A Novel Thin-Layer Flow Cell Sensor System Based on BDD Electrode for Heavy Metal Ion Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Apparatus
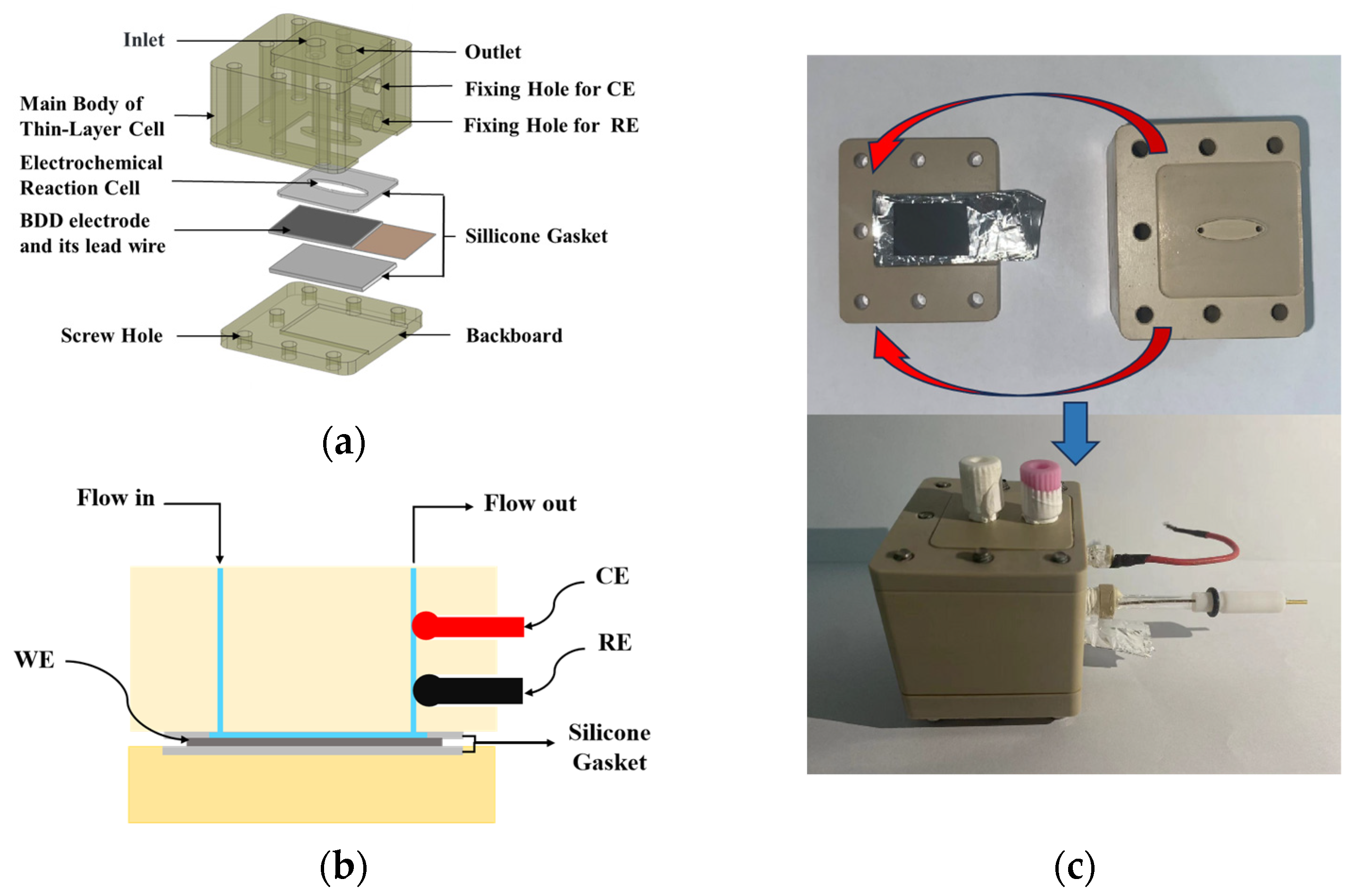
2.3. Design of the Thin-Layer Flow Cell
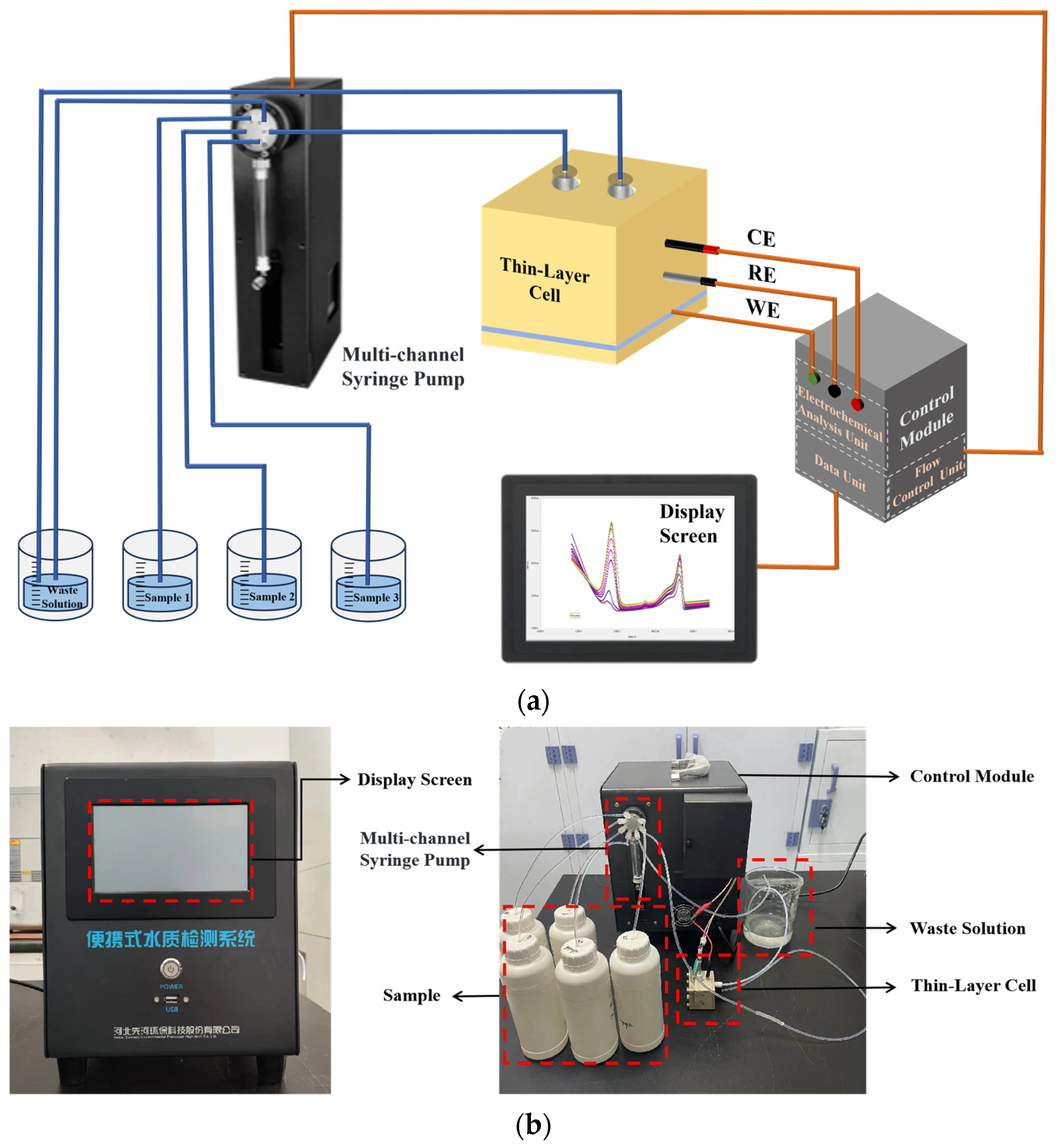
2.4. Design of the Fluidic Automatic Detection System
2.5. Pretreatment for the BDD Electrode
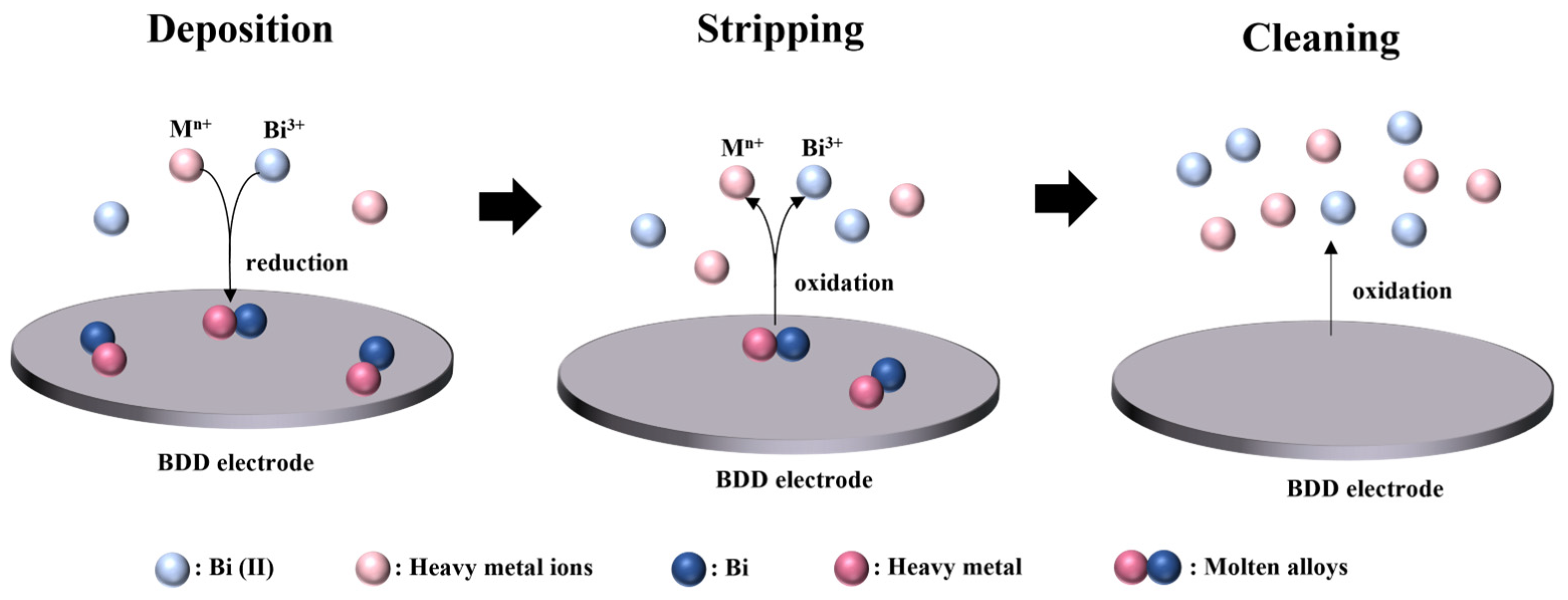
2.6. Experimental Procedures
3. Results and Discussion
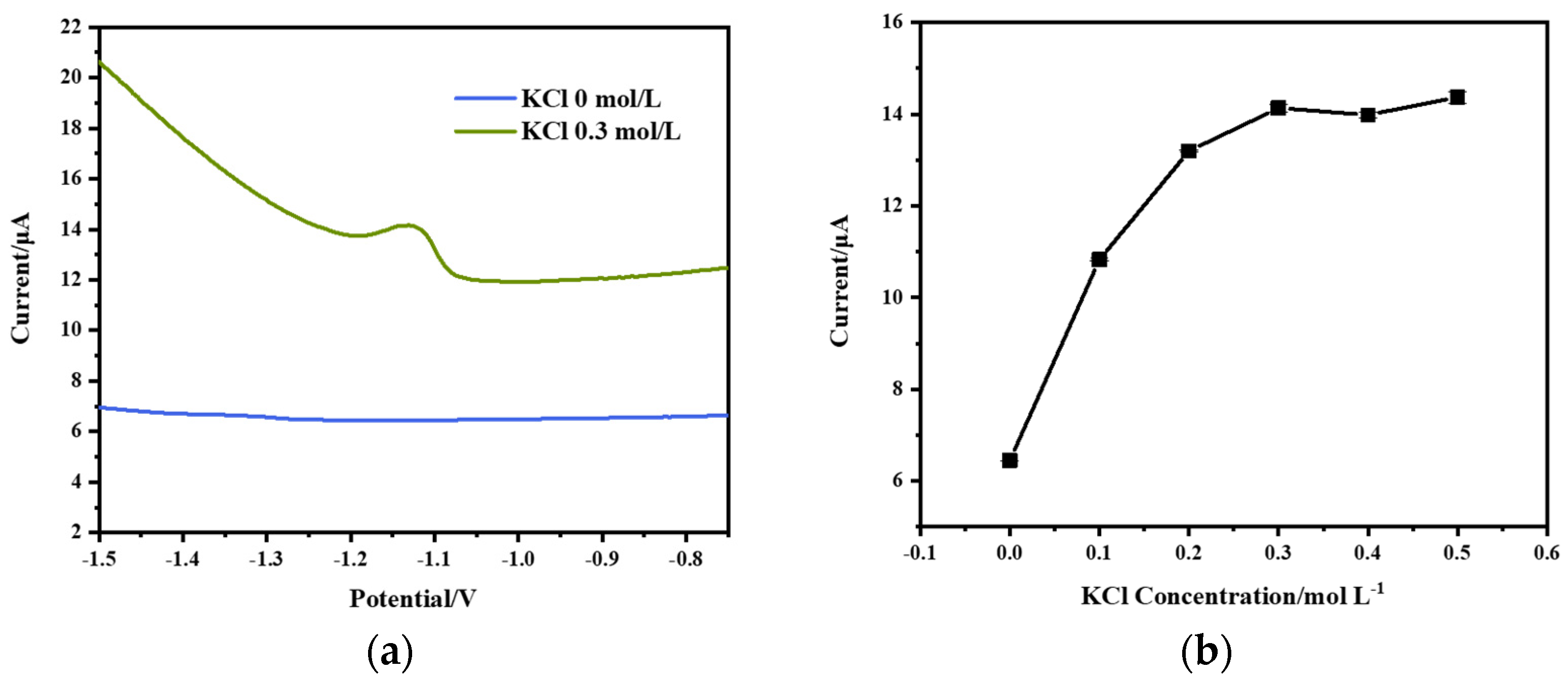
3.1. Effect of KCl Concentration
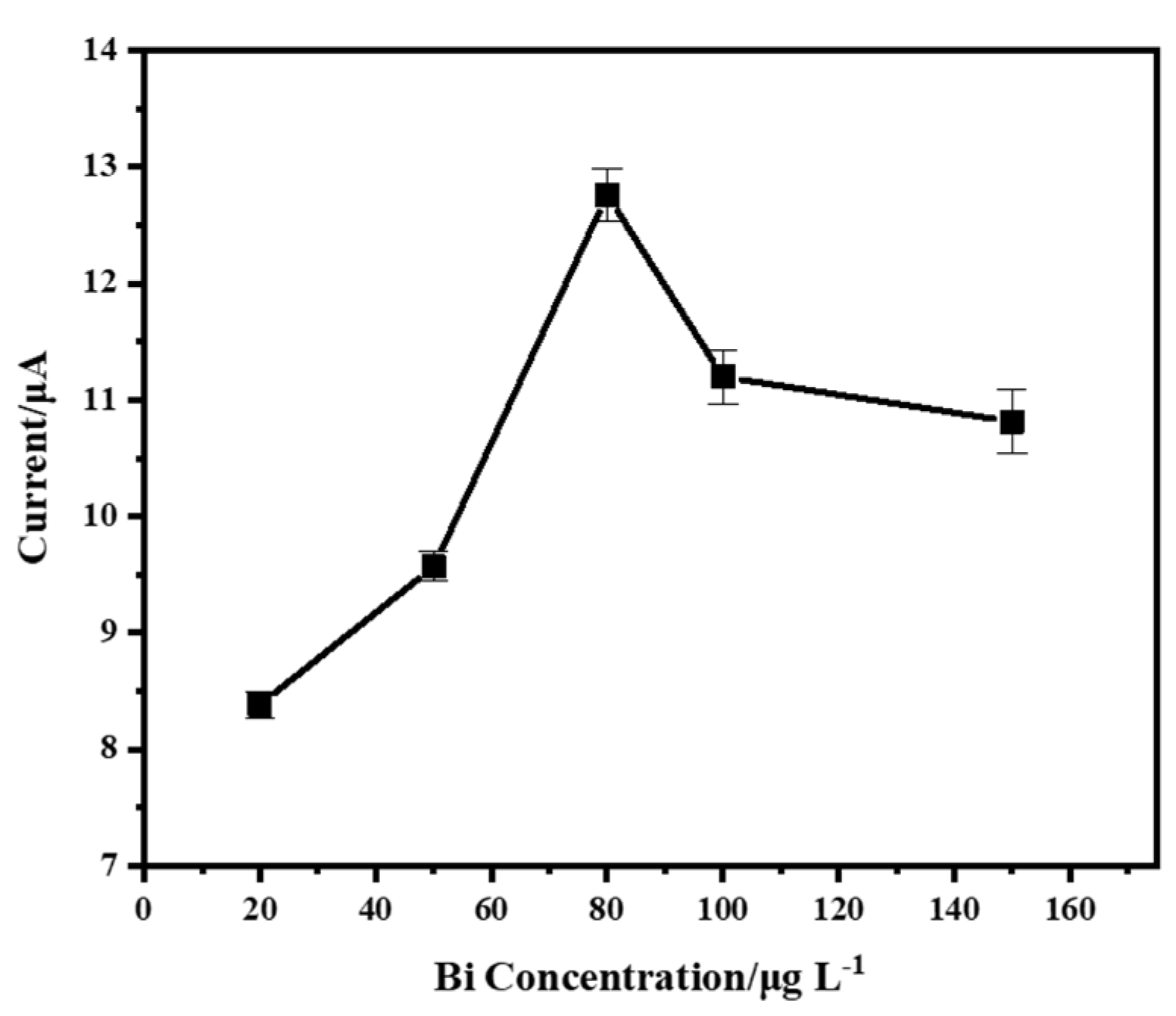
3.2. Effect of the Concentration of Bismuth Ion
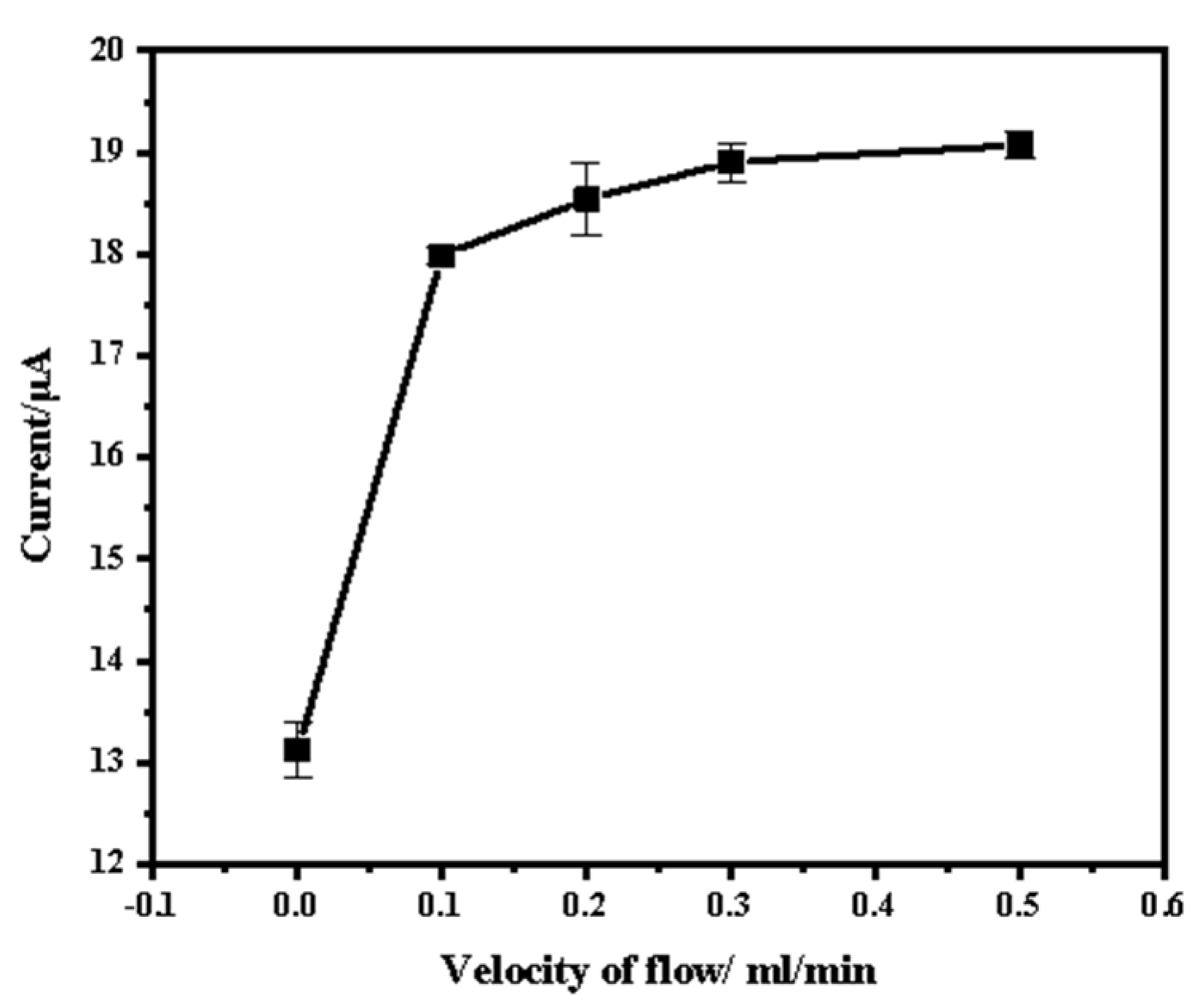
3.3. Effect of Flow Rate
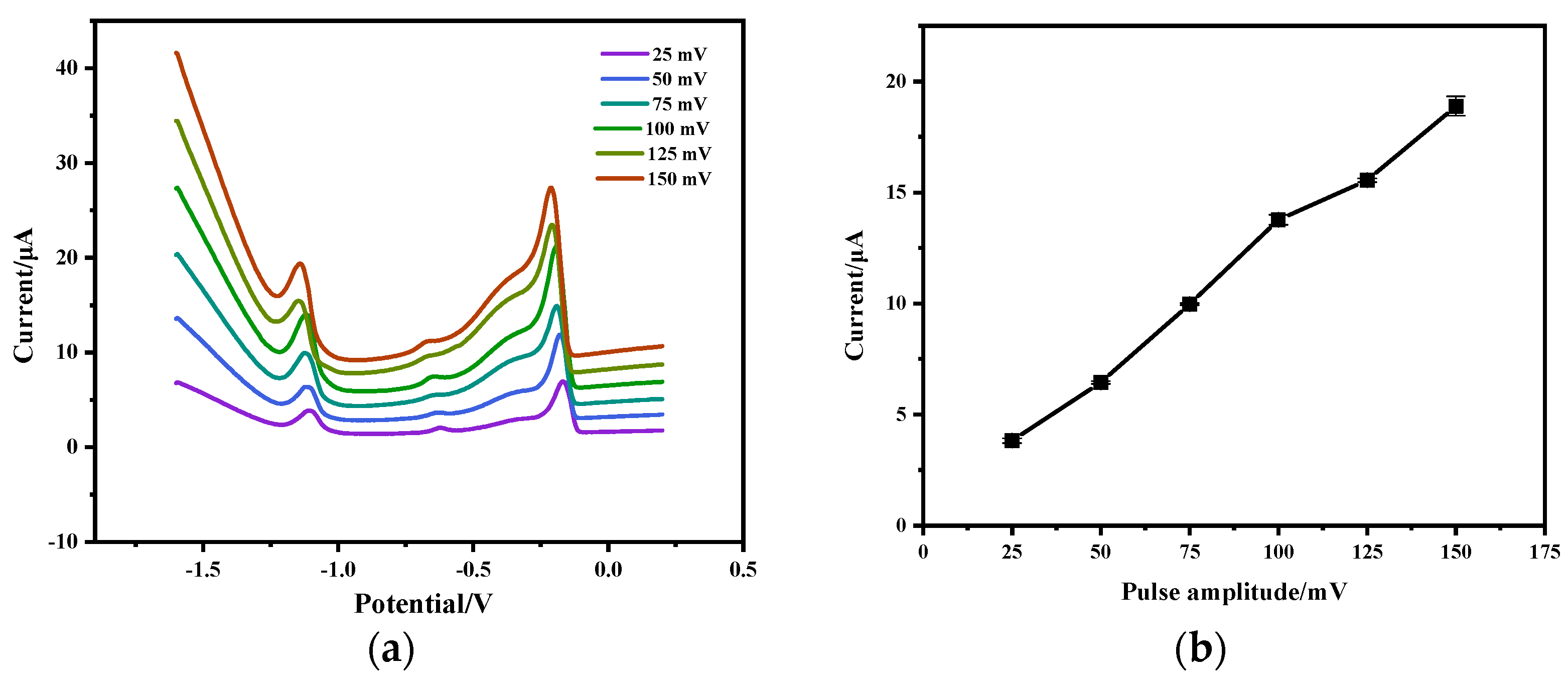
3.4. Effect of SWV Pulse Amplitude
3.5. Effect of Deposition Potential and Deposition Time
3.6. Effect of Cleaning Potential and Cleaning Time
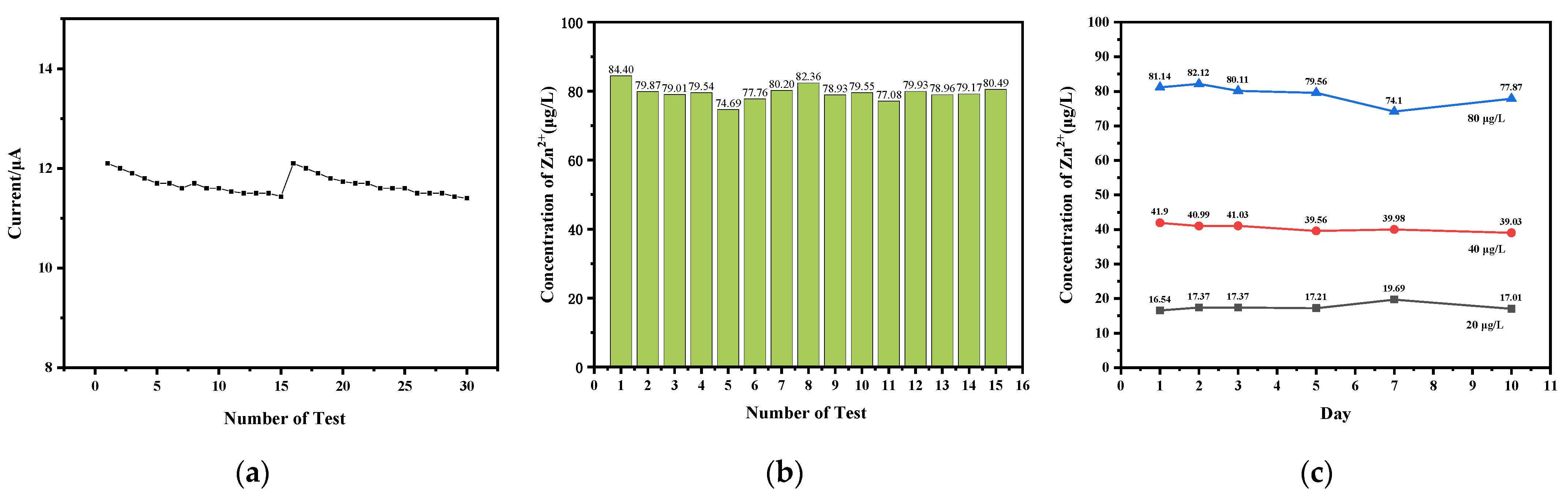
3.7. Analysis of Sensing Performance
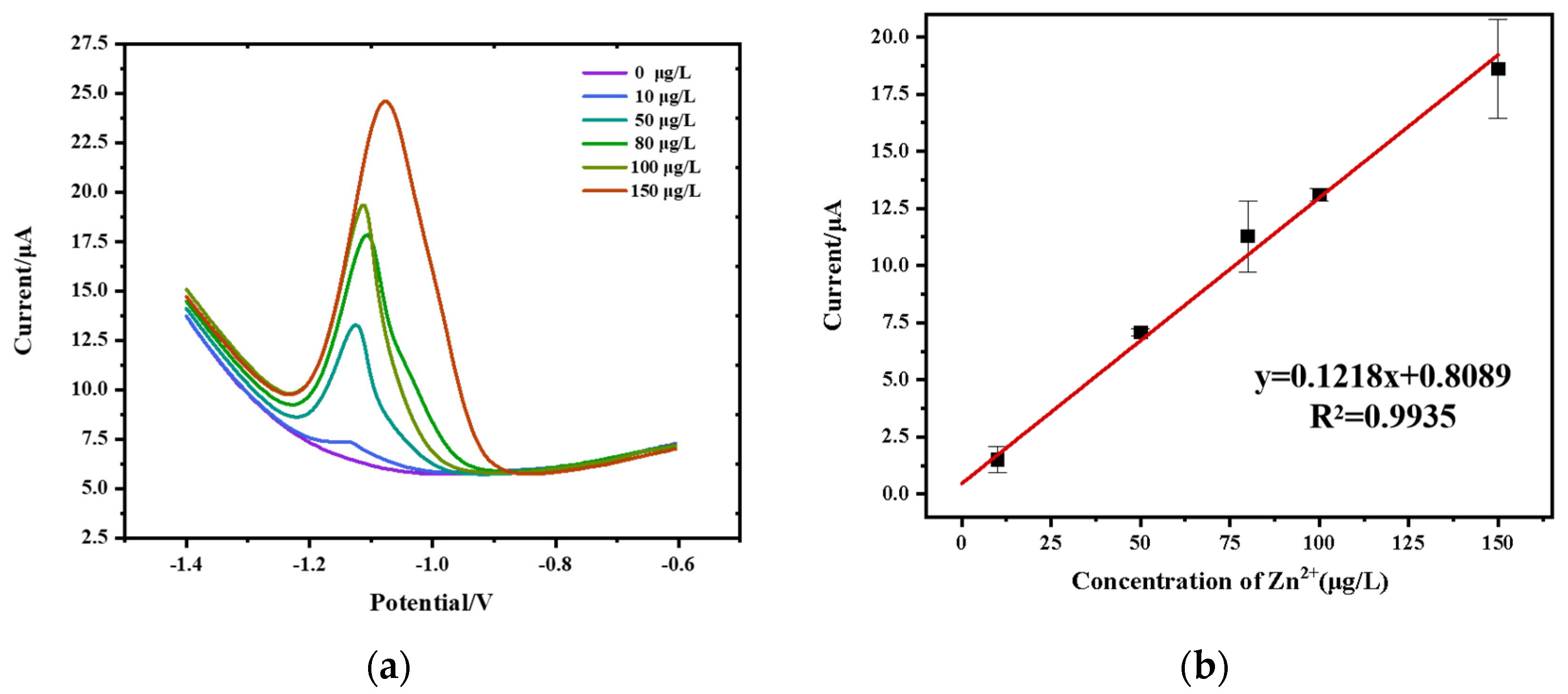
3.8. Real Sample Detection
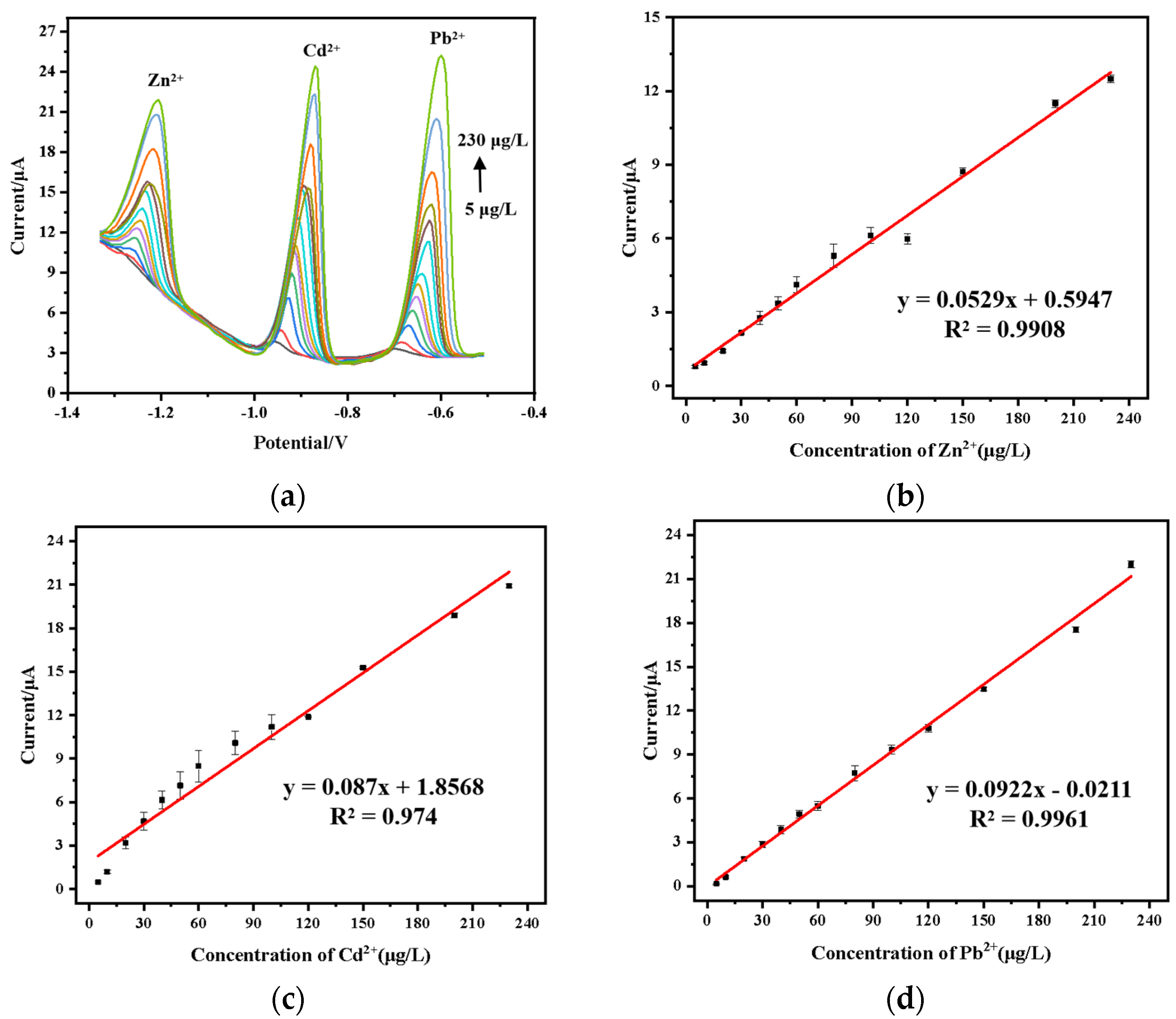
3.9. Simultaneous Detection of Pb2+, Cd2+ and Zn2+
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tonha, M.S.; Garnier, J.; Araujo, D.F.; Cunha, B.C.A.; Machado, W.; Dantas, E.; Araujo, R.; Kutter, V.T.; Bonnet, M.P.; Seyler, P. Behavior of metallurgical zinc contamination in coastal environments: A survey of Zn from electroplating wastes and partitioning in sediments. Sci. Total Environ. 2020, 743, 140610. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Walgama, C.; Narcisse, V.E.F.; Grant, C. Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review. Sensors 2023, 23, 9080. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Kaur, G. Zinc finger peptide based optic sensor for detection of zinc ions. Biosens. Bioelectron. 2016, 86, 466–471. [Google Scholar] [CrossRef]
