancements in Biomaterial Fabrication Technologies
2.7. Nanotechnology, Hybrid Biomaterials, and Bioprinting—The Next Frontier
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abune, L.; Wang, Y. Affinity Hydrogels for Protein Delivery. Trends Pharmacol. Sci. 2021, 42, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-based biomaterials for protein delivery. Med. Drug Discov. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Ye, Q.N.; Wang, Y.; Shen, S.; Xu, C.F.; Wang, J. Biomaterials-Based Delivery of Therapeutic Antibodies for Cancer Therapy. Adv. Healthc. Mater. 2021, 10, e2002139. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Sethiya, N.K.; Gulbake, A.S.; Mehra, N.K.; Murty, U.S.N.; Gulbake, A. A review on albumin as a biomaterial for ocular drug delivery. Int. J. Biol. Macromol. 2021, 191, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Merivaara, A.; Zini, J.; Koivunotko, E.; Valkonen, S.; Korhonen, O.; Fernandes, F.M.; Yliperttula, M. Preservation of biomaterials and cells by freeze-drying: Change of paradigm. J. Control. Release 2021, 336, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Liu, Z. Protein-Engineered Biomaterials for Cancer Theranostics. Adv. Healthc. Mater. 2018, 7, e1800913. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 155. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Kokare, C.R. Potential biomaterials and experimental animal models for inventing new drug delivery approaches in the neurodegenerative disorder: Multiple sclerosis. Brain Res. 2024, 1822, 148674. [Google Scholar] [CrossRef]
- Nayab, D.E.; Din, F.U.; Ali, H.; Kausar, W.A.; Urooj, S.; Zafar, M.; Khan, I.; Shabbir, K.; Khan, G.M. Nano biomaterials-based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: An up-to-date perspective. J. Nanobiotechnol. 2023, 21, 477. [Google Scholar] [CrossRef]
- Agrawal, M.; Prathyusha, E.; Ahmed, H.; Dubey, S.K.; Kesharwani, P.; Singhvi, G.; Naidu, V.G.M.; Alexander, A. Biomaterials in treatment of Alzheimer’s disease. Neurochem. Int. 2021, 145, 105008. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cui, W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv. Drug Deliv. Rev. 2021, 176, 113885. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Chen, S.-H. Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Mukunda, D.C.; Rodrigues, J.; Gail D’souza, M.; Gangadharan, G.; Pai, A.R.; Mahato, K.K. A comprehensive review of protein misfolding disorders, underlying mechanism, clinical diagnosis, and therapeutic strategies. Ageing Res. Rev. 2023, 90, 102017. [Google Scholar] [CrossRef] [PubMed]
- Dembélé, J.; Liao, J.H.; Liu, T.P.; Chen, Y.P. Overcoming Cytosolic Delivery Barriers of Proteins Using Denatured Protein-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Annabi, N.; Tamayol, A.; Oklu, R.; Ghanem, A.; Khademhosseini, A. Adenosine-associated delivery systems. J. Drug Target. 2015, 23, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, G.; Yao, X.; Zhou, H.; Lyu, B.; Pei, S.; Wen, P. Microneedle-based insulin transdermal delivery system: Current status and translation challenges. Drug Deliv. Transl. Res. 2022, 12, 2403–2427. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 413–428. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Wang, J.; Liu, C.; Kong, N.; Farokhzad, O.C.; Tao, W. Oral Insulin Delivery Platforms: Strategies To Address the Biological Barriers. Angew. Chem. (Int. Ed. Engl.) 2020, 59, 19787–19795. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from Nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yuvienco, C.; Montclare, J.K. Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials 2017, 134, 91–116. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H. Advanced trends in protein and peptide drug delivery: A special emphasis on aquasomes and microneedles techniques. Drug Deliv. Transl. Res. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Chapa-Villarreal, F.A.; Miller, M.; Rodriguez-Cruz, J.J.; Pérez-Carlos, D.; Peppas, N.A. Self-assembled block copolymer biomaterials for oral delivery of protein therapeutics. Biomaterials 2023, 300, 122191. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Lillard, J.W., Jr. Past, present, and future technologies for oral delivery of therapeutic proteins. J. Pharm. Sci. 2008, 97, 2497–2523. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- “Biomaterials.” National Institute of Biomedical Imaging and Bioengineering, U.S. Department of Health and Human Services. 2017. Available online: www.nibib.nih.gov/science-education/science-topics/biomaterials (accessed on 10 February 2024).
