Co3O4 Nanostructured Sensor for Electrochemical Detection of H2O2 as a Stress Biomarker in Barley: Fe3O4 Nanoparticles-Mediated Enhancement of Salt Stress Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
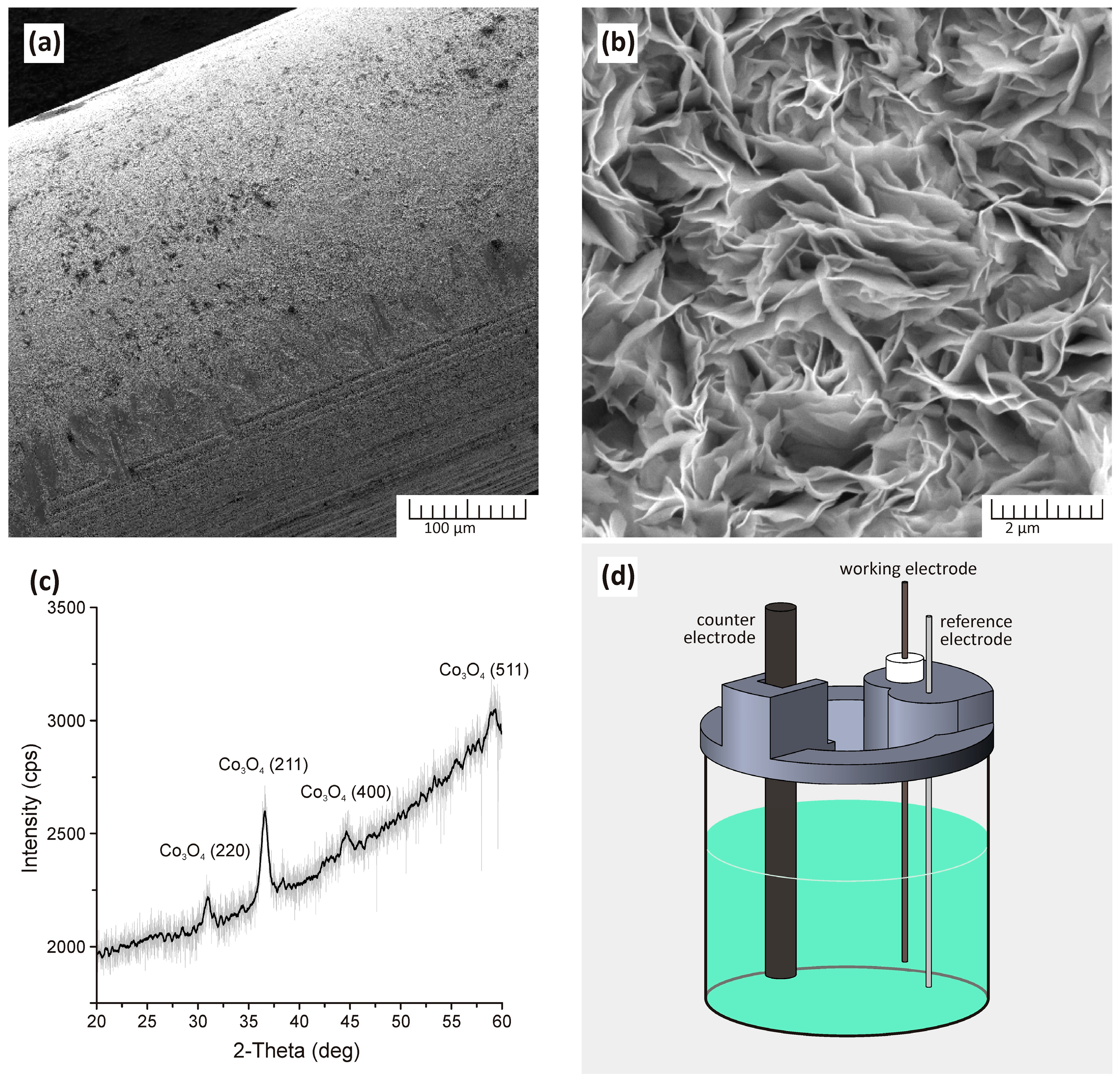
2.2. Synthesis and Characterization of Fe3O4 Nanoparticles
2.3. Barley Seedling Cultivation and Sample Preparation
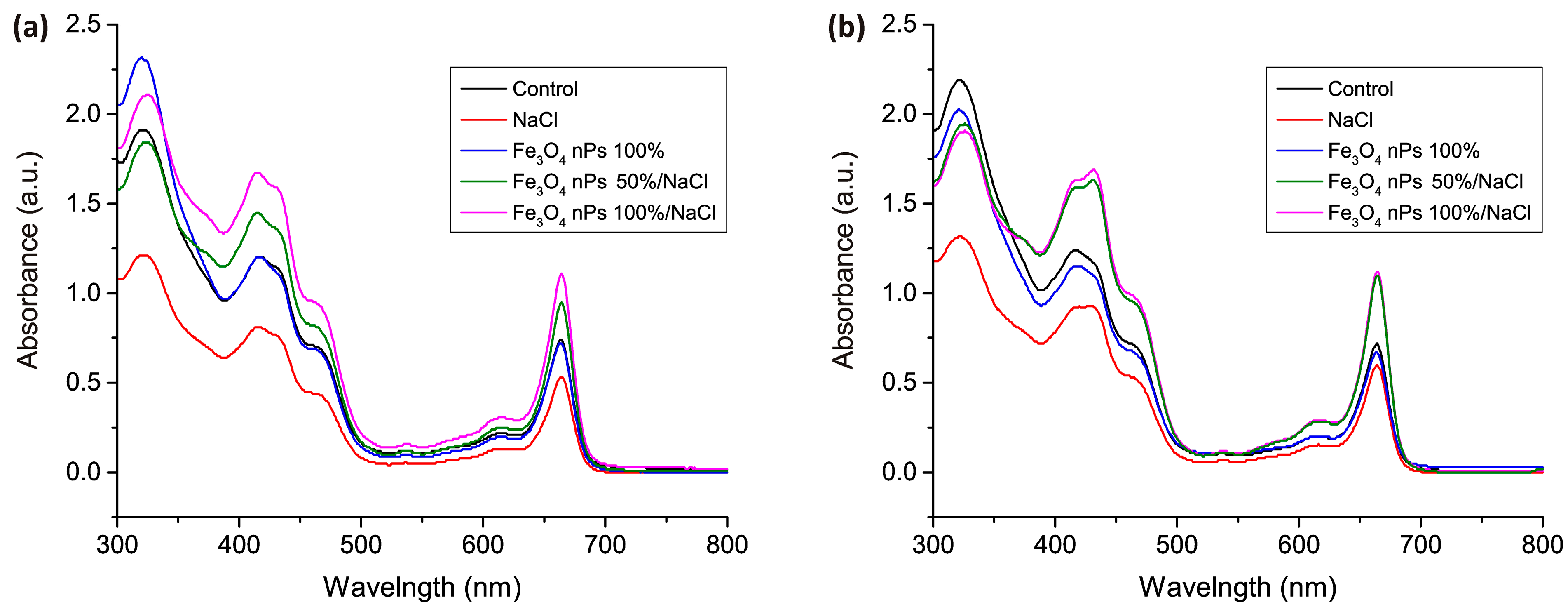
2.4. Optical Mesurements
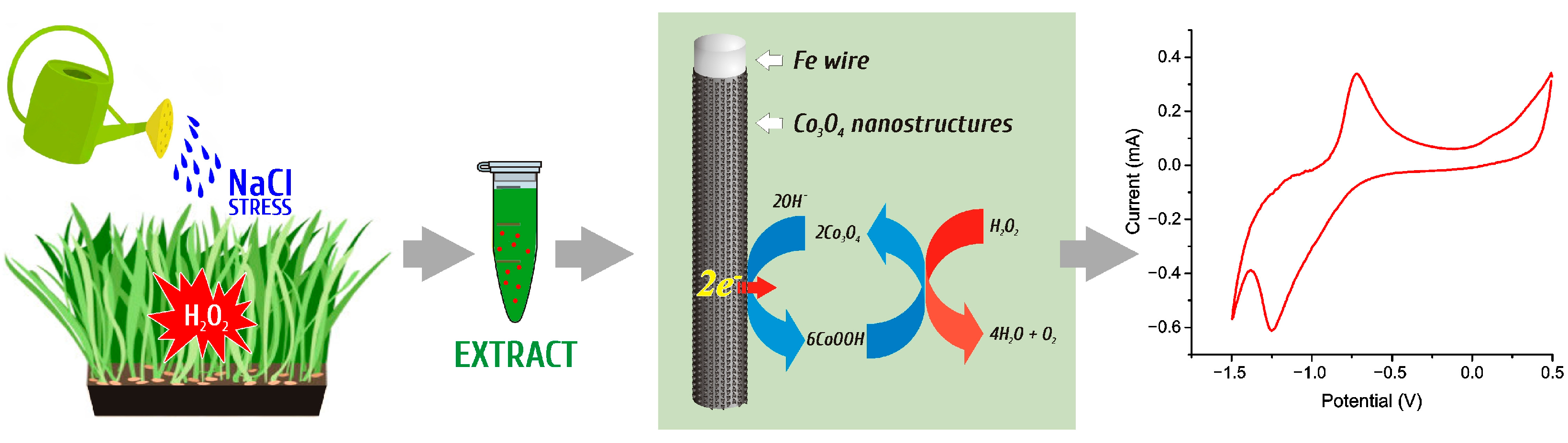
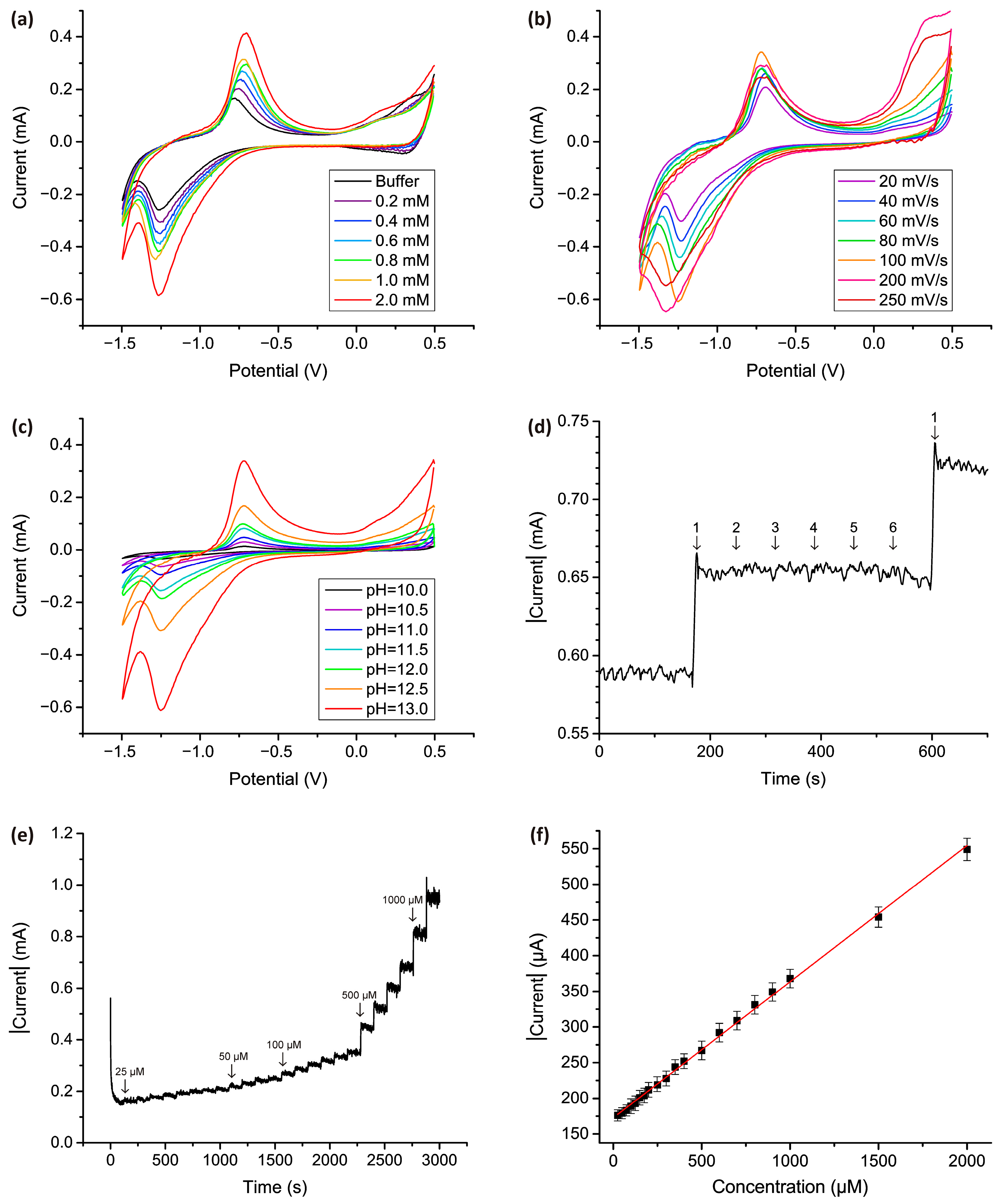
2.5. Co3O4 Nanostructured Electrode Preparation and Electrochemical Measurements for H2O2 Detection
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Element | Control (Weight %) | NaCl (Weight %) | nPs 100% (Weight %) | nPs 50%/NaCl (Weight %) | nPs 100%/NaCl (Weight %) |
|---|---|---|---|---|---|
| C | 53.11 | 50.40 | 50.23 | 50.59 | 49.59 |
| O | 37.19 | 32.22 | 40.41 | 39.19 | 37.19 |
| Na | 0.31 | 3.43 | 0.20 | 0.99 | 2.25 |
| Mg | 0.61 | 0.25 | 0.77 | 0.67 | 0.65 |
| Si | 0.09 | 0.04 | 0.04 | 0.37 | 0.23 |
| P | 1.26 | 0.66 | 1.30 | 0.79 | 0.85 |
| S | 1.40 | 0.54 | 1.07 | 0.70 | 0.46 |
| Cl | 0.44 | 7.77 | 0.54 | 3.71 | 4.86 |
| K | 3.97 | 3.96 | 3.75 | 1.22 | 2.58 |
| Ca | 1.46 | 0.60 | 1.54 | 1.55 | 1.17 |
| Fe | 0.04 | 0.02 | 0.06 | 0.10 | 0.06 |
| Cu | 0.12 | 0.11 | 0.09 | 0.12 | 0.11 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
References
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Gebresenbet, G.; Bosona, T.; Patterson, D.; Persson, H.; Fischer, B.; Mandaluniz, N.; Chirici, G.; Zacepins, A.; Komasilovs, V.; Pitulac, T.; et al. A concept for application of integrated digital technologies to enhance future smart agricultural systems. Smart Agric. Technol. 2023, 5, 100255. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Enhancing smart farming through the applications of Agriculture 4.0 technologies. Int. J. Intell. Netw. 2022, 3, 150–164. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent Advances in Nanoparticle-Mediated Co-Delivery System: A Promising Strategy in Medical and Agricultural Field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef] [PubMed]
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: Food security implication and challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Garg, V.K.; Chhillar, A.; Jogender, R. Recent advances in nanotechnology for the improvement of conventional agricultural systems: A Review. Plant Nano Biol. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Şahin, E.Ç.; Aydın, Y.; Utkan, G.; Uncuoğlu, A.A. Chapter 22—Nanotechnology in agriculture for plant control and as biofertilizer. In Synthesis of Bionanomaterials for Biomedical Applications; Ozturk, M., Roy, A., Bhat, R.A., Vardar-Sukan, F., Tonelli, F.M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 469–492. [Google Scholar] [CrossRef]