- Ringgit, G.; Siddiquee, S.; Saallah, S.; Lal, M.T.M. A sensitive and rapid determination of zinc ion (Zn2+) using electrochemical sensor based on f-MWCNTs/CS/PB/AuE in drinking water. Sci. Rep. 2022, 12, 18582. [Google Scholar] [CrossRef] [PubMed]
- Ata, S.; Wattoo, F.H.; Ahmed, M.; Wattoo, M.H.S.; Tirmizi, S.A.; Wadood, A. A method optimization study for atomic absorption spectrophotometric determination of total zinc in insulin using direct aspiration technique. Alex. J. Med. 2019, 51, 19–23. [Google Scholar] [CrossRef]
- Dasbasi, T.; Sacmaci, S.; Cankaya, N.; Soykan, C. A new synthesis, characterization and application chelating resin for determination of some trace metals in honey samples by FAAS. Food Chem. 2016, 203, 283–291. [Google Scholar] [CrossRef]
- Guzikowski, W.; Szynkowska, M.I.; Motak-Pochrzęst, H.; Pawlaczyk, A.; Sypniewski, S. Trace elements in seminal plasma of men from infertile couples. Clin. Res. Arch. Med. Sci. 2015, 11, 591–598. [Google Scholar] [CrossRef]
- Thabit, T.; Shokr, S.A.; Elgeddawy, D.I.H.; El-Naggar, M.A.H. Determination of Heavy Metals in Wheat and Barley Grains Using ICP-MS/MS. J. AOAC Int. 2020, 103, 1277–1281. [Google Scholar] [CrossRef]
- Li, G.; Brockman, J.D.; Lin, S.-W.; Schell, L.A.; Robertson, J.D. Measurement of the Trace Elements Cu, Zn, Fe, and Mg and the Ultratrace Elements Cd, Co, Mn, and Pb in Limited Quantity Human Plasma and Serum Samples by Inductively Coupled Plasma-Mass Spectrometry. Am. J. Anal. Chem. 2012, 3, 646–650. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Opoka, W.; Jakubowska, M.; Baś, B.; Sowa-Kućma, M. Development and Validation of an Anodic Stripping Voltammetric Method for Determination of Zn2+ Ions in Brain Microdialysate Samples. Biol. Trace Elem. Res. 2010, 142, 671–682. [Google Scholar] [CrossRef][Green Version]
- Kato, D.; Kamata, T.; Kato, D.; Yanagisawa, H.; Niwa, O. Au Nanoparticle-Embedded Carbon Films for Electrochemical As3+ Detection with High Sensitivity and Stability. Anal. Chem. 2016, 88, 2944–2951. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Bi, Z.; Shan, W.; Zhu, B.; Zhou, L.; Yu, L.; Zhang, H.; Xia, S.; Qiu, M. Ti3C2-MXene@N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals. J. Electroanal. Chem. 2022, 911, 116239. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, C.; Wang, W.; Wang, J.; Chen, S.; Xu, H.; Zhuiykov, S. Self-Assembled Co3O4/GO Composites for Excellent Electrochemical Detection of Heavy-Metal Ions. J. Electrochem. Soc. 2021, 168, 083503. [Google Scholar] [CrossRef]
- Lei, P.; Zhou, Y.; Zhao, S.; Dong, C.; Shuang, S. Carbon-supported X-manganate (XNi, Zn, and Cu) nanocomposites for sensitive electrochemical detection of trace heavy metal ions. J. Hazard. Mater. 2022, 435, 129036. [Google Scholar] [CrossRef]
- Dueraning, A.; Kanatharana, P.; Thavarungkul, P. An environmental friendly electrode and extended cathodic potential window for anodic stripping voltammetry of zinc d-etection. Electrochim. Acta 2016, 221, 133–143. [Google Scholar] [CrossRef]