- Wu, J.; Sahoo, J.K.; Li, Y.; Xu, Q.; Kaplan, D.L. Challenges in delivering therapeutic peptides and proteins: A silk-based solution. J. Control. Release Off. J. Control. Release Soc. 2022, 345, 176–189. [Google Scholar] [CrossRef]
- Choi, S.M.; Chaudhry, P.; Zo, S.M.; Han, S.S. Advances in protein-based materials: From origin to novel biomaterials. Adv. Exp. Med. Biol. 2018, 1078, 161–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A Review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Aktar, M.N.; Hossain, M.S.; Sarkar, N.; Islam, M.R.; Arafat, M.E.; Bhowmik, S.; Yusa, S.-I. Recent Advances in Micro- and Nano-Drug Delivery Systems Based on Natural and Synthetic Biomaterials. Polymers 2023, 15, 4563. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Zou, Y.; Hu, J.; Li, Y.; Cheng, Y. Natural polyphenols in drug delivery systems: Current status and future challenges. Giant 2020, 3, 100022. [Google Scholar] [CrossRef]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Q.; Lin, J.; Cai, Z.; Liao, G.; Wang, K.; Bai, L.; Zhao, P.; Yu, Z. Recent Advance in Polymer Based Microspheric Systems for Controlled Protein and Peptide Delivery. Curr. Med. Chem. 2019, 26, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Aiswarya, T.T.; Mirza, I.; Saha, S. Biocompatible polymers and their applications. Encycl. Mater. Plast. Polym. 2022, 2, 796–819. [Google Scholar] [CrossRef]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Vunjak-Novakovic, G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res. 2015, 5, 101–115. [Google Scholar] [CrossRef]
- Chander, S.; Kulkarni, G.T.; Dhiman, N.; Kharkwal, H. Protein-Based Nanohydrogels for Bioactive Delivery. Front. Chem. 2021, 9, 573748. [Google Scholar] [CrossRef]
- Petrak, K. Essential properties of drug-targeting delivery systems. Drug Discov. Today 2005, 10, 1667–1673. [Google Scholar] [CrossRef]
- Huang, W.; Rollett, A.; Kaplan, D.L. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015, 12, 779–791. [Google Scholar] [CrossRef]
- Nie, T.; Wang, W.; Liu, X.; Wang, Y.; Li, K.; Song, X.; Zhang, J.; Yu, L.; He, Z. Sustained Release Systems for Delivery of Therapeutic Peptide/Protein. Biomacromolecules 2021, 22, 2299–2324. [Google Scholar] [CrossRef]
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen based biomaterials for tissue engineering applications: A Review. In Lecture Notes in Earth System Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Khopade, S.; Gomte, S.S.; Janrao, C.; Bavaskar, A.; Agnihotri, T.G.; Jain, A.; Khatik, R. Peptide and protein delivery through cellulose, hyaluronic acid, and heparin. In Peptide and Protein Drug Delivery Using Polysaccharides; Academic Press: Cambridge, MA, USA, 2024; pp. 75–113. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, J.Z.; Fei, X.; Li, Y.; Song, Y.; Qian, Z.; Peng, Q. Can nanoparticles and nano–protein interactions bring a bright future for insulin delivery? Acta Pharm. Sin. B 2021, 11, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regen-erative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Lebeau, J.; Efromson, J.P.; Lynch, M.D. A Review of the Biotechnological Production of Methacrylic Acid. Front. Bioeng. Biotechnol. 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Karnoosh-Yamchi, J.; Mobasseri, M.; Akbarzadeh, A.; Davaran, S.; Ostad-Rahimi, A.R.; Hamishehkar, H.; Salehi, R.; Bahmani, Z.; Nejati-Koshki, K.; Darbin, A.; et al. Preparation of pH sensitive insulin-loaded nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol. Biol. Rep. 2014, 41, 6705–6712. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Saptaji, K.; Iza, N.R.; Widianingrum, S.; Mulia, V.K.; Setiawan, I. Poly(2-hydroxyethyl methacrylate) hydrogels for contact lens applications–a review. Makara J. Sci. 2021, 25, 3–154. [Google Scholar] [CrossRef]