- Gorantla, S.; Wadhwa, G.; Shailja Jain, S.; Sankar, S.; Kshitij Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent advances in nanocarriers for nutrient delivery. Drug Deliv. Transl. Res. 2022, 12, 2359–2384. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.A.; Fahmy, H.M.; Arafa, F.N.; Allah, M.Y.; Tawfik, Y.M.; Halwany, K.K.; El-Ashmanty, B.A.; Al-Anany, F.S.; Mohamed, M.A.; Bassily, M.E. Nanotechnology in pest management: Advantages, applications, and challenges. Int. J. Trop. Insect Sci. 2023, 43, 1387–1399. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Chhipa, H. Nano-based pesticides: Challenges for pest and disease management. Euro-Mediterr. J. Environ. Integr. 2021, 6, 69. [Google Scholar] [CrossRef]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Ray, M.K.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Chakrabartty, I.; Kungwani, N.A.; Avula, S.K.; Panda, J.; Pudake, R.N. Nanotechnology as a Promising Tool against Phytopathogens: A Futuristic Approach to Agriculture. Agriculture 2023, 13, 1856. [Google Scholar] [CrossRef]
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of Zinc Oxide and Silicon Dioxide Nanoparticles on Physiological, Yield, and Water Use Efficiency Traits of Potato Grown under Water Deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Ma, T.; Liang, Y.; Huo, Z.; Yang, F. Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Agronomy 2023, 13, 3060. [Google Scholar] [CrossRef]
- Goyal, V.; Rani, D.; Ritika; Mehrotra, S.; Deng, C.; Wang, Y. Unlocking the Potential of Nano-Enabled Precision Agriculture for Efficient and Sustainable Farming. Plants 2023, 12, 3744. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, T.; Zhu, K.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; Zhang, Z.; Wang, Z.; Wang, B.; et al. Effects of Salt Stress on Grain Yield and Quality Parameters in Rice Cultivars with Differing Salt Tolerance. Plants 2023, 12, 3243. [Google Scholar] [CrossRef]
- Junedi, M.A.; Mukhopadhyay, R.; Manjari, K.S. Alleviating salinity stress in crop plants using new engineered nanoparticles (ENPs). Plant Stress 2023, 9, 100184. [Google Scholar] [CrossRef]
- Seeda, A.; Abou El-Nour, E.-Z.; Abdallah, M.; El-Bassiouny, H.; El-Monem, A. Impacts of Salinity Stress on Plants and Their Tolerance Strategies: A Review. Middle East J. Appl. Sci. 2022, 12, 282–400. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.K.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S.; et al. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2021, 172, 1336–1351. [Google Scholar] [CrossRef]
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglansmicrocarpa L. Seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Loudari, A.; Mayane, A.; Zeroual, Y.; Colinet, G.; Oukarroum, A. Photosynthetic performance and nutrient uptake under salt stress: Differential responses of wheat. Front. Plant Sci. 2022, 13, 1038672. [Google Scholar] [CrossRef]