- Scandurra, A.; Mirabella, S. Square Wave Anodic Stripping Voltammetry Applied to a Nano-Electrode for Trace Analysis of Pb(II) and Cd(II) Ions in Solution. IEEE Sens. J. 2021, 21, 22134–22142. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Simon, N.; Decorse-Pascanut, C.; Bouttemy, M.; Etcheberry, A.; Li, M.; Boukherroub, R.; Szunerits, S. Comparison of the chemical composition of boron-doped diamond surfaces upon different oxidation processes. Electrochim. Acta 2009, 54, 5818–5824. [Google Scholar] [CrossRef]
- Zhu, R.; Deng, Z.; Wang, Y.; Zhou, K.; Yu, Z.; Ma, L.; Wei, Q. A nanoporous diamond particle microelectrode and its surface modification. Electrochim. Acta 2022, 430, 141015. [Google Scholar] [CrossRef]
- Kondo, T. Recent electroanalytical applications of boron-doped diamond electrodes. Curr. Opin. Electrochem. 2022, 32, 100891. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Rehacek, V.; Hotovy, I.; Marton, M.; Mikolasek, M.; Michniak, P.; Vincze, A.; Kromka, A.; Vojs, M. Voltammetric characterization of boron-doped diamond electrodes for electroanalytical applications. J. Electroanal. Chem. 2020, 862, 114020. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Tang, M.; Huang, L.; Zhang, Z.; Liu, C.; Lu, X.; Hunter, K.W.; Chen, G. A New Electrochemical System Based on a Flow-Field Shaped Solid Electrode and 3D-Printed Thin-Layer Flow Cell: Detection of Pb2+ Ions by Continuous Flow Accumulation Square-Wave Anodic Stripping Voltammetry. Anal. Chem. 2017, 89, 5024–5029. [Google Scholar] [CrossRef]
- Ngoensawat, U.; Pisuchpen, T.; Sritana-anant, Y.; Rodthongkum, N.; Hoven, V.P. Conductive electrospun composite fibers based on solid-state polymerized Poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of metal ions. Talanta 2022, 241, 123253. [Google Scholar] [CrossRef]