- Bhatti, S.S.; Singh, J. 3D printing of Biomaterials for Biomedical Applications: A Review. Int. J. Interact. Des. Manuf. (IJIDeM) 2023. [Google Scholar] [CrossRef]
- Davis, B.K. Control of diabetes with polyacrylamide implants containing insulin. Experientia 1972, 28, 348. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jones, C.N.; Revzin, A.; Tae, G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010, 31, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Huerta-López, C.; Alegre-Cebollada, J. Protein Hydrogels: The Swiss Army Knife for Enhanced Mechanical and Bioactive Properties of Biomaterials. Nanomaterials 2021, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef]
- Abune, L.; Davis, B.; Wang, Y. Aptamer-functionalized hydrogels: An emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1731. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, J.; Cai, F.; Zhang, F.; Yang, M.; Bi, S.; Gui, R.; Li, Y.; Xia, Y. Aptamer-functionalized hydrogel as effective anti-cancer drugs delivery agents. Colloids Surf. B Biointerfaces 2015, 134, 40–46. [Google Scholar] [CrossRef]
- Kang, H.; Trondoli, A.C.; Zhu, G.; Chen, Y.; Chang, Y.J.; Liu, H.; Huang, Y.F.; Zhang, X.; Tan, W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano 2011, 5, 5094–5099. [Google Scholar] [CrossRef]
- Bae, K.H.; Kurisawa, M. Emerging hydrogel designs for controlled protein delivery. Biomater. Sci. 2016, 4, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B Rev. 2010, 16, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Khan, R.S.; Rather, A.H.; Beigh, M.A.; Sheikh, F.A. Local dual delivery therapeutic strategies: Using biomaterials for advanced bone tissue regeneration. J. Control. Release Off. J. Control. Release Soc. 2021, 339, 143–155. [Google Scholar] [CrossRef]
- Piotrowicz, A.; Shoichet, M.S. Nerve guidance channels as drug delivery vehicles. Biomaterials 2006, 27, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Mukai, S.; Sasaki, Y.; Akiyoshi, K. Nanogel tectonics for tissue engineering: Protein delivery systems with Nanogel chaperones. Adv. Healthc. Mater. 2018, 7, e1800729. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Anooj, E.; Charumathy, M.; Sharma, V.; Vibala, B.V.; Gopukumar, S.T.; Jainab, S.I.B.; Vallinayagam, S. Nanogels: An overview of properties, biomedical applications, Future Research Trends and developments. J. Mol. Struct. 2021, 1239, 130446. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Research 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvi-ronments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric System for Dual Growth Factor Delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Micro-spheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and Design of Polymeric Surfactant-Based Drug Delivery Systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active Targeting Schemes for Nanoparticle Systems in Cancer Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Poly-meric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(Lactide-Co-Glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of Poly(Lactic-Co-Glycolic Acid) Microspheres: Effect of Copolymer Composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Con-trolled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2012, 64 (Suppl. S1), 37–48. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from Lipid Derivatives of Water-Soluble Polymers as Delivery Systems for Poorly Soluble Drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Block Copolymer Micelles as Long-Circulating Drug Vehicles. Adv. Drug Deliv. Rev. 1996, 16, 295–309. [Google Scholar] [CrossRef]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for Peptide and Protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Mahler, H.C.; Friess, W.; Grauschopf, U.; Kiese, S. Protein Aggregation: Pathways, Induction Factors and Analysis. J. Pharm. Sci. 2009, 98, 2909–2934. [Google Scholar] [CrossRef]