- Cruz, J.L.; Coelho, E.F.; Coelho Filho, M.; Santos, A.A. Salinity reduces nutrients absorption and efficiency of their utilization in cassava plants. Ciência Rural 2018, 48, e20180351. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Cho, U.-H.; Seo, N.-H. Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Oxidative Stress: Biology, Physiology and Mitigation. Plants 2022, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, S.; Steppe, K.; Eloudi, M.; Mechi, L.; Bahrini, I.; Van Labeke, M.-C. Salt Stress Induced Changes in Photosynthesis and Metabolic Profiles of One Tolerant (‘Bonica’) and One Sensitive (‘Black Beauty’) Eggplant Cultivars (Solanum melongena L.). Plants 2022, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chong, P.; Zhao, M. Effect of salt stress on the photosynthetic characteristics and endogenous hormones, and: A comprehensive evaluation of salt tolerance in Reaumuria soongorica seedlings. Plant Signal. Behav. 2022, 17, e2031782. [Google Scholar] [CrossRef] [PubMed]
- Holsteens, K.; De Jaegere, I.; Wynants, A.; Prinsen, E.L.J.; Van de Poel, B. Mild and severe salt stress responses are age-dependently regulated by abscisic acid in tomato. Front. Plant Sci. 2022, 13, 982622. [Google Scholar] [CrossRef] [PubMed]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Asghar, S.; Bibi, S.; Mukhtiar, A.; Zia, M.A.; Naqve, M.; Mahmood, A.; Javaid, M.M.; Azeem, M. Chapter—Mechanism and Approaches to Enhance Salt Stress Tolerance in Crop Plants. In Climate-Resilient Agriculture, Vol. 2; Hasanuzzaman, M., Ed.; Springer: Cham, Switzerland, 2023; pp. 407–426. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.-S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Strokina, V.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) and salinity on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum). Funct. Plant Biol. 2023, 50, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, H.; Hejabi, M.; Salari, H.; Oloumi, H. Effect of iron oxide and zinc oxide nanoparticles on growth improvement and tolerance to salinity stress in tomato plants. J. Ethno Pharm. Product. 2023, 3, 31–45. [Google Scholar] [CrossRef]
- El-Saber, M.M.; Mahdi, A.A.; Hassan, A.H.; Farroh, K.Y.; Osman, A. Effects of magnetite nanoparticles on physiological processes to alleviate salinity induced oxidative damage in wheat. J. Sci. Food Agric. 2021, 101, 5550–5562. [Google Scholar] [CrossRef] [PubMed]
- Adeoke, O.; Huseynova, N.; Kalilov, R.; Arahov, N.; Jafarzadeh, B.; Abdullayeva, N.; Azizov, I. Comparative impact of nanoparticles on salt resistance of wheat plants. MethodsX 2023, 11, 102371. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Xie, Y. Stimulatory Effect of Fe3O4 Nanoparticles on the Growth and Yield of Pseudostellaria heterophylla via Improved Photosynthetic Performance. HortScience 2021, 56, 753–761. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Govea-Alcaide, E.; Masunaga, S.H.; Fajardo-Rosabal, L.; Effenberger, F.; Rossi, L.M.; Jardim, R.F. Impact of Fe3O4 nanoparticle on nutrient accumulation in common bean plants grown in soil. SN Appl. Sci. 2019, 1, 308. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Rehman, S.U.; Shujah, S.; Rha, E.S.; Jamil, M. Bacillus subtilis Synthesized Iron Oxide Nanoparticles (Fe3O4 NPs) Induced Metabolic and Anti-Oxidative Response in Rice (Oryza sativa L.) under Arsenic Stress. Toxics 2022, 10, 618. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Li, Z.; Gao, M.; Li, S.; Guo, L.; She, Z.; Zhao, Y.; Zheng, D.; Jin, C.; et al. Magnetic Fe3O4 nanoparticles induced effects on performance and microbial community of activated sludge from a sequencing batch reactor under long-term exposure. Bioresour. Technol. 2017, 225, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Lian, X.; Liu, H.; Guo, Z.Z.; Huang, H.H.; Lei, Y.; Peng, H.P.; Chen, W.; Lin, X.H.; Liu, A.L.; et al. A colorimetric assay for sensitive detection of hydrogen peroxide and glucose in microfluidic paper-based analytical devices integrated with starch-iodide-gelatin system. Talanta 2019, 200, 511–517. [Google Scholar] [CrossRef]
- Leichnitz, S.; Heinrich, J.; Kulak, N. A Fluorescence Assay for the Detection of Hydrogen Peroxide and Hydroxyl Radicals generated by Metallonucleases. Chem. Commun. 2018, 54, 13411–13414. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 Determination by the I3– Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ostaszewski, R.; Wilk, M. Efficient assay for the detection of hydrogen peroxide via estimation of the enzyme promiscuous activity in the perhydrolysis reaction. ChemBioChem 2020, 22, 1464–1469. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and application of electrochemical sensor for analyzing hydrogen peroxide in food system and biological samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Ahmad, I.; Hussain, S.; Asif, M.; Pan, Y.; Zairov, R.; Alothman, A.A.; Ouladsmane, M.; Ullah, Z.; Ullah, N.; et al. Ag-Cu nanoalloys: An electrochemical sensor for H2O2 detection. Surf. Interfaces 2023, 36, 102616. [Google Scholar] [CrossRef]
- Oh, D.E.; Lee, C.-S.; Kim, T.W.; Jeon, S.; Kim, T.H. A Flexible and Transparent PtNP/SWCNT/PET Electrochemical Sensor for Nonenzymatic Detection of Hydrogen Peroxide Released from Living Cells with Real-Time Monitoring Capability. Biosensors 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Nishan, U.; Haq, S.U.; Rahim, A.; Asad, M.; Badshah, A.; Ali Shah, A.U.; Iqbal, A.; Muhammad, N. Ionic-Liquid-Stabilized TiO2 Nanostructures: A Platform for Detection of Hydrogen Peroxide. ACS Omega 2021, 6, 32754–32762. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khaliq, N.; Khan, A.A.; Khan, M.; Ali, G.; Maqbool, M. Synthesis, characterization and electrochemical analysis of TiO2 nanostructures for sensing l-Cysteine and hydrogen peroxide. Phys. E Low Dimens. Syst. Nanostruct. 2021, 128, 114541. [Google Scholar] [CrossRef]
- Tao, Z.; Si, H.; Zhang, X.; Liao, J.; Lin, S. Highly sensitive and selective H2O2 sensors based on ZnO TFT using PBNCs/Pt-NPs/TNTAs as gate electrode. Sens. Actuators B Chem. 2021, 349, 130791. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Alsareii, S.A. A Highly Efficient Nonenzymatic Hydrogen Peroxide Electrochemical Sensor Using Mesoporous Carbon Doped ZnO Nanocomposite. J. Electrochem. Soc. 2021, 168, 027512. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdul Hamid, M.A.; Shamsudin, R.; Othman, N.K.; Kar Keng, L. Amperometric Non-Enzymatic Hydrogen Peroxide Sensor Based on Aligned Zinc Oxide Nanorods. Sensors 2016, 16, 1004. [Google Scholar] [CrossRef]