- Hui, X.; Sharifuzzaman, M.; Sharma, S.; Xuan, X.; Zhang, S.; Ko, S.G.; Yoon, S.H.; Park, J.Y. High-Performance Flexible Electrochemical Heavy Metal Sensor Based on Layer-by-Layer Assembly of Ti3C2Tx/MWNTs Nanocomposites for Noninvasive Detection of Copper and Zinc Ions in Human Biofluids. ACS Appl. Mater. Interfaces 2020, 12, 48928–48937. [Google Scholar] [CrossRef]
- Shao, Y.; Dong, Y.; Bin, L.; Fan, L.; Wang, L.; Yuan, X.; Li, D.; Liu, X.; Zhao, S. Application of gold nanoparticles/polyaniline-multi-walled carbon nanotubes modified screen-printed carbon electrode for electrochemical sensing of zinc, lead, and copper. Microchem. J. 2021, 170, 106726. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Luo, H.; Zhao, J.; Bi, J.; Wu, G. Review—Ion Interference and Elimination in Electrochemical Detection of Heavy Metals Using Anodic Stripping Voltammetry. J. Electrochem. Soc. 2023, 170, 057507. [Google Scholar] [CrossRef]
- Levey, K.J.; Edwards, M.A.; White, H.S.; Macpherson, J.V. Simulation of the cyclic voltammetric response of an outer-sphere redox species with inclusion of electrical double layer structure and ohmic potential drop. Phys. Chem. Chem. 2023, 25, 7832–7846. [Google Scholar] [CrossRef] [PubMed]
- Crew, A.; Cowell, D.C.; Hart, J.P. Development of an anodic stripping voltammetric assay using a disposable mercury-free screen-printed carbon electrode for the determination of zinc in human sweat. Talanta 2008, 75, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Edmund, J.F.D.; Juan, G.L.; Neil, V.R.; Richard, G.C. How Much Supporting Electrolyte Is Required to Make a Cyclic Voltammetry Experiment Quantitatively “Diffusional”? A Theoretical and Experimental Investigation. J. Phys. Chem. C 2009, 113, 11157–11171. [Google Scholar] [CrossRef]