- Ratanji, K.D.; Derrick, J.P.; Dearman, R.J.; Kimber, I. Immunogenicity of Therapeutic Proteins: Influence of Aggregation. J. Immunotoxicol. 2014, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, M.E.M.; Hilario, E.; Jacobson, F. Protein Aggregation and Bioprocessing. AAPS J. 2006, 8, E572–E579. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Schwartz, A.G.; Xia, Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, Y.; Li, Y.; Zhao, P.; Zhu, K.; Chen, W. A Facile Technique to Prepare Biodegrada-ble Coaxial Electrospun Nanofibers for Controlled Release of Bioactive Agents. J. Control. Release 2005, 108, 237–243. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly(Ethylene-Co-Vinylacetate), Poly(Lactic Acid), and a Blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-Caprolactone) Microfiber and Mul-tilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Narang, N.; Sharma, J. Sublingual mucosa as a route for systemic drug delivery. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. S2), 18–22. [Google Scholar]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral Strip Technology: Overview and Future Potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Appli-cations. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applica-tions. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Micro-gels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Nanogels in the race for drug delivery. Nanomedicine 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, A.L.; Bhattacharya, A.; Montclare, J.K. Protein-Based Biomaterials for Thera-peutic and Diagnostic Applications. Prog. Biomed. Eng. 2022, 4, 012003. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Gagner, J.E.; Kim, W.; Chaikof, E.L. Designing protein-based biomaterials for medical applications. Acta Biomater. 2014, 10, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, L.; Cheng, X.; Li, Y.; Cheng, Y. Aminoglycoside-Based Biomaterials: From Mate-rial Design to Antibacterial and Gene Delivery Applications. Adv. Funct. Mater. 2021, 31, 2103718. [Google Scholar] [CrossRef]
- Chambre, L.; Martín-Moldes, Z.; Parker, R.N.; Kaplan, D.L. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv. Drug Deliv. Rev. 2020, 160, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.P.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self/nonself 2010, 1, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Arkin, S.; Cocea, L.; Devanarayan, V.; Kirshner, S.; Kromminga, A.; Quarmby, V.; Richards, S.; Schneider, C.K.; Subramanyam, M.; et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014, 16, 658–673. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Turner, M.R.; Balu-Iyer, S.V. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J. Pharm. Sci. 2018, 107, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, N.L.; Balu-Iyer, S.V. Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Nasiri, H.; Valedkarimi, Z.; Aghebati-Maleki, L.; Majidi, J. Antibody-drug conjugates: Promising and efficient tools for targeted cancer therapy. J. Cell. Physiol. 2018, 233, 6441–6457. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Zhu, Y.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Heymann, D.; Pradal, G.; Benahmed, M. Cellular mechanisms of calcium phosphate ceramic degradation. Histol. Histopathol. 1999, 14, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Espanol, M.; Beats, S.; Ginebra, M.P. In vitro degradation of calcium phosphates: Effect of multiscale porosity, textural properties and composition. Acta Biomater. 2017, 60, 81–92. [Google Scholar] [CrossRef]