- Gu, F.; Di, M.; Han, D.; Hong, S.; Wang, Z. Atomically Dispersed Au on In2O3 Nanosheets for Highly Sensitive and Selective Detection of Formaldehyde. ACS Sensors 2020, 5, 2611–2619. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Zhang, L.; Su, H.; Liu, X.; Liu, J. A sensitive H2O2 biosensor based on carbon nanotubes/tetrathiafulvalene and its application in detecting NADH. Anal. Biochem. 2020, 589, 113493. [Google Scholar] [CrossRef]
- Patel, V.; Saha, D.; Kruse, P.; Selvaganapathy, P.R. Reagent-Free Hydrogen Peroxide Sensing Using Carbon Nanotube Chemiresistors with Electropolymerized Crystal Violet. ACS Appl. Nano Mater. 2022, 5, 3957–3966. [Google Scholar] [CrossRef]
- Alencar, L.M.; Silva, A.W.B.N.; Trindade, M.A.G.; Salvatierra, R.V.; Martins, C.A.; Souza, V.H.R. One-step synthesis of crumpled graphene fully decorated by copper-based nanoparticles: Application in H2O2 sensing. Sens. Actuators B Chem. 2022, 360, 131649. [Google Scholar] [CrossRef]
- Yeh, I.H.; Tadepalli, S.; Liu, K.K. Au@Ag nanostructures for the sensitive detection of hydrogen peroxide. Sci. Rep. 2022, 12, 19661. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, Z.; Yu, Y.; Shi, L. A novel non-enzymatic hydrogen peroxide sensor based on poly-melamine film modified with platinum nanoparticles. RSC Adv. 2014, 4, 45185–45190. [Google Scholar] [CrossRef]
- Sequeira, C.A.C. Silver Nanoparticles for Hydrogen Peroxide Sensors. Biomed. J. Sci. Technol. Res. 2021, 40, 32438–32443. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Zhang, T.; Ju, Z.; Ji, X.; Cui, Y.; Wang, L.; Xiao, H. Pd nanoparticles stabilized by bitter gourd polysaccharide with peroxidase properties for H2O2 detection. Int. J. Biol. Macromol. 2023, 233, 123513. [Google Scholar] [CrossRef]
- Roy, K.; Ghosh, C.K.; Sarkar, C.K. Rapid detection of hazardous H2O2 by biogenic copper nanoparticles synthesized using Eichhornia crassipes extract. Microsyst. Technol. 2019, 25, 1699–1703. [Google Scholar] [CrossRef]
- Tong, P.; Asif, M.; Ajmal, M.; Aziz, A.; Sun, Y. A Multicomponent Polymer-Metal-Enzyme System as Electrochemical Biosensor for H2O2 Detection. Front. Chem. 2022, 10, 874965. [Google Scholar] [CrossRef]
- Murugan, P.; Sundramoorthy, A.K.; Nagarajan, R.D.; Atchudan, R.; Shanmugam, R.; Ganapathy, D.; Arya, S.; Alothman, A.A.; Ouladsmane, M. Electrochemical Detection of H2O2 on Graphene Nanoribbons/Cobalt Oxide Nanorods-Modified Electrode. J. Nanomater. 2022, 2022, 9866111. [Google Scholar] [CrossRef]
- Trujillo, R.M.; Barraza, D.E.; Zamora, M.L.; Cattani-Scholz, A.; Madrid, R.E. Nanostructures in Hydrogen Peroxide Sensing. Sensors 2021, 21, 2204. [Google Scholar] [CrossRef]
- Kogularasu, S.; Govindasamy, M.; Chen, S.-M.; Akilarasan, M.; Mani, V. 3D graphene oxide-cobalt oxide polyhedrons for highly sensitive non-enzymatic electrochemical determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 253, 773–783. [Google Scholar] [CrossRef]
- Barkaoui, S.; Haddaoui, M.; Dhaouadi, H.; Raouafi, N.; Touati, F. Hydrothermal synthesis of urchin-like Co3O4 nanostructures and their electrochemical sensing performance of H2O2. J. Solid State Chem. 2015, 228, 226–231. [Google Scholar] [CrossRef]