- Gerent, G.G.; Spinelli, A. Environmentally-friendly in situ plated bismuth-film electrode for the quantification of the endocrine disruptor parathion in skimmed milk. J. Hazard. Mater. 2016, 308, 157–163. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Zhao, S.; Cui, G. Simultaneous determination of trace Pb(II), Cd(II), and Zn(II) using an integrated three-electrode modified with bismuth film. Microchem. J. 2021, 168, 106390. [Google Scholar] [CrossRef]
- Ribeiro, M.M.A.C.; Rocha, R.G.; Munoz, R.A.A.; Richter, E.M. A Batch Injection Analysis System with Square-wave Voltammetric Detection for Fast and Simultaneous Determination of Zinc and Ascorbic Acid. Electroanalysis 2020, 33, 90–96. [Google Scholar] [CrossRef]
- Thanh, N.M.; Van Hop, N.; Luyen, N.D.; Phong, N.H.; Toan, T.T.T. Simultaneous Determination of Zn(II), Cd(II), Pb(II), and Cu(II) Using Differential Pulse Anodic Stripping Voltammetry at a Bismuth Film-Modified Electrode. Adv. Mater. Sci. Eng. 2019, 2019, 1826148. [Google Scholar] [CrossRef]
- Hassan, K.M.; Gaber, S.E.; Altahan, M.F.; Azzem, M.A. Single and simultaneous voltammetric sensing of lead(II), cadmium(II) and zinc(II) using a bimetallic Hg-Bi supported on poly(1,2-diaminoanthraquinone)/glassy carbon modified electrode. Sens. Bio-Sens. Res. 2020, 29, 100369. [Google Scholar] [CrossRef]
- Seanghirun, W.; Samoson, K.; Cotchim, S.; Kongkaew, S.; Limbut, W. Green electrochemical sensor for Zn(II) ions detection in human seminal fluid. Microchem. J. 2020, 157, 104958. [Google Scholar] [CrossRef]