- Schaefer, S.; Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity. Adv. Eng. Mater. 2011, 13, 342–350. [Google Scholar] [CrossRef]
- Andrée, L.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Designing biomaterials for the delivery of RNA therapeutics to stimulate bone healing. Mater. Today Bio 2021, 10, 100105. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.P. Protein Adsorption on Biomaterial Surfaces: Subsequent Conformational and Biological Consequences—A Review. J. Surf. Sci. Technol. 2020, 36, 7–38. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein interactions with polymer coatings and biomaterials. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 8004–8031. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Khurana, V.; Patel, S.; Mitra, A.K. Long-term delivery of protein therapeutics. Expert Opin. Drug Deliv. 2015, 12, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Agnieray, H.; Glasson, J.L.; Chen, Q.; Kaur, M.; Domigan, L.J. Recent developments in sustainably sourced protein-based biomaterials. Biochem. Soc. Trans. 2021, 49, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Wren, S.; Minelli, C.; Pei, Y.; Akhtar, N. Evaluation of particle size techniques to support the development of manufacturing scale nanoparticles for application in pharmaceuticals. J. Pharm. Sci. 2020, 109, 2284–2293. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based Ocular Drug Delivery Systems: Emerging Fabrication Strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef]
- Haq-Siddiqi, N.A.; Britton, D.; Kim Montclare, J. Protein-engineered biomaterials for cartilage therapeutics and repair. Adv. Drug Deliv. Rev. 2023, 192, 114647. [Google Scholar] [CrossRef]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Uzunalli, G.; Guler, M.O. Peptide gels for controlled release of proteins. Ther. Deliv. 2020, 11, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A., IV; Acharya, S.; Gadodia, T.; Shukla, S.; Harshita, J.; Akre, C.; Khare, M.; Huse, S. A Review on Techniques and Biomaterials Used in 3D Bioprinting. Cureus 2022, 14, e28463. [Google Scholar] [CrossRef] [PubMed]
- Dorogin, J.; Townsend, J.M.; Hettiaratchi, M.H. Biomaterials for protein delivery for complex tissue healing responses. Biomater. Sci. 2021, 9, 2339–2361. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Almquist, B.D. Spatiotemporal delivery of bioactive molecules for wound healing using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2020, 161–162, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, M.; Forootanfar, H.; Ramakrishna, S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110521. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadehmoghadam, S.; Dong, Y.; Davies, I.J. Modeling Electrospun nanofibers: An overview from theoretical, empirical, and numerical approaches. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 901–915. [Google Scholar] [CrossRef]
- Komatsu, T. Protein-based smart microtubes and nanotubes as ultrasmall biomaterials. Chem. Lett. 2020, 49, 1245–1255. [Google Scholar] [CrossRef]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Teal, C.J.; Lu, S.P.; Shoichet, M.S. Engineering hydrogels for affinity-based release of therapeutic proteins. Chem. Mater. 2024, 36, 614–641. [Google Scholar] [CrossRef]