- Kokina, I.; Plaksenkova, I.; Galek, R.; Jermal’onoka, M.; Kirilova, E.; Gerbreders, V.; Krasovska, M.; Sledevskis, E. Genotoxic Evaluation of Fe3O4 Nanoparticles in Different Three Barley (Hordeum vulgare L.) Genotypes to Explore the Stress-Resistant Molecules. Molecules 2021, 26, 6710. [Google Scholar] [CrossRef]
- Kokina, I.; Mickeviča, I.; Jahundoviča, I.; Ogurcovs, A.; Krasovska, M.; Jermaļonoka, M.; Mihailova, I.; Tamanis, E.; Gerbreders, V. Plant Explants Grown on Medium Supplemented with Fe3O4 Nanoparticles Have a Significant Increase in Embryogenesis. J. Nanomater. 2017, 2017, 4587147. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mizers, V.; Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Mihailova, I.; Ogurcovs, A.; Bulanovs, A.; Gerbreders, A. Non-Enzymatic Co3O4 Nanostructure-Based Electrochemical Sensor for H2O2 Detection. Latvian J. Phys. Technic. Sci. 2023, 60, 63–84. [Google Scholar] [CrossRef]
- Mihailova, I.; Krasovska, M.; Sledevskis, E.; Gerbreders, V.; Mizers, V.; Ogurcovs, A. Assessment of Oxidative Stress by Detection of H2O2 in Rye Samples Using a CuO- and Co3O4-Nanostructure-Based Electrochemical Sensor. Chemosensors 2023, 11, 532. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Elhag, S.; AlSalhi, M.S.; Nur, O.; Willander, M. Effect of Urea on the Morphology of Co3O4 Nanostructures and Their Application for Potentiometric Glucose Biosensor. Electroanalysis 2014, 26, 1773–1781. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Liu, J.; Guo, H.; Liu, C. Highly sensitive H2O2 sensor based on Co3O4 hollow sphere prepared via a template-free method. Electrochim. Acta 2015, 182, 613–620. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Huang, N.M. Morphology dependent electrocatalytic properties of hydrothermally synthesized cobalt oxide nanostructures. Ceramics Int. 2015, 41, 13210–13217. [Google Scholar] [CrossRef]
- Mihailova, I.; Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Mizers, V.; Bulanovs, A.; Ogurcovs, A. A non-enzymatic electrochemical hydrogen peroxide sensor based on copper oxide nanostructures. Beilstein J. Nanotechnol. 2022, 13, 424–436. [Google Scholar] [CrossRef]
- Ullah, R.; Rasheed, M.A.; Abbas, S.; Rehman, K.-U.; Shah, A.; Ullah, K.; Khan, Y.; Bibi, M.; Ahmad, M.; Ali, G. Electrochemical sensing of H2O2 using cobalt oxide modified TiO2 nanotubes. Curr. Appl. Phys. 2022, 38, 40–48. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Heli, H.; Pishahang, J. Cobalt oxide nanoparticles anchored to multiwalled carbon nanotubes: Synthesis and application for enhanced electrocatalytic reaction and highly sensitive nonenzymatic detection of hydrogen peroxide. Electrochim. Acta 2014, 123, 518–526. [Google Scholar] [CrossRef]
- Medhi, A.; Giri, M.K.; Mohanta, D. Non-enzymatic electrochemical detection of H2O2 using Ni(OH)2 nanoparticles. Mater. Today Proc. 2022, 68, 262–267. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, G.; Yi, H.; Jia, G.; Li, Z.; Yuan, C.; Bai, Y.; Fu, D. Interfacial Engineering of Hierarchical Transition Metal Oxide Heterostructures for Highly Sensitive Sensing of Hydrogen Peroxide. Small 2018, 14, 1703713. [Google Scholar] [CrossRef]