- Ai, Y.; Yan, L.; Zhang, S.; Ye, X.; Xuan, Y.; He, S.; Wang, X.; Sun, W. Ultra-sensitive simultaneous electrochemical detection of Zn(II), Cd(II) and Pb(II) based on the bismuth and graphdiyne film modified electrode. Microchem. J. 2023, 184, 108186. [Google Scholar] [CrossRef]
- Vanderlaan, E.L.; Nolan, J.K.; Sexton, J.; Evans-Molina, C.; Lee, H.; Voytik-Harbin, S.L. Development of electrochemical Zn2+ sensors for rapid voltammetric detection of glucose-stimulated insulin release from pancreatic beta-cells. Biosens. Bioelectron. 2023, 235, 115409. [Google Scholar] [CrossRef] [PubMed]
- Mirceski, V.; Sebez, B.; Jancovska, M.; Ogorevc, B.; Hocevar, S.B. Mechanisms and kinetics of electrode processes at bismuth and antimony film and bare glassy carbon surfaces under square-wave anodic stripping voltammetry conditions. Electrochim. Acta 2013, 105, 254–260. [Google Scholar] [CrossRef]


| Electrode Type | LOD (μg/L) | Sensitivity (μA·L·μg−1) | Linear Range (μg/L) | Deposition Time (s) | RSD (%) | Reference |
|---|---|---|---|---|---|---|
| PEDOT/PVA/AgNPs/SPCE | 6.000 | 0.041 | 10–80 | 240 | - | [27] |
| Ti3C2Tx/MWNTs/Au | 1.500 | 0.040 | 200–600 | 120 | - | [28] |
| AuNPs/PANI-MWCNTs/SPCE | 0.039 | 0.619 | 1–180 | 400 | 3.48 | [29] |
| Bi/screen-printed gold electrode | 0.050 | - | 1–120 | 180 | 2.05 | [35] |
| BDD | 13.00 | 0.060 | 33–1300 | 10 | 5.60 | [36] |
| Bi film electrode/glassy carbon electrode | 1.070 | 0.091 | 5–110 | 120 | 4.74 | [37] |
| Hg-Bi/PDAAQ/GC | 0.169 | 7.342 | 0.1–100 | 300 | 4.74 | [38] |
| Bi/graphene oxide/glassy carbon electrode | 6.000 | 0.402 | 20–8000 | 480 | 8.70 | [39] |