- Ji, X.; Li, Q.; Song, H.; Fan, C. Protein-mimicking nanoparticles in Biosystems. Adv. Mater. 2022, 34, 2201562. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Yeung, V.; Ross, A.; Kahale, F.; Boychev, N.; Kuang, L.; Chen, L.; Ciolino, J.B. Advances in biomaterials for the treatment of retinoblastoma. Biomater. Sci. 2022, 10, 5391–5429. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Mitragotri, S.; Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: Dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 2020, 12, 6318–6333. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Rehman, K.; Xia, J.; Shabbir, M.; Zaman, M.; Liang, Y.; Duan, L. Biomaterial-assisted targeted and controlled delivery of CRISPR/Cas9 for precise gene editing. Biomater. Sci. 2023, 11, 3762–3783. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Shahrivarkevishahi, A.; Herbert, F.C.; Wijesundara, Y.H.; Gassensmith, J.J. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1735. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Quadros, M.; Momin, M.; Verma, G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater. Sci. Eng. C 2021, 121, 111875. [Google Scholar] [CrossRef] [PubMed]
- Um, W.; Gupta, A.; Song, S.H.; Kim, C.H.; Park, J.H. Biomaterials as antigen delivery carrier for cancer immunotherapy. Macromol. Res. 2021, 29, 834–842. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhen, X.; Wu, W.; Jiang, X. Responsive boron biomaterials and their biomedical applications. Sci. China Chem. 2020, 63, 648–664. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent advances in silk sericin/calcium phosphate biomaterials. Front. Mater. 2020, 7, 24. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate-Containing Biocomposites and Hybrid Biomaterials for Biomedical Applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef]
- Erezuma, I.; Eufrasio-da-Silva, T.; Golafshan, N.; Deo, K.; Mishra, Y.K.; Castilho, M.; Gaharwar, A.K.; Leeuwenburgh, S.; Dolatshahi-Pirouz, A.; Orive, G. Nanoclay Reinforced Biomaterials for Mending Musculoskeletal Tissue Disorders. Adv. Healthc. Mater. 2021, 10, e2100217. [Google Scholar] [CrossRef]
- Cao, D.; Ding, J. Recent advances in regenerative biomaterials. Regen. Biomater. 2022, 9, rbac098. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ouyang, L.; Xu, R.; Yang, Y.; Sun, W. Responsive biomaterials for 3D bioprinting: A Review. Mater. Today 2022, 52, 112–132. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol. Biol. 2012, 899, 1–26. [Google Scholar] [CrossRef] [PubMed]
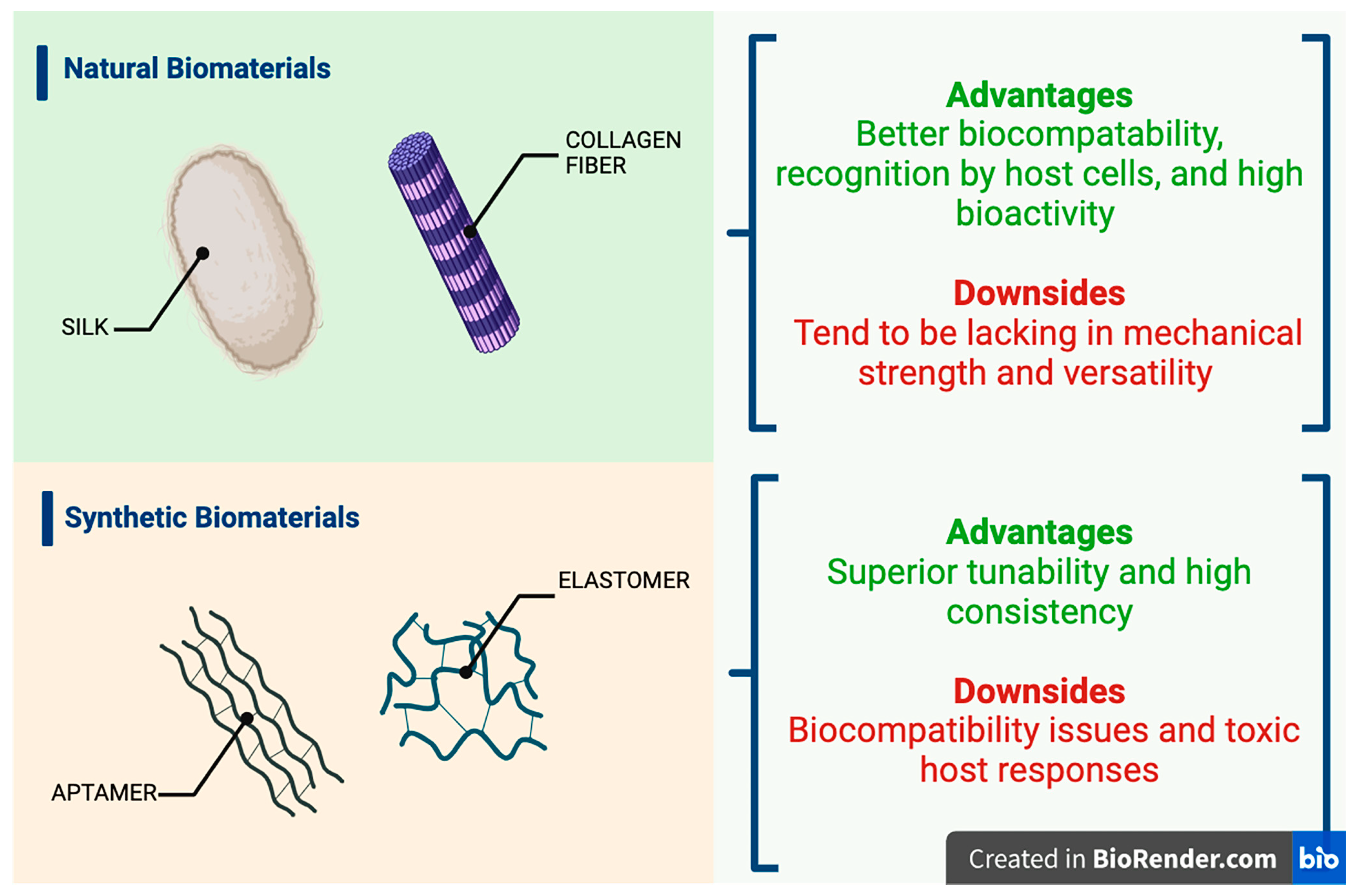

| Natural Carriers | Results Based on Chemical Structure | Synthetic Carriers | Results Based on Chemical Structure |
| Silk Fibroin |
| Poly (ethylene glycol) (PEG) |
|
| Cellulose |
| Poly (vinyl alcohol) (PVA) |
|
| Heparin |
| Methacrylic acid (MAA), |
|
| Hyaluronic Acid |
| N-isopropyl acrylamide (NIPAAm) |
|
| Starch |
| Polyacrylamide (PAA) |
|
| Chitosan |
| 2-hydroxyethyl methacrylate (HEMA) |
|
| Biomaterials | Advantages | Disadvantages |
|---|---|---|
| Traditional Hydrogels | ||
| Affinity Hydrogels | ||
| Scaffolds | ||
| Nanoparticles | ||
| Microparticles | ||
| Micelles | ||
| Aggregates | ||
| Electrospun Fibers | ||
| Buccal/Sublingual Films | ||
| Liposomes | ||
| Nanogels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorantla, A.; Hall, J.T.V.E.; Troidle, A.; Janjic, J.M. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines 2024, 15, 533. https://doi.org/10.3390/mi15040533
Gorantla A, Hall JTVE, Troidle A, Janjic JM. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines. 2024; 15(4):533. https://doi.org/10.3390/mi15040533
Chicago/Turabian StyleGorantla, Amogh, Jacques T. V. E. Hall, Anneliese Troidle, and Jelena M. Janjic. 2024. "Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation" Micromachines 15, no. 4: 533. https://doi.org/10.3390/mi15040533
APA StyleGorantla, A., Hall, J. T. V. E., Troidle, A., & Janjic, J. M. (2024). Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines, 15(4), 533. https://doi.org/10.3390/mi15040533