- Mai, L.N.T.; Bui, Q.B.; Bach, L.G.; Nhac-Vu, H.-T. A novel nanohybrid of cobalt oxide-sulfide nanosheets deposited three-dimensional foam as efficient sensor for hydrogen peroxide detection. J. Electroanal. Chem. 2020, 857, 113757. [Google Scholar] [CrossRef]
- Bohlooli, F.; Yamatogi, A.; Mori, S. Manganese oxides/carbon nanowall nanocomposite electrode as an efficient non-enzymatic electrochemical sensor for hydrogen peroxide. Sens. Biosen. Res. 2021, 31, 100392. [Google Scholar] [CrossRef]


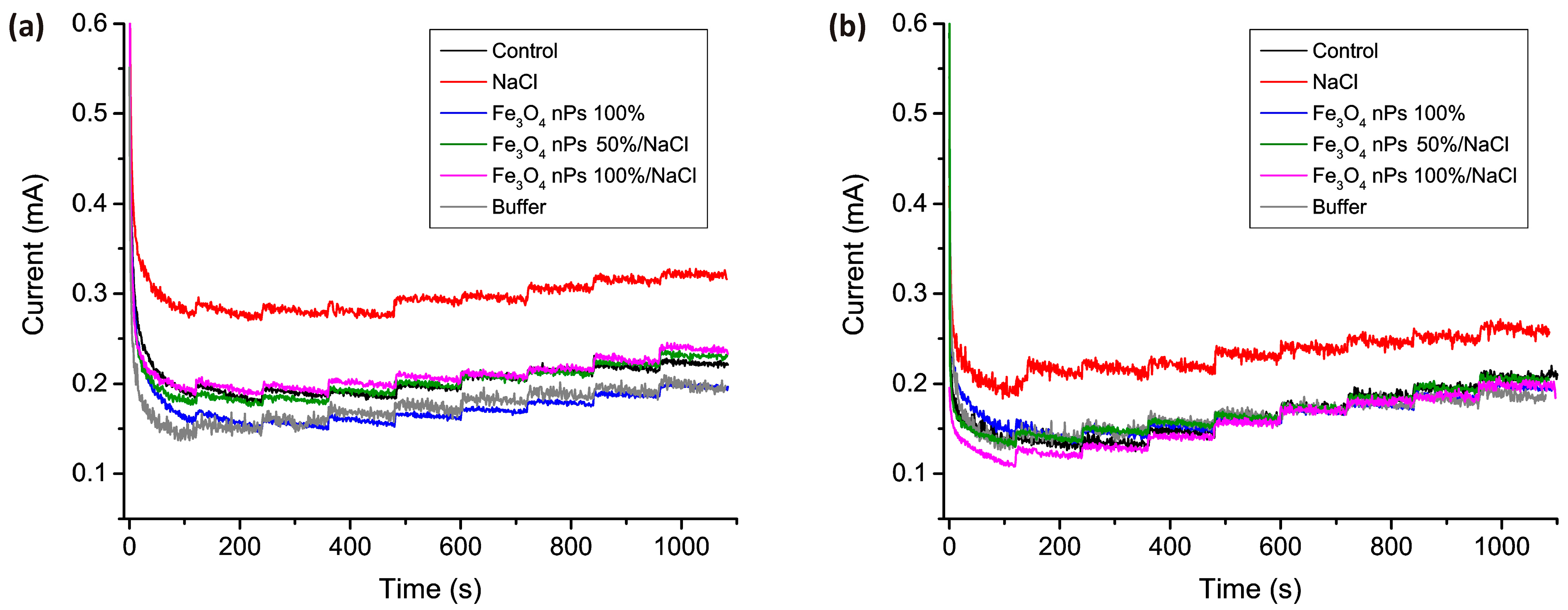
| Sample | First Leaf Length (cm), Average and Maximal | Total Length of Green Part (cm), Average and Maximal | Fresh Weight of 10 Plants (g) | Dry Weight of 10 Plants (g) | ||
|---|---|---|---|---|---|---|
| Control | 10.5 | 12.0 | 21.0 | 22.0 | 1.82 | 0.12 |
| NaCl | 9.0 | 10.5 | 22.0 | 26.0 | 1.77 | 0.14 |
| nPs 100% | 9.5 | 12.0 | 22.0 | 24.0 | 1.81 | 0.15 |
| nPs 50%/NaCl | 10.0 | 11.5 | 24.0 | 27.0 | 2.43 | 0.16 |
| nPs 100%/NaCl | 11.0 | 12.0 | 26.0 | 29.0 | 2.37 | 0.16 |
| 3 Weeks | A (λ = 645 nm) | A (λ = 663 nm) | A (λ = 480 nm) | Chl(α), mg/g FW | Chl(β), mg/g FW | Chl(α + β), mg/g FW | Carot., mg/g FW |
|---|---|---|---|---|---|---|---|
| Control | 0.34 | 0.74 | 0.48 | 0.3393 | 0.1729 | 0.5121 | 0.0139 |
| NaCl | 0.22 | 0.53 | 0.29 | 0.2456 | 0.1023 | 0.3478 | 0.0084 |
| nPs 100% | 0.33 | 0.72 | 0.45 | 0.3303 | 0.1675 | 0.4976 | 0.0129 |
| nPs 50%/NaCl | 0.39 | 0.94 | 0.55 | 0.4356 | 0.1813 | 0.6167 | 0.0163 |
| nPs 100%/NaCl | 0.47 | 1.1 | 0.64 | 0.5082 | 0.2246 | 0.7326 | 0.0186 |
| 4 weeks | A (λ = 645 nm) | A (λ = 663 nm) | A (λ -= 480 nm) | Chl(α), mg/g FW | Chl(β), mg/g FW | Chl(α + β), mg/g FW | Carot., mg/g FW |
| Control | 0.32 | 0.71 | 0.48 | 0.3262 | 0.1602 | 0.4863 | 0.0143 |
| NaCl | 0.25 | 0.59 | 0.36 | 0.2728 | 0.1186 | 0.3913 | 0.0107 |
| nPs 100% | 0.31 | 0.67 | 0.47 | 0.3070 | 0.1585 | 0.4654 | 0.0139 |
| nPs 50%/NaCl | 0.43 | 1.09 | 0.67 | 0.5075 | 0.1898 | 0.6971 | 0.0208 |
| nPs 100%/NaCl | 0.44 | 1.11 | 0.69 | 0.5165 | 0.1952 | 0.7116 | 0.0214 |
| Electrode | Sensitivity | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Co3O4/TiO2 NTs | 39.53 μA·mM−1·cm−2 | 1.27–26.80 mM | 6.71 μM | [83] |
| [Co(pbda)(4,4-bpy)(2H2O)]n/GCE | 83.10 μA·mM−1·cm−2 | 50–9000 μM | 3.76 μM | [84] |
| Co3O4 /MWCNTs/CPE | 729.7 μA·mM−1 | 20–430 μM | 2.46 μM | [85] |
| Ni(OH)2 nPs | 1660 μA·mM−1·cm−2 | 30–320 μM | 26.4 μM | [86] |
| CuO/CoO | 6349 μA·mM−1 | 2–4000 μM | 1.4 μM | [87] |
| CoO-CoS/NF | 590 μA·mM−1 | 2–954 μM | 0.890 μM | [88] |
| MnOx/CNW | 698.6 μA·mM−1· cm−2 | 40–10.230 μM | 0.55 μM | [89] |
| Co3O4 nPTLS | 201 µA·mM−1 | 25–5000 μM | 5.2 µM | This work |
| 3 Weeks | ||||||||
| Buffer | Control | NaCl | ||||||
| Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) |
| 25 | 25 | 0 | 25 | 30 | 5 | 25 | 221 | 196 |
| 50 | 50 | 0 | 50 | 25 | −25 | 50 | 237 | 187 |
| 75 | 75 | 0 | 75 | 60 | −15 | 75 | 250 | 175 |
| 100 | 100 | 0 | 100 | 102 | 2 | 100 | 320 | 220 |