| Bi/bismuth and graphdiyne/GCE | 0.010 | - | 0.065–65 | 150 | 2.03 | [40] |
| Ex-situ Bi/Nafion/glassy carbon electrode | 2.300 | 0.045 | 2.5–500 | 360 | 11.9 | [41] |
| BDD | 2.100 | 0.122 | 10–150 | 60 | 1.60 | This work |
| Sample | Added (μg/L) | Detected (μg/L) | Recovery (%) |
|---|---|---|---|
| Water 1 | 0 | - | - |
| 80 | 85.14 | 106 | |
| 120 | 141.39 | 117 | |
| 200 | 214.86 | 107 | |
| Water 2 | 0 | - | - |
| 80 | 87.15 | 108 | |
| 100 | 91.34 | 92 | |
| 120 | 125.40 | 104 | |
| Water 3 | 0 | - | - |
| 50 | 53.38 | 106 | |
| 80 | 90.64 | 113 | |
| 120 | 141.88 | 118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, D.; Zhai, J.; Shen, Z.; Wang, Q.; Wei, S.; Li, Y.; Bian, C. A Novel Thin-Layer Flow Cell Sensor System Based on BDD Electrode for Heavy Metal Ion Detection. Micromachines 2024, 15, 363. https://doi.org/10.3390/mi15030363
Xiao D, Zhai J, Shen Z, Wang Q, Wei S, Li Y, Bian C. A Novel Thin-Layer Flow Cell Sensor System Based on BDD Electrode for Heavy Metal Ion Detection. Micromachines. 2024; 15(3):363. https://doi.org/10.3390/mi15030363
Chicago/Turabian StyleXiao, Danlin, Junfeng Zhai, Zhongkai Shen, Qiang Wang, Shengnan Wei, Yang Li, and Chao Bian. 2024. "A Novel Thin-Layer Flow Cell Sensor System Based on BDD Electrode for Heavy Metal Ion Detection" Micromachines 15, no. 3: 363. https://doi.org/10.3390/mi15030363
APA StyleXiao, D., Zhai, J., Shen, Z., Wang, Q., Wei, S., Li, Y., & Bian, C. (2024). A Novel Thin-Layer Flow Cell Sensor System Based on BDD Electrode for Heavy Metal Ion Detection. Micromachines, 15(3), 363. https://doi.org/10.3390/mi15030363