| 125 | 125 | 0 | 125 | 129 | 4 | 125 | 348 | 223 |
| 150 | 150 | 0 | 150 | 160 | 10 | 150 | 379 | 229 |
| 175 | 175 | 0 | 175 | 200 | 25 | 175 | 424 | 249 |
| 200 | 200 | 0 | 200 | 230 | 30 | 200 | 454 | 254 |
| nPs 100% | nPs 50%/NaCl | nPs 100%/NaCl | ||||||
| Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) |
| 25 | 28 | 3 | 25 | 30 | 5 | 25 | 0 | −25 |
| 50 | 55 | 5 | 50 | 65 | 15 | 50 | 30 | −20 |
| 75 | 70 | −5 | 75 | 80 | 5 | 75 | 60 | −15 |
| 100 | 107 | 7 | 100 | 105 | 5 | 100 | 95 | −5 |
| 125 | 125 | 0 | 125 | 129 | 4 | 125 | 133 | 8 |
| 150 | 155 | 5 | 150 | 160 | 10 | 150 | 155 | 5 |
| 175 | 185 | 10 | 175 | 200 | 25 | 175 | 193 | 18 |
| 200 | 220 | 20 | 200 | 230 | 30 | 200 | 210 | 10 |
| 4 weeks | ||||||||
| Buffer | Control | NaCl | ||||||
| Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) |
| 25 | 25 | 0 | 25 | 82 | 57 | 25 | 561 | 536 |
| 50 | 50 | 0 | 50 | 103 | 53 | 50 | 578 | 528 |
| 75 | 75 | 0 | 75 | 93 | 28 | 75 | 590 | 515 |
| 100 | 100 | 0 | 100 | 151 | 51 | 100 | 630 | 530 |
| 125 | 125 | 0 | 125 | 169 | 44 | 125 | 643 | 518 |
| 150 | 150 | 0 | 150 | 213 | 63 | 150 | 692 | 542 |
| 175 | 175 | 0 | 175 | 241 | 66 | 175 | 756 | 581 |
| 200 | 200 | 0 | 200 | 261 | 61 | 200 | 777 | 577 |
| nPs 100% | nPs 50%/NaCl | nPs 100%/NaCl | ||||||
| Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) | Added (µM) | Found (µM) | Excess (µM) |
| 25 | 15 | −10 | 25 | 70 | 45 | 25 | 113 | 88 |
| 50 | 44 | −6 | 50 | 96 | 46 | 50 | 115 | 65 |
| 75 | 50 | −25 | 75 | 103 | 28 | 75 | 139 | 64 |
| 100 | 76 | −24 | 100 | 154 | 54 | 100 | 184 | 84 |
| 125 | 98 | −27 | 125 | 168 | 43 | 125 | 170 | 45 |
| 150 | 135 | −15 | 150 | 213 | 63 | 150 | 213 | 63 |
| 175 | 170 | −5 | 175 | 225 | 50 | 175 | 291 | 116 |
| 200 | 190 | −10 | 200 | 298 | 98 | 200 | 347 | 147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Mihailova, I.; Mizers, V. Co3O4 Nanostructured Sensor for Electrochemical Detection of H2O2 as a Stress Biomarker in Barley: Fe3O4 Nanoparticles-Mediated Enhancement of Salt Stress Tolerance. Micromachines 2024, 15, 311. https://doi.org/10.3390/mi15030311
Gerbreders V, Krasovska M, Sledevskis E, Mihailova I, Mizers V. Co3O4 Nanostructured Sensor for Electrochemical Detection of H2O2 as a Stress Biomarker in Barley: Fe3O4 Nanoparticles-Mediated Enhancement of Salt Stress Tolerance. Micromachines. 2024; 15(3):311. https://doi.org/10.3390/mi15030311
Chicago/Turabian StyleGerbreders, Vjaceslavs, Marina Krasovska, Eriks Sledevskis, Irena Mihailova, and Valdis Mizers. 2024. "Co3O4 Nanostructured Sensor for Electrochemical Detection of H2O2 as a Stress Biomarker in Barley: Fe3O4 Nanoparticles-Mediated Enhancement of Salt Stress Tolerance" Micromachines 15, no. 3: 311. https://doi.org/10.3390/mi15030311
APA StyleGerbreders, V., Krasovska, M., Sledevskis, E., Mihailova, I., & Mizers, V. (2024). Co3O4 Nanostructured Sensor for Electrochemical Detection of H2O2 as a Stress Biomarker in Barley: Fe3O4 Nanoparticles-Mediated Enhancement of Salt Stress Tolerance. Micromachines, 15(3), 311. https://doi.org/10.3390/mi15030311







